Abstract
Introduction:
Randomized clinical trials showed that bortezomib, in addition to conventional chemotherapy, improves survival and disease progression in multiple myeloma (MM) patients not eligible for stem cell transplantation. The aim of this retrospective population-based cohort study is the evaluation of both clinical and economic profile of bortezomib-based versus conventional chemotherapy in daily clinical practice.
Methods:
Healthcare utilization databases of six Italian regions were used to identify adult patients with non-transplant MM, who started a first-line therapy with bortezomib-based or conventional chemotherapy. Patients were matched by propensity score and were followed from treatment start until death, lost to follow-up or study end-point. Overall survival (OS) and restricted mean survival time (RMST) were estimated using the Kaplan–Meier method. Association between first-line treatment and risk of death was estimated by a conditional Cox proportional regression model. Average mean cumulative costs were estimated and compared between groups.
Results:
In the period 2010–2016, 3509 non-transplant MM patients met the inclusion criteria, of which 1157 treated with bortezomib-based therapy were matched to 1826 treated with conventional chemotherapy. Median OS and RMST were 33.9 and 27.9 months, and 42.9 and 38.4 months, respectively, in the two treatment arms. Overall, these values corresponded to a HR of death of 0.79 (95% CI 0.71–0.89) over a time horizon of 84 months. Average cumulative cost were 83,839 € and 54,499 €, respectively, corresponding to an incremental cost-effectiveness ratio of 54,333 € per year of life gained, a cost coherent with the willingness-to-pay thresholds frequently adopted from Western countries.
Conclusions:
These data suggested that, in a large cohort of non-transplant MM patients treated outside the experimental setting, first-line treatment with bortezomib-based therapy was associated with a favourable effectiveness and cost-effectiveness profile.
Keywords: multiple myeloma, bortezomib, effectiveness, cost-effectiveness, real-world
Introduction
Multiple myeloma (MM) accounts for 1% of all cancers and about 10% of all haematological malignancies.1 The incidence in Europe is 4.5–6.0/100,000 per year, with a median age at diagnosis ranging from 65 to 70 years.2 In Italy, MM accounts for about 5700 incident cases and 3200 deaths per year. Survival estimates at 1 and 5 years are ~80% and ~51%, respectively.3
Treatment of MM mainly depends on the patient’s age. In young patients (aged ⩽65 years) in good clinical condition (fit patients), induction followed by high-dose therapy with autologous stem cell transplantation is the standard treatment.4,5 In older patients (aged >65 years), oral combinations of melphalan and prednisone plus novel agents are considered as standards of care in Europe. Bortezomib (Velcade®, Jannsen-Cilag, Belgium) is the first proteasome inhibitor approved in 2003 by the US Food and Drug Administration (FDA) for the treatment of relapsed and/or refractory MM patients progressing after two prior therapies. In 2008 the FDA, and in mid-2009 the Italian Medicine Agency (Agenzia Italiana del Farmaco, AIFA), approved bortezomib also for the treatment of previously untreated MM patients ineligible for stem cell transplantation. This approval was granted after the pivotal phase III VISTA trial showed that 334 previously untreated MM patients not eligible for stem cell transplantation treated with bortezomib in addition to conventional chemotherapy were associated with a 39% reduction of death (p < 0.001), as compared with 338 controls, after 2 years of follow-up.6 Two updated follow-up of the VISTA trial, prolonged to 48 and 60 months, confirmed the main result, showing, respectively, 35% (p < 0.001)7 and 31% reduced risk of death (p < 0.001) in patients treated with bortezomib.8 Despite the efficacy of bortezomib in the prevention of disease recurrence and death that was impressively reported in randomized clinical trials (RCTs), no evidence about its added value is available in real-world populations of patients not eligible for stem cell transplantation. Therefore, the present study aims at evaluating the long-term overall survival (OS) and costs associated with initial treatment with bortezomib-based therapy, compared with conventional chemotherapy, in a wide population-based cohort of non-transplant MM patients in clinical practice. The study is a part of an Italian project funded by AIFA and the Health Department of the Sardinia Region, which supported the so-called FABIO programme (Biologic Drugs in Oncology, the Italian acronym being Farmaci Biologici in Oncologia). The FABIO project aimed at evaluating the profile of safety, effectiveness and cost-effectiveness of biologic drugs approved for treating cancer.
Methods
Setting
The Italian National Health Service (NHS) provides universal and free-of-charge healthcare services considered essential, including cancer medicaments. The service is administered within each of the 21 Italian regions by an automated system of healthcare utilization (HCU) databases that collect a variety of individual-patient-level information, for each of the beneficiaries of the NHS. The FABIO programme was conducted by retrieving HCU data from six Italian regions localized at Northern (Lombardy), Central (Lazio and Marche), Southern (Abruzzo) and Insular (Sardinia and Sicily) Italy. Overall, data covered more than 25 million beneficiaries of the Italian NHS, nearly 42% of the entire Italian population. Details of regional HCU databases of Italy and of the FABIO network have been previously reported.9 Specific diagnostic and therapeutic codes used for this study are reported in Supplementary material (Table S1).
Cohort selection, exposure definition and follow-up
All NHS beneficiaries with a diagnosis of MM were selected during a recruitment period that varied in a time-span comprised between 2010 and 2016, based on data availability of participating regions. The date of the first hospital admission for MM was defined as the ‘index date’. In order to select only incident cases, patients who received diagnosis of malignancy and/or underwent chemotherapy within 5 years before the index date were excluded. Patients younger than 18 years at index date and those who died during the index hospitalization were further excluded. Among the remaining patients, only those who did not undergo stem cell transplantation after the index date and who started drug therapy within 6 months after the index date were included in the study cohort.
The date of the first cancer drug dispensation after MM diagnosis was defined as ‘treatment start’. Cohort members were classified as exposed to first-line bortezomib-based therapy or to conventional chemotherapy, according whether during 42 days following the treatment start (i.e. the duration of a treatment cycle with bortezomib10) they did or did not receive at least a bortezomib dispensation, respectively.
Each cohort member accumulated person-years of follow-up from the date of treatment start until death (i.e. the outcome of interest), lost to follow-up (i.e. emigration), or study end-point, whichever came first. Study end-point was the last date with data available within each region (i.e. in a time-span comprised between 31 December 2016 and 30 June 2018).
Baseline characteristics
Baseline covariates included age, gender and year of MM diagnosis. In addition, a previous history of diabetes, bone, renal, circulatory, pulmonary, and stomach disease, mental disorders, as well as a previous history of treatment with bisphosphonates, was assessed for all cohort patients in the 3 years preceding the index date.
Matching cohort arms
To reduce the between treatments heterogeneity, a propensity score (PS) matched analysis was performed.11 A multivariable logistic regression was used to model the probability of being treated with bortezomib (i.e. the PS), given a set of covariates. The latter were those above-listed as baseline characteristics, in addition to the number of hospitalizations, outpatient services and drug prescriptions in the year before the index date. Each patient belonging to the bortezomib arm (index case) was matched with up to two patients randomly selected from those on standard arm with the same PS value of the corresponding index case, with a tolerated difference of ±0.01.
Statistical analyses
Between-arm differences in baseline characteristics were tested by the chi-square statistics. Overall survival was estimated by using the Kaplan–Meier (KM) estimator. To increase the precision of the estimates, between-region summarized KM curves were estimated. As regional data were not available to be analysed in a pooled analysis, a method for reconstructing individual patients’ data starting from each regional KM curve was applied. Briefly, digital software was used to read the coordinates of KM curves within each region. Information on the number of patients still alive at each year of follow-up and the total number of deaths was used to solve the inverted KM equation, which allowed reconstruction of regional data for each arm, so obtaining pooled individual patients’ data.9,12 Median survivals and restricted mean survival times (RMST) were reported as descriptive measures of survival in the two treatment arms. RMST, that is the area under the KM curve, represents the average survival time experienced by cohort members.13,14 The association between exposure to bortezomib and risk of death was estimated by means of a conditional Cox proportional hazard model. Estimates were expressed as Hazard Ratio (HR), along with 95% Confidence Intervals (CI). As regional data were not available to be analysed in a pooled analysis, the so-called two-stage meta-analysis was performed to increase the precision of the estimates.15 Briefly, the proportional hazard model was first fitted separately within each region, and then between-region summarized HR was estimated by means of a fixed-effect model, using the inverse variance weighting.16 Between-regions heterogeneity was evaluated by using the chi-square statistics17 and was measured through the I2 index,18 which measures the percentage of variation across the regions due to heterogeneity.
Finally, limited to data from the Lombardy Region (i.e. the largest Region among those included in the FABIO programme) cumulative healthcare cost (CHC) in both treatment arms were calculated by means of the Bang and Tsiatis estimator,19 a method that takes into account censored cost data. For each patient, CHC were calculated by summing up direct costs sustained by the NHS for both inpatient and outpatient services, and drug dispensations supplied during follow-up. The incremental cost-effectiveness ratio (ICER) was measured by dividing the differences in healthcare costs (CHC) and health-related outcomes (measured by the RMST) between the two treatment arms (bortezomib arm and conventional chemotherapy). The ICER represents the healthcare expenditure expected to be saved (or added, depending on the sign) for gaining 1 year of life due to starting therapy with bortezomib. Non-parametric bootstrap method based on 1000 re-samples20 was used to explore the uncertainty in the cost-effectiveness estimates.21
Sensitivity analyses
Two sensitivity analyses were performed in order to assess the robustness of the main results. These analyses were only performed on data from Lombardy Region. First, in the study cohort were included all patients who started cancer treatment after the index date, not limiting the inclusion only to patients treated within 6 months from index date. Second, because of the arbitrariness in the choice of the time-window used for defining first-line therapy (42 days), a shorter time-window of 21 days was considered for classifying patients into first-line bortezomib of conventional chemotherapy arm.
All analyses were performed using the Statistical Analysis System Software (version 9.4; SAS Institute, Cary, NC, USA). Statistical significance was set at the 0.05 level. All p-values were two-sided.
The study protocol was approved by the Ethical Committee of the University of Milano-Bicocca (number ‘Prot. 506_2016’), which established the study (i) to be exempt from informed consent (according to General Authorization for the Processing of Personal Data for Scientific Research Purposes Issued by the Italian Privacy Authority on August 10, 2018; https://www.gpdp.it/web/guest/home/docweb/-/docweb-display/docweb/9124510), (ii) provides sufficient guarantees of anonymizing individual records, and (iii) was designed according to quality standards of good practice of observational research based on secondary data.
Results
Study cohort
Out of 15,643 MM patients identified during the recruitment period from the six regions participating in the study, 7575 were adult incident cases, surviving after the index hospitalization. Among the 6187 (81.7%) patients who did not undergo stem cell transplantation, 3509 were treated within 6 months after diagnosis with either bortezomib-based therapy (n = 1325, 37.8%) or conventional chemotherapy (n = 2184, 62.2%). Finally, 1157 patients treated with bortezomib-based therapy were matched to 1826 patients treated with conventional chemotherapy. The process of selection of the study cohort is reported in Figure 1 (region-specific data are reported in Supplementary material, Figure S1).
Figure 1.
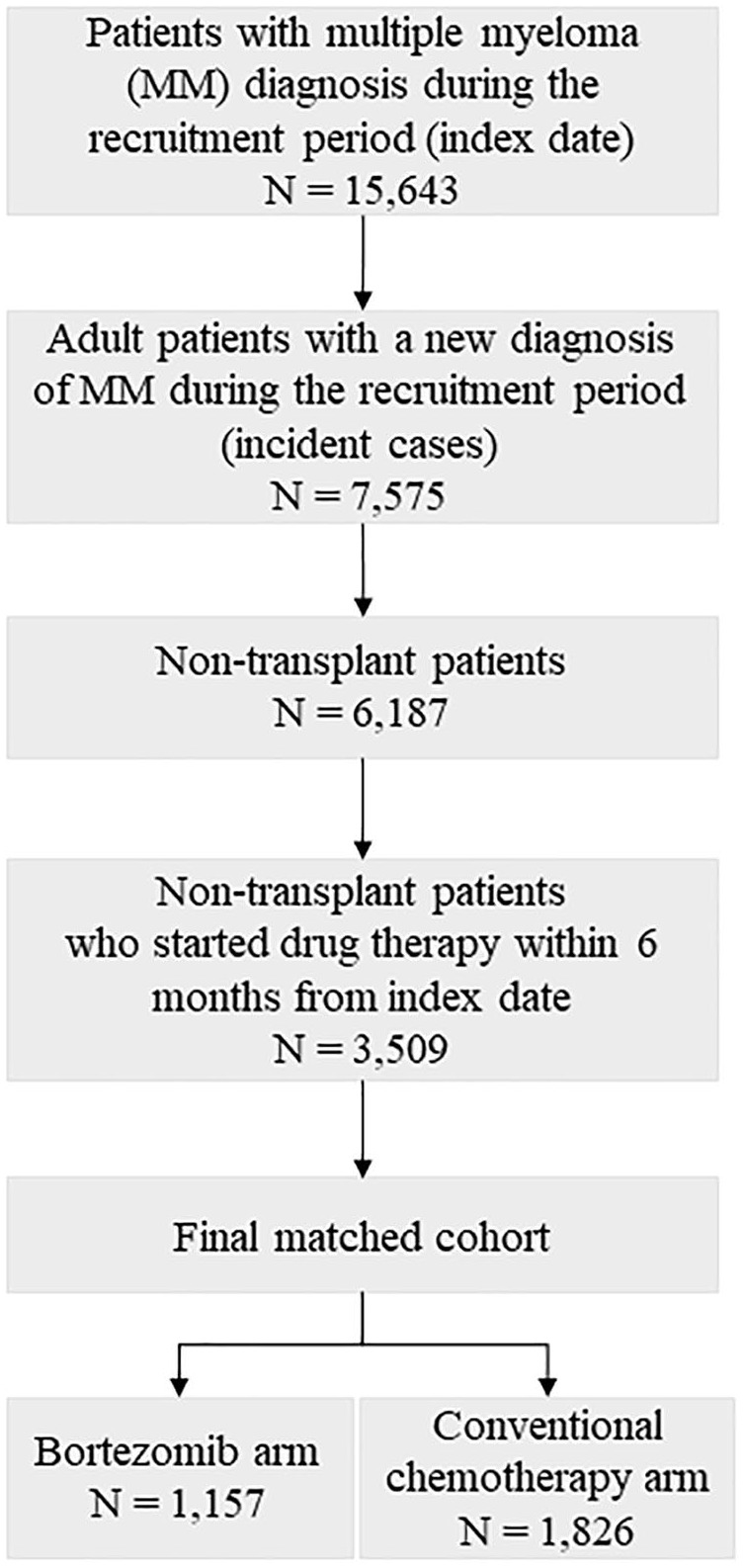
Flow-chart of inclusion and exclusion criteria. FABIO project, Italy, 2010–2016.
In the unmatched cohort, patients treated with bortezomib-based therapy were younger (p < 0.001) and had a lower prevalence of bone (p = 0.047), circulatory (p < 0.001) and pulmonary disease (p < 0.001), and there was a lower prevalence of users of bisphosphonates (p = 0.006). On the contrary, they had a higher prevalence of renal disorders (p = 0.012). However, after matching, no between-arm differences were observed. Baseline characteristics of both unmatched and matched cohorts are reported in Table 1.
Table 1.
Comparison between baseline characteristics of multiple myeloma patients belonging to the unmatched and propensity score (PS)-matched cohorts on first-line treatment with bortezomib-based or conventional chemotherapy alone. FABIO project, Italy, 2010–2016.
| Unmatched cohort members |
PS-matched cohort members |
|||
|---|---|---|---|---|
| Bortezomib-based therapy (n = 1325) | Conventional chemotherapy (n = 2184) | Bortezomib-based therapy (n = 1157) | Conventional chemotherapy (n = 1826) | |
| Gender | ||||
| Men | 678 (51.2) | 1079 (49.4) | 581 (50.2) | 935 (51.2) |
| Women | 647 (48.8) | 1105 (50.6) | 576 (49.8) | 891 (48.8) |
| p-value† | 0.311 | 0.599 | ||
| Age at diagnosis (years) | ||||
| <60 | 99 (7.5) | 169 (7.7) | 59 (5.1) | 103 (5.6) |
| 60–69 | 328 (24.8) | 375 (17.2) | 273 (23.6) | 489 (26.8) |
| 70–79 | 716 (54.0) | 902 (41.3) | 661 (57.1) | 998 (54.7) |
| ⩾80 | 182 (13.7) | 738 (33.8) | 164 (14.2) | 236 (12.9) |
| p-value† | <0.001 | 0.187 | ||
| Bone diseases | 236 (17.8) | 449 (20.6) | 216 (18.7) | 313 (17.1) |
| p-value† | 0.046 | 0.287 | ||
| Renal diseases | 365 (27.5) | 519 (23.8) | 283 (24.5) | 412 (22.6) |
| p-value† | 0.012 | 0.232 | ||
| Circulatory diseases | 481 (36.3) | 938 (43.0) | 418 (36.1) | 613 (33.6) |
| p-value† | <0.001 | 0.152 | ||
| Pulmonary diseases | 50 (3.8) | 195 (8.9) | 46 (4.0) | 85 (4.7) |
| p-value† | <0.001 | 0.378 | ||
| Diabetes | 239 (18.0) | 408 (18.7) | 208 (18.0) | 379 (20.8) |
| p-value† | 0.634 | 0.063 | ||
| Mental disorders | 35 (2.6) | 76 (3.5) | 30 (2.6) | 33 (1.8) |
| p-value† | 0.169 | 0.146 | ||
| Stomach | 64 (4.8) | 112 (5.1) | 208 (18.0) | 379 (20.8) |
| p-value† | 0.659 | 0.063 | ||
| Use of bisphosphonates | 119 (9.0) | 261 (12.0) | 109 (9.4) | 186 (10.2) |
| p-value† | 0.006 | 0.495 | ||
Chi-square test.
Overall survival
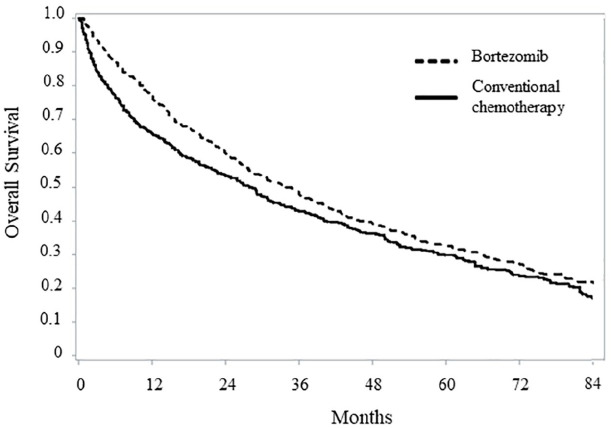
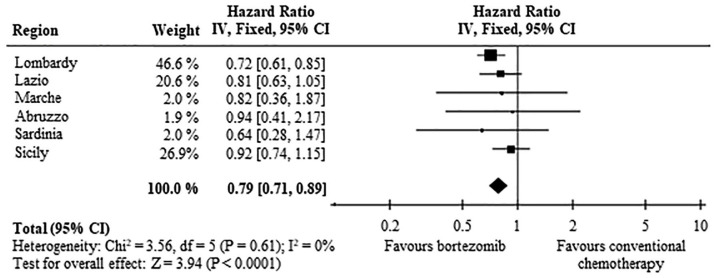
After a mean follow-up of 21.4 months, 780 (67.4%) and 1222 (66.9%) deaths were observed among patients on bortezomib-based and conventional chemotherapy, respectively. The pattern of OS among the two treatment arms is shown in Figure 2. Survival estimates at 12, 36 and 60 months from the date of treatment start were 76.8%, 47.7% and 32.9%, among patients on bortezomib therapy, compared with 66.0%, 42.8% and 29.8% among those on conventional chemotherapy. Median OS was 33.9 and 27.9 months, respectively (p < 0.001), and RMST was 42.9 and 38.4 months, respectively (p = 0.001). Overall, these data correspond to a HR of death of 0.79 (95% CI 0.71–0.89) (Figure 3). Region-specific survival curves are reported in Supplementary Material, Figure S2, confirming, overall, the national data.
Figure 2.
Comparison between Kaplan–Meier overall survival curves of non-transplant multiple myeloma patients on first-line treatment with bortezomib-based or conventional chemotherapy. FABIO project, Italy, 2010–2016.
Cohort members selected with a propensity score matched design were included in this analysis.
Figure 3.
Forest plot of the summarized associations between first-line treatment with bortezomib, compared with conventional chemotherapy, and risk of death. Estimates are shown for each participant region, and by summarizing region-specific hazard ratios.
Healthcare costs
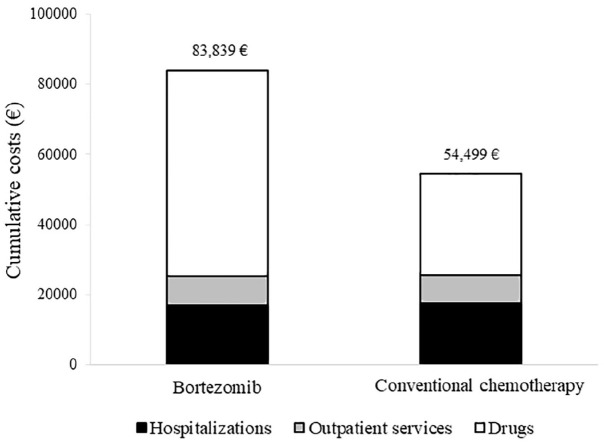
Cumulative NHS healthcare costs according to therapeutic strategy are shown in Figure 4. Overall, 82,808 € and 54,154 € were spent, on average, for each patient belonging to bortezomib and standard arms, respectively, over a time horizon of 84 months after starting therapy. The average cost of a patient on treatment with bortezomib therapy included 17,140 € for hospitalization, 8127 € for outpatients services and 58,572 € for drugs. Corresponding figures for a patient on treatment with standard chemotherapy were 17,479 €, 8104 € and 28,916 €, respectively. The cost-effectiveness profile is shown in Figure 5. The ICER value indicated an average cost of 54,333 € for each year of life gained by the treatment with bortezomib. The ICER value was suggestive of clinical effectiveness (longer survival for patients on bortezomib), constrained to higher healthcare costs, in all of the 1000 bootstrap replications.
Figure 4.
Comparison between cumulative per capita healthcare costs sustained by the NHS for caring non-transplant multiple myeloma patients on first-line treatment with bortezomib or conventional chemotherapy. FABIO project, Italy, 2010–2016.
Cohort members selected with a propensity score matched design were included in this analysis. The Bang and Tsiatis estimator was used for estimating cumulative costs (see text).
Figure 5.
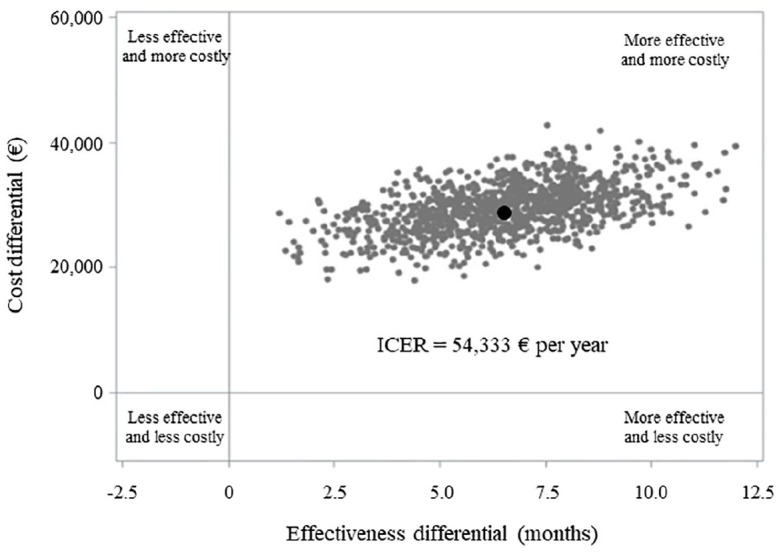
ICER scatterplot comparing non-transplant multiple myeloma patients on first-line treatment with bortezomib or conventional chemotherapy. FABIO project, Lombardy Region, 2010–2015.
Costs were calculated from the amount that the Regional Health Authority reimbursed to health providers. The average survival time was calculated by means of the restricted mean survival time.
The incremental cost-effectiveness ratio (ICER) was measured by dividing the differences in healthcare costs and health-related outcomes between the two treatment arms (i.e. bortezomib and conventional chemotherapy). Non-parametric bootstrap method based on 1000 re-samples was used to explore the uncertainty in the estimates of cost-effectiveness. The black circle represents the ICER observed in our cohort.
Sensitivity analyses
In the main analysis on Lombardy data, 603 patients treated with bortezomib were 1:1 matched to patients treated with conventional chemotherapy, and the HR of death was 0.72 (0.61–0.85). These results did not change substantially after performing sensitivity analyses. When all patients treated after diagnosis were included in the study cohort (without restricting the entry cohort to those who started therapy within 6 months from diagnosis), 701 matched pairs generated a HR of death equal to 0.70 (0.59–0.82). Further, when a period of 21 days was considered for defining the first-line treatment with either bortezomib or conventional chemotherapy (instead of 42 days), 524 matched pairs gave a HR of death equal to 0.76 (0.63–0.91).
Discussion
The present study assessed the long-term survival of non-transplant MM patients, in a large population-based study involving six different geographic areas in Italy. In this setting, patients treated with first-line bortezomib were associated with a HR of death of 0.79 (95% CI 0.71–0.89), as compared with those treated with conventional chemotherapy. These results are coherent with those observed in the pivotal VISTA trial.6 However, as patients included in our study were older (the median age was 74 versus 71 years) and had more concomitant conditions than those included in the trial, a lower OS was observed in our cohort. To the best of our knowledge, the current study represents the only evidence available in the scientific literature about the effectiveness of bortezomib, outside the experimental setting. Only a prospective observational post-marketing study carried out in Germany showed that bortezomib-containing regimens, as compared with standard care, increased overall response rates from 50.0% to 65.9%. However, given the small sample size (353 patients treated with bortezomib-containing regimens and 37 patients treated with conventional chemotherapy), the authors could not perform any statistical test to compare the observed differences.22 Thus, the evidence on the favourable effect of bortezomib are limited to experimental designs. Two recent network meta-analyses of RCTs showed that bortezomib-containing regimens were superior to conventional chemotherapy in improving OS, indicating a large convergence of the results coming from RCTs.23,24 Despite this, a prospective clinical cohort study involving Cancer Registries in Germany classified 285 non-transplant MM patients in trial-eligible and trial-ineligible patients, showing that about one-third of the cohort had baseline characteristics that would have precluded their inclusion in clinical trials. As expected, survival was considerably shorter for trial-ineligible patients.25 An evaluation of the clinical impact of bortezomib in the real-world clinical practice was, therefore, essential in this context. Other than the clinical impact, the current study also aimed at evaluating the economic impact of bortezomib on the NHS. A systematic review of published cost-effectiveness analysis showed that bortezomib-based therapy was cost-effective for previously untreated stem cell transplantation-ineligible patients, as compared with conventional chemotherapy, in the USA, Canada, the UK and Sweden.26 In particular, the USA study found an incremental cost per life-year gained of 86,213 $.27 In our cohort, the mean cost for each life-year gained with bortezomib treatment was 54,333 €, a cost coherent with the willingness-to-pay thresholds ranging from €50 thousands to $100 thousands per year of life gained frequently adopted in Western countries.28,29
Our study has several strengths. First, it represents the first study carried out in a real-world setting that evaluated the effectiveness and the cost-effectiveness profile of bortezomib-based therapy, as compared with conventional chemotherapy. Second, the study cohort included patients resident and diagnosed in several geographical areas of Italy, including Northern, Central, Southern and Insular regions, guaranteeing the representativeness of routine clinical practice in Italy and the generalizability of the results, and reflecting the potential heterogeneity in the management of MM. Indeed, the study cohort included all the potential NHS beneficiaries who had a new diagnosis of MM during the recruitment period, with no restriction on age and comorbidities, and the target population from which the study cohort was selected represents almost 42% of the Italian population. Finally, the extended follow-up allowed a long-term evaluation of survival and costs associated with MM patients.
On the other hand, the main weakness of this study is the paucity of data on individual traits, clinical features and drug patterns and regimens, which may result in systematic bias due to confounding. Indeed, as in all observational studies, treatment arms may be unbalanced for some characteristics also associated with the baseline risk of death. Factors such as ethnicity or socioeconomic status can be confidently excluded because Italy population is largely Caucasian and free-of-pay access to cancer care is ensured for all NHS beneficiaries. In addition, in order to better take into account measurable confounding, a PS matching design was used. Other unmeasured factors, however, might affect our conclusions, including clinical features (i.e. performance status or the stage of the disease) and therapeutic regimens (i.e. doses and combination therapies), which were not available in administrative databases.
In conclusion, the current study supports the available evidence about a favourable effect on OS of first-line bortezomib-based therapy, as compared with conventional chemotherapy, in a large real-world cohort of non-transplant MM patients. In addition, the results also confirm the cost-effective profile of bortezomib. However, the recent approval of new therapeutic regimens that combine bortezomib with melphalan and prednisone (VMP) or with lenalidomide and dexamethasone (VRd) require future real-world studies evaluating the value of bortezomib in these new therapeutic strategies.
Supplemental Material
Supplemental material, sj-pdf-1-tah-10.1177_2040620721996488 for Bortezomib-based therapy in non-transplant multiple myeloma patients: a retrospective cohort study from the FABIO project by Matteo Franchi, Claudia Vener, Donatella Garau, Ursula Kirchmayer, Mirko Di Martino, Marilena Romero, Ilenia De Carlo, Salvatore Scondotto, Chiara Stival, Matteo Giovanni Della Porta, Francesco Passamonti and Giovanni Corrao in Therapeutic Advances in Hematology
Acknowledgments
The ‘FArmaci BIologici in Oncologia’ (FABIO) working group includes the following: Giovanni Corrao, Matteo Franchi and Ivan Merlo (Lab Healthcare Research & Pharmacoepidemiology, Department of Statistic and Quantitative Methods, University of Milano-Bicocca, Milan, Italy), Luca Merlino (Epidemiologic Observatory, Regional Welfare Service, Lombardy Region, Milan, Italy), Annalisa Trama, Pamella Minicozzi and Giovanni Apolone (Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy); Luigi Tarantini (Department of Cardiology, Azienda Ospedale San Martino, ASL n. 1, Belluno, Italy); Donatella Garau (General Directorate for Health, Sardinia Region, Italy); Ursula Kirchmayer, Mirko Di Martino and Adele Lallo (Department of Epidemiology ASL Roma 1, Lazio Regional Health Service, Rome, Italy); Marilena Romero (Department of Medical, Oral and Biotechnological Sciences – Section of Pharmacology and Toxicology, University of Chieti, Italy), Antonio D’Ettorre, Antonia Petrucci (Regional Health Authority, Abruzzo Region); Ilenia De Carlo, Luigi Patregnani and Christian Bogino (Regional Health Authority, Marche Region); Salvatore Scondotto and Walter Pollina Addario (Department of Health Services and Epidemiological Observatory, Regional Health Authority, Palermo, Sicily Region, Italy), Gianluca Trifirò (Department of Biomedical and Dental Sciences and Morphofunctional Imaging, University of Messina, Messina, Italy); Anna Rosa Marra (Italian Medicine Agency (Agenzia Italiana del Farmaco, AIFA), Rome, Italy). Matteo Giovanni Della Porta (Humanitas Clinical and Research Hospital – IRCCS, Milan, Italy); Francesco Passamonti (University of Insubria and ASST Sette Laghi, Ospedale di Circolo of Varese, Varese, Italy).
Footnotes
Conflict of interest statement: Giovanni Corrao received research support from the European Community (EC), the Italian Agency of Drugs (AIFA), and the Italian Ministry for University and Research (MIUR). He took part in a variety of projects that were funded by pharmaceutical companies (i.e. Novartis, GSK, Roche, AMGEN and BMS). He also received honoraria as a member of the advisory board to Roche. The other authors declare that they have no conflicts of interest to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a research grant from the AIFA – the Italian Medicines Agency – (project AIFA – Fondi Farmacovigilanza Attiva; CUP code H56J16000500005) and from the Sardinia Region (CUP code H56D15000030005). Data analyses were performed at the Laboratory of Healthcare Research & Pharmacoepidemiology, University of Milano-Bicocca with grants from the Italian Ministry of Education, University and Research (‘Fondo d’Ateneo per la Ricerca’ portion, year 2018).
ORCID iD: Matteo Franchi  https://orcid.org/0000-0001-9620-8057
https://orcid.org/0000-0001-9620-8057
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Matteo Franchi, Laboratory of Healthcare Research & Pharmacoepidemiology, Department of Statistics and Quantitative Methods, University of Milano-Bicocca, Building U7, Via Bicocca degli Arcimboldi 8, Milan, 20126, Italy; National Centre for Healthcare Research and Pharmacoepidemiology, Milan, Italy.
Claudia Vener, Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy.
Donatella Garau, General Directorate for Health, Sardinia Region, Italy.
Ursula Kirchmayer, Department of Epidemiology ASL Roma 1, Lazio Regional Health Service, Rome, Lazio, Italy.
Mirko Di Martino, Department of Epidemiology ASL Roma 1, Lazio Regional Health Service, Rome, Lazio, Italy.
Marilena Romero, Department of Medical, Oral and Biotechnological Sciences – Section of Pharmacology and Toxicology, University of Chieti, Italy.
Ilenia De Carlo, Regional Centre of Pharmacovigilance, Regional Health Authority, Marche Region, Ancona, Italy.
Salvatore Scondotto, Department of Health Services and Epidemiological Observatory, Regional Health Authority, Palermo, Sicily Region, Palermo, Italy.
Chiara Stival, National Centre for Healthcare Research and Pharmacoepidemiology, Milan, ItalyLaboratory of Healthcare Research & Pharmacoepidemiology, Department of Statistics and Quantitative Methods, University of Milano-Bicocca, Milan, Lombardia, Italy.
Matteo Giovanni Della Porta, Humanitas Clinical and Research Hospital – IRCCS and Department of Biomedical Sciences, Humanitas University, Rozzano, Italy.
Francesco Passamonti, Department of Medicine and Surgery, University of Insubria and ASST Sette Laghi, Ospedale di Circolo of Varese, Varese, Lombardia, Italy.
Giovanni Corrao, National Centre for Healthcare Research and Pharmacoepidemiology, Milan, Italy; Laboratory of Healthcare Research & Pharmacoepidemiology, Department of Statistics and Quantitative Methods, University of Milano-Bicocca, Milan, Lombardia, Italy.
References
- 1. Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol 2016; 91: 719–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 2009; 23: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. AIRTUM/AIOM. I numeri del cancro in Italia 2019, https://www.registri-tumori.it/cms/pubblicazioni/i-numeri-del-cancro-italia-2019 (accessed 17 November 2020).
- 4. Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med 1996; 335: 91–97. [DOI] [PubMed] [Google Scholar]
- 5. Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 2003; 348: 1875–1883. [DOI] [PubMed] [Google Scholar]
- 6. San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med 2008; 359: 906–917. [DOI] [PubMed] [Google Scholar]
- 7. Mateos MV, Richardson PG, Schlag R, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol 2010; 28: 2259–2266. [DOI] [PubMed] [Google Scholar]
- 8. San Miguel JF, Schlag R, Khuageva NK, et al. Persistent overall survival benefit and no increased risk of second malignancies with bortezomib-melphalan-prednisone versus melphalan-prednisone in patients with previously untreated multiple myeloma. J Clin Oncol 2013; 31: 448–455. [DOI] [PubMed] [Google Scholar]
- 9. Franchi M, Garau D, Kirchmayer U, et al. Effectiveness and costs associated to adding cetuximab or bevacizumab to chemotherapy as initial treatment in metastatic colorectal cancer: results from the observational FABIO project. Cancers (Basel) 2020; 12: 839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. European Medicine Agency. Bortezomib – Velcade, https://www.ema.europa.eu/en/medicines/human/EPAR/velcade (accessed 17 November 2020).
- 11. Haukoos JS, Lewis RJ. The propensity score. JAMA 2015; 314: 1637–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012; 12: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim DH, Uno H, Wei LJ. Restricted mean survival time as a measure to interpret clinical trial results. JAMA Cardiol 2017; 2: 1179–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao L, Claggett B, Tian L, et al. On the restricted mean survival time curve in survival analysis. Biometrics 2016; 72: 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scotti L, Rea F, Corrao G. One-stage and two-stage meta-analysis of individual participant data led to consistent summarized evidence: lessons learned from combining multiple databases. J Clin Epidemiol 2018; 95: 19–27. [DOI] [PubMed] [Google Scholar]
- 16. Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987; 9: 1–30. [DOI] [PubMed] [Google Scholar]
- 17. Cochran WG. The combination of estimates from different experiments. Biometrics 1954; 10: 101–129. [Google Scholar]
- 18. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bang H, Tsiatis AA. Median regression with censored cost data. Biometrics 2002; 58: 643–649. [DOI] [PubMed] [Google Scholar]
- 20. Wang H, Zhao H. A study on confidence intervals for incremental cost-effectiveness ratios. Biom J 2008; 50: 505–514. [DOI] [PubMed] [Google Scholar]
- 21. Canivet C, Costa N, Ory-Magne F, et al. Clinical impact and cost-effectiveness of an education program for PD patients: a randomized controlled trial. PLoS One 2016; 11: e0162646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Knauf W, Tapprich C, Schlag R, et al. Bortezomib-containing regimens are effective in multiple myeloma–results of a non-interventional phase IV study. Oncol Res Treat 2015; 38: 167–173. [DOI] [PubMed] [Google Scholar]
- 23. Sekine L, Ziegelmann PK, Manica D, et al. Upfront treatment for newly diagnosed transplant-ineligible multiple myeloma patients: a systematic review and network meta-analysis of 14,533 patients over 29 randomized clinical trials. Crit Rev Oncol Hematol 2019; 143: 102–116. [DOI] [PubMed] [Google Scholar]
- 24. Blommestein HM, van Beurden-Tan CHY, Franken MG, et al. Efficacy of first-line treatments for multiple myeloma patients not eligible for stem cell transplantation: a network meta-analysis. Haematologica 2019; 104: 1026–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Knauf W, Aldaoud A, Hutzschenreuter U, et al. Survival of non-transplant patients with multiple myeloma in routine care differs from that in clinical trials-data from the prospective German tumour registry lymphatic neoplasms. Ann Hematol 2018; 97: 2437–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen W, Yang Y, Chen Y, et al. Cost-effectiveness of bortezomib for multiple myeloma: a systematic review. Clinicoecon Outcomes Res 2016; 8: 137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oster G, Berger A, Bornheimer R, et al. Cost-effectiveness of lenalidomide and bortezomib in patients with previously untreated multiple myeloma (MM). Blood 2013; 122: 5604. [Google Scholar]
- 28. Di Tanna GL, Bychenkova A, O’Neill F, et al. Evaluating cost-effectiveness models for pharmacologic interventions in adults with heart failure: a systematic literature review. Pharmacoeconomics 2019; 37: 359–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eichler HG, Kong SX, Gerth WC, et al. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health 2004; 7: 518–528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tah-10.1177_2040620721996488 for Bortezomib-based therapy in non-transplant multiple myeloma patients: a retrospective cohort study from the FABIO project by Matteo Franchi, Claudia Vener, Donatella Garau, Ursula Kirchmayer, Mirko Di Martino, Marilena Romero, Ilenia De Carlo, Salvatore Scondotto, Chiara Stival, Matteo Giovanni Della Porta, Francesco Passamonti and Giovanni Corrao in Therapeutic Advances in Hematology