Key Points
Question
Are opioid use disorder (OUD) medications associated with alcohol-related poisonings, falls, and injuries in persons with OUD and co-occurring alcohol use?
Findings
This case-control cohort study of 13 335 individuals with OUD from a large, nationally representative data set found that patients prescribed OUD medication had significant reductions in alcohol-related acute events.
Meaning
The use of OUD medication in opioid-dependent patients with co-occurring alcohol use may hold promise in reducing alcohol-related acute events, but more investigation is needed.
This case-control cohort study examines whether the use of medications to treat opioid use disorder is associated with decreased risk for alcohol-related falls or injuries and poisonings in persons with opioid use disorder with and without co-occurring alcohol use disorder.
Abstract
Importance
Persons with opioid use disorder (OUD) and co-occurring alcohol use disorder (AUD) are understudied and undertreated. It is unknown whether the use of medications to treat OUD is associated with reduced risk of alcohol-related morbidity.
Objective
To determine whether the use of OUD medications is associated with decreased risk for alcohol-related falls, injuries, and poisonings in persons with OUD with and without co-occurring AUD.
Design, Setting, and Participants
This recurrent-event, case-control, cohort study used prescription claims from IBM MarketScan insurance databases from January 1, 2006, to December 31, 2016. The sample included persons aged 12 to 64 years in the US with an OUD diagnosis and taking OUD medication who had at least 1 alcohol-related admission. The unit of observation was person-day. Data analysis was performed from June 26 through September 28, 2020.
Exposures
Days of active OUD medication prescriptions, with either agonist (ie, buprenorphine or methadone) or antagonist (ie, oral or extended-release naltrexone) treatments compared with days without OUD prescriptions.
Main Outcomes and Measures
The primary outcome was admission for any acute alcohol-related event defined by International Classification of Diseases, Ninth Revision and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes. Conditional logistic regression was used to compare OUD medication use between days with and without an alcohol-related event. Stratified analyses were conducted between patients with OUD with and without a recent AUD diagnostic code.
Results
There were 8 424 214 person-days of observation time among 13 335 participants who received OUD medications and experienced an alcohol-related admission (mean [SD] age, 33.1 [13.1] years; 5884 female participants [44.1%]). Agonist treatments (buprenorphine and methadone) were associated with reductions in the odds of any alcohol-related acute event compared with nontreatment days, with a 43% reduction for buprenorphine (odds ratio [OR], 0.57; 95% CI, 0.52-0.61) and a 66% reduction for methadone (OR, 0.34; 95% CI, 0.26-0.45). The antagonist treatment naltrexone was associated with reductions in alcohol-related acute events compared with nonmedication days, with a 37% reduction for extended-release naltrexone (OR, 0.63; 95% CI, 0.52-0.76) and a 16% reduction for oral naltrexone (OR, 0.84; 95% CI, 0.76-0.93). Naltrexone use was more prevalent among patients with OUD with recent AUD claims than their peers without AUD claims.
Conclusions and Relevance
These findings suggest that OUD medication is associated with fewer admissions for alcohol-related acute events in patients with OUD with co-occurring AUD.
Introduction
Over the last decade, an unprecedented epidemic of drug-related poisonings has affected countries spanning North America and Europe. Recent estimates of unintentional poisoning deaths (19.1 deaths per 100 000) have eclipsed those of falls (11.4 deaths per 100 000) and motor vehicle traffic deaths (11.6 deaths per 100 000).1 This is evidenced by an all-time high of 70 980 overdose deaths in the US in 2019.2 Although the medical community has largely focused on the central role of opioid use disorder (OUD), polysubstance use has been underrecognized, even though most patients with substance use disorder use multiple substances,3 and the presence of multiple substance use disorders is associated with worse treatment outcomes.4,5,6
Alcohol is the most commonly misused substance, both as a standalone drug and in the context of polysubstance use.5 More than 25% of patients with OUD exhibit problematic alcohol use, and, therefore, mitigation of risk for incident or recurrent alcohol use disorder (AUD) presents a salient problem in the treatment of OUD.5,7,8,9 As central nervous system depressants,10 alcohol and opioids can be lethal in combination, leading to increased overdoses and mortality among patients with OUD,11,12 in addition to increased health care utilization.13,14,15 Because problematic alcohol and opioid use are traditionally studied as independent conditions, limited evidence-based strategies exist for mitigation of alcohol-related risk among patients with OUD.
Despite the therapeutic challenge of treating alcohol and opioid co-use, there is reason to believe that existing OUD medications may hold promise in treating comorbid AUD, thus potentially reducing alcohol-related risk among patients with AUD. For instance, some treatment options for dual-diagnosis patients overlap, such as naltrexone, which holds dual US Food and Drug Administration (FDA) indications for OUD and AUD treatment and has been found to be used more commonly in patients with both OUD and AUD than patients with OUD but without comorbid AUD (Mintz CM, Presnall NJ, Xu KY, et al, unpublished data, November 2020). However, few studies have directly evaluated the efficacy of OUD medications on alcohol-related outcomes among patients with OUD,15 and the limited research base has been marked by inconsistent results. For instance, although an open randomized study16 suggested that methadone and buprenorphine suppressed alcohol intake in heroin users, other analyses17 have not replicated these effects, with some studies18 showing increased problematic use of alcohol after initiation of opioid agonist treatment. Existing studies16,17,19 are also characterized by short follow-up periods, modest sample sizes, and self-reported data.
Given the research gaps on alcohol-related outcomes among patients with OUD, our study used the IBM MarketScan Commercial and Multi-State Medicaid databases (2006-2016) to evaluate the association of 3 OUD medication treatments—buprenorphine, methadone, and naltrexone—with emergency and inpatient admissions for acute alcohol-related events, specifically falls, injuries, and poisonings in patients with OUD.
Methods
Study Overview
This case-control cohort study used a repeated-event, case-control design using the MarketScan pharmaceutical claims data. We studied whether alcohol-related acute medical events were associated with OUD pharmacologic treatment. This design used each subject as his or her own control by comparing OUD medication use (exposure) at the time of event with exposure during control periods. Thus, the exposure variable was various types of OUD pharmacologic treatments, and the outcome variable was alcohol-related acute events. We also examined whether patients with known recent AUD claims were more likely to receive naltrexone, given its dual FDA indications for AUD and OUD, as opposed to buprenorphine and methadone, and whether the association between OUD medication and alcohol-related events varies by the presence of recent AUD claims.
Study Setting
The MarketScan databases represent claims from both employer-sponsored and Medicaid insurance holders in the US.20 This captures longitudinal information on inpatient and outpatient health care utilization, diagnosis codes based on the International Classification of Diseases, Ninth Revision (ICD-9) and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10), and procedure codes. Prescription medication data provided information on medication quantity, dosing, and fill dates. Commercial data were available from January 1, 2006, to December 31, 2016. Medicaid data were available from January 1, 2011, to December 31, 2016. Because all data were deidentified, our study was exempt from human subjects review and the need for informed consent by the institutional review board at Washington University. This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) and RECORD-PE reporting guidelines.
Participants
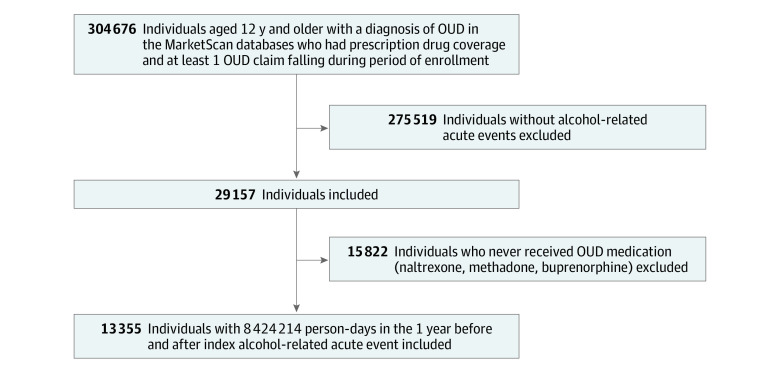
Our analytic sample was derived from a previously described cohort of 304 676 individuals in the US, aged 12 to 64 years, who had prescription drug coverage, a diagnosis of OUD, at least 1 OUD claim during enrollment, and continuous insurance enrollment.20 Figure 1 depicts the derivation of our final analytical sample. Because we used a case-control design, we limited analyses to the 29 157 individuals with alcohol-related acute events (ie, 275 519 individuals were ineligible because they did not experience the outcome of interest). The first event for each person subsequent to initiating OUD treatment was defined as the index event. The index event date was used to generate a longitudinal data set at the day level containing all days in which a given individual was observed (ie, covered by insurance), up to 1 year before and after the index event; that is, the final data set contained 1 record per day per person, with the units of observation being person-days. Our decision to choose a 2-year maximum observation window was guided by the mean length of time individuals were observed in our data set, which was approximately 2 years. The selection of a standard, 2-year observation window also helps fulfill the case-control design’s requirement of a stable within-person probability of exposure over a defined period.21
Figure 1. Flowchart for Development of Analytical Sample.
Chart shows derivation of the study’s final analytical sample during follow-up. After application of inclusion and exclusion criteria, there were 13 335 unique patients with opioid use disorder (OUD), all of whom had received OUD medication during insurance enrollment. The analytical sample was subsequently restricted to observations within 1 year before and 1 year after index alcohol-related acute event to decrease heterogeneity in observation time, contributing a total of 8 424 214 person-days in the study database.
Finally, we further limited our analyses to individuals who received OUD medication at least once, thus excluding 15 822 individuals who never received any pharmacotherapy for OUD (buprenorphine, naltrexone, and methadone). Participants were permitted to contribute multiple events as long as they fell within a maximum of 1 year before and after the index event.
Variables
Our primary outcome was emergency department visit or inpatient hospitalization for any alcohol-related acute event during insurance enrollment. Such events were defined on the basis of established methods and ICD-9 and ICD-10 codes22 as either (1) 100% alcohol-attributable acute events (eg, alcohol poisoning) or (2) health care encounters involving an alcohol-related diagnosis (eg, alcoholic liver disease or chronic alcohol dependence) in combination with acute events that are known to be associated with excess alcohol use (eg, falls, injuries, and poisonings).22
Our primary exposure variable was OUD medication by days, operationalized as days characterized by the presence or absence of prescription coverage or procedure codes for buprenorphine, methadone, or naltrexone (oral or extended-release). Specific national drug codes (buprenorphine and oral naltrexone) and procedure codes (methadone and extended-release naltrexone) can be found elsewhere.20 We assumed that the prescription was filled on the date after the prescription was received by the pharmacy and taken until the last day supplied. We assumed that extended-release naltrexone was active for 28 days from the date of injection.
Because the MarketScan data do not differentiate between patients who were receiving naltrexone for OUD as opposed to AUD, we further stratified between patients with existing AUD diagnoses at the time of OUD pharmacologic treatment initiation or during the 6 months preceding treatment initiation, and those without recent AUD diagnoses. AUD diagnoses were identified by insurance claims using ICD-9 and ICD-10 codes during any inpatient or outpatient encounter.
For the purposes of sample description, we also obtained data on time-invariant variables, such as age, sex, and insurance status, which were compared among the subsamples with and without recent AUD claims. Because the case-control design allows for individuals to serve as their own control, we did not adjust for these time-invariant variables in our models. However, we included time-varying covariates corresponding to prescriptions for medications commonly found in alcohol and drug poisonings23; these included muscle relaxants, antispasmodics, opioids (not buprenorphine or methadone), antidepressants, mood stabilizers, antipsychotics, nonsteroidal anti-inflammatory drugs, and benzodiazepines (eTable 1 in the Supplement). The logic for including these was 2-fold. First, any interaction between these medications and OUD medications may be associated with the point estimates for the OUD medications. Second, use of these medications may serve as proxy variables for comorbidities that can be measured with greater frequency than diagnoses.
Statistical Analysis
We calculated descriptive statistics for the primary analytical sample using χ2 and Kruskal-Wallis tests, which were stratified by recent AUD claims. For our primary analysis, we used a case-control approach described by Allison et al24 that includes all observed days limited to 365 days before and after the index alcohol-related acute event already described. Thus, case days included the index event and any subsequent event, whereas control days included all other days within the observation window. An overview of repeated-event, case-control design is illustrated in the eFigure in the Supplement. Models to estimate the odds ratio (OR) describing the association between events and OUD medication exposure days were estimated using conditional logistic regression, with each person serving as the stratification (conditioning) variable. Medication days were coded as 1, and nonmedication days were coded as 0 (reference group). This approach is analogous to a stratified Cox-regression with recurrent events.24 As a recurrent-event approach, our analysis allowed for incorporation of time (days since index event) as a covariate, mitigating secular confounding that is often a concern with single-event, case-control studies.24,25,26 We controlled for secular time trends using restricted cubic splines.27 Unadjusted models included only OUD medications, whereas adjusted models controlled for exposure medications commonly associated with alcohol poisonings.23 All P values were derived from 2-sided tests, with significance reached at P < .05. Statistical analysis was performed using SAS statistical software version 9.4 (SAS Institute) from June 26 through September 28, 2020.
Results
Sample and Treatment Characteristics
The final study sample contained 13 335 unique patients with OUD (mean [SD] age, 33.1 [13.1] years; 5884 female participants [44.1%]; mean observation time, 627.6 days; 8 424 214 person-days of observation time) who received OUD medications and had at least 1 alcohol-related acute event. Table 1 summarizes descriptive statistics at the individual participant level, with stratification by the presence of recent AUD claims. In total, 19.6% of the sample (2615 individuals) had claims for more than 1 event. Of all participants, 6299 (47.2%) received buprenorphine in the year before and after the index event; in comparison, 667 (5.0%) received methadone, 1096 (8.2%) received extended-release naltrexone, and 3236 (24.3%) received oral naltrexone. Although all participants had received OUD medication during insurance enrollment, 2037 (15.3%) did not receive OUD medication during the 1 year before and after index event that constituted our study’s observation window.
Table 1. Treatment Characteristics at the Individual Participant Level During 1 Year Before and After Index Alcohol-Related Acute Eventa.
| Characteristic | Participants, No. (%) | P value | ||
|---|---|---|---|---|
| Total (N = 13 335) | OUD with recent AUD claims (n = 7462) | OUD without recent AUD claims (n = 5873) | ||
| OUD medication use during 1 y before and 1 y after index alcohol-related acute event | ||||
| Buprenorphine | 6299 (47.2) | 3134 (42.0) | 3165 (53.9) | <.001 |
| Methadone | 667 (5.0) | 298 (4.0) | 369 (6.3) | <.001 |
| Naltrexone extended release | 1096 (8.2) | 737 (9.9) | 359 (6.1) | <.001 |
| Naltrexone oral | 3236 (24.3) | 2399 (32.2) | 837 (14.3) | <.001 |
| None | 2037 (15.3) | 894 (12.0) | 1143 (19.5) | <.001 |
| Use of medications commonly found in association with alcohol poisonings during 1 y before and 1 y after index alcohol-related acute event | ||||
| Muscle relaxant | 4579 (34.3) | 2462 (33.0) | 2117 (36.1) | <.001 |
| Antispasmodic | 1667 (12.5) | 912 (12.2) | 755 (12.9) | .27 |
| Opioid (not or buprenorphine or methadone) | 8543 (64.1) | 4812 (64.5) | 3731 (63.5) | .25 |
| Antidepressant | 10533 (79.0) | 6159 (82.5) | 4374 (74.5) | <.001 |
| Mood stabilizer | 5891 (44.2) | 3790 (50.8) | 2101 (35.8) | <.001 |
| Antipsychotic | 5938 (44.5) | 3521 (47.2) | 2417 (41.2) | <.001 |
| Nonsteroidal anti-inflammatory drug | 6312 (47.3) | 3583 (48.0) | 2729 (46.5) | .08 |
| Benzodiazepine | 7552 (56.6) | 4359 (57.7) | 3193 (42.3) | <.001 |
| Sociodemographic characteristics | ||||
| Sex | ||||
| Male | 7451 (55.9) | 4235 (56.8) | 3216 (54.7) | .02 |
| Female | 5884 (44.1) | 3227 (43.3) | 2657 (45.2) | |
| Insurance | ||||
| Private insurance | 9276 (69.6) | 5322 (71.3) | 3954 (67.3) | <.001 |
| Medicaid | 4059 (30.4) | 2140 (28.7) | 1919 (32.7) | |
| Year of birth | 1977 | 1975 | 1979 | |
| Age, mean (SD), y | 33.1 (13.1) | 34.9 (12.9) | 30.8 (13.1) | <.001 |
| Alcohol-related acute events, No. | ||||
| 1 | 10 720 (80.4) | 5501 (73.7) | 5219 (88.9) | <.001 |
| 2 | 1639 (12.3) | 1167 (15.6) | 472 (8.0) | |
| ≥3 | 976 (7.3) | 794 (10.6) | 182 (3.1) | |
Abbreviations: AUD, alcohol use disorder; OUD, opioid use disorder.
This table illustrates OUD treatment characteristics at the individual participant level among those with a history of alcohol-related acute events during the study’s observation window (1 year before and after index event), stratified by recent AUD claims. The prevalence of using specific medications of interest within the study’s observation period is also depicted. The bottom of the table depicts demographic characteristics for participants, as well as number of alcohol-related acute events.
A total of 55.9% of the sample (7462 participants) had a recent AUD claim before or at start of OUD treatment, defined by diagnoses within 6 months of the start of treatment. These patients were less likely to receive agonist therapy than their peers without AUD claims (42.0% [3134 participants] vs 53.9% [3165 participants] for buprenorphine; 4.0% [298 participants] vs 6.3% [369 participants] for methadone). Conversely, they were more likely to receive naltrexone (oral naltrexone, 32.2% [2399 participants] vs 14.3% [837 participants]; extended-release naltrexone, 9.9% [737 participants] vs 6.1% [359 participants]). With regard to sociodemographic characteristics, individuals with recent AUD claims were slightly older (mean [SD] age, 34.9 [12.9] vs 30.8 [13.1] years), more likely to be male (4235 [56.8%] vs 3216 [54.7%] men), less likely to be enrolled in Medicaid (2140 [28.7%] vs 1919 [32.7%] participants), and were more likely to incur multiple alcohol-related events (1967 [26.2%] vs 654 [11.1%] events) (Table 1). Participants with recent AUD claims also had more prescriptions for antidepressants (6156 [82.5%] vs 4374 [74.5%] participants), mood stabilizers (3790 [50.8%] vs 2101 [35.8%] participants), antipsychotics (3521 [47.2%] vs 2417 [41.2%] participants), and benzodiazepines (4359 [57.7%] vs 3193 [42.2%] participants) than participants without AUD claims.
Table 2 depicts the rate of alcohol-related events, per 100 days, stratified by OUD medication exposure and by recent AUD claims. In general, event rates were lower for buprenorphine or methadone treatment days than for days when patients were treated with either naltrexone formulation or for nonmedication days. For nontreatment days, 0.22% of days overall (mean, 535.0 days per person), 0.25% of days for those with active AUD claims (mean, 534.6 days per person), and 0.18% of days among those without active AUD claims (mean, 535.6 days per person) were marked by alcohol-related acute events. For antagonist medications, 0.29% (mean, 15.7 days per person) of oral naltrexone treatment days (0.28% [mean, 8.2 days per person] for those with active AUD claims and 0.29% [mean, 21.5 days per person] for those without active AUD claims) and 0.19% (mean, 6.4 days per person) of extended-release naltrexone treatment days (0.20% [mean, 7.5 days per person] for those with AUD claims and 0.18% [mean, 5.1 days per person] for those without AUD claims) were marked by events. Among agonist medications, 0.11% (mean, 8.4 days per person) of methadone treatment days (0.11% [mean, 5.8 days per person] of days for those with AUD claims and 0.12% [mean, 11.7 days per person] of days for those without AUD claims) and 0.15% (mean, 62.7 days per person) of buprenorphine treatment days (0.13% of days [mean, 48.9 days per person] for those with AUD claims and 0.16% [mean, 80.3 days per person] for those without AUD claims) were marked by alcohol-related acute events.
Table 2. Rate of Alcohol-Related Events Associated With OUD Medications, Stratified by Recent AUD Claimsa.
| Variable | Total (N = 8 424 214 person-days among 13 335 individuals) | OUD with recent AUD claims (n = 4 629 076 person-days among 7462 individuals) | OUD without recent AUD claims (n = 3 795 138 person-days among 5873 individuals) | |||
|---|---|---|---|---|---|---|
| Mean d/person, No. | Rate of alcohol-related events/100 d | Mean d/person, No. | Rate of alcohol-related events/100 d | Mean d/person, No. | Rate of alcohol-related events/100 d | |
| Treatment | ||||||
| Buprenorphine | 62.7 | 0.15 | 48.9 | 0.13 | 80.3 | 0.16 |
| Methadone | 8.4 | 0.11 | 5.8 | 0.11 | 11.7 | 0.12 |
| Naltrexone extended-release | 6.4 | 0.19 | 7.5 | 0.20 | 5.1 | 0.18 |
| Naltrexone oral | 15.7 | 0.29 | 21.5 | 0.29 | 8.2 | 0.28 |
| Nonmedication | 535.0 | 0.22 | 534.6 | 0.25 | 535.6 | 0.18 |
| All observation days | 627.6 | 0.21 | 617.5 | 0.24 | 640.4 | 0.18 |
Abbreviations: AUD, alcohol use disorder; OUD, opioid use disorder.
This table depicts the percentage of insurance coverage days entailing an alcohol-related acute event, which is compared among days when participants were receiving buprenorphine, methadone, naltrexone (extended release or oral), and nonmedication treatment days. These analyses were further stratified between persons who had recent AUD claims and those without recent AUD claims.
Association of OUD Medication With Alcohol-Related Acute Events
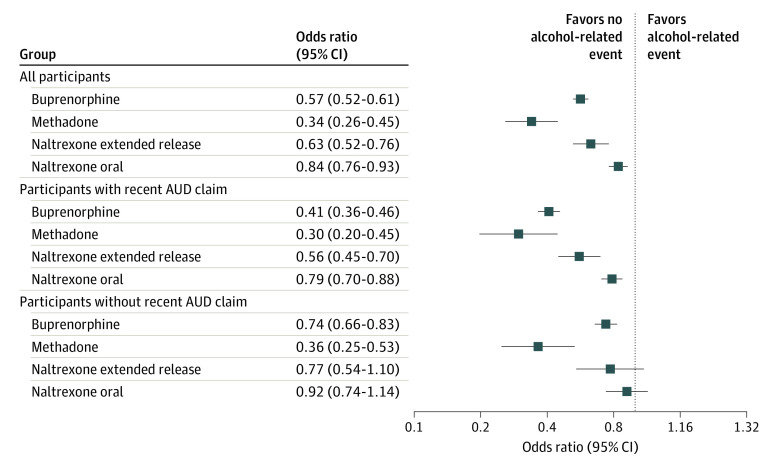
Figure 2 shows the adjusted ORs of admissions for alcohol-related acute events associated with days using OUD medications vs nonmedication days. A full listing of point estimates and 95% CIs is enumerated in eTable 2 (unadjusted models) and eTable 3 (adjusted models) in the Supplement, with the association between OUD medication and alcohol-related acute events unchanged between unadjusted and adjusted analyses. Covariate adjustment had little impact on the other parameters in the model. Among agonist medications, buprenorphine was associated with a 43% reduction (OR, 0.57; 95% CI, 0.52-0.61) and methadone was associated a 66% reduction (OR, 0.34; 95% CI, 0.26-0.45) in adjusted odds of alcohol-related acute events. Among antagonist medication, oral naltrexone was associated with a 37% reduction (OR, 0.63; 95% CI, 0.52-0.76) and extended-release naltrexone was associated with a 16% reduction (OR, 0.84; 95% CI, 0.76-0.93) in the adjusted odds of such events.
Figure 2. Adjusted Odds of Alcohol-Related Acute Events Associated With Opioid Use Disorder (OUD) Treatment Days Compared With Nontreatment Days.
Plot shows adjusted odds of alcohol-related acute events associated with OUD treatment days compared with nontreatment days, stratified for all participants, patients with OUD with recent alcohol use disorder (AUD) claims, and patients with OUD without recent AUD claims.
In the stratified analysis, buprenorphine’s protective associations against alcohol-related acute events were more pronounced among patients with OUD with recent AUD claims (OR, 0.41; 95% CI, 0.36-0.46) than among peers without such claims (OR, 0.74; 95% CI, 0.66-0.83). In comparison, methadone exhibited similar protective associations in patients with OUD with (OR, 0.30; 95% CI, 0.20-0.45) and without (OR, 0.36; 95% CI, 0.25-0.53) recent AUD claims. The protective associations of extended-release naltrexone (OR, 0.56; 95% CI, 0.45-0.70) and oral naltrexone (OR, 0.79; 95% CI, 0.70-0.88) were significant only among patients with OUD with AUD, as opposed to peers without claims; however, differences in adjusted ORs by AUD status were not significant because of wide 95% CIs among patients with AUD. Across all analyses, the 2 forms of naltrexone did not significantly differ from one another in their association with alcohol-related acute events.
Discussion
Our study shows that OUD treatment days were associated with reductions in overall odds of admissions for alcohol-related acute events (ie, falls, injuries, and poisonings). We observed risk reductions of 43% for buprenorphine and 66% for methadone treatment days. Extended-release and oral naltrexone treatment days were associated with 37% and 16% reductions, respectively. Naltrexone use was more prevalent among patients with OUD with existing AUD claims than their peers without such claims, consistent with naltrexone’s dual FDA indications of naltrexone for both AUD and OUD treatment. However, naltrexone and buprenorphine showed similar overall protective outcomes against alcohol-related acute events.
Interestingly, our findings illustrate that the greatest overall reduction in alcohol-related acute events were observed with methadone. Time-varying confounding cannot be ruled out because these results can be partly explained by the structure of opioid treatment programs (ie, methadone clinics), which frequently monitor alcohol and illicit drug use with urine drug screens. The effectiveness of OUD medications in AUD may be more limited when it is not accompanied by psychosocial interventions, such as relapse-prevention therapy or counseling focused on medication adherence,28 which are seen in opioid treatment programs that dispense methadone. The association between methadone treatment days and alcohol-related events warrants further investigation.
Of note, our results should be interpreted in conjunction with known differences in medication adherence. The true practical effectiveness of antagonist therapies may be hindered by lower medication adherence associated with naltrexone compared with agonist treatments in the OUD population. For instance, substantially greater medication adherence has been observed for buprenorphine treatment (median filled prescriptions of 19 months) compared with both extended-release (9 months) and oral (5 months) naltrexone,29 with patients with OUD found to show similar adherence to methadone and buprenorphine.30 As our study evaluates ORs that describe protective outcomes associated with medication at a given point in time, the protective outcomes observed in association with buprenorphine and methadone treatment days are likely to be amplified by their higher adherence rates compared with naltrexone. However, our analyses rely on the implicit assumption that risks associated with medication or lack of medication are constant over time. More investigation is needed to evaluate how these risks might vary before, during, and after OUD treatment, in addition to investigating the comparative effectiveness of buprenorphine, methadone, and naltrexone in suppressing alcohol use in patients with OUD.
Limitations and Strengths
There are several limitations to note. First, although the MarketScan data benefit from a large, national sample with ample longitudinal follow-up, measurement error cannot be ruled out, such that medication coverage does not always reflect actual consumption. Furthermore, patients with OUD without observed AUD claims may have a history of treated or untreated AUD not reflected in recent insurance claims. However, we observed a higher prevalence of naltrexone use in patients with AUD claims, and we also observed significant differences in OUD medication treatment outcomes between patients with OUD with recent AUD claims and peers without such claims, suggesting that the distinction between these 2 groups may be clinically meaningful and thus warrants more research. Second, unmeasured time-varying factors associated with alcohol-related admissions and OUD medication exposure can introduce confounding in case-control designs. We mitigated this, however, by including calendar time and time from event as covariates and restricting participants to 2-year observation periods surrounding the index event in order to reduce heterogeneity in observation time. We also used bidirectional sampling in order to reduce overlap bias resulting from control period selection as a function of event time.25 Third, even though self-matching is used by the case-control design, residual confounding by unmeasured time-invariant variables cannot be ruled out. Fourth, the MarketScan data only encompass insured patients with observed alcohol-related acute events, which limits our study’s generalizability.
Despite these limitations, our study was strengthened by its repeated-event, case-control design, allowing for reduction of selection and sampling bias stemming from conventional observational study recruitment. In addition, we controlled for conditions leading to alcohol-related events via adjustment for medications commonly found in association with such events. The protective associations of buprenorphine, methadone, and naltrexone were not significantly changed in the adjusted analyses, providing evidence of robustness to measured confounders.
Conclusions
The findings of this study suggest that OUD medications are associated with decreased hospital admissions for alcohol-related acute events among persons with OUD. Although naltrexone use was more prevalent among patients with OUD with recent AUD claims, agonist treatments (buprenorphine and methadone) also were associated with fewer alcohol-related events. The present study raises key questions about the potential of OUD medications for reducing alcohol-related events and highlights the need for additional research in this area.
eTable 1. Classification of Medications Commonly Found in Association With Alcohol Poisoning
eTable 2. Odds of Alcohol-Related Acute Events Associated with OUD Medication Treatment Days Without Adjustment for Treatment Days Encompassing Medications Commonly Found in Association With Alcohol Poisoning
eTable 3. Odds of Alcohol-Related Acute Events Associated With OUD Medication Treatment Days, With Adjustment for Treatment Days Encompassing Medications Commonly Found in Association with Alcohol Poisoning
eFigure. Overview of Case-Crossover Design
References
- 1.Centers for Disease Control and Prevention, National Center for Health Statistics FactStats: accidents or unintentional injuries. Updated November 13, 2020. Accessed January 21, 2021. https://www.cdc.gov/nchs/fastats/accidental-injury.htm
- 2.Ahmad FB, Rossen LM, Sutton P; National Center for Health Statistics Provisional drug overdose death counts. Published 2020. Accessed January 21, 2021. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- 3.Cicero TJ, Ellis MS, Kasper ZA. Polysubstance use: a broader understanding of substance use during the opioid crisis. Am J Public Health. 2020;110(2):244-250. doi: 10.2105/AJPH.2019.305412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Degenhardt L, Grebely J, Stone J, et al. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. Lancet. 2019;394(10208):1560-1579. doi: 10.1016/S0140-6736(19)32229-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassan AN, Le Foll B. Polydrug use disorders in individuals with opioid use disorder. Drug Alcohol Depend. 2019;198:28-33. doi: 10.1016/j.drugalcdep.2019.01.031 [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Min JE, Krebs E, et al. Polydrug use and its association with drug treatment outcomes among primary heroin, methamphetamine, and cocaine users. Int J Drug Policy. 2017;49:32-40. doi: 10.1016/j.drugpo.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomes T, Khuu W, Martins D, et al. Contributions of prescribed and non-prescribed opioids to opioid related deaths: population based cohort study in Ontario, Canada. BMJ. 2018;362:k3207. doi: 10.1136/bmj.k3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78-82. doi: 10.1016/j.drugalcdep.2018.12.030 [DOI] [PubMed] [Google Scholar]
- 9.Winstanley EL, Stover AN, Feinberg J. Concurrent alcohol and opioid use among harm reduction clients. Addict Behav. 2020;100:106027. doi: 10.1016/j.addbeh.2019.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White JM, Irvine RJ. Mechanisms of fatal opioid overdose. Addiction. 1999;94(7):961-972. doi: 10.1046/j.1360-0443.1999.9479612.x [DOI] [PubMed] [Google Scholar]
- 11.Coffin PO, Galea S, Ahern J, Leon AC, Vlahov D, Tardiff K. Opiates, cocaine and alcohol combinations in accidental drug overdose deaths in New York City, 1990-98. Addiction. 2003;98(6):739-747. doi: 10.1046/j.1360-0443.2003.00376.x [DOI] [PubMed] [Google Scholar]
- 12.Hser YI, Mooney LJ, Saxon AJ, et al. High mortality among patients with opioid use disorder in a large healthcare system. J Addict Med. 2017;11(4):315-319. doi: 10.1097/ADM.0000000000000312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank D, Mateu-Gelabert P, Guarino H, et al. High risk and little knowledge: overdose experiences and knowledge among young adult nonmedical prescription opioid users. Int J Drug Policy. 2015;26(1):84-91. doi: 10.1016/j.drugpo.2014.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hickman M, Lingford-Hughes A, Bailey C, Macleod J, Nutt D, Henderson G. Does alcohol increase the risk of overdose death: the need for a translational approach. Addiction. 2008;103(7):1060-1062. doi: 10.1111/j.1360-0443.2008.02134.x [DOI] [PubMed] [Google Scholar]
- 15.Witkiewitz K, Vowles KE. Alcohol and opioid use, co-use, and chronic pain in the context of the opioid epidemic: a critical review. Alcohol Clin Exp Res. 2018;42(3):478-488. doi: 10.1111/acer.13594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soyka M Alcohol use disorders in opioid maintenance therapy: prevalence, clinical correlates and treatment. Eur Addict Res. 2015;21(2):78-87. doi: 10.1159/000363232 [DOI] [PubMed] [Google Scholar]
- 17.Srivastava A, Kahan M, Ross S. The effect of methadone maintenance treatment on alcohol consumption: a systematic review. J Subst Abuse Treat. 2008;34(2):215-223. doi: 10.1016/j.jsat.2007.04.001 [DOI] [PubMed] [Google Scholar]
- 18.Dong H, Hayashi K, Milloy MJ, et al. Changes in substance use in relation to opioid agonist therapy among people who use drugs in a Canadian setting. Drug Alcohol Depend. 2020;212:108005. doi: 10.1016/j.drugalcdep.2020.108005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caputo F, Addolorato G, Domenicali M, et al. ; Services for Addiction Treatment . Short-term methadone administration reduces alcohol consumption in non-alcoholic heroin addicts. Alcohol Alcohol. 2002;37(2):164-168. doi: 10.1093/alcalc/37.2.164 [DOI] [PubMed] [Google Scholar]
- 20.Mintz CM, Presnall NJ, Sahrmann JM, et al. Age disparities in six-month treatment retention for opioid use disorder. Drug Alcohol Depend. 2020;213:108130. doi: 10.1016/j.drugalcdep.2020.108130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang SV, Gagne JJ, Glynn RJ, Schneeweiss S. Case-crossover studies of therapeutics: design approaches to addressing time-varying prognosis in elderly populations. Epidemiology. 2013;24(3):375-378. doi: 10.1097/EDE.0b013e31828ac9cb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention Alcohol-related disease impact (ARDI) application. Updated September 3, 2020. Accessed January 21, 2021. https://nccd.cdc.gov/DPH_ARDI/default/default.aspx
- 23.Castle IJ, Dong C, Haughwout SP, White AM. Emergency department visits for adverse drug reactions involving alcohol: United States, 2005 to 2011. Alcohol Clin Exp Res. 2016;40(9):1913-1925. doi: 10.1111/acer.13167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allison P, Christakis N. Fixed-effects methods for the analysis of nonrepeated events. Sociol Methodol. 2006;36(1):155-172. doi: 10.1111/j.1467-9531.2006.00177.x [DOI] [Google Scholar]
- 25.Mittleman MA, Mostofsky E. Exchangeability in the case-crossover design. Int J Epidemiol. 2014;43(5):1645-1655. doi: 10.1093/ije/dyu081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hallas J, Pottegård A, Wang S, Schneeweiss S, Gagne JJ. Persistent user bias in case-crossover studies in pharmacoepidemiology. Am J Epidemiol. 2016;184(10):761-769. doi: 10.1093/aje/kww079 [DOI] [PubMed] [Google Scholar]
- 27.Jensen AK, Gerds TA, Weeke P, Torp-Pedersen C, Andersen PK. On the validity of the case-time-control design for autocorrelated exposure histories. Epidemiology. 2014;25(1):110-113. doi: 10.1097/EDE.0000000000000001 [DOI] [PubMed] [Google Scholar]
- 28.Dugosh K, Abraham A, Seymour B, McLoyd K, Chalk M, Festinger D. A systematic review on the use of psychosocial interventions in conjunction with medications for the treatment of opioid addiction. J Addict Med. 2016;10(2):93-103. doi: 10.1097/ADM.0000000000000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan JR, Schackman BR, Weinstein ZM, Walley AY, Linas BP. Overdose following initiation of naltrexone and buprenorphine medication treatment for opioid use disorder in a United States commercially insured cohort. Drug Alcohol Depend. 2019;200:34-39. doi: 10.1016/j.drugalcdep.2019.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinsky S, Houck PR, Mayes K, Loveland D, Daley D, Schuster JM. A comparison of adherence, outcomes, and costs among opioid use disorder Medicaid patients treated with buprenorphine and methadone: a view from the payer perspective. J Subst Abuse Treat. 2019;104:15-21. doi: 10.1016/j.jsat.2019.05.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Classification of Medications Commonly Found in Association With Alcohol Poisoning
eTable 2. Odds of Alcohol-Related Acute Events Associated with OUD Medication Treatment Days Without Adjustment for Treatment Days Encompassing Medications Commonly Found in Association With Alcohol Poisoning
eTable 3. Odds of Alcohol-Related Acute Events Associated With OUD Medication Treatment Days, With Adjustment for Treatment Days Encompassing Medications Commonly Found in Association with Alcohol Poisoning
eFigure. Overview of Case-Crossover Design