Abstract
Background
Total shoulder arthroplasty has been demonstrated to be an effective treatment for arthritis of the glenohumeral joint. Prior studies have identified longer operative times as a risk factor for complications after numerous types of procedures. We hypothesized that increased operative time, in 20-min intervals, would be associated with complications following total shoulder arthroplasty.
Methods
Patients undergoing total shoulder arthroplasty from 2006 to 2015 were identified from the ACS-NSQIP database. Patient demographic information, perioperative parameters, and 30-day outcomes were retrieved. Pearson's Chi-square test and multivariate Poisson regression with robust error variance were used to analyze the relationship of operative time and outcomes.
Results
A total of 10,082 patients were included. Multivariate analysis revealed that for each increase in 20 min of operative time, there were significantly increased rates of any complication (relative risk (RR) 1.24, 95% confidence interval (CI) 1.19–1.26), anemia requiring transfusion (RR 1.33, 95%CI 1.26–1.4), peripheral nerve injury (RR 1.88, 95%CI 1.53–2.31), and urinary tract infection (RR 1.24, 95%CI 1.09–1.41).
Discussion
This study indicates that increasing operative time confers increased risk for postoperative complications following total shoulder arthroplasty. We anticipate the results of this manuscript will be used for provider education, policy decision-making, and potentially to derive algorithms that can improve safety and efficiency in total shoulder arthroplasty.
Level of evidence
III.
Keywords: operative time, total shoulder, arthroplasty, outcomes, complications
Introduction
Total shoulder arthroplasty (TSA) has quickly evolved1,2 into an effective and reliable treatment option that offers significant improvements in pain, function, and satisfaction in patients with degenerative glenohumeral pathology.3–5 Due to innovations in surgical techniques and implants, as well as an aging population, the number of total shoulder arthroplasties being performed annually will continue to rise.6–8 Given this increasing incidence, it has become increasingly important to understand the perioperative (pre-, intra-, and postoperative) risk factors for complications in those undergoing this procedure.
Understanding various patient- and pathology-related risk factors is important for both improving outcomes, as well as determining risk stratification in health policy, such as with bundled payments. There have been many risk factors identified as being associated with increased complications after TSA,2,9 including increasing American Society of Anesthesiology (ASA) scores,10,11 increasing body mass index (BMI),12–14 preoperative anemia, 11 steroid use, 11 hypoalbuminemia, 15 several medical comorbidities,11,16,17 revision TSA, 14 and increased operative time.11,16 Unfortunately, many of these risk factors are not modifiable and therefore offer little opportunity for intervention aimed at risk reduction.
Operative time, on the other hand, represents a surgeon-specific, potentially modifiable risk factor. Operative time has been shown to be an important consideration in various surgeries, 18 including shoulder arthroscopy, 19 as well as total hip and knee arthroplasty. 20 Two studies on TSA have demonstrated increased risk with operative times longer than 2 h 11 or longer than 174 min. 16 While these studies identify increased operative time as a potential risk factor, both are limited by their analysis and methodology. Both studies compare only long versus short cases (at arbitrarily established cutoffs 11 ) and are generally limited by their reporting only a general increased risk in complications. Thus, there is a need for further clarification with regard to the nature and magnitude of the risk that increased operative time poses to patients.
We hypothesize that each additional 20-min increase in operative time in patients undergoing TSA is a risk factor for increased postoperative complications, even when controlling for confounding variables.
Methods
Data collection
Patient data for this study was obtained from the American College of Surgeons-National Surgery Quality Improvement Program (ACS-NSQIP) database, an international registry that includes preoperative and 30-day outcome data for patients undergoing surgical operations at voluntarily participating academic and community institutions. The database is now well established and is extensively utilized in the orthopedic literature.11,16,20–23 By employing highly trained clinical reviewers, conducting audits, and utilizing multiple methods of data collection (chart review, phone calls, written questionnaires), the database is able to maintain a 95% data capture rate.
In the current investigation, the NSQIP database was searched from 2006 to 2015 for patients with the Current Procedural Terminology (CPT) code 23472 (arthroplasty, glenohumeral joint; total shoulder (glenoid and proximal humeral replacement (e.g. total shoulder)). Because separate CPT codes are not available for varying prosthesis options, this would include anatomic TSA, reverse TSA, and total shoulder resurfacing. We then excluded patients using the following criteria: systemic sepsis on admission, prior operation within 30 days of TSA, emergency case, concurrent procedure (including removal of hardware), disseminated cancer, and open wound or infection. Revision cases and hemiarthroplasties were excluded given that only CPT code 23472 was used. Additionally, in order to limit the effect of extreme outliers, patients with operative times less than 30 min and greater than 310 min were excluded—this ultimately omitted only 23 patients. Those with missing operative time were excluded. Other missing variables that were present were coded with missing variables in the analysis. This included the following: 62 patients (0.6%) with missing age, six patients (0.05%) without sex, 237 patients (2.3%) with either missing height or weight data precluding calculation of BMI, 75 patients (0.7%) with missing functional status, and 13 patients (0.1%) with missing ASA classification.
Baseline patient characteristics
Demographic data were collected, including sex, age, race, ASA classification, smoking status, and BMI. Additionally, we assessed baseline comorbidity information, including history of congestive heart failure, diabetes mellitus (DM), hypertension (HTN), chronic obstructive pulmonary disease (COPD), dyspnea on exertion, and functional status. These were controlled for in the multivariable model.
Outcome and complication data
Using the NSQIP database, we were able to collect information on outcomes and complications up to 30-days postoperative. The following complications were assessed: anemia requiring transfusion, peripheral nerve injury, urinary tract infection (UTI), pneumonia, surgical site infection (SSI), cardiac arrest requiring cardiopulmonary resuscitation, cerebrovascular accident, deep vein thrombosis, wound dehiscence, myocardial infarction (MI), pulmonary embolism (PE), renal insufficiency, sepsis, unplanned reintubation, extended hospital length of stay (LOS; ≥4 days), and mortality. All complications were then analyzed as a conglomerate (this constituted the primary outcome of the study). Additionally, we analyzed Clavien-Dindo IV complications separately. This group included life-threatening complications that cause end-organ dysfunction. In the present study, cardiac arrest, MI, septic shock, PE, and renal failure were included as Clavien-Dindo IV complications. Lastly, 30-day readmission and reoperation data were also collected and analyzed.
Statistical analysis
Statistical analysis was performed using IBM SPSS Software (Armonk, New York, NY, Version 25). In order to first ascertain the association of operative time with baseline patient characteristics, the Pearson's Chi-square test was performed, with a p value of less than 0.05 considered statistically significant. The Pearson's Chi-square test was then performed to determine if there was an association of postoperative complications with operative time. Then, in order to control for possible confounding variables, Poisson regression with robust error variance was performed to test for an association of operative time with postoperative complications. This was undertaken using a model controlling for all the variables listed in the “Baseline Patient Characteristics” section.
Results
Baseline patient information
From 2006 to 2015, 10,467 patients who underwent TSA were identified using the NSQIP database; after exclusion criteria were applied, 10,082 patients remained for analysis. The majority (69%) of patients were between the ages of 60 and 79 years old (mean 69.15, range: 23–89), with a slight female predominance (56%). Most patients were either overweight or obese as measured by BMI (32% and 25.6%, respectively; mean 31.21). Nearly all included patients had an ASA classification of 3 or less (97.5%; 45.7% class 2, 49.6% class 3). Included patients had the following comorbid conditions: positive smoking status (10.1%), diabetes (17.1%), COPD (6.1%), HTN (67.3%), dyspnea on exertion (6.9%), bleeding disorder (2.8%), and dependent functional status (3%) (Table 1).
Table 1.
Operative time by demographic, comorbidity, and procedural variables.
| Variable | Number of patients (n, %) | Operative time ± SD (min) | p value (bivariate analysis) |
|---|---|---|---|
| Overall | 10,082 | 113.8 ± 42.4 | |
| Age | |||
| 18–59 | 1538, 15.3 | 122.3 ± 45.0 | <0.001 |
| 60–69 | 3365, 33.6 | 115.8 ± 42.7 | |
| 70–79 | 3605, 36.0 | 110.6 ± 40.8 | |
| ≥80 | 1512, 15.1 | 108.7 ± 40.8 | |
| Sex | |||
| Male | 4410, 43.8 | 118.5 ± 43.8 | <0.001 |
| Female | 5666, 56.2 | 110.2 ± 40.9 | |
| BMI | |||
| <18.5 | 60, 0.6 | 105.2 ± 43.3 | <0.001 |
| 18.5–24.9 | 1616, 16.4 | 109.5 ± 41.6 | |
| 25.0–29.9 | 3227, 32.8 | 112.1 ± 43.0 | |
| 30.0–34.9 | 2589, 26.3 | 115.5 ± 41.8 | |
| 35.0–39.9 | 1345, 13.7 | 117.5 ± 41.9 | |
| ≥40.0 | 1008, 10.2 | 117.1 ± 43.4 | |
| ASA class | |||
| 1 | 200, 2.0 | 114.2 ± 44.3 | 0.312 |
| 2 | 4610, 45.8 | 113.4 ± 42.6 | |
| 3 | 5003, 49.7 | 114.0 ± 42.2 | |
| 4 | 256, 2.5 | 118.4 ± 42.0 | |
| mFI | |||
| 0 | 2878, 28.5 | 115.3 ± 43.9 | 0.001 |
| 1 | 5189, 51.5 | 112.4 ± 41.8 | |
| ≥2 | 2015, 20.0 | 115.5 ± 41.7 | |
| Current smoker | |||
| No | 9059, 89.9 | 113.5 ± 42.5 | 0.008 |
| Yes | 1023, 10.1 | 117.2 ± 41.9 | |
| Diabetes mellitus | |||
| No | 8361, 82.9 | 113.5 ± 42.5 | 0.053 |
| Yes | 1721, 17.1 | 115.6 ± 41.8 | |
| COPD | |||
| No | 9465, 93.9 | 113.8 ± 42.4 | 0.882 |
| Yes | 617, 6.1 | 114.0 ± 42.1 | |
| Hypertension | |||
| No | 3294, 32.7 | 114.7 ± 43.6 | 0.174 |
| Yes | 6788, 67.3 | 113.4 ± 41.9 | |
| Dyspnea on exertion | |||
| No | 9386, 93.1 | 114.02 ± 42.5 | 0.094 |
| Yes | 696, 6.9 | 111.2 ± 41.7 | |
| Bleeding disorder | |||
| No | 9804, 97.2 | 113.9 ± 42.4 | 0.601 |
| Yes | 278, 2.8 | 112.5 ± 42.7 | |
| Functionally Dept. | |||
| No | 9702, 97.0 | 114.0 ± 42.5 | 0.147 |
| Yes | 305, 3.0 | 110.4 ± 44.2 |
ASA: American Society of Anesthesiology; BMI: body mass index; COPD: chronic obstructive pulmonary disease; mFI: modified frailty index.
Operative time
Mean operative time for all included patients was 114.47 ± 47.4 min. After patients with operative time <30 min and >310 min were excluded, the mean operative time was 113.83 ± 42.4 min. Median operative time was 108 min and the interquartile range was 54 min (25th percentile = 83 min, 75th percentile = 137 min). Operative time was significantly longer when the patient was younger, male, had a higher BMI, and a smoker (Table 1). A histogram of the distribution of operative times can be seen in Figure 1. Each 20-min interval represents 17.5% of the mean operative time.
Figure 1.
Histogram demonstrating the distribution of operative times included in the current investigation.
Operative time and outcomes
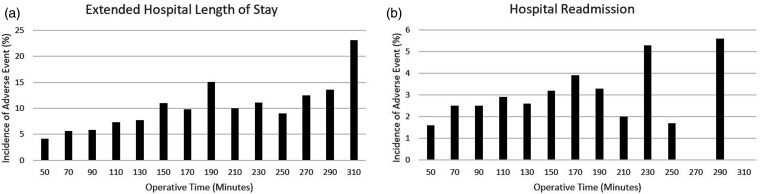
Operative time was analyzed in 20-min increments, thus the presented relative risks should be interpreted as the risk of each additional 20 min of operative time. First, an unadjusted bivariate analysis was conducted. This identified the following as being significantly associated with operative time: anemia requiring transfusion, peripheral nerve injury, pneumonia, SSI, UTI, any complication and extended hospital stay (>4 days; p < 0.05; Table 2). When controlling for demographic and comorbid data, the following remained significantly associated with operative time (increase in 20-min): anemia requiring transfusion, peripheral nerve injury, UTI, and the rate of any complication (p < 0.001; Table 3, Figure 2). An increase in operative time by 20 min was also associated with a 9% increased risk of readmission and a 22% increased risk for extended LOS (Table 3. Figure 3).
Table 2.
Bivariate analysis exploring the association of 20-min increases in operative time with adverse events following total shoulder arthroplasty.
| Bivariate (unadjusted) analysis |
||||
|---|---|---|---|---|
| Postoperative outcome | Rate (n, %) | RR | 95% CI | p value |
| Significantly associated*: | ||||
| Any complication | 613 (6.1%) | 1.21 | 1.15–1.26 | <0.001 |
| Anemia requiring transfusion | 370 (3.7%) | 1.27 | 1.21–1.34 | <0.001 |
| Peripheral nerve injury | 18 (0.2%) | 1.86 | 1.48–2.32 | <0.001 |
| Urinary tract infection | 74 (0.7%) | 1.20 | 1.06–1.36 | 0.005 |
| Pneumonia | 52 (0.5%) | 0.85 | 0.73–0.98 | 0.030 |
| Surgical site infection | 26 (0.3%) | 1.19 | 1.01–1.39 | 0.038 |
| Hospital LOS ≥4 days | 805 (8.0%) | 1.17 | 1.13–1.22 | <0.001 |
| Not significantly associated: | ||||
| Cardiac arrest requiring CPR | 6 (0.1%) | 1.05 | 0.75–1.45 | 0.789 |
| Cerebrovascular accident | 9 (0.1%) | 0.91 | 0.70–1.18 | 0.476 |
| Death | 19 (0.2%) | 0.95 | 0.68–1.33 | 0.754 |
| Deep vein thrombosis | 35 (0.3%) | 0.97 | 0.79–1.18 | 0.736 |
| Dehiscence | 9 (0.1%) | 1.12 | 0.82–1.53 | 0.472 |
| Myocardial infarction | 21 (0.2%) | 1.35 | 0.91–1.43 | 0.246 |
| Pulmonary embolism | 34 (0.3%) | 1.13 | 0.92–1.39 | 0.238 |
| Renal insufficiency | 10 (0.1%) | 1.09 | 0.81–1.47 | 0.560 |
| Sepsis | 19 (0.2%) | 1.21 | 0.93–1.57 | 0.158 |
| Unplanned intubation | 17 (0.2%) | 1.01 | 0.83–1.24 | 0.915 |
| Clavien-Dindo IV | 78 (0.8%) | 1.10 | 0.97–1.25 | 0.148 |
| Hospital Readmission | 247 (2.4%) | 1.06 | 0.99–1.14 | 0.099 |
RR: relative risk; CI: confidence interval; LOS: length of stay; n: number of patients; CPR: cardiopulmonary resuscitation.
p value < 0.05 considered significant. Values in bold represent those that are statistically significant.
Table 3.
Multivariable analysis of the association of 20-min interval increases in operative time with adverse events following total shoulder arthroplasty.
| Multivariable (adjusted) analysis |
|||
|---|---|---|---|
| Postoperative outcome: | RR | 95% CI | p value |
| Any complication | 1.24 | 1.19–1.26 | <0.001 |
| Anemia requiring transfusion | 1.33 | 1.26–1.40 | <0.001 |
| Peripheral nerve injury | 1.88 | 1.53–2.31 | <0.001 |
| Urinary tract infection | 1.24 | 1.09–1.41 | 0.001 |
| Pneumonia | 0.9 | 0.77–1.04 | 0.153 |
| Surgical site infection | 1.12 | 0.95–1.32 | 0.195 |
| Hospital readmission | 1.09 | 1.01–1.16 | 0.018 |
| Hospital LOS ≥4 days | 1.22 | 1.18–1.27 | <0.001 |
RR: relative risk; CI: confidence interval; LOS: length of stay. Values in bold represent those that are statistically significant.
Figure 2.
Incidence of adverse outcome as it related to operative time in 20-min intervals. (a) UTI, (b) any complication, (c) anemia requiring transfusion, and (d) peripheral nerve injury.
Figure 3.
Incidence of increased length of stay (a) and hospital readmission (b) as it relates to increased operative time.
Discussion
TSA is a successful procedure, the demand for which continues to rise.6,7 This increase in utilization highlights the importance of identifying risk factors for complications in order to minimize adverse outcomes. Many risk factors that have previously been identified are patient-specific, and include BMI, 12 DM, 24 age, 25 worker's compensation, 26 depression and anxiety,27,28 smoking, 29 among others.11,16,17 Alternatively, operative duration is of interest as it is a surgeon-specific risk factor that is potentially modifiable. This has received only minimal attention in the literature.11,17
The current investigation found that patients who were younger, male, smokers, and obese had significantly longer operative times when compared to patients who did not meet these criteria. This study identifies operative time as an important surgeon-specific risk factor with important implications for patient outcomes. After controlling for confounding variables, we found with each 20-min increase in operative time, there was a 24% increased risk of any complication, 33% increased risk of anemia requiring transfusion, 88% increased risk of peripheral nerve injury, 24% increased risk of UTI, 9% increased risk of hospital readmission, and 22% increased risk of extended hospital LOS. These results are not entirely dissimilar to those found by Bohl et al. 20 in their study on operative time in primary total hip and knee arthroplasty.
The results of our study were not entirely unexpected. It has been well documented that in many operations, including TSA, the incidence of all complications rise with increasing operative time.18,20 In this study, we were able to identify specific complications that increased with prolonged surgical duration. One of these was anemia requiring transfusion. This is intuitive as prolonged operative time allows for more opportunity for blood loss. Anemia and resultant allogenic blood transfusion has negative implications that have been well described. 30 In their article, Grier et al. suggest that allogenic transfusion may confer increased risk even out to two years postoperatively. 30 Given this finding, the current investigation likely underestimates the ultimate downstream effect of this complication. Additionally, when prolonged operative time is anticipated, surgeons are more inclined to use Foley catheters, or if using the catheter only for the duration of the procedure, a prolonged operation translates to a longer time with an indwelling Foley catheter. Catheters have been repeatedly associated with infection and may be responsible for the increased incidence of UTI in our cohort. 31 Peripheral nerve injury was also significantly increased in our investigation. This complication was essentially non-existent in cases that lasted less than 2.5 h but very high in cases lasting between 290 and 310 min. Given this distribution, it is likely that prolonged retraction and prolonged periods of the operative arm being held in external rotation during long procedures contributed to peripheral nerve injury. 32 While caution should always be exercised, overzealous retraction in particular must be avoided with longer procedural times.
While not the primary aim of our study, the patient factors that were associated with an increased operative time were notable, and included younger age, male, obesity, and positive smoker status. Similar to the study on TKA and THA by Bohl et al., 20 increasing BMI is associated with prolonged operative time, likely due to the added difficulty of the surgical exposure. A similar mechanism may be the reason for prolonged operative time in male patients. On average, males have greater muscle mass than females, which can often make obtaining adequate exposure more challenging. This may potentially be extrapolated to younger patients as well. Additionally, younger patients (<60 years old) may represent cases of more severe pathology given that many patients and surgeons attempt to delay operating on younger patients for as long as possible. The association with smoking status is less clear.
While our results suggest that shorter operative times lead to lower rates of postoperative complications, the speed of the operation should not be prioritized over surgical technique. Instead, the focus should be on improved team efficiency. This should include experienced staff members (nurses, scrub technologists, anesthesiologist, etc.) all of whom are familiar with the procedure and, when possible, the surgeon. Team members unfamiliar with the procedure, instrumentation, or set up can significantly prolong operative time which, as this study demonstrates, has implications for the patient's outcome. While anesthesia time was not included in this analysis, the anesthesiologist likely plays a role in operative time via diligent blood pressure control and appropriate hypotensive anesthesia. 33 Managing blood pressure is known to contribute to less blood loss, and consequently, better operative field visualization. This improved visualization could help speed the operation. This can be accomplished through a multimodal approach and usually involves pressure control and the administration of tranexamic acid.34,35
The importance of surgeon volume should also not be ignored as the relationship between surgeon volume and outcomes has been repeatedly demonstrated in various orthopedic operations,36–38 including TSA.39–41 While this data was not available for analysis in our study, Singh et al. found that operative time was significantly lower when performed at high volume institutions by high volume surgeons, with these surgeons operating 30–50 min faster than the lower volume surgeons. 40 Therefore, given our series' use of 20-min intervals, patients treated by a low volume (and consequently slower) surgeon may be expected to have an increased risk (1.5–2.5 times those reported) for each 20-min increment in the current investigation. Taken together, these findings suggest a role for surgeon specialization and that patients undergoing TSA may be best served by an efficient, high volume surgeon. Lastly, while operative time is potentially modifiable to some degree, certain aspects of operative time are not modifiable. Many factors not captured in this analysis could contribute to increased surgical time and include case complexity (including issues like preoperative deformity, bone loss, muscle mass in men, bone quality, etc.), time needed for safe surgical approach, surgical approach chosen, cemented versus uncemented technique, and specific prosthesis being used. The inability to include these variables must be considered and the results should be interpreted in this context.
In addition to those mentioned above, there are several limitations to the current investigation, many of which are inherent to retrospectively analyzing data from a large database. First, as this is a general surgical database, the outcomes available for analysis are primarily limited to medical complications; many outcomes of interest (i.e. mechanical failure, implant position, pain relief, patient reported outcomes, postoperative instability, and revision rates) are not available for analysis. Similarly, component position, which could conceivably be compromised if the procedure is rushed, cannot be examined. It is important to note that while shorter operative times may indeed decrease medical complication rates, this must not be at the expense of a technically sound operation. Another consideration is that because the NSQIP database tracks complications and outcomes only for 30 days postoperatively, the actual rates of complications may be higher as midterm and long-term complications are not captured. Additionally, given that patients are filed by CPT codes in the NSQIP and due to the nonspecific nature of the CPT code for TSA (CPT 23472), there was no differentiation between anatomic and reverse total shoulder arthroplasties, or total shoulder resurfacing. Lastly, it is true that operative time may be difficult to truly isolate. 42 Many factors associated with longer operative time are also associated with worse outcomes. However, our analysis controlled for available patient demographic and comorbid information in an attempt to limit the effect of confounding variables.
In conclusion, the results of this investigation suggest that operative time in TSA is an important surgeon-related risk factor for increased morbidity. While there are multiple patient factors that may impact operative time, there are also likely modifiable aspects such as surgical technique, surgeon experience, and operative team efficiency. Given these findings, it is critical for surgeons to focus on optimization of operating room efficiency so as to decrease operative time to the shortest possible duration without compromising the technical integrity of the operation. We anticipate the results of this manuscript will be used for provider education, policy decision-making, and perhaps algorithms that could improve safety and efficiency after shoulder arthroplasty.
Acknowledgements
This paper is not based on a previous communication to a society or meeting.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was deemed IRB exempt.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed Consent: As this was an investigation on a large database, informed consent was not applicable.
References
- 1.Zilber S. Shoulder arthroplasty: historical considerations. Open Orthop J 2017; 11: 1100–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohsali KI, Wirth MA, Rockwood CA, Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am 2006; 88: 2279–2292. [DOI] [PubMed] [Google Scholar]
- 3.Deshmukh AV, Koris M, Zurakowski D, et al. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg 2005; 14: 471–479. [DOI] [PubMed] [Google Scholar]
- 4.Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg 2002; 11: 130–135. [DOI] [PubMed] [Google Scholar]
- 5.Bryant D, Litchfield R, Sandow M, et al. A comparison of pain, strength, range of motion, and functional outcomes after hemiarthroplasty and total shoulder arthroplasty in patients with osteoarthritis of the shoulder. a systematic review and meta-analysis. J Bone Joint Surg Am 2005; 87: 1947–1956. [DOI] [PubMed] [Google Scholar]
- 6.Zmistowski B, Padegimas EM, Howley M, et al. Trends and variability in the use of total shoulder arthroplasty for medicare patients. J Am Acad Orthop Surg 2018; 26: 133–141. [DOI] [PubMed] [Google Scholar]
- 7.Day JS, Lau E, Ong KL, et al. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg 2010; 19: 1115–1120. [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Wise BL, Zhang Y, et al. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am 2011; 93: 2249–2254. [DOI] [PubMed] [Google Scholar]
- 9.Bohsali KI, Bois AJ, Wirth MA. Complications of shoulder arthroplasty. J Bone Joint Surg Am 2017; 99: 256–269. [DOI] [PubMed] [Google Scholar]
- 10.Johnson CC, Sodha S, Garzon-Muvdi J, et al. Does preoperative American Society of Anesthesiologists score relate to complications after total shoulder arthroplasty?. Clin Orthop Relat Res 2014; 472: 1589–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anthony CA, Westermann RW, Gao Y, et al. What are risk factors for 30-day morbidity and transfusion in total shoulder arthroplasty? A review of 1922 cases. Clin Orthop Relat Res 2015; 473: 2099–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner ER, Houdek MT, Schleck C, et al. Increasing body mass index is associated with worse outcomes after shoulder arthroplasty. J Bone Joint Surg Am 2017; 99: 929–937. [DOI] [PubMed] [Google Scholar]
- 13.Statz JM, Wagner ER, Houdek MT, et al. Outcomes of primary reverse shoulder arthroplasty in patients with morbid obesity. J Shoulder Elbow Surg 2016; 25: e191–198. [DOI] [PubMed] [Google Scholar]
- 14.Saltzman BM, Chalmers PN, Gupta AK, et al. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2014; 23: 1647–1654. [DOI] [PubMed] [Google Scholar]
- 15.Garcia GH, Fu MC, Dines DM, et al. Malnutrition: a marker for increased complications, mortality, and length of stay after total shoulder arthroplasty. J Shoulder Elbow Surg 2016; 25: 193–200. [DOI] [PubMed] [Google Scholar]
- 16.Waterman BR, Dunn JC, Bader J, et al. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg 2015; 24: 24–30. [DOI] [PubMed] [Google Scholar]
- 17.Chalmers PN, Gupta AK, Rahman Z, et al. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty 2014; 29: 856–860. [DOI] [PubMed] [Google Scholar]
- 18.Daley BJ, Cecil W, Clarke PC, et al. How slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee Surgical Quality Collaborative. J Am Coll Surg 2015; 220: 550–558. [DOI] [PubMed] [Google Scholar]
- 19.Martin CT, Gao Y, Pugely AJ, et al. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg 2013; 22: 1667–1675 e1661. [DOI] [PubMed] [Google Scholar]
- 20.Bohl DD, Ondeck NT, Darrith B, et al. Impact of operative time on adverse events following primary total joint arthroplasty. J Arthroplasty 2018; 33: 2256–2262 e2254. [DOI] [PubMed] [Google Scholar]
- 21.Wilson JM, Holzgrefe RE, Staley CA, et al. Use of a 5-Item Modified Frailty Index for risk stratification in patients undergoing surgical management of distal radius fractures. J Hand Surg Am 2018; 43: 701–709. [DOI] [PubMed] [Google Scholar]
- 22.Segal DN, Wilson JM, Staley C, et al. The 5-Item Modified Frailty Index is predictive of 30-day postoperative complications in patients undergoing kyphoplasty vertebral augmentation. World Neurosurg 2018; 116: e225–e231. [DOI] [PubMed] [Google Scholar]
- 23.Segal DN, Wilson JM, Staley C, et al. The 5-Item Modified Frailty Index Is Predictive of 30-Day Postoperative Complications in Patients Undergoing Kyphoplasty Vertebral Augmentation. World Neurosurg 2018; 116: e225–e231. [DOI] [PubMed] [Google Scholar]
- 24.Ponce BA, Menendez ME, Oladeji LO, et al. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg 2014; 23: 671–678. [DOI] [PubMed] [Google Scholar]
- 25.Wagner ER, Houdek MT, Schleck CD, et al. The role age plays in the outcomes and complications of shoulder arthroplasty. J Shoulder Elbow Surg 2017; 26: 1573–1580. [DOI] [PubMed] [Google Scholar]
- 26.Jawa A, Dasti UR, Fasulo SM, et al. Anatomic total shoulder arthroplasty for patients receiving workers' compensation. J Shoulder Elbow Surg 2015; 24: 1694–1697. [DOI] [PubMed] [Google Scholar]
- 27.Bot AG, Menendez ME, Neuhaus V, et al. The influence of psychiatric comorbidity on perioperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg 2014; 23: 519–527. [DOI] [PubMed] [Google Scholar]
- 28.Mollon B, Mahure SA, Ding DY, et al. The influence of a history of clinical depression on peri-operative outcomes in elective total shoulder arthroplasty: a ten-year national analysis. Bone Joint J 2016; 98-B: 818–824. [DOI] [PubMed] [Google Scholar]
- 29.Hatta T, Werthel JD, Wagner ER, et al. Effect of smoking on complications following primary shoulder arthroplasty. J Shoulder Elbow Surg 2017; 26: 1–6. [DOI] [PubMed] [Google Scholar]
- 30.Grier AJ, Bala A, Penrose CT, et al. Analysis of complication rates following perioperative transfusion in shoulder arthroplasty. J Shoulder Elbow Surg 2017; 26: 1203–1209. [DOI] [PubMed] [Google Scholar]
- 31.Nicolle LE. Catheter-related urinary tract infection. Drugs Aging 2005; 22: 627–639. [DOI] [PubMed] [Google Scholar]
- 32.Nagda SH, Rogers KJ, Sestokas AK, et al. Neer Award 2005: peripheral nerve function during shoulder arthroplasty using intraoperative nerve monitoring. J Shoulder Elbow Surg 2007; 16: S2–58. [DOI] [PubMed] [Google Scholar]
- 33.Juelsgaard P, Larsen UT, Sorensen JV, et al. Hypotensive epidural anesthesia in total knee replacement without tourniquet: reduced blood loss and transfusion. Reg Anesth Pain Med 2001; 26: 105–110. [DOI] [PubMed] [Google Scholar]
- 34.Vara AD, Koueiter DM, Pinkas DE, et al. Intravenous tranexamic acid reduces total blood loss in reverse total shoulder arthroplasty: a prospective, double-blinded, randomized, controlled trial. J Shoulder Elbow Surg 2017; 26: 1383–1389. [DOI] [PubMed] [Google Scholar]
- 35.Kuo LT, Hsu WH, Chi CC, et al. Tranexamic acid in total shoulder arthroplasty and reverse shoulder arthroplasty: a systematic review and meta-analysis. BMC Musculoskelet Disord 2018; 19: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basques BA, Bitterman A, Campbell KJ, et al. Influence of surgeon volume on inpatient complications, cost, and length of stay following total ankle arthroplasty. Foot Ankle Int 2016; 37: 1046–1051. [DOI] [PubMed] [Google Scholar]
- 37.Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med 1979; 301: 1364–1369. [DOI] [PubMed] [Google Scholar]
- 38.Laucis NC, Chowdhury M, Dasgupta A, et al. Trend toward high-volume hospitals and the influence on complications in knee and hip arthroplasty. J Bone Joint Surg Am 2016; 98: 707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain N, Pietrobon R, Hocker S, et al. The relationship between surgeon and hospital volume and outcomes for shoulder arthroplasty. J Bone Joint Surg Am 2004; 86: 496–505. [DOI] [PubMed] [Google Scholar]
- 40.Singh A, Yian EH, Dillon MT, et al. The effect of surgeon and hospital volume on shoulder arthroplasty perioperative quality metrics. J Shoulder Elbow Surg 2014; 23: 1187–1194. [DOI] [PubMed] [Google Scholar]
- 41.Ramkumar PN, Navarro SM, Haeberle HS, et al. Evidence-based thresholds for the volume-value relationship in shoulder arthroplasty: outcomes and economies of scale. J Shoulder Elbow Surg 2017; 26: 1399–1406. [DOI] [PubMed] [Google Scholar]
- 42.Wu XD, Hu KJ, Tian M, et al. Letter to the Editor on Impact of operative time on adverse events following primary total joint arthroplasty. J Arthroplasty 2018; 33: 2701–2702. [DOI] [PubMed] [Google Scholar]