Abstract
Background
Tranexamic acid efficacy on clinically relevant adverse outcomes in patients undergoing shoulder arthroplasty has been contradictory. The aim of this review was to analyze whether tranexamic acid administration could decrease transfusions, infection and hematoma formation in patients undergoing shoulder arthroplasty.
Methods
PubMed, EMBASE, and the Cochrane Library were searched up to May 2019 for randomized controlled trials comparing tranexamic acid to placebo in shoulder arthroplasty. Random-effect models were performed to meta-analyze the evidence. Trial sequential analysis was used to calculate and to establish the conclusiveness of the evidence derived from the meta-analysis.
Results
Four randomized controlled trials comprising 375 patients were included. Meta-analysis showed no effect of tranexamic acid on transfusion rate (RR = 0.48 (adjusted 95% CI 0.05 to 3.85)). The possible effect of tranexamic acid on hematoma formation or infection rates after shoulder arthroplasty is non-estimable with the current evidence. The sample size necessary to reliably determine if tranexamic acid decreases transfusions, infection rates and hematoma formation is not available from the current literature as determined by the trial sequential analysis.
Discussion
While tranexamic acid has proven its efficacy in decreasing blood loss in shoulder arthroplasty, this meta-analysis of randomized controlled trials clarifies that there is currently no conclusive evidence for a positive effect of tranexamic acid upon transfusion rate, infection rates or hematoma formation in patients undergoing primary shoulder arthroplasty.
Keywords: Shoulder arthroplasty, bleeding, transfusion, infection, hematoma, tranexamic acid, trial sequential analysis, efficacy
Introduction
Patients undergoing shoulder arthroplasty (SA) may experience variable degrees of perioperative blood loss, which in the most severe cases, may result in hematoma formation, 1 acute symptomatic anemia, and the need for allogenic blood transfusions.2–4 Although hematoma requiring intervention is a very rare event after SA with a reported rate of 0.3%, 1 the need for blood transfusion is more common with an incidence of 4.3% to 11.3%.3–8 Besides the costs, allogeneic blood transfusion can be associated with rare but serious complications including allergic and immune-mediated reactions, hemodynamic overload, and risk of blood borne infections. 9 Therefore, strategies to decrease the need for allogenic blood transfusion and prevent infection or hematoma formation after SA are warranted.
Tranexamic acid (TXA) is a synthetic analog of the amino acid lysine that serves as an antifibrinolytic agent by reversibly binding to lysine receptor sites on plasminogen reducing the conversion of plasminogen to plasmin and thus preventing fibrin degradation. 10 TXA has been used in the prevention and treatment of excessive bleeding in several clinical settings. 10 Previous studies and meta-analyses of the use of TXA in total hip arthroplasty (THA) and total knee arthroplasty (TKA) have shown that TXA is a safe and cost-effective perioperative method of reducing blood loss and transfusion requirements during or after the procedure.11,12 The current evidence from several meta-analyses of the efficacy of TXA in SA13–18 demonstrate that TXA is effective in decreasing blood loss as measured by drain output, change in hemoglobin (Hb) or total calculated blood loss. However, the results of the efficacy of TXA on clinically relevant adverse outcomes such as transfusions or postoperative infection in SA have been contradictory.
When evaluating the cost-effectiveness of TXA and when generating recommendations regarding the routine administration of TXA in SA the most critical endpoints are whether its use reduces clinically relevant events such as transfusions, delayed recovery, hematoma formation or subsequent infection rate. Therefore, the aim of this study was to synthesize and clarify the conclusiveness of the evidence regarding the efficacy of TXA on blood transfusion, postoperative infection and hematoma formation in patients undergoing SA. These findings may aid to guide future research and may be helpful when generating recommendations upon the adoption of the perioperative use of TXA in SA.
Methods
The methodology described in in the Cochrane Handbook for Systematic Reviews of Interventions 19 was followed to conduct this review and is reported in accordance to the PRISMA statement. 20
Eligibility criteria
Randomized controlled trials (RCTs) with no restrictions on language, or publication status comparing the efficacy of TXA irrespective of the dose or route of administration with placebo or no intervention, in patients who underwent primary or revision total SA (anatomic or reverse) irrespective of the patient’s age or indication for SA.
Outcome measures
Primary outcome was blood transfusion. Secondary outcomes were postoperative infection, hematoma formation, and hematoma formation requiring surgical intervention.
Information sources and search strategy
Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and EMBASE were searched up to May 2019 through the strategy described in Supplementary File 1. To identify ongoing clinical trials and unpublished trials the U.S. National Institutes of Health trials registry and the World Health Organization International Clinical Trials Registry Platform were searched. A manual search of references of all included trials, pertinent reviews, and previous meta-analyses was performed for additional references.
Data collection and analysis
Selection of the studies
Two independent review authors screened all abstracts identified by the search strategy and excluded those clearly irrelevant. Then the full texts of all potentially relevant trials were formally assessed for eligibility against the criteria outlined above. All disagreements were resolved by discussion with a third review author.
Data extraction
Using prepared data-extraction spreadsheet forms, two of the review authors extracted data in duplicate according to Cochrane guidelines. 19 Review authors resolved disagreements by consensus.
Risk of bias assessment
The methodological quality of the included trials was assessed independently by any two of the review authors using the Cochrane Risk of bias tool and the results were reported in a “Risk of bias” table. 21
Grading quality of evidence
The GRADE approach was used to rate the quality of evidence of each outcome as “high”, ‘moderate’, ‘low’, or ‘very-low’.
Data analysis and synthesis
Meta-analysis was performed using the DerSimonian-Laird random-effect model to calculate the relative risks (RRs) and corresponding 95% CI. Random model was selected a priori due to the clinical heterogeneity across the included RCTs. Heterogeneity among trials was quantified with inconsistency factor (I2). 22 A constant continuity correction of 0.5 23 was applied in both-armed zero-events trials (i.e. trials with no events in either the TXA arm or the control arm) to include them in the analysis. Meta-analysis was performed using Stata 14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
Trial sequential analysis
Meta-analyses are prone to random errors resulting in statistically significant treatment effects that do not truly exist (type I error) or overlooking clinically important treatment effects that do (type II error). Trial sequential analysis is a cumulative random-effect meta-analysis methodology24,25 to control for the risk of random errors in meta-analyses. 26 This methodology enables the estimation of the required meta-analysis sample size–optimal information size (OIS) to obtain sufficient statistical power and adjusts the thresholds for statistical significance when the OIS has not been reached. 25 Trial sequential analysis was performed in the present meta-analysis by using the Trial Sequential Analysis software 0.9 (Copenhagen Trials Unit, Denmark). Assumptions made to estimate the OIS for each outcome are described in Table 1.
Table 1.
Assumptions for optimal information size estimations. a
| Outcome | Assumptions |
||||
|---|---|---|---|---|---|
| Minimally important effect | Control event proportion | Measure of heterogeneity | Amount of heterogeneity | Power | |
| Transfusion rates | 60% RRR b | 3.57% c | D2 | 20% | 80% |
| Infection rates | 25% RRR d | 4% e | D2 | 20% | 80% |
| Hematoma formation | 25% RRR d | 30% f | D2 | 20% | 80% |
| Hematoma formation requiring surgery | 25% RRR d | 0.30% g | D2 | 20% | 80% |
All the estimations were derived to yield “moderate” meta-analytic evidence ensuring a maximum type I error (α) of 5%, and a maximum type II error of 20% (i.e. 80% power) and assuming that 20% of the total variation in the meta-analysis would be explained by variation across trials (heterogeneity measured with D2).
60% relative risk reduction (RRR) was selected based on previous literature that suggest this number as the threshold where routinely TXA use turns into a cost-effective intervention. 37
Mean value of control arms of the included RCTs.
25% RRR selected a priori. This is a reasonable expectation of acute treatments if they are to translate into patient-important benefits.
Selected a priori based on the literature.
Mean value of the control arms of the included RCTs and values reported in the literature.
Selected a priori based on the literature. 1
Results
Search results
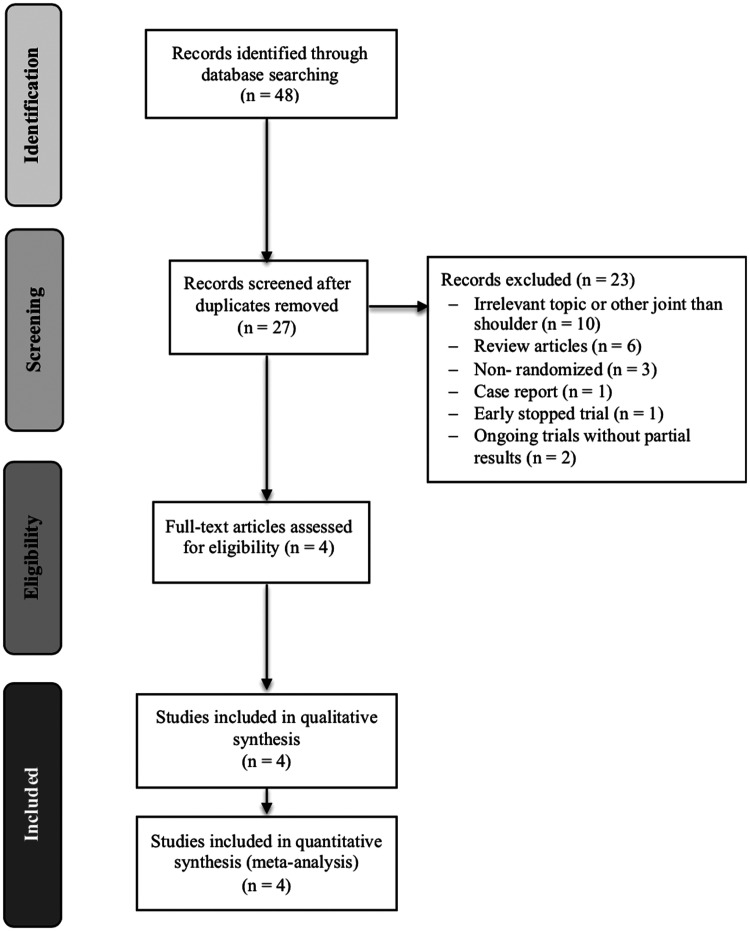
Figure 1 shows the study flow diagram. From the 27 studies reviewed after duplicate removal, we considered six studies eligible. Two ongoing trials (NCT01937559, KCT0002073) were identified and were not included because partial results were not available at the moment of development of this review. Four published RCTs’ patients underwent full-text review and were included.27–30
Figure 1.
Study selection flow diagram.
Included studies
Overall the included trials recruited 375 patients: 188 randomized to TXA and 187 to placebo. Only primary total SA cases were included (118 anatomic and 257 reverse). Of note, while these trials had blood loss as the primary outcome, none included as a primary outcome blood transfusion, hematoma formation or postoperative infection. The characteristics of the included studies are summarized in Table 2 and the description of the protocols of transfusion utilized by each trial is presented in Table 3.
Table 2.
Characteristics of the included trials.
| Author | Year | Country | Setting | Inclusion period | Participants |
Intervention |
Outcomes |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Procedure performed | Indication for SA | Mean age (years) per study arm | Males (%) per study arm | Dose | Route of administration | Control | Primary | Secondary | |||||
| Gillespie et al. 29 | 2015 | USA | Multi- center | October 2012– June 2014 | 111 TXA = 56 Control = 55 | TSA = 44 RTSA = 67 | OA | TXA = 66.4 Control = 67.9 | TXA = 41.1 Control = 47.2 | Single dose 2 g of TXA in 100 mL NS for 5 min | Topical | Placebo. 100 mL NS for 5 min | (1) Postoperative drain output (mL) at 24 h | (1) Change in hemoglobin (2) Rate of transfusion (3) Other complications. |
| Pauzenberger et al. 28 | 2017 | Austria | Single center | July– December 2015 | 54 TXA = 27 Control = 27 | Stemless TSA = 26 RTSA = 28 | N/S | TXA = 70.3 Control = 71.3 | TXA = 67 Control ;= 74 | Two doses 1 g of TXA in 100 mL NS prior to skin incision and 1 g of TXA in 100 mL NS during wound closure | Intravenous | Placebo. 100 mL NS prior to skin incision and 100 mL NS during wound closure | (1) Postoperative drain blood loss (mL) at 24 h | (1) Total calculated blood loss (2) Number of transfusions |
| Vara et al. 30 | 2017 | USA | Single center | September 2013– December 2015 | 102 TXA = 53 Control = 49 | RTSA = 102 | MRCT with or without OA | TXA = 66 Control = 67 | TXA = 49 Control = 38 | Two doses 10 mg/kg of TXA 60 min before surgery and 10 mg/kg of TXA at wound closure | Intravenous | Placebo. 100 mL NS prior to skin incision and 100 mL NS at wound closure | (1) Total calculated blood loss (2) Total hemoglobin loss (3) Total drain output | (1) Number of transfusions (2) DVT, PE, MI hematoma and infection |
| Cvetanovich et al. 27 | 2018 | USA | Single center | September 2015– November 2016 | 108 TXA = 52 Control = 56 | TSA = 44 RTSA = 67 | N/S | TXA = 65.2 Control = 67.7 | TXA = 50 Control = 44.2 | Single dose 1 g of TXA in 10 mL NS administered 10 min before incision | Intravenous | Placebo. 10 mL NS administered 10 min before incision | (1) Total calculated blood loss | (1) Transfusion rates (2) Hemoglobin loss (3) Intraoperative blood loss (4) Hospital length of stay. (5) Postoperative complications, |
TSA: anatomic total shoulder arthroplasty; RTSA: reverse total arthroplasty; TXA: tranexamic acid; NS: normal saline; N/S: not specified; OA: osteoarthritis; MRCT: massive rotator cuff tear; DVT: deep venous thrombosis; PE: pulmonary embolism; MI: myocardial infarction; SSI: surgical site infection.
Table 3.
Protocol of transfusion of the included trials.
| Study | Transfusion trigger |
|---|---|
| Gillespie et al. 29 | Hb level < 7.0 g/dL or Hb level > 7.1 g/dL and < 9.0 g/dL with accompanying signs and symptoms of acute blood loss anemia, as demonstrated by tachycardia (heart rate > 100 beats/min), hypotension (systolic blood pressure < 100 mm Hg), or subjective complaints of light-headedness or dizziness that did not resolve after administration of intravenous fluids. |
| Pauzenberger et al. 28 | Hb level < 8.0 g/dL or Hb level > 8.0 g/dL and < 10.0 g/dL with on-going blood loss or symptoms related to anemia. |
| Vara et al. 30 | Hb level < 7.0 g/dL or Hb level > 7.1 g/dL and < 9.0 g/dL with symptoms of anemia, other than low Hb, including fatigue, palpitation, pallor, tachycardia, or tachypnea. |
| Cvetanovich et al. 27 | Hb level < 7.0 g/dL or higher Hb values only for specific medical indications specified by the consulting hospitalist attending |
Study quality
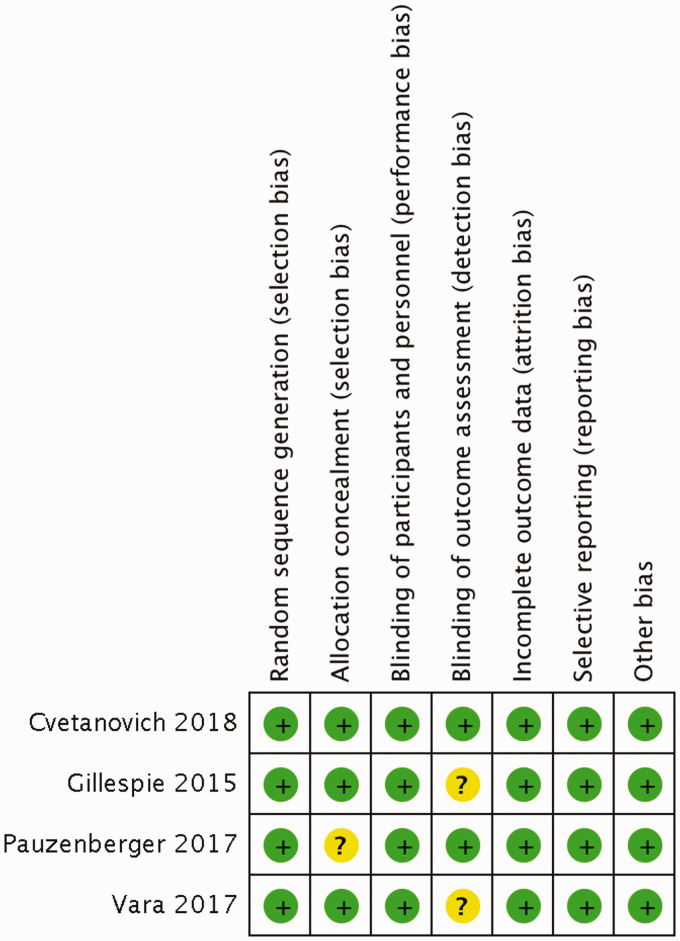
Overall the included studies had a low risk of bias. Risk-of-bias assessments are presented in Figure 2. Detailed authors’ judgements for the risk of bias are presented in the “Risk of bias” tables available in Supplementary File 2.
Figure 2.
Risk of bias summary: review authors’ judgements about each “Risk of bias” item for each included trial. “+” represents low risk of bias; “?” represents unclear risk of bias; “−” represents high risk of bias.
Primary outcome
Blood transfusion
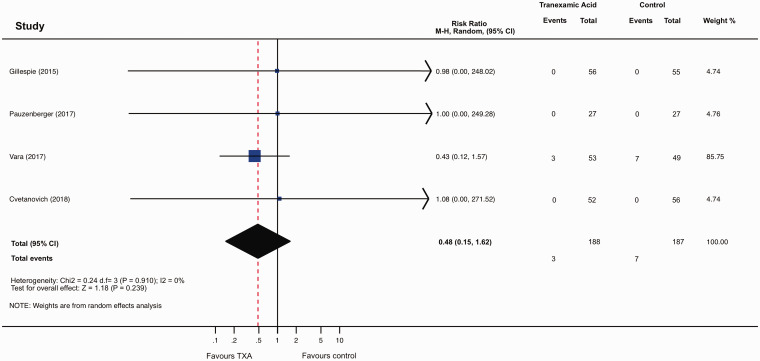
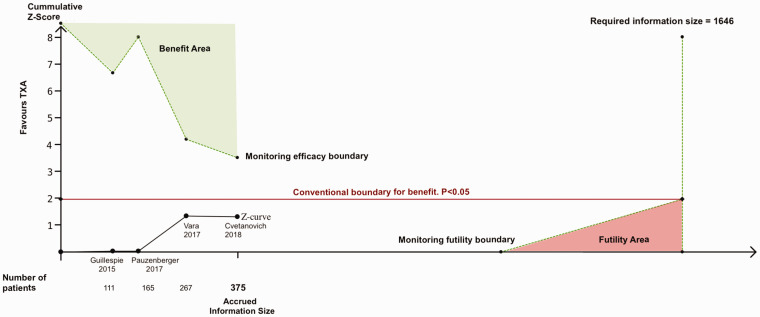
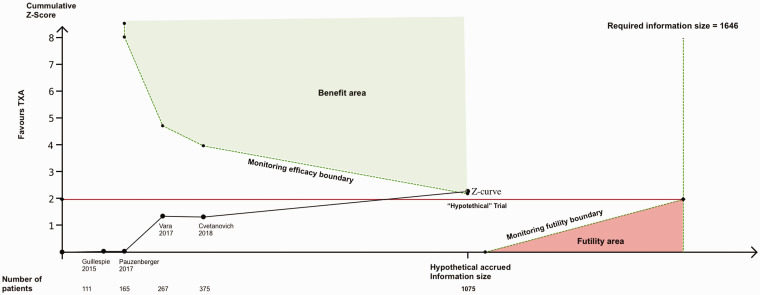
Data on transfusion were provided in the four trials (375 patients). Conventional meta-analysis showed no significant difference in the total number of participants transfused with blood between those treated with TXA and those given placebo (RR 0.48; 95% CI 0.15 to 1.62, p = 0.239, I2 = 0%; Figure 3). Trial sequential analysis estimated that 1646 patients would be required to reliably assess the evidence of the effect of TXA on transfusions in SA. The current evidence has accrued 23% (375/1646) of that OIS. Adjusted 95% CI of the trial sequential analysis was 0.05 to 3.85, which is wider than that of conventional meta-analysis to account for the sparse available sample size. The estimate of the effect of TXA on transfusions did not cross either of the trial sequential analysis monitoring boundaries (Figure 4). These findings suggest that the current evidence for the efficacy of TXA on transfusions in SA is neither reliable nor definitive, leaving the meta-analysis inconclusive. Trial sequential analysis estimated that a new RCT would have to include approximately 700 patients (350 randomized to TXA, and 350 randomized to placebo) for the meta-analysis to cross the efficacy monitoring boundary for moderate evidence (Figure 5). The quality of the evidence for this outcome in accordance with the GRADE approach was judged to be low owing to the small number of participants and the inconclusive results found with the trial sequential analysis.
Figure 3.
Forest plot from conventional random effects meta-analysis. Relative risk < 1 favors the conclusion that TXA reduces risk of transfusion compared with placebo; relative risk > 1 favors the conclusion that TXA increases risk of transfusion compared with placebo. M-H: Mantel-Haenszel; CI: confidence interval; df: degrees of freedom.
Figure 4.
Trial sequential analysis for moderate evidence (α = 5%, β = 20%) of the effect of TXA on blood transfusions. Z-curve (black solid line) represents the cumulative effect of the included studies. Z-score + 1.96 (red solid line) represents the “conventional” efficacy boundary (i.e. p < 0.05) and it was not crossed by the Z-curve. The monitoring boundaries (green dotted lines) were based on the estimated required sample size of 1646 patients. The cumulative Z-curve does not cross either of the two monitoring boundaries (i.e. efficacy and futility boundaries), and thus, there is no statistical evidence to support or reject a treatment effect of TXA on transfusions and more evidence is needed.
Figure 5.
Trial sequential analysis monitoring boundaries for moderate evidence (α = 5%, β = 20%) after adding a “hypothetical trial” with 700 patients. The cumulative Z-curve (black solid line) crossed the monitoring efficacy boundary after adding a “hypothetical trial” with an intervention effect estimate of a 60% relative risk reduction, a transfusion rate of 3.57% in the control group and 700 patients (350 in each group). This result demonstrates that under the event rate assumptions made for the sample size calculation, a new trial would need to include 700 patients (350 in each group) to make the meta-analysis conclusive with moderate statistical support.
Secondary outcomes
Postoperative infection
Two of the included trials27,30 (210 patients) reported data on this outcome. No infection events occurred in any of the trials and therefore the effect of TXA on postoperative infection was non-estimable. Trial sequential analysis estimated that 2856 patients would be required to reliably assess the effect of TXA on postoperative infection. The accrued sample size in the literature for SA so far is only 7% (210/2856) of that estimation.
Hematoma formation
Only one trial 28 (54 patients) reported data on this outcome and therefore meta-analysis was not conducted. Trial sequential analysis estimated that the required sample size for reliable evidence of the effect of TXA on hematoma formation after SA was 893 patients, of which 6% (54/893) has been accrued so far.
Hematoma requiring surgical intervention
No data was found for this outcome in any of the trials. Trial sequential analysis estimated that 39,156 patients would be required to reliably assess the effect of TXA on this outcome.
Discussion
In the present meta-analysis, we did not find a difference in transfusion rates between patients undergoing primary SA receiving TXA versus placebo. However, trial sequential analysis highlighted that the required sample size (1646 patients) to reliably assess the effect of TXA on transfusions in SA had not been reached, indicating that the present meta-analysis is underpowered to be conclusive. Using trial sequential analysis, it was estimated that approximately 700 patients would need to be randomized in future trials to have a conclusive result of the efficacy of TXA on transfusions in primary SA. For the secondary outcomes of hematoma formation or postoperative infection the current data in the literature was also insufficient to conduct a meta-analysis of the effects of TXA upon these variables.
The small sample size and low number of events in the outcomes of interest of the present meta-analysis can be explained by several factors. First, none of the included RCTs considered clinically relevant events such as transfusion, postoperative infection or hematoma formation as primary outcomes and thus their sample sizes were not calculated to detect differences in those events. Second, in all included RCTs restrictive transfusion thresholds were utilized and patients at higher risk of transfusion (e.g. revision SA, low preoperative hemoglobin/hematocrit, ischemic heart disease) were excluded. As a result, the number of events of transfusion was very low with a transfusion rate in the control group of the RCTs (0% to 3.7%) much lower than that reported in the literature for the general population of patients undergoing SA (4.3% to 11.3%).2–8,31
All the RCTs included in this meta-analysis used blood loss as the primary endpoint. While some authors argue that blood loss may be considered as the most valid and clinically significant outcome for assessment of the efficacy of antifibrinolytic therapy, 32 there are recognized limitations of the use of blood loss as the only primary clinical endpoint. Firstly, perioperative bleeding is a natural continuous variable and although there is general agreement that less bleeding is better, all patients bleed after surgery and it remains unclear at what point bleeding becomes clinically significant. Second, it has been proven that there is no lineal relationship between bleeding and clinically relevant adverse outcomes and that even relatively large differences in bleeding (e.g. 150 mL vs. 300 mL) are not necessarily accompanied by a clinically relevant difference in outcome. 33 Third, the relevance of bleeding does not depend only in its amount but also in its location and in patient-related factors. For example, a relatively limited amount of bleeding that leads to hematoma formation may be a determinant of an adverse outcome. Similarly, patients’ tolerance to the anemia resulting from the bleeding is variable and may partially depend on the preoperative Hb level.
Given these limitations, it is required a standardized definition of perioperative bleeding after SA which considers not only the amount of blood loss but the presence of clinically relevant events such as transfusion, hematoma formation, infection, fatigue, pain, and delayed recovery. Since to date there is no such definition, the assessment of the efficacy of antifibrinolytic therapy requires the direct evaluation of the impact on clinically adverse relevant outcomes.
Relation to other meta-analyses
Previous meta-analyses have consistently shown a significant effect of TXA in decreasing blood loss and Hb change after primary SA. Pooled mean differences reported in those meta-analyses ranged between 172 and 267 mL for total blood loss, between 95 and 133 mL for drain output and between 0.5 and 0.9 g/dL for Hb change (Table 4). However, the clinical significance of these differences is arguable considering that the magnitude of the effect sizes is less than 50% of those reported in THA and TKA, 11 but more importantly, considering that the effect of such decreases in blood loss on the decrease of transfusion requirements has been contradictory.
Table 4.
Summary of the findings of previous meta-analyses of tranexamic acid in shoulder arthroplasty.
| Variable |
Sun et al.
16
|
He et al.
18
|
Yu et al.
15
|
Kirsch et al.
13
|
Kuo et al.
14
|
Box et al.
17
|
|---|---|---|---|---|---|---|
| Included studies | Gillespie et al., 29 Friedman (2016),42 Abildgaard (2016), a, 43 Vara et al. 30 | Gillespie et al., 29 Friedman (2016),42 Abildgaard (2016), a, 43 Vara et al. 30 | Gillespie et al., 29 Friedman (2016),42 Abildgaard (2016),43 Vara et al. 30 | Gillespie et al., 29 Friedman (2016),42 Abildgaard (2016),43 Vara et al., 30 Pauzenberger et al. 28 | Gillespie et al., 29 Friedman (2016),42 Abildgaard (2016),43 Vara et al., 30 Pauzenberger et al., 28 Kim (2017) b, 44 | Gillespie et al., 29 Friedman (2016),42 Abildgaard (2016),43 Vara et al., 30 Pauzenberger et al., 28 Kim (2017)44 |
| Design of the included studies | 2 RCT 2 Non-RCT | 2 RCT 2 Non-RCT | 2 RCT 2 Non-RCT | 3 RCT 2 Non-RCT | 3 RCT 3 Non-RCT | 3 RCT 3 Non-RCT |
| Level of evidence | III | III | III | III | III | III |
| Total shoulders (#) | 484 | 484 | 484 | 632 | 680 | 680 |
| Control (#) | 234 | 234 | 234 | 313 | 337 | 337 |
| TXA (#) | 250 | 250 | 250 | 319 | 343 | 343 |
| Hemoglobin change | ||||||
| Estimated effect of TXA (95% CI) | MD −0.88 g/dL (−1.33 to −0.44) c | MD −0.53 g/dL (−0.83 to −0.23) c | MD −0.71 g/dL (−0.91 to −0.5) c | MD −0.64 g/dL (−0.84 to −0.44) c | MD −0.64 g/dL (−0.81 to −0.46) c | MD −0.63 g/dL (−0.87 to −0.39) c |
| Total blood loss | ||||||
| Estimated effect of TXA (95% CI) | MD −172.2 mL (−308.9 to −35.5) c | MD −267.1 mL (−385.1 to −149.1) c | MD −226.8 mL (−301.4 to −152.3) c | NR | MD −249.2 mL (−338.7 to −159.7) c | MD −231.9 mL (−334.2 to −129.5) c |
| Drain output | ||||||
| Estimated effect of TXA (95% CI) | NR | WMD −119.3 mL (−165.6 to −73.1) c | MD −133.2 mL (−194.7 to −71.8) c | MD −116.8 mL (−139.2 to −94.4) c | MD −95.4 mL (−139.9 to −50.9) c | MD −112.1 mL (−182.3 to −41.8) c |
| Allogenic blood transfusions | ||||||
| Transfusion rate controls | 5.24% | 5.24% | 5.24% | 4.79% | 6.39% | 4.45% |
| Transfusion rate TXA | 2.40% | 2.40% | 2.40% | 2.19% | 2.19% | 2.04% |
| Estimated effect of TXA (95% CI) | OR 0.34 (0.13 to 0.91) c | RR = 0.45 (0.21–0.99) c | RD = −3% (−6% to 0%) | RR = 0.45 (0.18 to 1.09) | RR 0.34 (0.14 to 0.79) c | RD = −3% (−5% to 0%) |
| Statistical model for meta-analysis | Random-effect model | Random-effect model | Fixed-effect model | Random-effect model | Random-effect model | Fixed-effect model |
| Management of both armed zero event studies when estimating the pooled effect | Included | Included | Included | Excluded | Excluded | Included |
RCT: randomized controlled trial; MD: mean difference; WMD: weighted mean difference; OR: odds ratio; RR: risk ratio; RD: risk difference.
Patients with reverse total shoulder arthroplasty from this study were not included in the meta-analysis.
This study was not included when pooling the effect.
Statistically significant difference.
Three meta-analyses14,16,18 reported significant reduction of blood transfusions after SA with TXA, whereas other three13,15,17 did not find significant differences on transfusions after SA between TXA and placebo (Table 4). Possible reasons for these contradictory results include the inclusion of retrospective non-randomized studies, the use of different pooling methodologies to produce a combined estimate (i.e. random vs. fixed meta-analytical models), the selection of different measures of the effect (e.g. risk ratio, odds ratio, risk difference), the exclusion of studies with no events of transfusion when pooling the combined effect and the lack of methods to controlling the risk of random errors given the scarce data and repetitive testing on accumulating data.
The present meta-analysis clarifies these contradictory results, showing that the current evidence of the effect of TXA in transfusion rates in SA is inconclusive and the OIS to have firm evidence is far from reached. These findings illustrate how the conclusiveness and credibility of statistically significant meta-analyses with too few participants or events is poor, and as a result intervention effects are often spuriously overestimated (type I errors).
Strengths and limitations of this review
This is the first systematic review and meta-analysis of RCTs on perioperative TXA in SA focused only on high-quality RCTs and clinically relevant adverse outcomes. This meta-analysis is unique in the use of trial sequential analysis which accounts for sparse data and repetitive testing on accumulating data. Applying Cochrane and PRISMA methodologies is another strength of this systematic review. Limitations of our review include the clinical heterogeneity which could have resulted from different transfusion thresholds and TXA regimes between trials, and the limited or inexistent data on the secondary outcomes in the available trials. Although random models were performed to account for this variation, clinical heterogeneity may increase the risk of type II error.
The estimations of the OIS made with trial sequential analysis are only reliable to the extent that the assumptions (i.e. control group incidence rate and intervention effect) are a good approximation of the “truth”. Control group incidence rate and intervention effect assumed in the present analysis were presented transparently and represent what it might realistically be expected given the current data. Despite these limitations, trial sequential analysis represents one of several new developments in interpreting the utility of meta-analyses24,25,34,35 and is more informative than subjective assessments of the conclusiveness of the evidence.
Unanswered questions and future perspectives
Whether TXA should be used routinely in all patients undergoing SA is still debatable. While the results of this review show that the effect of TXA in transfusion rates, hematoma formation or infection rates in primary SA is inconclusive, an absence of evidence is not evidence of absence of effect. It is possible that TXA shows a conclusive beneficial effect on the clinically relevant outcomes when a meta-analysis would eventually reach the OIS.
A population-based retrospective study using national claims data from 82,512 patients undergoing SA reported that TXA was associated with a 36% decrease in transfusion risk (OR, 0.64; 95% CI, 0.52 to 0.77; p < 0.05) after adjustment for relevant covariates. 36 However, retrospective studies may have severe limitations to adjust for all the confounding effects of pre- and intraoperative variables and even the most complete databases do not include a number of potentially important variables. The most important variable among these is the clinical judgement of the attending physician making the decision regarding the transfusion. In addition, a positive statistically significant finding such as a RR reduction does not alone provide sufficient evidence that the intervention should be universally adopted or that it will be cost-saving. 37 Baseline event rates need to be carefully considered when evaluating a new intervention effect. 38 In THA and TKA, cost-analysis have shown that TXA will be cost-saving only if it reduces transfusions and if the baseline risk of blood transfusion is larger than 25% 37 which are conditions not fulfilled in SA.
The results of this meta-analysis demonstrate that in a subgroup of patients undergoing SA such as those selected in the included RCTs (i.e. primary SA without risk factors for transfusions), transfusions rates are very low ranging from 0% to 3.7%. Other recent studies on primary total SA and shoulder surface replacement have also reported transfusion rates lower than 4%.39,40 As a result, the effectiveness and cost-effectiveness of TXA in terms of decreasing the need of transfusions would be marginal in this specific subgroup of patients, as it would be required to treat a very high number of patients with TXA for preventing only one event of transfusion. These conclusions may not apply to patients with a higher baseline risk of transfusion such as revision arthroplasty, arthroplasty for fractures or patients with preoperative anemia, in whom TXA may be more effective. Further studies would be necessary to evaluate the clinical efficacy of TXA in these subgroups of patients. A recent retrospective study evaluated the efficacy of TXA in SA patients with preoperative anemia. 41 These authors found that the transfusion rate in this group of patients was 25% and TXA was associated with a reduction of 80% in transfusion rates. If the main aim with perioperative use of TXA is decreasing transfusions, it is possible that TXA in SA is a cost-effective intervention only in selected subgroups of patients.
On the other side, TXA may be beneficial for patients undergoing SA even if it has no or minimal effect in transfusion or infection rates. Decreased bleeding could possibly lead to lower rates of hematoma formation, which can theoretically also improve outcomes by decreasing pain and improving postoperative therapy. Nevertheless, there is no evidence of the effect of TXA on pain, range of motion or postoperative therapy after SA. In order to evaluate other benefits of TXA in SA it is recommended that future investigations of the efficacy of TXA in SA collect data on clinical outcomes including pain, postoperative rehabilitation, and patient-reported outcomes.
The question of whether TXA is effective in decreasing blood loss in SA is already resolved.13–18 However, whether TXA remains as a cost-effective intervention even if it has no or minimal effect on transfusion rates, the best dose and route of administration of TXA in SA and the effect of TXA on subpopulations of SA patients with differential risks are still unanswered questions. There is a need for large multicenter pragmatic RCTs to examine the effectiveness of TXA on clinically relevant outcomes in a heterogeneous group of patients undergoing SA, including patients with a higher baseline risk of transfusion (e.g. revision SA, SA for fractures, preoperative anemia). Similarly, there is a need for a consensus to define and validate an evaluation system for assessing hematoma formation after SA and to delineate a universal definition of perioperative bleeding in SA which precisely describe and quantify bleeding in SA.
Conclusions
The currently available RCTs on the perioperative administration of TXA do not answer whether its use yields better, worse, or equivalent results compared with placebo in terms of blood transfusions, hematoma formation, and postoperative infection in SA. A much larger number of patients would be required to answer the effect of TXA upon these important clinical outcomes. Until more evidence becomes available, centers considering routine use of TXA in SA use should consider their costs, baseline risk of transfusion, hematoma formation, and infection rates and monitor the impact of TXA use on these outcomes to determine the cost-effectiveness of TXA in their own clinical environments.
Supplemental Material
Supplemental material, SEL896794 Supplemental Material1 for Inconclusive evidence for the efficacy of tranexamic acid in reducing transfusions, postoperative infection or hematoma formation after primary shoulder arthroplasty: A meta-analysis with trial sequential analysis by Jorge Rojas, Uma Srikumaran and Edward G McFarland in Shoulder & Elbow
Supplemental material, SEL896794 Supplemental Material2 for Inconclusive evidence for the efficacy of tranexamic acid in reducing transfusions, postoperative infection or hematoma formation after primary shoulder arthroplasty: A meta-analysis with trial sequential analysis by Jorge Rojas, Uma Srikumaran and Edward G McFarland in Shoulder & Elbow
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Each author certifies that he has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements) that might pose a conflict of interest in connection with the submitted article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jorge Rojas https://orcid.org/0000-0002-1714-9261
Supplemental material: Supplemental material is available at: http://journals.sagepub.com
References
- 1.Cheung EV, Sperling JW, Cofield RH. Infection associated with hematoma formation after shoulder arthroplasty. Clin Orthop Relat Res 2008; 466: 1363–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling JW, Duncan SFM, Cofield RH, et al. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg 2005; 14: 599–601. [DOI] [PubMed] [Google Scholar]
- 3.Padegimas EM, Clyde CT, Zmistowski BM, et al. Risk factors for blood transfusion after shoulder arthroplasty. Bone Joint J 2016; 98-B: 224–228. [DOI] [PubMed] [Google Scholar]
- 4.Ryan DJ, Yoshihara H, Yoneoka D, et al. Blood transfusion in primary total shoulder arthroplasty: incidence, trends, and risk factors in the United States from 2000 to 2009. J Shoulder Elbow Surg 2015; 24: 760–765. [DOI] [PubMed] [Google Scholar]
- 5.Makhni EC, Trofa DP, Watling JP, et al. Risk factors associated with blood transfusion after shoulder arthroplasty. JSES Open Access 2017; 1: 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anthony CA, Westermann RW, Gao Y, et al. What are risk factors for 30-day morbidity and transfusion in total shoulder arthroplasty? a review of 1922 cases. Clin Orthop Relat Res 2015; 473: 2099–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg 2013; 22: 233–239. [DOI] [PubMed] [Google Scholar]
- 8.Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution’s experience and review of the literature. J Shoulder Elbow Surg 2014; 23: 43–48. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen HJ. Detrimental effects of perioperative blood transfusion. Br J Surg 1995; 82: 582–587. [DOI] [PubMed] [Google Scholar]
- 10.Reed MR, Woolley LCT. Uses of tranexamic acid. Contin Educ Anaesth Crit Care Pain 2015; 15: 32–37. [Google Scholar]
- 11.Melvin JS, Stryker LS, Sierra RJ. Tranexamic acid in hip and knee. J Am Acad Orthop Surg 2015; 23: 732–740. [DOI] [PubMed] [Google Scholar]
- 12.Ker K, Edwards P, Perel P, et al. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. Brit Med J 2012; 344: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirsch JM, Bedi A, Horner N, et al. Tranexamic acid in shoulder arthroplasty a systematic review and meta-analysis. JBJS Rev 2017; 5: e3–e3. [DOI] [PubMed] [Google Scholar]
- 14.Kuo L-T, Hsu W-H, Chi C-C, et al. Tranexamic acid in total shoulder arthroplasty and reverse shoulder arthroplasty: a systematic review and meta-analysis. BMC Musculoskelet Disord 2018; 19: 60–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu B-F, Yang G-J, Li Q, et al. Tranexamic acid decreases blood loss in shoulder arthroplasty: a meta-analysis. Medicine 2017; 96: e7762–e7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun C, Zhang L, Mi L, et al. Efficiency and safety of tranexamic acid in reducing blood loss in total shoulder arthroplasty. Medicine 2017; 96: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Box HN, Tisano BS, Khazzam M. Tranexamic acid administration for anatomic and reverse total shoulder arthroplasty: a systematic review and meta-analysis. JSES Open Access 2018; 2: 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He J, Wang X, Yuan G-H, et al. The efficacy of tranexamic acid in reducing blood loss in total shoulder arthroplasty. Medicine 2017; 96: e7880–e7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JPT, Thomas J, Chandler J (eds) Cochrane handbook for systematic reviews of interventions. Version 5.1.0. London: The Cochrane Collaboration, 2011.
- 20.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100–e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 23.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 2004; 23: 1351–1375. [DOI] [PubMed] [Google Scholar]
- 24.Wetterslev J, Thorlund K, Brok J, et al. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008; 61: 64–75. [DOI] [PubMed] [Google Scholar]
- 25.Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol 2017; 17: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner RM, Bird SM, Higgins JPT. The impact of study size on meta-analyses: examination of underpowered studies in Cochrane reviews. PLoS One 2013; 8: e59202–e59202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cvetanovich GL, Fillingham YA, O’Brien M, et al. Tranexamic acid reduces blood loss after primary shoulder arthroplasty: a double-blind, placebo-controlled, prospective, randomized controlled trial. JSES Open Access 2018; 2: 23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pauzenberger L, Domej MA, Heuberer PR, et al. The effect of intravenous tranexamic acid on blood loss and early postoperative pain in total shoulder arthroplasty. Bone Joint J 2017; 99-B: 1073–1079. [DOI] [PubMed] [Google Scholar]
- 29.Gillespie R, Shishani Y, Joseph S, et al. Neer Award 2015: a randomized, prospective evaluation on the effectiveness of tranexamic acid in reducing blood loss after total shoulder arthroplasty. J Shoulder Elbow Surg 2015; 24: 1679–1684. [DOI] [PubMed] [Google Scholar]
- 30.Vara AD, Koueiter DM, Pinkas DE, et al. Intravenous tranexamic acid reduces total blood loss in reverse total shoulder arthroplasty: a prospective, double-blinded, randomized, controlled trial. J Shoulder Elbow Surg 2017; 26: 1383–1389. [DOI] [PubMed] [Google Scholar]
- 31.Kandil A, Griffin J, Novicoff W, et al. Blood transfusion after total shoulder arthroplasty: which patients are at high risk? Int J Shoulder Surg 2016; 10: 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNicol ED, Tzortzopoulou A, Schumann R, et al. Antifibrinolytic agents for reducing blood loss in scoliosis surgery in children. Cochrane Database Syst Rev 2016; 9: CD006883–CD006883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranucci M. Outcome measures and quality markers for perioperative blood loss and transfusion in cardiac surgery. Can J Anesth 2016; 63: 169–175. [DOI] [PubMed] [Google Scholar]
- 34.Roshanov PS, Dennis BB, Pasic N, et al. When is a meta-analysis conclusive? A guide to trial sequential analysis with an example of remote ischemic preconditioning for renoprotection in patients undergoing cardiac surgery. Nephrol Dial Transplant 2017; 32: ii23–ii30. [DOI] [PubMed] [Google Scholar]
- 35.Thorlund K, Devereaux PJ, Wetterslev J, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol 2009; 38: 276–286. [DOI] [PubMed] [Google Scholar]
- 36.Anthony S, Patterson D, Cagle P, et al. Utilization and real-world effectiveness of tranexamic use in shoulder arthroplasty. J Am Acad Orthop Surg 2019; 1: 736–742. [DOI] [PubMed] [Google Scholar]
- 37.Slover J, Bosco J. Cost analysis of use of tranexamic acid to prevent major bleeding complications in hip and knee arthroplasty surgery. Am J Orthop 2014; 43: E217–220. [PubMed] [Google Scholar]
- 38.Sinclair JC, Cook RJ, Guyatt GH, et al. When should an effective treatment be used? Derivation of the threshold number needed to treat and the minimum event rate for treatment. J Clin Epidemiol 2001; 54: 253–262. [DOI] [PubMed] [Google Scholar]
- 39.Dacombe PJ, Kendall JV, McCann PAS, et al. Blood transfusion rates following shoulder arthroplasty in a high volume UK centre and analysis of risk factors associated with transfusion. Shoulder Elbow 2018; 11: 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh VK, Trehan R, Banerjee S, et al. Blood loss and the need for transfusion after shoulder surface replacement. Shoulder Elbow 2013; 5: 100–105. [Google Scholar]
- 41.Clay TB, Lawal AS, Wright TW, et al. Tranexamic acid use is associated with lower transfusion rates in shoulder arthroplasty patients with preoperative anaemia. Shoulder Elbow. Epub ahead of print 2019. DOI: 10.1177/1758573219841058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedman RJ, Gordon E, Butler RB, et al. Tranexamic acid decreases blood loss after total shoulder arthroplasty. J Shoulder Elbow Surg 2016; 25: 614–618. [DOI] [PubMed]
- 43.Abildgaard JT, McLemore R and Hattrup SJ. Tranexamic acid decreases blood loss in total shoulder arthroplasty and reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2016; 25: 1643–1648. [DOI] [PubMed]
- 44.Kim SH, Jung WI, Kim YJ, et al. Effect of Tranexamic Acid on Hematologic Values and Blood Loss in Reverse Total Shoulder Arthroplasty. Biomed Res Int 2017; 2017. DOI: 10.1155/2017/9590803. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, SEL896794 Supplemental Material1 for Inconclusive evidence for the efficacy of tranexamic acid in reducing transfusions, postoperative infection or hematoma formation after primary shoulder arthroplasty: A meta-analysis with trial sequential analysis by Jorge Rojas, Uma Srikumaran and Edward G McFarland in Shoulder & Elbow
Supplemental material, SEL896794 Supplemental Material2 for Inconclusive evidence for the efficacy of tranexamic acid in reducing transfusions, postoperative infection or hematoma formation after primary shoulder arthroplasty: A meta-analysis with trial sequential analysis by Jorge Rojas, Uma Srikumaran and Edward G McFarland in Shoulder & Elbow