Abstract
Skin cancer is caused by abnormal proliferation, gene regulation and mutation of epidermis cells. Compound C is commonly used as an inhibitor of AMP-activated protein kinase (AMPK), which serves as an energy sensor in cells. Recently, compound C has been reported to induce apoptotic and autophagic death in various skin cancer cell lines via an AMPK-independent pathway. However, the signaling pathways activated in compound C-treated cancer cells remain unclear. The present oligodeoxynucleotide-based microarray screening assay showed that the mRNA expression of the zinc-finger transcription factor early growth response-1 (EGR-1), which helps regulate cell cycle progression and cell survival, was significantly upregulated in compound C-treated skin cancer cells. Compound C was demonstrated to induce EGR-1 mRNA and protein expression in a time and dose-dependent manner. Confocal imaging showed that compound C-induced EGR-1 protein expression was localized in the nucleus. Compound C was demonstrated to activate extracellular signal-regulated kinase (ERK) phosphorylation. Inhibition of this compound C-induced ERK phosphorylation downregulated the mRNA and protein expression of EGR-1. In addition, removal of compound C-induced reactive oxygen species (ROS) not only decreased ERK phosphorylation, but also inhibited compound C-induced EGR-1 expression. A functional assay showed that knock down of EGR-1 expression in cancer cells decreased the survival rate while also increasing caspase-3 activity and apoptotic marker expression after compound C treatment. However, no difference in autophagy marker light chain 3-II protein expression was observed between compound C-treated control cells and EGR-1-knockdown cells. Thus, it was concluded that that EGR-1 may antagonize compound C-induced apoptosis but not compound C-induced autophagy through the ROS-mediated ERK activation pathway.
Keywords: early growth response-1, adenosine 5′ monophosphate-activated protein kinase, compound C
Introduction
Compound C (6-[4-(2-piperidin-1-yl-ethoxy)-phenyl] 3-pyridin-4-yl-pyrazolo[1,5-a] pyrimidine) is a small molecule with cell-permeable and selective ATP-competitive functions that inhibits AMP-activated protein kinase (AMPK) and interferes with AMPK metabolic regulation (1). Compound C has been reported to attenuate metformin-induced apoptosis via AMPK inhibition (2). In contrast, it has been suggested that compound C treatment directly induces apoptosis in hepatoma, glioma and breast carcinoma cells (2–4). In addition, compound C has been described to inhibit AMPK-dependent autophagy in yeast, hepatocytes and HeLa cells (5). Therefore, controversially, compound C has dual roles in autophagic protection against compound C-induced apoptosis in glioma cancer cells, and autophagic cell death in colorectal cancer cells (6,7). This evidence indicates that compound C has variable effects on apoptosis and the type of autophagy in dose-, cell type- and/or context-dependent manners.
The transcription factor early growth response-1 (EGR-1) is a member of the immediate-early family of genes and encodes a 59-kDa phosphoprotein (8). Numerous factors, such as growth factors, cytokines, radiation, injury and mechanical stress, can induce EGR-1 protein expression (9). There are five serum response elements (SREs) in the EGR-1 promoter region, and the level of EGR-1 transcription is most commonly mediated by transcription factors in the Elk-1 family, which are activated by the MAP kinase family. CREB-binding protein (CBP) and serum response factor (SRF) associate with Elk-1 to form the ternary complex factor that binds to SREs (9,10). There are several specificity protein 1 (SP1) consensus sequences, a putative AP-1-binding site, functional cAMP regulatory elements (CREs) and a functional NF-κB-binding site in the EGR-1 promoter region (10). EGR1 transcription is also self-regulated via binding to functional EGR-1 binding sites (EBS). The targeting of EGR-1 by E26 transformation-specific transcription factors is involved in hematopoiesis, angiogenesis and neoplasia (11). The EGR-1 promoter contains two activating transcription factor 5 (ATF5) consensus sequences that are activated by ATF5 in fibroblasts (10). Moreover, there are two functional non-consensus binding sites for the tumor suppressor protein p53. The binding of p53 to the EGR-1 promoter in response to DNA damage leads to sustained expression of EGR-1 protein and induction of apoptosis (12). The activity and stability of the EGR-1 protein are regulated by post-translational modifications. Acetylation improves EGR-1 protein stability and may facilitate cell survival, whereas phosphorylation of EGR-1 protein in response to stress may favor cell death (13). Sumoylation directs EGR-1 protein to the nucleus (14), and the short half-life of EGR-1 protein is a result of ubiquitination and degradation by the proteasome (15).
EGR-1 protein is associated with cell proliferation and the regulation of apoptosis as it belongs to a network of tumor suppressor genes that include p53 and p73, which promote apoptosis in cancer cells in response to stress and DNA damage (16–19). In contrast, EGR-1 protein specifically promotes prostate cancer progression. The mRNA expression of EGR-1 is higher in prostate adenocarcinoma tissue compared with normal tissues. The degree of differentiation of carcinoma cells is inversely correlated with the levels of EGR-1 mRNA and protein expression (20). Evidence indicates that the role of EGR-1 protein in prostate cancer could be due, at least in part, to an interaction with the androgen receptor (21). Thus, while EGR-1 protein mediates apoptosis in response to stress and DNA damage by regulating a tumor suppressor network, it also promotes the proliferation of prostate cancer cells via a mechanism that is not fully understood. EGR-1 protein was reported to bind to the promoter of the autophagy gene light chain 3B (LC3B), increasing LC3B expression and promoting autophagy. Knocking down EGR-1 expression inhibits cigarette smoke extract-induced autophagy and protects epithelial cells from cigarette smoke extract-induced apoptosis (22).
In our previous study, we demonstrated that compound C induced not only autophagy but also apoptosis in skin cancer cells (23). The present study showed that compound C could induce EGR-1 gene expression through the reactive oxygen species (ROS)-mediated extracellular signal-regulated kinase (ERK) activation pathway, which provided a protective response against compound C-induced apoptosis. Thus, targeting EGR-1 expression may increase the antitumor efficacy of compound C in skin cancer.
Materials and methods
Reagents and antibodies
Compound C was purchased from Calbiochem; Merck KGaA. Metformin, N-acetylcysteine (NAC) and U0126 were obtained from Sigma-Aldrich; Merck KGaA. Cells were treated with 40 µM compound C for 0, 1, 2, 4, 6, 8, 12, or 24 h, or 0, 1, 5, 10, 20, or 40 µM compound C for 6 or 8 h. Cells were treated with 4 mM metformin for 0, 1, 2, 4, 6, 8, 12, or 24 h, or 0, 1, 5, 10, 20, or 40 µM compound C for 6 or 8 h. Cells were treated with 2 mM NAC or 10 µM U0126 for 1 h before compound treatment. An antibody specific for LC3 (cat. no. NB600-1384) was purchased from Novus Biologicals, LLC. Antibodies specific for p53 (cat. no. 9282), cleaved caspase-3 (cat. no. 9661), caspase-9 (cat. no. 7237), poly ADP-ribose polymerase (PARP) (cat. no. 9541), EGR-1 (cat. no. 4154), AMPKα (cat. no. 2532), phosphorylated-AMPKα (Thr172) (cat. no. 2535), phosphorylated-ERK (cat. no. 9101) and ERK (cat. no. 4695) were purchased from Cell Signaling Technology, Inc. An antibody specific for β-actin (cat. no. sc-47778) was purchased from Santa Cruz Biotechnology, Inc.. All antibodies were 1000-fold dilution for detection.
Cell culture
A basal cell carcinoma (BCC) cell line (BCC-1/KMC) was cultured in Roswell Park Memorial Institute-1640 (RPMI-1640) (Gibco; Thermo Fisher Scientific, Inc.) medium supplemented with 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.). The human keratinocyte cell line HaCaT and human melanoma cell lines C32 and A375 were obtained from Bioresource Collection and Research Center. C32 and A375 cells were cultured in Minimum Essential Medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS. HaCaT cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS.
Cell viability assay
Cell viability was evaluated with a trypan blue exclusion assay (Gibco; Thermo Fisher Scientific, Inc.). Cells were seeded at 2×105 cells/well in a 6-well plate before compound C treatment. After compound C treatment for 48 h, the cells were collected and stained with trypan blue for 3 min at room temperature to count the number of viable cells under a light microscope (Carl Zeiss AF).
ROS detection
Cells were pretreated with 2 mM NAC for 1 h at 37°C and then treated with compound C for 4 h. After compound C treatment, the cells were stained with 1 µM CM-H2DCFDA (Invitrogen; Thermo Fisher Scientific, Inc.) for 30 min at 37°C and the stained cells were detected with a BD FACSCalibur™ cytometer and analyzed with BD CellQuest™ Pro, Version 6.0 (Beckman Coulter, Inc.).
Cell cycle analysis
Cells treated with compound C for 48 h were harvested and fixed in 70% ethanol at 4°C for overnight. After centrifugation (300 × g) at 4°C for 5 min, the cell pellets were resuspended in a buffer containing phosphate-buffered saline (PBS), 0.05% RNase A and 40 µg/ml propidium iodide (PI) and incubated at 37°C for 30 min. After staining, the cells were collected and resuspended in PBS. The fluorescence emitted from the PI-DNA complexes following laser excitation of the fluorescent dye was quantified using a BD FACSCalibur™ cytometer and analyzed with BD CellQuest™ Pro version 6.0 (Beckman Coulter, Inc.).
Protein immunoblotting
Cells were harvested, and whole-cell extracts were prepared with PRO-PREP protein extraction reagent (Level Biotechnology, Inc.). Protein samples (30 µg/lane) were resolved by electrophoresis with 15% SDS-acrylamide gel and transferred to polyvinylidene difluoride membranes by electroblotting. Then, the blots were blocking with 5% bovine serum albumin (BSA) (Gibco; Thermo Fisher Scientific, Inc.) for 1 h at room temperature. After blocking, the blots were incubated overnight at 4°C in PBS supplemented with 0.05% Tween-20 (TBST) containing primary antibodies as aforementioned. After washing three times (10 min/time at room temperature) with TBST, the blots were incubated for 1 h at room temperature with a corresponding horseradish peroxidase-coupled anti-rabbit or anti-mouse secondary antibody (Pierce). After washing three times (10 min/time at room temperature) with TBST, the blots were visualized with SuperSignal West Pico ECL reagents (Pierce), and chemiluminescence was detected by exposing the membranes to Kodak-X-Omat film (Sigma-Aldrich; Merck KGaA). The levels of the β-actin signal were used to verify equal protein loading among all lanes.
Reverse transcription PCR
Total RNA of whole cell extract was isolated using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) reagent. First-strand cDNA was synthesized from total RNA using the Sprint PowerScript PrePrimed Single Shots kit (Takara Bio, Inc.). PCR was performed using cDNA (25 cycles at 94°C for 30 sec, 55°C for 30 sec and 68°C for 1 min) and Titanium Taq DNA polymerase (Takara Bio, Inc.). The primer pairs were as follows: Human EGR-1 forward, 5′-CTGCGACATCTGTGGAAGAA-3′ and reverse, 5′-TGTCCTGGGAGAAAAGGTTG-3′; and GAPDH forward, 5′-ACCACAGTCCATGCCATCAC-3′ and reverse, 5′-TCCACCACCCTGTTGCTGT-3′. All PCR products were separated by 2% agarose gel electrophoresis and visualized with ethidium bromide.
Luciferase reporter assay
The human EGR-1 promoter sequence containing the region from-714 bases to the translational start site was amplified from the pDRIVE-hEGR-1 plasmid (InvivoGen) by PCR as described in a previous report (24). This plasmid was co-transfected with the pGL4.74 plasmid containing the Renilla luciferase gene (Promega Corporation) into cells and incubated for 48 h. The transfected cells were treated with compound C and then harvested. Supernatants were collected for a Dual-Glo luciferase assay to evaluate promoter activity. Luciferase activity was measured with a dual-luciferase reporter assay system (Promega Corporation). Light units were normalized to those corresponding to Renilla luciferase activity. Final data were presented as the fold change in the EGR-1 luciferase activity compared with untreated group.
Immunocytochemistry
Cells (5×104 cells per chamber) were cultured on two-chamber slides and then treated with compound C. The treated cells were fixed in 4% formaldehyde in PBS overnight at 4°C. For intracellular staining, the cells were blocked with 2% normal horse serum (Invitrogen; Thermo Fisher Scientific, Inc.) for 30 min at room temperature, incubated with antibodies against EGR-1 in PBS containing 0.2% Triton X-100 (PBST), and then incubated with a goat fluorescein isothiocyanate-conjugated anti-rabbit IgG antibody (Sigma-Aldrich; Merck KGaA). After washing with PBST, the cells were mounted using an antifade, DAPI-containing, water-based mounting medium (Vector Laboratories, Inc.). For Magic Red staining, cells were seeded at 5×104 cells/well in a 6-well plate containing one 22×22-mm glass coverslip in each well (Sigma-Aldrich; Merck KGaA), stained with a Magic Red assay kit (ImmunoChemistry Technologies, LLC) at 37°C for 1 h and washed with PBS before image capture. Before fluorescent image capture, the coverslips were detached from the 6-well plate and sealed on glass microscope slides. All fluorescent images were captured by confocal microscopy (Olympus Corporation).
Construction of RNA interference (RNAi) vectors and introduction of RNAi vectors into BCC cells
Human short hairpin shAMPKα1/2 and control shRNA plasmids (Santa Cruz Biotechnology, Inc.) were transfected at 37°C into BCC cells using jetPEI™ (Polyplus-transfection®) for 48 h and then selected with 10 µg/ml puromycin to obtain stably transfected clones. The stable clones were maintained with RMPI medium containing 10 µg/ml puromycin. The level of AMPK-knockdown in the stable clones was analyzed by immunoblotting with specific AMPKα and phosphorylated-AMPKα (Thr172) antibodies. To construct an EGR-1 RNAi vector, a DNA fragment containing a human U6 promoter followed by an EGR-1-specific shRNA sequence (5′-CACCGACCCTAAGCTGGAGGAGATGATTCAAGAGATCATCTCCTCCAGCTTAGGGTTTTTTTG-3′) was retrieved from the CMV-/Hu6-RNAi plasmid by HindIII and EcoRI double digestion. This DNA fragment was then inserted into the mammalian expression vector pcDNA3, which resulted in a plasmid carrying the U6 promoter driving the expression of the EGR-1-specific shRNA. This allowed for the selection of stably transfected clones. An EGFP RNAi vector was employed as the negative control for EGR-1-knockdown. The EGR-1 RNAi and EGFP RNAi vectors were subsequently transfected into BCC cells using jetPEI™, followed by 800 µg/ml G418 (Invitrogen) treatment for 48 h post-transfection to select stably transfected clones. The degree of EGR-1 knockdown in these stable clones was verified by protein immunoblotting analysis of endogenous EGR-1 protein expression using anti-human EGR-1 antibodies after compound C treatment. The method of protein immunoblotting analysis was performed as aforementioned.
Oligodeoxynucleotide-based microarray screening assay
The BCCs were treated with 40 µM compound C for 4 h, and the control and compound C-treated cells were then harvested for total RNA extraction. Total RNA was isolated using TRIzol reagent. The concentration and purity of RNAs were checked to confirm OD260/OD280 (>1.8) and OD260/OD230 (>1.6) using an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.). In total, 1 µg of RNA was prepared for fluorescent antisense RNA (aRNA) targets using the OneArray® Amino Allyl aRNA Amplificant kit (Phalanx Biotech Group) and Cy5 dye (Cyvita). The fluorescent targets were hybridized to the Human Whole Genome OneArray® with a Phalanx Hybridization system (Phalanx Biotech Group) (25). The signal intensity of each spot was analyzed with the Rosetta Resolver system® (Rosetta Biosoftware), and the normalized intensity of each spot was translated into gene expression by log2 ratios compared with the control. The distribution of 11,909 normalized plots, which were represented by 11,909 normalized genes, was represented by scatter plots using the Nested graph family in GraphPad Prism 8 (GraphPad Software, Inc.).
Ingenuity pathway analysis® (IPA)
After the oligodeoxynucleotide-based microarray screening assay, the resultant gene lists were used with a decision-tree-based classifier method to analyze the microarray data (26). The selected genes were used to confirm the relationship with BCC cells. The genetic pathways were analyzed by IPA (Ingenuity Systems; QIAGEN) with species and confidence levels.
Statistical analysis
All statistical results are representative of three independent experiments. Data are presented as mean ± SEM. Data between were analyzed with one-way or two-way ANOVA tests for multiple comparisons, and P<0.05 was considered to indicate a statistically significant difference. The statistical analysis was performed using GraphPad Prism 8 (GraphPad Software, Inc.).
Results
Compound C increases the expression of EGR-1 in skin cancer cell lines
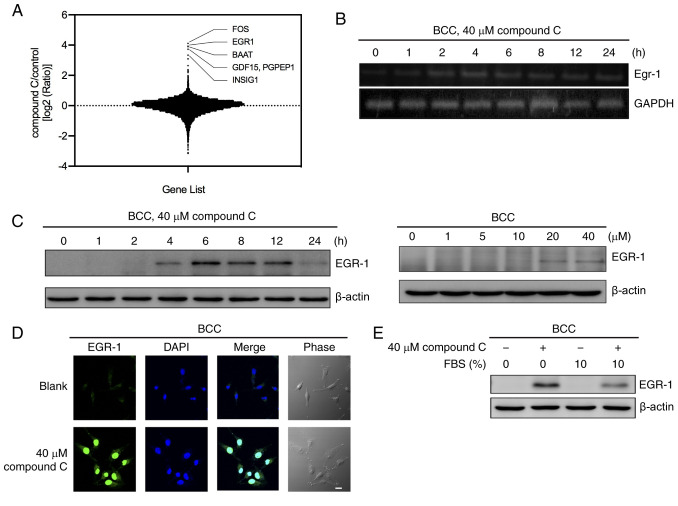
Several studies have indicated that the AMPK inhibitor compound C induces apoptosis and autophagy in an AMPK-independent manner (4,6,7,27). To evaluate the putative mechanisms that regulate compound C-induced apoptosis and autophagy in cancer cells, an oligodeoxynucleotide-based microarray screening assay was used to identify compound C-induced gene expression. Then, 11,909 normalized genes were surveyed and the top five were found to be FOS, EGR-1, BAAT, GDF15 and INSIG1 (Fig. 1A and Table SI.).
Figure 1.
Compound C induces EGR-1 expression in BCC cells. (A) BCC cells were treated with 40 µM compound C for 4 h, and the mRNA expression levels of genes were detected with an oligodeoxynucleotide-based microarray assay. (B) BCC cells were treated with 40 µM compound C for 0, 1, 2, 4, 6, 8, 12 or 24 h, and the mRNA expression levels of EGR-1 were detected by reverse transcription PCR. (C) BCC cells were treated with 40 µM compound C for 0, 1, 2, 4, 6, 8, 12 or 24 h or with 0, 1, 5, 10, 20 or 40 µM compound C for 6 h. Protein expression levels of EGR-1 were detected by immunoblotting. (D) BCC cells were treated with 0 or 40 µM compound C for 6 h. (E) BCC cells were treated with 40 µM compound C and culture medium with or without 10% FBS for 6 h. Scale bar, 20 µm. Protein expression level of EGR-1 was detected by immunoblotting. EGR-1, early growth response-1; BCC, basal cell carcinoma; FBS, fetal bovine serum.
Next, the selected genes were input into IPA and found that TP53-EGR-1 pathway was associated with compound C treatment in BCC cells (Fig. S1A). We had previously demonstrated that compound C-induced apoptosis in skin cancer cells was p53-dependent (23). Hence, EGR-1 was selected as the targeted gene for further investigation. The expression of the EGR-1 gene was confirmed by RT-PCR compared with mock DMSO-treated cells (0 h) (Fig. 1B). After treatment with compound C, BCC cells showed enhanced EGR-1 mRNA expression after 2 h, with expression peaking at 4 h and then gradually declining (Fig. 1B). The protein expression of EGR-1 was upregulated by compound C treatment in a similar time- and dose-dependent manner, although different kinetics were observed in the BCC, C32 and A375 cell lines (Figs. 1C and S1B). Furthermore, the protein expression of EGR-1 was induced when treated with a high concentration of compound C (40 µM) (Fig. 1C).
EGR-1 was also localized in the nucleus after compound C treatment, as demonstrated by immunofluorescence staining (Figs. 1D and S1C). Growth factors and cytokines have been reported to induce the expression of EGR-1 (11). To rule out the possibility that growth factors and cytokines involved in EGR-1 induction, the expression of EGR-1 was examined in culture medium with or without FBS. As shown in Fig. 1E, the EGR-1 expression in the BCC cell line was increased after compound C treatment regardless of whether FBS was added to the culture medium. This result indicated that FBS did not contribute to compound C-mediated EGR-1 induction. Thus, it was concluded that a high dose of compound C may induce EGR-1 mRNA and protein expression in skin cancer cells.
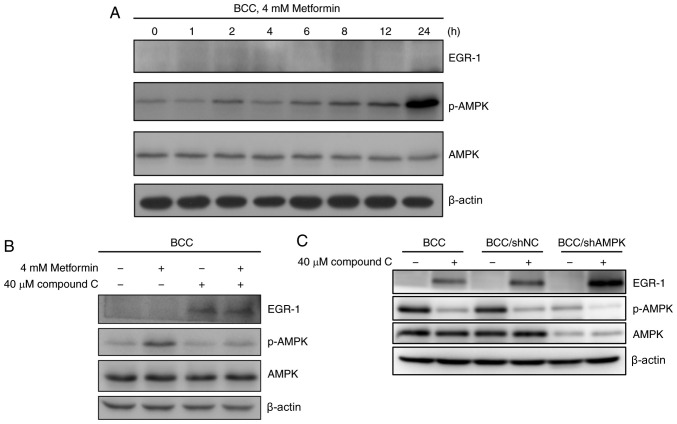
Compound C-induced EGR-1 expression is independent of AMPK activation
Compound C is a commonly used AMPK inhibitor, and activation of AMPK also reportedly induces the expression of EGR-1 and its target dual specificity phosphatase 4 (28). To evaluate the role of AMPK activation in the regulation of EGR1 expression, BCC cells were treated with metformin, an AMPK activator, to evaluate whether the activation of AMPK (p-AMPK) can control EGR-1 expression. Metformin induced the activation of AMPK but had no effect on the expression of EGR-1 in skin cancer cells (Figs. 2A and S2). Co-treatment with metformin and compound C did not change the level of compound C-induced EGR-1 expression in skin cancer cells (Figs. 2B and S2). To confirm whether AMPK is involved in compound C-induced EGR-1 production, EGR-1 in stable AMPK-knockdown BCC cells (shAMPK) treated with compound C were examined. In control and AMPK-knockdown BCC cells without compound C treatment, EGR-1 expression was not induced, indicating that AMPK may be dispensable in the regulation of EGR-1 expression in un-stimulated conditions (Fig. 2C). Expression of EGR-1 was highly induced in AMPK-knockdown BCC cells treated with compound C, revealing that compound C-induced EGR-1 expression may be controlled by other pathways and further enhanced in the AMPK-knockdown condition.
Figure 2.
AMPK activation is not involved in compound C-induced EGR-1 expression in BCC cells. (A) BCC cells were treated with 4 mM metformin for 0, 1, 2, 4, 6, 8, 12 or 24 h. (B) BCC cells were cotreated with or without 4 mM metformin and 40 µM compound C for 6 h. (C) BCC, BCC/shNC and BCC/shAMPK cells were treated with 40 µM compound C for 6 h. Expression levels of EGR-1, AMPK, p-AMPK and β-actin were detected by immunoblotting. AMPK, AMP-activated protein kinase; BCC, basal cell carcinoma; p-, phosphorylated; NC, negative control; sh, short hairpin.
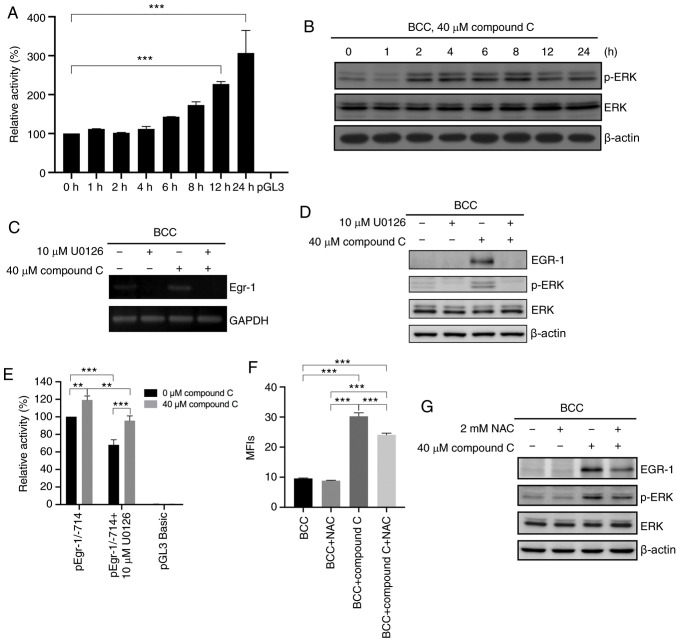
Compound C upregulates EGR-1 mRNA expression via the ROS-mediated ERK signaling pathway
Further investigation into whether compound C induces EGR-1 expression through transcriptional regulation of the EGR-1 gene was conducted. Using a luciferase reporter assay, EGR1 promoter activity was shown to be increased by 1.3-, 1.6-, 2.3-, and 3.1-fold at 6, 8, 12 and 24 h, respectively, in cells treated with compound C (Fig. 3A). This result confirmed that compound C could induce EGR-1 expression at the mRNA level. As the transcription of EGR-1 is reportedly regulated by the ERK signaling pathway in cancer cells (20), it was evaluated whether compound C-induced EGR-1 transcriptional regulation was mediated by the ERK signaling pathway. First, compound C was demonstrated to significantly induce ERK phosphorylation in skin cancer cells (Figs. 3B and S3A). Next, the MEK/ERK inhibitor U0126 was used to evaluate whether the ERK signaling pathway was involved in compound C-induced EGR-1 expression in BCC, C32 and A375 cells. Pretreatment with U0126 decreased the basal-level mRNA expression of EGR-1 and inhibited the compound C-induced mRNA and protein expression of EGR-1 and p-ERK (Fig. 3C and D) in BCC cells. Similarly, U0126 pretreatment inhibited the compound C-induced protein expression of EGR-1 and p-ERK in C32 cells (Fig. S3B). In the promoter activity assay, pretreatment with U0126 not only decreased the promoter activity of EGR-1 in the compound C-treated pEgr-1/-714 group compared with only compound C-treated pEgr-1/-714 group, but also decreased the promoter activity of EGR-1 in the U0126-treated pEgr-1/-714 group compared with pEgr-1/-714 group (Fig. 3E). We previously demonstrated that compound C-induced p53-dependent apoptosis in skin cancer cells is mediated by compound C-induced ROS (23). Figs. 3F and S3C show that compound C induced ROS production in BCC and C32 cells, but pretreatment with NAC decreased the level of compound C-induced ROS production. Thus, it was evaluated whether compound C-induced ROS also promoted ERK activation and subsequent EGR-1 expression. As shown in Figs. 3G and S3D, the antioxidant NAC not only mitigated compound C-induced ERK phosphorylation but also inhibited EGR-1 expression in BCC and C32 cells. Thus, it was concluded that compound C upregulates EGR-1 mRNA expression via ROS-mediated ERK activation.
Figure 3.
Compound C upregulates EGR-1 mRNA expression via the ROS-mediated ERK signaling pathway. (A) BCC cells were co-transfected with pGL3-basic-hEGR-1-pro-1/-714 and pGL3 carrying the Renilla luciferase gene for 48 h and then incubated in medium containing 0% FBS and 40 µM compound C for 0, 1, 2, 4, 6, 8, 12 or 24 h, and analyzed with one-way ANOVA. (B) BCC cells were treated with 40 µM compound C for 0, 1, 2, 4, 6, 8, 12 or 24 h. Expression levels of p-ERK, ERK and β-actin were detected by immunoblotting. (C and D) BCC cells were pretreated with 10 µM U0126 for 1 h and then treated with 40 µM compound C for 6 h. mRNA and protein expression levels of EGR-1 were detected by reverse transcription PCR and immunoblotting, respectively. (E) BCC cells were co-transfected with pGL3-basic-hEGR-1-pro-1/-714 and pGL4.74 carrying the Renilla luciferase gene for 48 h. Cells were pretreated with 10 µM U0126 for 1 h in serum-free medium and then treated with 0 or 40 µM compound C for 6 h, and analyzed with two-way ANOVA. (F) BCC cells were pretreated with 2 mM NAC for 1 h and then treated with 40 µM compound C for 4 or 6 h to evaluate the ROS or protein expression level. This statistical result was analyzed using one-way ANOVA. (G) Expression levels of EGR-1, p-ERK, ERK and β-actin were detected by immunoblotting with specific antibodies. Data are expressed as the mean ± SEM. of three independent experiments. **P<0.01 and ***P<0.001 compared with the control. EGR-1, early growth response-1; BCC, basal cell carcinoma; NAC, N-acetyl-cysteine; p-, phosphorylated.
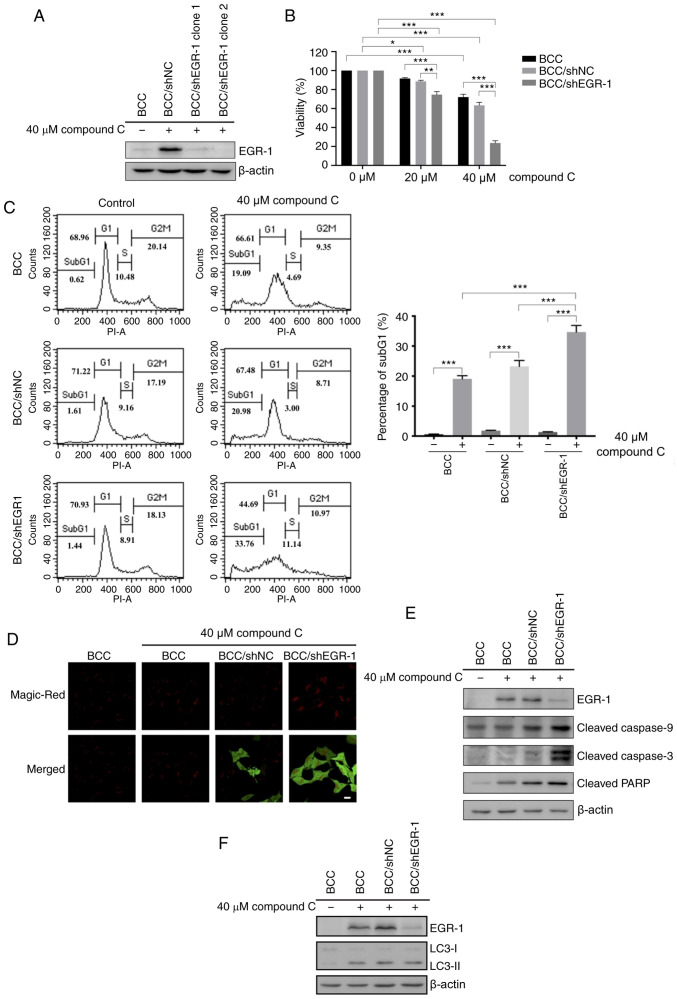
EGR-1 knockdown enhanced compound C-induced apoptosis
Previous studies found that compound C efficiently increases apoptotic and autophagic populations in skin cancer cells (23). A rapid and transient increase in EGR-1 expression was demonstrated during compound C treatment. Therefore, it was hypothesized that the apoptotic and autophagic effects of compound C may be regulated by EGR-1. To investigate this hypothesis, the effect of compound C on BCC cells with an EGR-1 functional deficiency was evaluated. RNAi was used to reduce the level of endogenous EGR-1, which was sufficient to abolish EGR-1 function. An EGR-1-specific shRNA vector carrying a human U6 promoter was transfected into BCC cells, and the stably transfected clones were screened by G418 selection. BCC cells stably expressing an EGFP RNAi vector were used as a negative control for EGR-1-knockdown, and the degree of EGR-1 knockdown in cells was validated by EGR-1 immunoblotting. The endogenous EGR-1 level in BCC/EGR-1-shRNA stable clones dropped to a barely detectable level after compound C treatment, which indicated the efficiency of EGR-1 knockdown (Fig. 4A). As expected, 40 µM compound C triggered a slight reduction in the number of cells transfected with the BCC/sh negative control (NC) (Fig. 4B). However, compound C decreased the BCC/EGR-1-shRNA clone cell numbers in a dose-dependent manner (Fig. 4B), suggesting that compound C-induced cell death was enhanced by EGR-1 knockdown.
Figure 4.
Expression of EGR-1 is important for the survival of BCC cells after compound C treatment. BCC cells were transfected with shRNA plasmids carrying green fluorescent protein. (A) Cells were treated with or without 40 µM compound C for 6 h. Protein expression level of EGR-1 was detected by immunoblotting. (B) Cells were treated with 0, 20 or 40 µM compound C for 48 h. (C) Cells were treated with 0 or 40 µM compound C for 48 h. Percentage of subG1 cells was measured by cell cycle analysis. (D) Cells were treated with 40 µM compound C for 6 h (E and F) Cells were treated with or without 40 µM compound C for 6 h. Two-way ANOVA was used for statistical analysis. *P<0.05, **P<0.01 and ***P<0.001 compared with the control. BCC, basal cell carcinoma; EGR-1, early growth response-1; PARP, ADP-ribose polymerase; LC3, light chain 3-II; sh, short hairpin. The value of scale bar was 20 µm.
Then, apoptosis was characterized by a DNA content assay, revealing that ~32% of the apoptosis observed for the BCC/EGR-1-shRNA clone was caused by compound C (Fig. 4C). Caspase-3 activity was also increased in the BCC/EGR1-shRNA clone compared with BCC control cells after compound C treatment (Fig. 4D). Consistently, the cleaved caspase-9, caspase-3 and PARP levels were increased in the BCC/EGR1-shRNA clone after compound C treatment (Fig. 4E). However, there was no difference in the protein expression of the autophagy induction marker light chain 3-II (LC3-II) between the BCC/shNC and BCC/EGR-1-shRNA cells (Fig. 4F). Collectively, these data revealed that EGR-1 can protect against compound C-induced apoptosis but not against compound C-induced autophagy in skin cancer cells.
Discussion
In our previous study, we demonstrated that compound C-induced apoptosis in human skin cancer cells is p53-dependent (23). The present oligodeoxynucleotide-based microarray assay showed that the EGR-1 gene was one of highest upregulated genes in BCC cells after compound C treatment. The results of IPA indicated that TP53 and EGR-1 expression might be connected in BCC cells with compound C treatment. The report that p53 gene expression is under EGR-1 regulation may indicate the possibility that EGR-1 is involved in compound C-induced p53-dependent apoptosis (17). However, according to the result of Figure S4, compound C treatment still induced p53 expression in EGR-1-knockdown BCC cells. This result indicated that p53 gene expression cannot be controlled by EGR-1 in compound C-treated BCC cells, and therefore EGR-1 may play another role in compound C-induced apoptosis in BCC cells. Thus, understanding the role of EGR-1 in cancer could improve cancer therapeutic methods. The expression of EGR-1 increased after compound C treatment in BCC, C32 and A375 cells, and the EGR-1 protein was also localized in the nucleus. These results indicated that compound C-induced EGR-1 expression and localization are dose-dependent in different skin cancer cells. The kinetic expression patterns of EGR-1 may be related to the different genetic backgrounds in different cells (13–15,29–33). The present study demonstrated that the expression level of EGR-1 after compound C treatment was higher in serum-free medium compared with in serum. EGR-1 is a zinc-finger transcription factor with EBS, with a CRE-binding site, serum response elements/erythroblast transformation-specific-binding site and NF-κB-like-binding site, ATF5 consensus sequences, an AP1 site and an SP1 site upstream of the promoter region (11). Growth factors activate the Ras/Raf/MAP kinase signaling pathway and facilitate formation of the Elk-1/CBP/SRF complex, which binds to the SRE functional element of the EGR-1 promoter to induce EGR-1 transcription (20). When cells are in the absence of growth factors, EGR-1 is induced, and the activities of JNK/SAPK and p38 are increased (22,34–36). Collectively, these results indicate that compound C is capable of inducing EGR-1 expression and regulating EGR-1 transcription in an environmental context-dependent manner in cancer cells.
In the absence of energy, AMPK activation induces EGR-1 expression in hepatocytes (28). A high-glucose state activates the protein kinase C (PKC)-ERK signaling pathway, inducing EGR-1 expression; however, valsartan, a Food and Drug Administration approved antihypertensive drug, activates the liver kinase B1-AMP-activated protein kinase signaling pathway and inhibits EGR-1 expression independent of the PKC-ERK signaling pathway in THP1 cells (37). These results indicate that the role of AMPK in EGR-1 expression might be dependent on cell type and environmental conditions. Compound C is a widely used AMPK inhibitor that has the ability to regulate apoptosis and autophagy in an AMPK-independent manner (1,4,6,7,16,27). The present results indicated that treatment with metformin did not induce EGR-1 expression. In addition, co-treatment with metformin and compound C did not decrease compound C-induced EGR-1 expression. These results showed that compound C-induced EGR-1 expression is AMPK activation-independent in skin cancer cells. However, the level of compound C-induced EGR-1 induction in AMPK-knockdown cells was higher compared with that in control cells. However, EGR-1 expression was not induced in control and AMPK-knockdown BCC cells without compound C treatment. This indicated that AMPK inhibition or knockdown is not enough to solely induce EGR-1 expression, and that compound C-induced EGR-1 expression may be controlled by other pathways and only enhanced by AMPK-knockdown conditions in skin cancer cells.
MAPK proteins, such as ERK1/2, JNK, and p38-MAPK, induce EGR-1 activation (20). In the present study, compound C can induce EGR-1 expression at the mRNA level. According to RT-PCR analysis and reporter assay evaluating the EGR-1 promoter, the present study hypothesized that ERK may regulate compound C-induced EGR-1 expression. Pretreatment with the ERK inhibitor U0126 decreased compound C-induced promoter activity and the mRNA and protein expression of EGR-1. Notably, pre-treatment with the ERK inhibitor U0126 decreased the promoter activity of the control group and compound C treatment group. This result implied that ERK might also be involved in EGR-1 mRNA expression at the post-transcriptional level. Additionally, it is possible that the regulatory sequences that are responsible for endogenous EGR-1 mRNA induction may not be contained in the cloned promoter of the luciferase reporter plasmid. These results consequently indicated that EGR-1 mRNA expression was upregulated by compound C via the ERK signaling pathway. In malignant tumor progression, cellular adhesion is lost and eventually promotes tumor cell migration or invasion into surrounding tissues (38). Hepatocyte growth factor can facilitate cellular mobility by triggering the ERK/EGR-1 signaling pathway to promote HepG2 cell scattering (39,40). U0126 and PD98059, ERK inhibitors, can inhibit the EGR-1 expression induced by neuron growth factor in PC-12 cells (41). In addition to the ERK signaling pathways involved in EGR-1 expression, the PI3K/Akt signaling pathway has been shown to modulate EGR-1 expression (36,42,43). Protein kinase A (PKA) activates CREB and cAMP response element-binding protein, binding to CREs to promote the transcription of target genes (44,45), and exendin-4 induces the expression of EGR-1 through the cAMP/PKA/CREB signaling pathway in type II diabetes (46,47). These studies demonstrate that numerous signaling pathways are involved in the modulation of EGR-1 expression. Whether these other signaling pathways, in addition to the ERK pathway, regulate compound C-induced EGR-1 expression requires further investigation.
ROS constitutes one of numerous extracellular and intracellular stimuli that activate the MAPK pathways for cell proliferation, differentiation, survival and death; such as the Raf-MEK-ERK axis activated in response to growth factor receptor stimulation by oxidative stress. ROS can also activate EGF and platelet derived growth-factor (PDGF) receptors to stimulate the Ras and ERK pathways in cancer cells (48,49). Our previous study demonstrated that compound C induced ROS production and caused apoptosis in skin cancer cells in a p53-dependent manner (23). The data from the present study further suggested that compound C-activated EGR-1 may act through ROS-mediated ERK activation in cancer cells.
An EGR-1-specific shRNA was used to knock down EGR-1 expression to evaluate whether the apoptotic and autophagic effects of compound C are regulated by EGR-1. Knocking down EGR-1 expression caused more compound C-induced cell death. Furthermore, caspase-3 activity and the expression of cleaved caspase-9, caspase-3 and PARP were significantly increased, but there was no difference in the protein expression of LC3-II between BCC/shNC control cells and BCC/EGR-1-shRNA cells. These results implied that the protective role of EGR-1 in compound C-induced cell death affects apoptosis but not autophagy in skin cancer cells. In HaCaT cells, cigarette smoke extracts and UVB exposure induces EGR-1 expression and then increases TNF-α secretion (50,51). UVB-induced EGR-1 expression is involved in the expression of DNA repair proteins that repair DNA damage and protect cells against apoptosis (52). The localization of EGR-1 in the nucleus after compound C treatment in BCC and HaCaT cells implied that EGR-1 may be related to DNA repair in compound C-induced apoptosis. Thus, the mechanism involving EGR-1 in the DNA repair system needs to be further investigated.
The present study found that the ROS-mediated ERK activation pathway may be a mechanism by which compound C induces EGR-1 expression. In EGR-1-knockdown cells, treatment with compound C increased caspase-3 activity and the expression of cleaved caspase-9, caspase-3 and PARP. Loss of EGR-1 causes pancreatic β-cell apoptosis induced by palmitic acid treatment (53). Overexpression of EGR-1 has been reported to promote cell proliferation and prevent cycle arrest and apoptosis in prostate carcinoma cells (54). Thus, one function of EGR-1 in various cell types is to protect against stress-induced death. The present study's results indicated that EGR-1 played a protective role in compound C-induced apoptosis. It was concluded that compound C has potential in skin cancer therapy and that EGR-1 may be a promising therapeutic target for increasing compound C sensitivity in skin cancer cells.
Supplementary Material
Acknowledgements
The authors would like to thanks Mrs. Mei-Chun Liu (Instrument Center of the Department of Medical Research of Taichung Veterans General Hospital) for helping confocal imaging analysis.
Funding Statement
This work was supported by grants from The Taichung Veterans General Hospital/National Chung Hsing University Joint Research Program (grant no. TCVGH-NCHU-1027603) and The Ministry of Science and Technology (grant no. MOST-108-2320-B-005-005-MY3). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Funding
This work was supported by grants from The Taichung Veterans General Hospital/National Chung Hsing University Joint Research Program (grant no. TCVGH-NCHU-1027603) and The Ministry of Science and Technology (grant no. MOST-108-2320-B-005-005-MY3). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors' contributions
KCC, MHT and JJS conceived the study. KCC, JSS and MHT curated the data. KCC, FWC, MHT and JJS did the formal analysis. JSS acquired the funding and was the project administrator. KCC and FWC did the investigation for the study and wrote the methodology. KCC, FWC, MHT and JJS provided the resources. MHT and JJS supervised the study. JJS validated the study. KCC and JJS visualized the study. KCC wrote and prepared the original draft. JJS reviewed the writing and edited. MHT and JJS confirm the authenticity of all raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae EJ, Cho MJ, Kim SG. Metformin prevents an adaptive increase in GSH and induces apoptosis under the conditions of GSH deficiency in H4IIE cells. J Toxicol Environ Health A. 2007;70:1371–1380. doi: 10.1080/15287390701434430. [DOI] [PubMed] [Google Scholar]
- 3.Vucicevic L, Misirkic M, Janjetovic K, Harhaji-Trajkovic L, Prica M, Stevanovic D, Isenovic E, Sudar E, Sumarac- Dumanovic M, Micic D, Trajkovic V. AMP-activated protein kinase-dependent and -independent mechanisms underlying in vitro antiglioma action of compound C. Biochem Pharmacol. 2009;77:1684–1693. doi: 10.1016/j.bcp.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Jin J, Mullen TD, Hou Q, Bielawski J, Bielawska A, Zhang X, Obeid LM, Hannun YA, Hsu YT. AMPK inhibitor compound C stimulates ceramide production and promotes Bax redistribution and apoptosis in MCF7 breast carcinoma cells. J Lipid Res. 2009;50:2389–2397. doi: 10.1194/jlr.M900119-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meley D, Bauvy C, Houben-Weerts JH, Dubbelhuis PF, Helmond MT, Codogno P, Meijer AJ. AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem. 2006;281:34870–34879. doi: 10.1074/jbc.M605488200. [DOI] [PubMed] [Google Scholar]
- 6.Vucicevic L, Misirkic M, Janjetovic K, Vilimanovich U, Sudar E, Isenovic E, Prica M, Harhaji-Trajkovic L, Kravic-Stevovic T, Bumbasirevic V, Trajkovic V. Compound C induces protective autophagy in cancer cells through AMPK inhibition-independent blockade of Akt/mTOR pathway. Autophagy. 2011;7:40–50. doi: 10.4161/auto.7.1.13883. [DOI] [PubMed] [Google Scholar]
- 7.Jang JH, Lee TJ, Yang ES, Min do S, Kim YH, Kim SH, Choi YH, Park JW, Choi KS, Kwon TK. Compound C sensitizes Caki renal cancer cells to TRAIL-induced apoptosis through reactive oxygen species-mediated down-regulation of c-FLIPL and Mcl-1. Exp Cell Res. 2010;316:2194–2203. doi: 10.1016/j.yexcr.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 8.Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987;238:797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- 9.Gashler A, Sukhatme VP. Early growth response protein 1 (Egr-1): Prototype of a zinc-finger family of transcription factors. Prog Nucleic Acid Res Mol Biol. 1995;50:191–224. doi: 10.1016/S0079-6603(08)60815-6. [DOI] [PubMed] [Google Scholar]
- 10.Aicher WK, Sakamoto KM, Hack A, Eibel H. Analysis of functional elements in the human Egr-1 gene promoter. Rheumatol Int. 1999;18:207–214. doi: 10.1007/s002960050086. [DOI] [PubMed] [Google Scholar]
- 11.Pagel JI, Deindl E. Early growth response 1-a transcription factor in the crossfire of signal transduction cascades. Indian J Biochem Biophys. 2011;48:226–235. [PubMed] [Google Scholar]
- 12.Yu J, Baron V, Mercola D, Mustelin T, Adamson ED. A network of p73, p53 and Egr1 is required for efficient apoptosis in tumor cells. Cell Death Differ. 2007;14:436–446. doi: 10.1038/sj.cdd.4402093. [DOI] [PubMed] [Google Scholar]
- 13.Yu J, de Belle I, Liang H, Adamson ED. Coactivating factors p300 and CBP are transcriptionally crossregulated by Egr1 in prostate cells, leading to divergent responses. Mol Cell. 2004;15:83–94. doi: 10.1016/j.molcel.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 14.Yu J, Zhang SS, Saito K, Williams S, Arimura Y, Ma Y, Ke Y, Baron V, Mercola D, Feng GS, et al. PTEN regulation by Akt-EGR1-ARF-PTEN axis. EMBO J. 2009;28:21–33. doi: 10.1038/emboj.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bae MH, Jeong CH, Kim SH, Bae MK, Jeong JW, Ahn MY, Bae SK, Kim ND, Kim CW, Kim KR, Kim KW. Regulation of Egr-1 by association with the proteasome component C8. Biochim Biophys Acta. 2002;1592:163–167. doi: 10.1016/S0167-4889(02)00310-5. [DOI] [PubMed] [Google Scholar]
- 16.Kim HS, Hwang JT, Yun H, Chi SG, Lee SJ, Kang I, Yoon KS, Choe WJ, Kim SS, Ha J. Inhibition of AMP-activated protein kinase sensitizes cancer cells to cisplatin-induced apoptosis via hyper-induction of p53. J Biol Chem. 2008;283:3731–3742. doi: 10.1074/jbc.M704432200. [DOI] [PubMed] [Google Scholar]
- 17.Krones-Herzig A, Mittal S, Yule K, Liang H, English C, Urcis R, Soni T, Adamson ED, Mercola D. Early growth response 1 acts as a tumor suppressor in vivo and in vitro via regulation of p53. Cancer Res. 2005;65:5133–5143. doi: 10.1158/0008-5472.CAN-04-3742. [DOI] [PubMed] [Google Scholar]
- 18.Das A, Chendil D, Dey S, Mohiuddin M, Mohiuddin M, Milbrandt J, Rangnekar VM, Ahmed MM. Ionizing radiation down-regulates p53 protein in primary Egr-1−/− mouse embryonic fibroblast cells causing enhanced resistance to apoptosis. J Biol Chem. 2001;276:3279–3286. doi: 10.1074/jbc.M008454200. [DOI] [PubMed] [Google Scholar]
- 19.Baron V, Adamson ED, Calogero A, Ragona G, Mercola D. The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGFbeta1, PTEN, p53, and fibronectin. Cancer Gene Ther. 2006;13:115–124. doi: 10.1038/sj.cgt.7700896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gitenay D, Baron VT. Is EGR1 a potential target for prostate cancer therapy? Future Oncol. 2009;5:993–1003. doi: 10.2217/fon.09.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang SZ, Abdulkadir SA. Early growth response gene 1 modulates androgen receptor signaling in prostate carcinoma cells. J Biol Chem. 2003;278:39906–39911. doi: 10.1074/jbc.M307250200. [DOI] [PubMed] [Google Scholar]
- 22.Lu C, Shi Y, Wang Z, Song Z, Zhu M, Cai Q, Chen T. Serum starvation induces H2AX phosphorylation to regulate apoptosis via p38 MAPK pathway. FEBS Lett. 2008;582:2703–2708. doi: 10.1016/j.febslet.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 23.Huang SW, Wu CY, Wang YT, Kao JK, Lin CC, Chang CC, Mu SW, Chen YY, Chiu HW, Chang CH, et al. p53 modulates the AMPK inhibitor compound C induced apoptosis in human skin cancer cells. Toxicol Appl Pharmacol. 2013;267:113–124. doi: 10.1016/j.taap.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Datta R, Rubin E, Sukhatme V, Qureshi S, Hallahan D, Weichselbaum RR, Kufe DW. Ionizing radiation activates transcription of the EGR1 gene via CArG elements. Proc Natl Acad Sci USA. 1992;89:10149–10153. doi: 10.1073/pnas.89.21.10149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kao JK, Wang SC, Ho LW, Huang SW, Lee CH, Lee MS, Yang RC, Shieh JJ. M2-like polarization of THP-1 monocyte-derived macrophages under chronic iron overload. Ann Hematol. 2020;99:431–441. doi: 10.1007/s00277-020-03916-8. [DOI] [PubMed] [Google Scholar]
- 26.Tsai MH, Wang HC, Lee GW, Lin YC, Chiu SH. A decision tree based classifier to analyze human ovarian cancer cDNA microarray datasets. J Med Syst. 2016;40:21. doi: 10.1007/s10916-015-0361-9. [DOI] [PubMed] [Google Scholar]
- 27.Yang WL, Perillo W, Liou D, Marambaud P, Wang P. AMPK inhibitor compound C suppresses cell proliferation by induction of apoptosis and autophagy in human colorectal cancer cells. J Surg Oncol. 2012;106:680–688. doi: 10.1002/jso.23184. [DOI] [PubMed] [Google Scholar]
- 28.Berasi SP, Huard C, Li D, Shih HH, Sun Y, Zhong W, Paulsen JE, Brown EL, Gimeno RE, Martinez RV. Inhibition of gluconeogenesis through transcriptional activation of EGR1 and DUSP4 by AMP-activated kinase. J Biol Chem. 2006;281:27167–27177. doi: 10.1074/jbc.M602416200. [DOI] [PubMed] [Google Scholar]
- 29.Liu DX, Qian D, Wang B, Yang JM, Lu Z. p300-Dependent ATF5 acetylation is essential for Egr-1 gene activation and cell proliferation and survival. Mol Cell Biol. 2011;31:3906–3916. doi: 10.1128/MCB.05887-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao X, Mahendran R, Guy GR, Tan YH. Protein phosphatase inhibitors induce the sustained expression of the Egr-1 gene and the hyperphosphorylation of its gene product. J Biol Chem. 1992;267:12991–12997. doi: 10.1016/S0021-9258(18)42372-1. [DOI] [PubMed] [Google Scholar]
- 31.Huang RP, Fan Y, deBelle I, Ni Z, Matheny W, Adamson ED. Egr-1 inhibits apoptosis during the UV response: Correlation of cell survival with Egr-1 phosphorylation. Cell Death Differ. 1998;5:96–106. doi: 10.1038/sj.cdd.4400322. [DOI] [PubMed] [Google Scholar]
- 32.Manente AG, Pinton G, Tavian D, Lopez-Rodas G, Brunelli E, Moro L. Coordinated sumoylation and ubiquitination modulate EGF induced EGR1 expression and stability. PLoS One. 2011;6:e25676. doi: 10.1371/journal.pone.0025676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varshavsky A. The ubiquitin system. Trends Biochem Sci. 1997;22:383–387. doi: 10.1016/S0968-0004(97)01122-5. [DOI] [PubMed] [Google Scholar]
- 34.Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 35.Lim CP, Jain N, Cao X. Stress-induced immediate-early gene, egr-1, involves activation of p38/JNK1. Oncogene. 1998;16:2915–2926. doi: 10.1038/sj.onc.1201834. [DOI] [PubMed] [Google Scholar]
- 36.Sarker KP, Lee KY. L6 myoblast differentiation is modulated by Cdk5 via the PI3K-AKT-p70S6K signaling pathway. Oncogene. 2004;23:6064–6070. doi: 10.1038/sj.onc.1207819. [DOI] [PubMed] [Google Scholar]
- 37.Ha YM, Park EJ, Kang YJ, Park SW, Kim HJ, Chang KC. Valsartan independent of AT1 receptor inhibits tissue factor, TLR-2 and −4 expression by regulation of Egr-1 through activation of AMPK in diabetic conditions. J Cell Mol Med. 2014;18:2031–2043. doi: 10.1111/jcmm.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birchmeier W, Birchmeier C. Epithelial-mesenchymal transitions in development and tumor progression. EXS. 1995;74:1–15. doi: 10.1007/978-3-0348-9070-0_1. [DOI] [PubMed] [Google Scholar]
- 39.Grotegut S, von Schweinitz D, Christofori G, Lehembre F. Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of Snail. EMBO J. 2006;25:3534–3545. doi: 10.1038/sj.emboj.7601213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vande Woude GF, Jeffers M, Cortner J, Alvord G, Tsarfaty I, Resau J. Met-HGF/SF: Tumorigenesis, invasion and metastasis. Ciba Found Symp. 1997;212:119–132. doi: 10.1002/9780470515457.ch8. 148-154. [DOI] [PubMed] [Google Scholar]
- 41.Harada T, Morooka T, Ogawa S, Nishida E. ERK induces p35, a neuron-specific activator of Cdk5, through induction of Egr1. Nat Cell Biol. 2001;3:453–459. doi: 10.1038/35074516. [DOI] [PubMed] [Google Scholar]
- 42.Guillemot L, Levy A, Raymondjean M, Rothhut B. Angiotensin II-induced transcriptional activation of the cyclin D1 gene is mediated by Egr-1 in CHO-AT(1A) cells. J Biol Chem. 2001;276:39394–39403. doi: 10.1074/jbc.M103862200. [DOI] [PubMed] [Google Scholar]
- 43.Cabodi S, Morello V, Masi A, Cicchi R, Broggio C, Distefano P, Brunelli E, Silengo L, Pavone F, Arcangeli A, et al. Convergence of integrins and EGF receptor signaling via PI3K/Akt/FoxO pathway in early gene Egr-1 expression. J Cell Physiol. 2009;218:294–303. doi: 10.1002/jcp.21603. [DOI] [PubMed] [Google Scholar]
- 44.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 45.Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White M, Montminy M. cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003;17:1575–1580. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang JH, Kim MJ, Ko SH, Jeong IK, Koh KH, Rhie DJ, Yoon SH, Hahn SJ, Kim MS, Jo YH. Upregulation of rat Ccnd1 gene by exendin-4 in pancreatic beta cell line INS-1: Interaction of early growth response-1 with cis-regulatory element. Diabetologia. 2006;49:969–979. doi: 10.1007/s00125-006-0179-6. [DOI] [PubMed] [Google Scholar]
- 47.Kang JH, Kim MJ, Jang HI, Koh KH, Yum KS, Rhie DJ, Yoon SH, Hahn SJ, Kim MS, Jo YH. Proximal cyclic AMP response element is essential for exendin-4 induction of rat EGR-1 gene. Am J Physiol Endocrinol Metab. 2007;292:E215–E222. doi: 10.1152/ajpendo.00181.2006. [DOI] [PubMed] [Google Scholar]
- 48.León-Buitimea A, Rodriguez-Fragoso L, Lauer FT, Bowles H, Thompson TA, Burchiel SW. Ethanol-induced oxidative stress is associated with EGF receptor phosphorylation in MCF-10A cells overexpressing CYP2E1. Toxicol Lett. 2012;209:161–165. doi: 10.1016/j.toxlet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lei H, Kazlauskas A. Growth factors outside of the platelet-derived growth factor (PDGF) family employ reactive oxygen species/Src family kinases to activate PDGF receptor alpha and thereby promote proliferation and survival of cells. J Biol Chem. 2009;284:6329–6336. doi: 10.1074/jbc.M808426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeong SH, Park JH, Kim JN, Park YH, Shin SY, Lee YH, Kye YC, Son SW. Up-regulation of TNF-alpha secretion by cigarette smoke is mediated by Egr-1 in HaCaT human keratinocytes. Exp Dermatol. 2010;19:e206–e212. doi: 10.1111/j.1600-0625.2009.01050.x. [DOI] [PubMed] [Google Scholar]
- 51.Kim JN, Kim HJ, Jeong SH, Kye YC, Son SW. Cigarette smoke-induced early growth response-1 regulates the expression of the cysteine-rich 61 in human skin dermal fibroblasts. Exp Dermatol. 2011;20:992–997. doi: 10.1111/j.1600-0625.2011.01380.x. [DOI] [PubMed] [Google Scholar]
- 52.Thyss R, Virolle V, Imbert V, Peyron JF, Aberdam D, Virolle T. NF-kappaB/Egr-1/Gadd45 are sequentially activated upon UVB irradiation to mediate epidermal cell death. EMBO J. 2005;24:128–137. doi: 10.1038/sj.emboj.7600501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheong MW, Kuo LH, Cheng YN, Tsai PJ, Ho LC, Tai HC, Chiu WT, Chen SH, Lu PJ, Shan YS, et al. Loss of Egr-1 sensitizes pancreatic β-cells to palmitate-induced ER stress and apoptosis. J Mol Med (Berl) 2015;93:807–818. doi: 10.1007/s00109-015-1272-4. [DOI] [PubMed] [Google Scholar]
- 54.Parra E, Ferreira J, Ortega A. Overexpression of EGR-1 modulates the activity of NF-κB and AP-1 in prostate carcinoma PC-3 and LNCaP cell lines. Int J Oncol. 2011;39:345–352. doi: 10.3892/ijo.2011.1047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.