Key Points
Question
In adults with overweight or obesity without diabetes, what effect does once-weekly subcutaneous semaglutide, 2.4 mg, have on body weight when added to intensive behavioral therapy with an initial low-calorie diet?
Findings
In this randomized clinical trial that included 611 adults with overweight or obesity, 68 weeks’ treatment with once-weekly subcutaneous semaglutide vs placebo, combined with intensive behavioral therapy (and a low-calorie diet for the initial 8 weeks), resulted in reductions in body weight of 16.0% vs 5.7%, respectively; the difference was statistically significant.
Meaning
When used as an adjunct to intensive behavioral therapy and initial low-calorie diet, once-weekly subcutaneous semaglutide produced significantly greater weight loss than placebo during 68 weeks in adults with overweight or obesity.
Abstract
Importance
Weight loss improves cardiometabolic risk factors in people with overweight or obesity. Intensive lifestyle intervention and pharmacotherapy are the most effective noninvasive weight loss approaches.
Objective
To compare the effects of once-weekly subcutaneous semaglutide, 2.4 mg vs placebo for weight management as an adjunct to intensive behavioral therapy with initial low-calorie diet in adults with overweight or obesity.
Design, Setting, and Participants
Randomized, double-blind, parallel-group, 68-week, phase 3a study (STEP 3) conducted at 41 sites in the US from August 2018 to April 2020 in adults without diabetes (N = 611) and with either overweight (body mass index ≥27) plus at least 1 comorbidity or obesity (body mass index ≥30).
Interventions
Participants were randomized (2:1) to semaglutide, 2.4 mg (n = 407) or placebo (n = 204), both combined with a low-calorie diet for the first 8 weeks and intensive behavioral therapy (ie, 30 counseling visits) during 68 weeks.
Main Outcomes and Measures
The co–primary end points were percentage change in body weight and the loss of 5% or more of baseline weight by week 68. Confirmatory secondary end points included losses of at least 10% or 15% of baseline weight.
Results
Of 611 randomized participants (495 women [81.0%], mean age 46 years [SD, 13], body weight 105.8 kg [SD, 22.9], and body mass index 38.0 [SD, 6.7]), 567 (92.8%) completed the trial, and 505 (82.7%) were receiving treatment at trial end. At week 68, the estimated mean body weight change from baseline was –16.0% for semaglutide vs –5.7% for placebo (difference, −10.3 percentage points [95% CI, −12.0 to −8.6]; P < .001). More participants treated with semaglutide vs placebo lost at least 5% of baseline body weight (86.6% vs 47.6%, respectively; P < .001). A higher proportion of participants in the semaglutide vs placebo group achieved weight losses of at least 10% or 15% (75.3% vs 27.0% and 55.8% vs 13.2%, respectively; P < .001). Gastrointestinal adverse events were more frequent with semaglutide (82.8%) vs placebo (63.2%). Treatment was discontinued owing to these events in 3.4% of semaglutide participants vs 0% of placebo participants.
Conclusions and Relevance
Among adults with overweight or obesity, once-weekly subcutaneous semaglutide compared with placebo, used as an adjunct to intensive behavioral therapy and initial low-calorie diet, resulted in significantly greater weight loss during 68 weeks. Further research is needed to assess the durability of these findings.
Trial Registration
ClinicalTrials.gov Identifier: NCT03611582
This randomized clinical trial compares the effects of once-weekly subcutaneous semaglutide vs placebo for weight management as an adjunct to intensive behavioral therapy with initial low-calorie diet in adults with overweight or obesity.
Introduction
Intensive behavioral interventions for obesity providing 14 or more counseling sessions in 6 months induce mean losses of 5% to 10% of baseline body weight.1 Weight loss can be increased by an additional 3 to 5 percentage points by including a low-calorie (1000-1200 kcal/d) portion-controlled diet composed of liquid shakes, meal bars, and prepared meals.2,3 Larger weight losses (eg, ≥10%) are desired because they produce greater improvements in several obesity-related cardiometabolic risk factors and diseases, including type 2 diabetes, hypertension, and sleep apnea.4,5,6,7,8
Antiobesity medications approved by the Food and Drug Administration also increase weight loss when used adjunctively with behavioral intervention. Once-daily liraglutide, 3.0 mg, a glucagon-like peptide 1 receptor agonist approved for weight management, added approximately 3 to 5 percentage points of additional weight loss to intensive behavioral therapy compared with behavioral therapy alone.9,10 Subcutaneous semaglutide is a long-acting glucagon-like peptide 1 receptor agonist approved for the treatment of type 2 diabetes at once-weekly doses of up to 1.0 mg,11 which reduces body weight by approximately 6% by 1 year in these patients.12,13 In a 52-week, phase 2 trial, semaglutide produced a mean loss of up to 13.8% of baseline body weight (with 0.4 mg once daily, equivalent to a weekly dose of 2.8 mg) compared with 2.3% for placebo (both combined with approximately monthly behavioral counseling) and demonstrated an acceptable tolerability profile.14 Semaglutide as a 2.4-mg once-weekly dose is being evaluated for weight management in the phase 3 Semaglutide Treatment Effect for People with obesity (STEP) program.15
The present clinical trial was designed to maximize weight loss in adults with overweight or obesity without diabetes. Its objective was to evaluate the effects on body weight and cardiometabolic risk factors of adding subcutaneous semaglutide, 2.4 mg, to intensive behavioral therapy, the latter of which was also combined with an initial 8-week low-calorie diet to boost total weight loss.
Methods
Study Design and Oversight
STEP 3 was a 68-week, randomized, double-blind, placebo-controlled, multicenter study conducted at 41 sites in the US from August 2018 to April 2020. The study design has been published.15 The protocol and amendments (available in Supplement 2) were approved by institutional review boards or independent ethics committees at each study site. The study was conducted according to consensus ethical principles derived from guidelines, including the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice Guideline, and applicable local laws and regulations. All participants provided written informed consent.
Participants
Eligible participants were aged 18 years or older, reported 1 or more unsuccessful dietary efforts to lose weight, and had either body mass index (BMI) of 27 or higher with at least 1 weight-related comorbidity (cardiovascular disease, dyslipidemia, hypertension, or obstructive sleep apnea) or BMI of 30 or higher. Participants were excluded if they had diabetes, glycated hemoglobin levels of 6.5% or more (≥48 mmol/mol), self-reported body weight change greater than 5 kg within 90 days before screening, or prior or planned obesity treatment with surgery or a weight loss device. Full eligibility criteria are provided in eAppendix 3 in Supplement 1. To meet regulatory requirements,16 race and ethnicity were recorded in this study and determined by the participant according to fixed selection categories (with the option of answering “other,” “not applicable,” or “unknown”).
Procedures
Participants were randomized 2:1 with a blocking schema (block size of 9) via an interactive web-response system to once-weekly subcutaneous semaglutide, 2.4 mg, or visually identical placebo for 68 weeks, with an additional 7 weeks’ off-treatment follow-up to monitor adverse events. Based on Food and Drug Administration recommendations,16 a 2:1 randomization was selected to ensure that approximately 3000 participants across the phase 3 clinical program were exposed to semaglutide, 2.4 mg. Semaglutide was initiated at 0.25 mg, with dose escalation every 4 weeks until the target dose of 2.4 mg/wk was reached at week 16 (eFigure 1 in Supplement 1). If participants did not tolerate the 2.4-mg dose, they were permitted to receive 1.7 mg instead (at the investigator’s discretion) and encouraged to make at least 1 attempt to reescalate to the 2.4-mg dose.
For the first 8 weeks after randomization, participants received a low-calorie diet (1000-1200 kcal/d) provided as meal replacements (eg, liquid shakes, meal bars, portion-controlled meals [provided by Nutrisystem, supplied by the sponsor]). Participants subsequently transitioned to a hypocaloric diet (1200-1800 kcal/d) of conventional food for the remainder of the 68 weeks, with prescribed calorie intake based on randomization body weight. At randomization, participants were prescribed 100 minutes of physical activity per week (spread across 4-5 days), which increased by 25 minutes every 4 weeks, to reach 200 min/wk. During the 68 weeks, participants were provided with 30 individual intensive behavioral therapy visits with a registered dietitian, who instructed them in diet, physical activity, and behavioral strategies. Details of these counseling visits, and of the assessment schedule, are provided in eAppendix 4 and eAppendix 5 in Supplement 1.
End Points
The co–primary end points, in the order planned for sequential hierarchic testing, were the percentage change in body weight and the proportion of participants who lost at least 5% of baseline weight by week 68 (eAppendix 6 in Supplement 1). Confirmatory secondary end points (in hierarchic testing order) included the proportions of participants achieving weight reductions of at least 10% or 15%, and the change from baseline to week 68 in waist circumference, systolic blood pressure, and physical functioning score assessed by the 36-Item Short Form Health Survey (SF-36), Acute Version (eAppendix 7 in Supplement 1). Additional supportive secondary and exploratory end points are listed in eAppendix 8 in Supplement 1. Treatment-emergent adverse events and serious adverse events were assessed throughout treatment and follow-up. Selected adverse events (eg, cardiovascular events, acute pancreatitis) and deaths were reviewed by an independent external event adjudication committee.
Statistical Analysis
A sample size of 600 participants was calculated to provide power of 86% for the 7 end points in the hierarchic testing procedure, with greater than 99% power for the co–primary end points (see eAppendix 6 in Supplement 1 for details of the statistical analysis and Supplement 3 for the full statistical analysis plan). Efficacy end points were analyzed with the full analysis set (ie, all participants randomly assigned to a treatment group regardless of whether they initiated treatment), and adverse event end points were analyzed with the safety analysis set (ie, all randomized participants exposed to at least 1 dose of randomized treatment). Observation periods included the in-trial period (while in trial, regardless of treatment discontinuation or rescue intervention) and the on-treatment period (in which any dose of trial product was administered within the previous 2 weeks for efficacy analyses, or within the previous 49 days for adverse event analyses [ie, any period of temporary treatment interruption with trial product was excluded]). The superiority of subcutaneous semaglutide to placebo for the primary and secondary confirmatory end points was assessed in hierarchic order, with superiority at a significance level of 5% required before testing of subsequent end points in the hierarchy. All results from statistical analyses are reported together with the associated 2-sided 95% CI and corresponding P value (significance defined as P < .05). Findings for analyses of supportive secondary end points should be interpreted as exploratory because of the potential for type I error due to multiple comparisons. All statistical analyses were performed with SAS version 9.4 TS1M5.
Two estimands were used to address different scientific questions, as described elsewhere.17,18 The primary hierarchic statistical analyses were based on the treatment policy estimand (similar to an intention-to-treat analysis), which quantified the average treatment effect among all randomized participants, regardless of adherence to treatment or initiation of rescue interventions (ie, antiobesity medications or bariatric surgery). Continuous and categorical end points were analyzed with analysis of covariance and logistic regression, respectively (both with randomized treatment as a factor and baseline value as a covariate). Missing data were imputed with a multiple imputation approach, similar to that described by McEvoy.19 Missing body weight measurements were imputed by sampling from available measurements at week 68 from participants receiving randomized treatment in the relevant randomized treatment group. Missing values were multiply imputed (× 1000). Each of the 1000 complete data sets was analyzed, resulting in 1000 estimates that were combined by using the formula by Rubin20 to obtain overall estimates.
The trial product estimand quantified the average treatment effect in all randomized participants, assuming they remained receiving randomized treatment for the duration of the trial (and without rescue intervention). For this estimand, continuous end points were analyzed with a mixed model for repeated measurements. Categorical end points were analyzed with logistic regression, with treatment as the only factor; for missing data, categorization was based on predicted values from the mixed model for repeated measurements. The trial product estimand, which models the data to assume that all participants were adherent to treatment, typically yields a higher estimate of weight loss than the treatment policy estimand, which includes data for all participants, regardless of treatment adherence. All reported results are for the treatment policy estimand, unless stated otherwise.
Results
Study Participants
From August 2018 to November 2018, 742 participants were screened, and 611 were randomized to treatment: 407 to semaglutide and 204 to placebo. Overall, 567 participants (92.8%) completed the trial, and 505 (82.7%) completed the trial in the on-treatment period (Figure 1). The proportion of participants permanently discontinuing trial product was similar between treatment groups (semaglutide, 16.7%; placebo, 18.6%) (eFigure 2 in Supplement 1). The most frequent reasons for permanent discontinuation were adverse events (semaglutide, 6.4%; placebo, 2.9%), lost to follow-up (semaglutide, 4.4%; placebo, 3.4%), and the category “other,” which included various personal reasons. Demographic and baseline clinical characteristics were similar for the 2 groups (Table 1). Most participants were women (81.0%) and White individuals (76.1%), with a mean age of 46 years. Mean body weight was 105.8 kg, mean BMI was 38.0, and mean waist circumference was 113.0 cm. At screening, 75.8% of participants had 1 or more comorbidities.
Figure 1. Participant Flow in the STEP 3 Trial of Semaglutide in Adults With Overweight or Obesity.
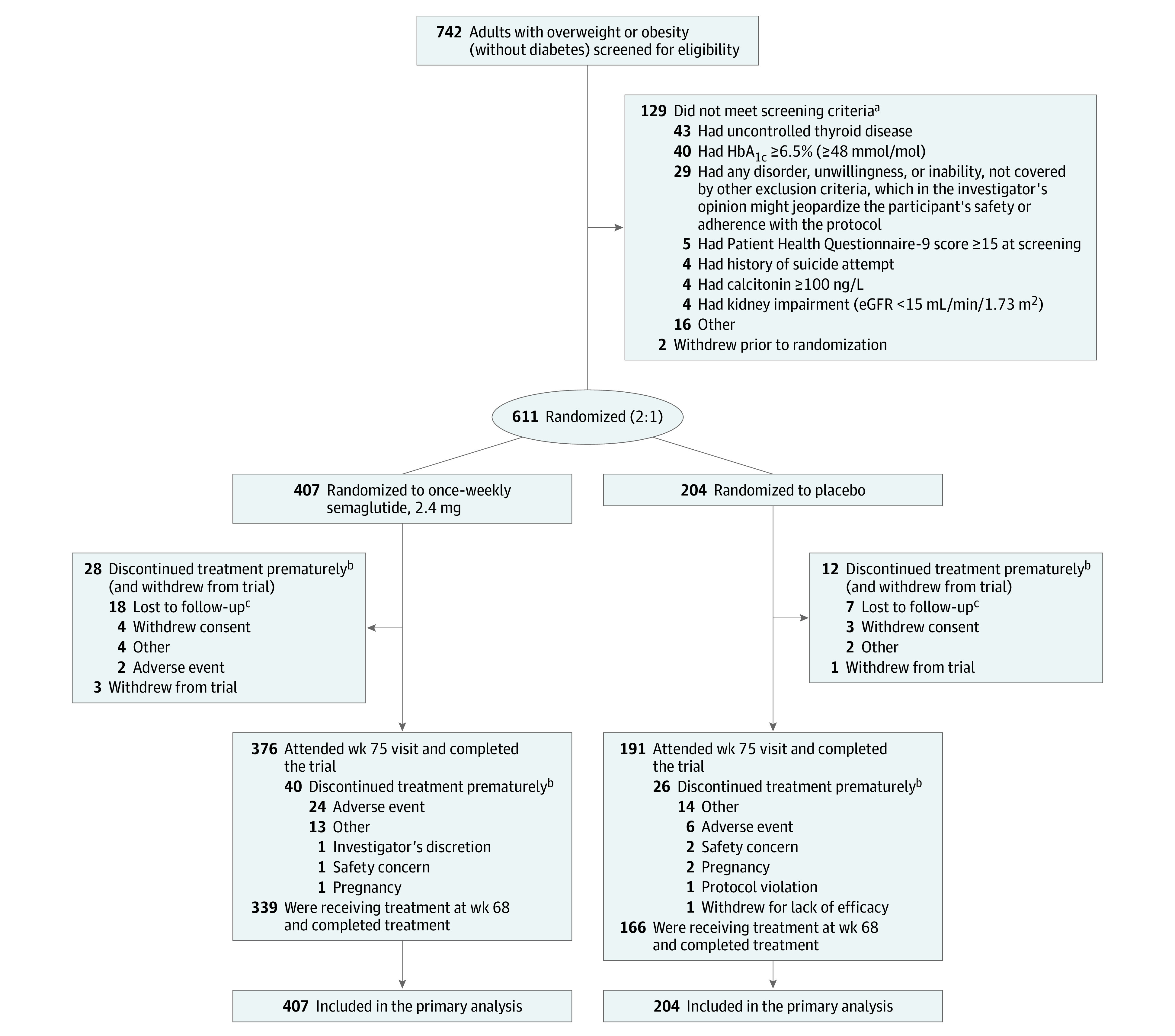
aParticipants could meet more than 1 exclusion or randomization criterion.
bAdverse events leading to permanent discontinuation of trial product (participants may have discontinued due to ≥1 adverse event): (1) gastrointestinal disorders: constipation, n = 2 (semaglutide); diarrhea/explosive diarrhea, n = 2 (semaglutide); eructation/belching, n = 1 (semaglutide); flatulence/excessive gas, n = 1 (semaglutide); nausea/worsening nausea, n = 7 (semaglutide); retching/dry heaves, n = 1 (semaglutide); and vomiting/worsening vomiting/recurrent vomiting, n = 6 (semaglutide). (2) General disorders and administration site conditions/hepatobiliary disorders: biliary colic/gallbladder pain, n = 1 (semaglutide); biliary dyskinesia, n = 1 (semaglutide); and injection site hematoma, n = 1 (placebo). (3) Infections and infestations: diverticulitis, n = 1 (placebo). (4) Injury, poisoning, and procedural complications: concussion, n = 1 (semaglutide). (5) Investigations: amylase increased/elevated amylase, n = 1 (semaglutide); blood creatine phosphokinase increased/elevated creatine kinase, n = 1 (semaglutide); and lipase increased/elevated lipase, n = 1 (semaglutide). (6) Metabolism and nutrition disorders: loss of appetite, n = 1 (semaglutide). (7) Musculoskeletal and connective tissue disorders: right-sided flank pain, n = 1 (placebo). (8) Nervous system disorders: headache, n = 1 (semaglutide); and worsening of migraine, n = 1 (semaglutide). (9) Psychiatric disorders: anxiety/worsening anxiety, n = 3 (semaglutide), n = 1 (placebo). (10) Skin and subcutaneous tissue disorders: hair thinning, n = 1 (semaglutide); hair loss, n = 1 (placebo); burning under skin of the right leg, n = 1 (semaglutide); and generalized pruritic rash, n = 1 (placebo).
cA total of 5.6% of participants were lost to follow-up. In the semaglutide group, 12 were lost to follow-up by week 38, and in the placebo group, 5 were lost to follow-up by week 25.
Table 1. Baseline Demographics and Clinical Characteristicsa.
| Characteristic | No. (%) | |
|---|---|---|
| Semaglutide, 2.4 mg (n = 407) | Placebo (n = 204) | |
| Age, mean (SD), y | 46 (13) | 46 (13) |
| Sex | ||
| Women | 315 (77.4) | 180 (88.2) |
| Men | 92 (22.6) | 24 (11.8) |
| Raceb | ||
| White | 307 (75.4) | 158 (77.5) |
| Black or African American | 80 (19.7) | 36 (17.6) |
| Other | 11 (2.7) | 4 (2.0) |
| Asian | 5 (1.2) | 6 (2.9) |
| Native Hawaiian or other Pacific Islander | 3 (0.7) | 0 |
| American Indian or Alaska Native | 1 (0.2) | 0 |
| Hispanic or Latino ethnic group | 75 (18.4) | 46 (22.5) |
| Body weight, mean (SD), kg | 106.9 (22.8) | 103.7 (22.9) |
| Body mass index, mean (SD) | 38.1 (6.7) | 37.8 (6.9) |
| Body mass index categories | ||
| ≥27-<30 (overweight) | 23 (5.7) | 15 (7.4) |
| ≥30-<35 (class 1 obesity) | 126 (31.0) | 58 (28.4) |
| ≥35-<40 (class 2 obesity) | 136 (33.4) | 76 (37.3) |
| ≥40 (class 3 obesity) | 122 (30.0) | 55 (27.0) |
| Waist circumference, mean (SD), cm | 113.6 (15.1) | 111.8 (16.2) |
| Comorbidities at screeningc | ||
| Dyslipidemia | 145 (35.6) | 67 (32.8) |
| Hypertension | 145 (35.6) | 67 (32.8) |
| Knee osteoarthritis | 76 (18.7) | 31 (15.2) |
| Asthma/COPD | 67 (16.5) | 25 (12.3) |
| Obstructive sleep apnea | 58 (14.3) | 19 (9.3) |
| Nonalcoholic fatty liver disease | 23 (5.7) | 12 (5.9) |
| Polycystic ovary syndrome | 17 (5.4) | 10 (5.6) |
| Coronary artery disease | 6 (1.5) | 4 (2.0) |
| No. of comorbidities at screeningc | ||
| None | 99 (24.3) | 49 (24.0) |
| 1 | 93 (22.9) | 53 (26.0) |
| 2 | 96 (23.6) | 43 (21.1) |
| 3 | 62 (15.2) | 38 (18.6) |
| 4 | 31 (7.6) | 14 (6.9) |
| ≥5 | 26 (6.4) | 7 (3.4) |
| Blood pressure, mean (SD), mm Hg | ||
| Systolic | 124 (15) | 124 (15) |
| Diastolic | 80 (10) | 81 (10) |
| Pulse, mean (SD), /min | 71 (10) | 71 (10) |
| Glycated hemoglobin, mean (SD), %d | 5.7 (0.3) | 5.8 (0.3) |
| Fasting plasma glucose, mean (SD), mg/dd | 93.9 (9.4) [n = 397] | 94.0 (9.8) [n = 200] |
| Fasting serum insulin, geometric mean pmol/L (CV)d | 90.1 (59.5) [n = 388] | 92.6 (61.0) [n = 194] |
| C-reactive protein, geometric mean (CV), mg/Ld | 4.52 (142.1) [n = 401] | 4.35 (129.9) [n = 202] |
| Fasting lipid profile, geometric mean (CV), mg/dLe | ||
| Cholesterol | [n = 401] | [n = 202] |
| Total | 185.4 (19.8) | 188.7 (20.6) |
| LDL | 107.7 (30.3) | 111.8 (31.2) |
| HDL | 51.6 (24.0) | 50.9 (22.6) |
| VLDL | 21.0 (49.7) | 21.7 (44.5) |
| Free fatty acids | 11.9 (59.4) [n = 388] | 11.1 (64.8) [n = 195] |
| Triglycerides | 107.9 (50.3) [n = 401] | 110.9 (44.4) [n = 202] |
| Kidney function | ||
| eGFR, geometric mean (CV), mL/min/1.73 m2 | 96.6 (21.3) | 96.5 (20.7) |
| Normal (eGFR ≥90 mL/min/1.73 m2) | 280 (68.8) | 133 (65.2) |
| Mild impairment (eGFR ≥60 –<90 mL/min/1.73 m2) | 118 (29.0) | 66 (32.4) |
| Moderate impairment (eGFR ≥30 –<60 mL/min/1.73 m2) | 9 (2.2) | 5 (2.5) |
| SF-36f | [n = 402] | [n = 203] |
| Physical functioning score | 51.9 (6.7) | 52.1 (6.8) |
| Component summary score | ||
| Physical | 51.6 (6.9) | 51.7 (7.3) |
| Mental | 55.7 (5.3) | 55.4 (6.1) |
Abbreviations: COPD, chronic obstructive pulmonary disease; CV, coefficient of variation (in percentage); eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SF-36, 36-Item Short Form Health Survey, Acute Version; VLDL, very low-density lipoprotein.
SI conversion factors: To convert values for glucose to mmol/L, multiply by 0.0555; and cholesterol to mmol/L, multiply by 0.0259. Body mass index is calculated as weight in kilograms divided by height in meters squared.
Body weight, vital signs, and glycated hemoglobin were assessed at screening and randomization; all other laboratory measurements were assessed at randomization only.
Race and ethnicity were determined by the participant according to fixed selection categories with options of “other,” “not applicable,” or “unknown.”
Comorbidities included dyslipidemia, hypertension, coronary artery disease, cerebrovascular disease, obstructive sleep apnea, impaired glucose metabolism, reproductive system disorders, liver disease, kidney disease, osteoarthritis, gout, thyroid disease, and asthma/COPD. Information about comorbidities judged to be relevant and significant for the trial population was collected at screening, using specific disease forms based on information from the participants (yes/no answers).
Normal value for glycated hemoglobin is <6.5%; for fasting plasma glucose, 74-99 mg/dL; for fasting serum insulin, 11-220 pmol/L (in women) and <218 pmol/L (in men); for C-reactive protein, <5 mg/L.
Normal values: total cholesterol, <199.6 mg/dL; LDL, <99.2 mg/dL; HDL, >59.9 mg/dL; VLDL, <30.1 mg/dL; free fatty acids, 2.8-25.4 mg/dL; and triglycerides, <150.4 mg/dL.
SF-36 is a measure of health-related quality of life and general health status. It uses a norm-based score: greater than and less than 50 are greater than and less than the average, respectively, found in the 2009 US general population. Further information on the SF-36 is provided in eAppendix 7 in Supplement 1.
Co–Primary End Points
At week 68, the estimated mean weight change from baseline was −16.0% with semaglutide vs –5.7% with placebo, both combined with intensive behavioral therapy and meal replacements (difference, −10.3 percentage points [95% CI, −12.0 to −8.6]; P < .001) (Table 2, Figure 2A, and eFigure 3 in Supplement 1). For the trial product estimand, corresponding changes were −17.6% with semaglutide vs −5.0% with placebo (difference, −12.7 percentage points [95% CI, −14.3 to −11.0]; P < .001) (eTable 1 and eFigure 4 in Supplement 1). See eFigure 5 in Supplement 1 for cumulative distribution function plots for weight change.
Table 2. Primary and Secondary End Points at Week 68a.
| End pointb | Semaglutide, 2.4 mg (n = 407) | Placebo (n = 204) | Difference (95% CI) | Odds ratio (95% CI) |
P value |
|---|---|---|---|---|---|
| Co–primary end pointsc | |||||
| Body weight, % reduction | –16.0 | –5.7 | –10.3 (–12.0 to –8.6) | <.001 | |
| Body weight reduction ≥5%, proportion of participants at week 68, % | 86.6 | 47.6 | 6.1 (4.0 to 9.3) | <.001 | |
| Confirmatory secondary end points | |||||
| Waist circumference, cm | –14.6 | –6.3 | –8.3 (–10.1 to –6.6) | <.001 | |
| Systolic blood pressure, mm Hg | –5.6 | –1.6 | –3.9 (–6.4 to –1.5) | .001 | |
| SF-36 physical functioning scored | 2.4 | 1.6 | 0.8 (–0.2 to 1.9) | .12 | |
| Body weight reduction ≥10%, proportion of participants at week 68, % | 75.3 | 27.0 | 7.4 (4.9 to 11.0) | <.001 | |
| Body weight reduction ≥15%, proportion of participants at week 68, % | 55.8 | 13.2 | 7.9 (4.9 to 12.6) | <.001 | |
| Supportive secondary end pointse | |||||
| Body weight reduction ≥20%, proportion of participants at week 68, % | 35.7 | 3.7 | 13.7 (6.2 to 30.3) | <.001 | |
| Body weight, kg | –16.8 | –6.2 | –10.6 (–12.5 to –8.8) | <.001 | |
| Body mass index | –6.0 | –2.2 | –3.8 (–4.4 to –3.1) | <.001 | |
| Glycated hemoglobin, percentage points | –0.51 | –0.27 | –0.24 (–0.29 to –0.19) | <.001 | |
| Fasting plasma glucose, mg/dL | –6.73 | –0.65 | –6.09 (–8.13 to –4.04) | <.001 | |
| Diastolic blood pressure, mm Hg | –3.0 | –0.8 | –2.2 (–3.9 to –0.6) | .008 | |
| SF-36d | |||||
| Physical component summary score | 3.0 | 2.3 | 0.7 (–0.5 to 1.9) | .27 | |
| Mental component summary score | –0.8 | –2.9 | 2.1 (0.5 to 3.6) | .011 | |
| Fasting values, % change at week 68f | |||||
| Serum insulin | –32.3 | –15.0 | –20.3 (–30.4 to –8.7)f | .001 | |
| Lipid profile | |||||
| Cholesterol | |||||
| Total | –3.8 | 2.1 | –5.8 (–8.4 to –3.2)f | <.001 | |
| HDL | 6.5 | 5.0 | 1.5 (–1.8 to 4.9)f | .39 | |
| LDL | –4.7 | 2.6 | –7.1 (–10.9 to –3.2)f | <.001 | |
| VLDL | –22.5 | –6.6 | –17.0 (–22.8 to –10.9)f | <.001 | |
| Free fatty acids | –11.9 | 4.0 | –15.3 (–25.0 to –4.3)f | .008 | |
| Triglycerides | –22.5 | –6.5 | –17.0 (–22.8 to –10.8)f | <.001 | |
| C-reactive protein, % change at week 68f | –59.6 | –22.9 | –47.6 (–55.0 to –39.0)f | <.001 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; SF-36, 36-Item Short Form Health Survey, Acute Version; VLDL, very low-density lipoprotein.
Values are estimated mean change from baseline to week 68 and estimated treatment difference (unless stated otherwise), based on the treatment policy estimand for the in-trial period (from randomization to last contact with the trial site, regardless of treatment discontinuation or rescue intervention) for the full analysis set, which includes all participants randomly assigned to a treatment group regardless of whether they initiated treatment; see eTable 1 in Supplement 1 for corresponding data for the trial product estimand (assesses treatment effect assuming all participants adhered to treatment and did not receive rescue intervention).
Continuous end points were analyzed with analysis of covariance, with randomized treatment as a factor and baseline end point value as a covariate, and a multiple imputation approach for missing data.15 Categorical end points were analyzed with logistic regression, with the same factor and covariate.
Baseline body weight was 106.9 kg (SD, 22.8) in the semaglutide group and 103.7 kg (SD, 22.9) in the placebo group.
SF-36 is a measure of health-related quality of life and general health status and uses a norm-based score. Norm-based scores greater than and less than 50 are greater than and less than the average, respectively, found in the 2009 US general population. Further information on the SF-36 is provided in eAppendix 7 in Supplement 1.
Supportive secondary end point analyses were not adjusted for multiplicity.
These parameters were initially analyzed on a log scale as estimated ratio to baseline (within treatment groups) and estimated treatment ratios (between treatment groups). For interpretation, these data are expressed as relative percentage change and estimated relative percentage difference between groups, respectively, and were calculated with the following formula: (estimated ratio – 1) × 100.
Figure 2. Body Weight–Related Efficacy End Points.
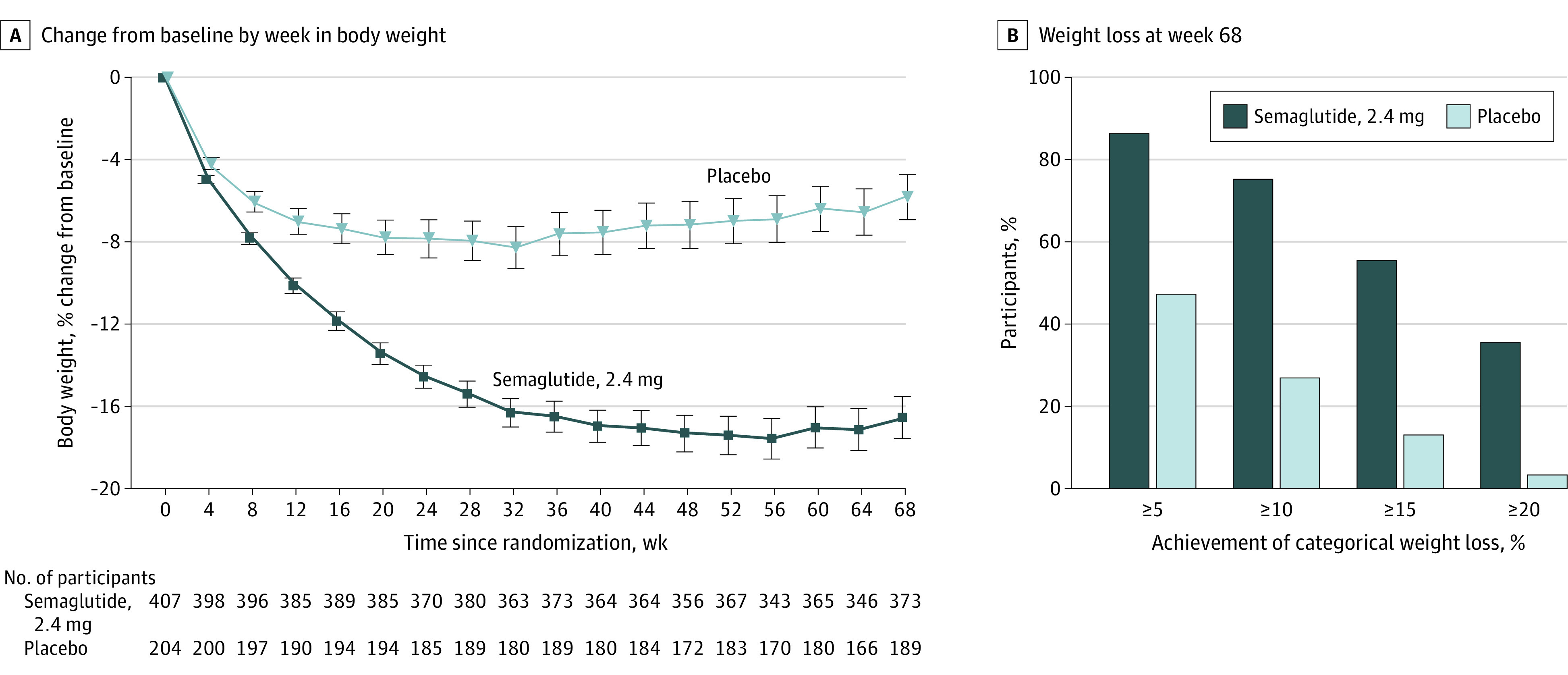
A, The observed mean percentage change in body weight over time for participants in the full analysis set for the in-trial period (from randomization to last contact with the trial site, regardless of treatment discontinuation or rescue intervention). Error bars represent 95% CIs of the mean. B, The observed proportions of participants attaining at least 5% (co–primary end point), 10%, 15%, and 20% reductions in baseline body weight by week 68 in the full analysis set. The proportions shown are cumulative, such that the 88.6% of semaglutide-treated participants who lost more than 5% of baseline body weight includes the 75.3% of participants who lost more than 10%, and so on. See eFigure 4 in Supplement 1 for corresponding on-treatment data (during treatment with the trial product [any dose of trial medication administered within the previous 2 weeks]).
Semaglutide-treated participants were significantly more likely to have lost at least 5% of baseline body weight at week 68 vs placebo (P < .001 for both estimands) (Table 2 and eTable 1 in Supplement 1), with 86.6% of participants in the semaglutide group vs 47.6% in the placebo group achieving this threshold (in-trial period) (Figure 2B and eFigure 4 in Supplement 1).
Confirmatory Secondary End Points
At week 68, participants in the semaglutide group were significantly more likely to have lost at least 10% or 15% of baseline body weight vs placebo (P < .001 for both estimands) (Table 2 and eTable 1 in Supplement 1). These thresholds were achieved by 75.3% vs 27.0% and 55.8% vs 13.2% of participants in the semaglutide and placebo groups, respectively (in-trial period) (Figure 2B and eFigure 4 in Supplement 1). Reductions at week 68 in waist circumference and systolic blood pressure were significantly greater with semaglutide than with placebo (difference, –8.3 cm [95% CI, –10.1 to –6.6]; P < .001 and –3.9 mm Hg [95% CI, –6.4 to –1.5]; P = .001, respectively) (Table 2 and eTable 1 in Supplement 1). Physical function (measured by the SF-36 physical functioning score) improved similarly in both groups from baseline to week 68 (difference, 0.8 [95% CI, –0.2 to 1.9]; P = .12) (Table 2; eTable 1 and eFigures 6 and 7 in Supplement 1).
Supportive Secondary End Points
Relative to the placebo group, participants in the semaglutide group were more likely to have lost 20% or more of baseline body weight by week 68 (Table 2 and eTable 1 in Supplement 1); 35.7% vs 3.7% achieved this weight-loss threshold with semaglutide vs placebo, respectively (in-trial period) (Figure 2B and eFigure 4 in Supplement 1). Semaglutide was associated with improvements vs placebo in BMI and diastolic blood pressure at week 68 (Table 2 and eTable 1 in Supplement 1). At week 68, levels of C-reactive protein and lipids had improved with semaglutide relative to placebo, with the exception of high-density lipoprotein cholesterol (Table 2 and eTable 1 in Supplement 1). Semaglutide was also associated with a reduction in glycated hemoglobin compared with placebo (Table 2 and eTable 1 in Supplement 1). From baseline to week 68, SF-36 physical component summary score improved similarly in both groups, whereas the mental component summary score favored semaglutide (Table 2; eTable 1 and eFigure 7 in Supplement 1).
Adverse Events
The proportion of participants reporting adverse events was similar in the semaglutide and placebo groups (95.8% and 96.1%, respectively). Gastrointestinal disorders (typically nausea, constipation, diarrhea, and vomiting) were the most frequent and occurred in more participants receiving semaglutide (82.8%) than placebo (63.2%) (Table 3). Most gastrointestinal events were mild to moderate and of relatively short duration (median duration of events: nausea [5 days in both groups], vomiting [2 days in both groups], diarrhea [3 days in both groups], and constipation [27 days with semaglutide vs 16 days with placebo]), and the majority of participants recovered without treatment discontinuation (eFigure 8 in Supplement 1). The proportion of participants experiencing nausea with semaglutide peaked at approximately 25% at week 20 and declined thereafter, remaining at approximately 15% for the duration of the study. At any given time during the study, the proportion of participants who experienced vomiting was less than 5% in both treatment groups.
Table 3. Adverse Eventsa.
| Event | Semaglutide, 2.4 mg (n = 407) | Placebo (n = 204) | ||||
|---|---|---|---|---|---|---|
| Participants, No. (%) | No. of events | Events per 100 patient-yearsb | Participants, No. (%) | No. of events | Events per 100 patient-yearsb | |
| Participants with ≥1 adverse eventc | 390 (95.8) | 4035 | 766.9 | 196 (96.1) | 1325 | 506.9 |
| Adverse events leading to treatment discontinuation | 24 (5.9) | 34 | 6.5 | 6 (2.9) | 6 | 2.3 |
| Gastrointestinal disorders | 14 (3.4) | 20 | 3.8 | 0 | ||
| Adverse events reported in ≥10% of participantsd | ||||||
| Nausea | 237 (58.2) | 511 | 97.1 | 45 (22.1) | 60 | 23 |
| Constipation | 150 (36.9) | 210 | 39.9 | 50 (24.5) | 62 | 23.7 |
| Diarrhea | 147 (36.1) | 307 | 58.3 | 45 (22.1) | 62 | 23.7 |
| Vomiting | 111 (27.3) | 212 | 40.3 | 22 (10.8) | 25 | 9.6 |
| Nasopharyngitis | 90 (22.1) | 128 | 24.3 | 49 (24.0) | 70 | 26.8 |
| Upper respiratory tract infection | 85 (20.9) | 115 | 21.9 | 44 (21.6) | 65 | 24.9 |
| Headache | 78 (19.2) | 123 | 23.4 | 20 (9.8) | 25 | 9.6 |
| Abdominal pain | 54 (13.3) | 76 | 14.4 | 10 (4.9) | 11 | 4.2 |
| Back pain | 54 (13.3) | 68 | 12.9 | 22 (10.8) | 24 | 9.2 |
| Dizziness | 52 (12.8) | 73 | 13.9 | 11 (5.4) | 14 | 5.4 |
| Fatigue | 52 (12.8) | 69 | 13.1 | 15 (7.4) | 19 | 7.3 |
| Flatulence | 47 (11.5) | 62 | 11.8 | 23 (11.3) | 24 | 9.2 |
| Gastroenteritis viral | 42 (10.3) | 47 | 8.9 | 13 (6.4) | 13 | 5 |
| Urinary tract infection | 42 (10.3) | 61 | 11.6 | 10 (4.9) | 11 | 4.2 |
| Abdominal distention | 41 (10.1) | 55 | 10.5 | 20 (9.8) | 28 | 10.7 |
| Sinusitis | 39 (9.6) | 51 | 9.7 | 26 (12.7) | 34 | 13 |
| Adverse events of intereste | ||||||
| Gastrointestinal disorders | 337 (82.8) | 1760 | 334.5 | 129 (63.2) | 333 | 127.4 |
| Psychiatric disorders | 60 (14.7) | 97 | 18.4 | 24 (11.8) | 31 | 11.9 |
| Cardiovascular disordersf | 40 (9.8) | 50 | 8.9 | 22 (10.8) | 27 | 9.5 |
| Allergic reactions | 35 (8.6) | 41 | 7.8 | 19 (9.3) | 19 | 7.3 |
| Injection site reactions | 22 (5.4) | 31 | 5.9 | 12 (5.9) | 16 | 6.1 |
| Gallbladder-related disorders | 20 (4.9) | 24 | 4.6 | 3 (1.5) | 3 | 1.1 |
| Cholelithiasis | 13 (3.2) | 13 | 2.5 | 2 (1.0) | 2 | 0.8 |
| Hepatic disorders | 8 (2.0) | 9 | 1.7 | 4 (2.0) | 5 | 1.9 |
| Malignant neoplasmsf | 3 (0.7) | 3 | 0.5 | 1 (0.5) | 1 | 0.4 |
| Hypoglycemia | 2 (0.5) | 2 | 0.4 | 0 | ||
| Acute pancreatitisg | 0 | 0 | ||||
| Acute renal failure | 0 | 0 | ||||
| Participants with ≥1 serious adverse eventh | 37 (9.1) | 55 | 10.5 | 6 (2.9) | 7 | 2.7 |
| Serious adverse events by SOC reported in >1% of participants | ||||||
| Hepatobiliary disorders | 10 (2.5) | 13 | 2.5 | 0 | ||
| Cholelithiasis | 7 (1.7) | 7 | 1.3 | 0 | ||
| Acute cholecystitis | 3 (0.7) | 3 | 0.6 | 0 | ||
| Cholecystitis | 2 (0.5) | 2 | 0.4 | 0 | ||
| Biliary dyskinesia | 1 (0.2) | 1 | 0.2 | 0 | ||
| Infections and infestations | 8 (2.0) | 12 | 2.3 | 0 | ||
| Appendicitis | 3 (0.7) | 4 | 0.8 | 0 | ||
| Abdominal abscess | 1 (0.2) | 1 | 0.2 | 0 | ||
| Cellulitis | 1 (0.2) | 1 | 0.2 | 0 | ||
| Viral gastroenteritis | 1 (0.2) | 1 | 0.2 | 0 | ||
| Large intestine infection | 1 (0.2) | 1 | 0.2 | 0 | ||
| Pelvic inflammatory disease | 1 (0.2) | 1 | 0.2 | 0 | ||
| Pneumonia | 1 (0.2) | 1 | 0.2 | 0 | ||
| Sepsis | 1 (0.2) | 1 | 0.2 | 0 | ||
| Urinary tract infection | 1 (0.2) | 1 | 0.2 | 0 | ||
| Musculoskeletal and connective tissue disorders | 5 (1.2) | 8 | 1.5 | 0 | ||
| Osteoarthritis | 3 (0.7) | 4 | 0.8 | 0 | ||
| Cervical spinal stenosis | 2 (0.5) | 2 | 0.4 | 0 | ||
| Back pain | 1 (0.2) | 1 | 0.2 | 0 | ||
| Intervertebral disk protrusion | 1 (0.2) | 1 | 0.2 | 0 | ||
Abbreviation: SOC, system organ class.
Adverse events that occurred in participants in the safety analysis set are included and presented by their preferred terms according to the Medical Dictionary for Regulatory Activities version 22.1. Events were included if the date of onset was during the on-treatment period (date of first trial product administration to date of last trial product administration, excluding potential off-treatment intervals triggered by at least 2 consecutive missed doses), unless specified otherwise. The investigator (with study staff) was responsible for detecting, documenting, recording, and following up on events that met the definition of an adverse event or serious adverse event. Events were detected from participant reports at clinic visits or by telephone.
Events per 100 patient-years are calculated as (number of events/patient years) × 100.
Includes serious adverse events.
Most common adverse events by preferred term reported in at least 10% of participants in either treatment group.
Identified via Medical Dictionary for Regulatory Activities searches.
Event occurred during the in-trial period (time from randomization to last contact with trial site, irrespective of treatment discontinuation or rescue intervention).
Event adjudication committee–confirmed event.
A serious adverse event was defined as an adverse event that fulfilled at least 1 of the following criteria: resulted in death, was life threatening, required inpatient hospitalization or prolongation of existing hospitalization, resulted in persistent disability/incapacity, was a congenital anomaly/birth defect, or was an important medical event.
Serious adverse events were reported in 9.1% and 2.9% of participants in the semaglutide and placebo groups, respectively (Table 3). More participants discontinued treatment due to adverse events in the semaglutide group (5.9%) compared with placebo (2.9%), mainly because of gastrointestinal events (Table 3). No deaths were reported during the study. Gallbladder-related disorders (mainly cholelithiasis) were reported in 20 participants (4.9%) treated with semaglutide and in 3 (1.5%) receiving placebo. Malignant neoplasms were reported in 3 semaglutide-treated participants (0.7%; basal cell carcinoma, breast cancer, and papillary thyroid cancer) and 1 placebo-treated participant (0.5%; invasive lobular breast carcinoma). There were no cases of acute pancreatitis, medullary thyroid carcinoma, or pancreatic cancer in either group.
At week 68, the estimated change in pulse from baseline was 3.1/min for semaglutide vs 2.1/min for placebo (trial product estimand difference, 1.0/min [95% CI, –0.7 to 2.6]) (eTable 2 in Supplement 1). At the follow-up visit (week 75) after the off-treatment period, mean pulse had neared the baseline level. Additional adverse event findings are described in Table 3 and eTable 2 in Supplement 1.
Discussion
In adults with overweight or obesity (without diabetes), once-weekly subcutaneous semaglutide increased mean weight loss by 10.3 percentage points compared with placebo when used adjunctively with intensive behavioral therapy combined with an initial low-calorie, meal-replacement program. A previous trial, which used a similar program of intensive behavioral therapy (delivered without meal replacements) combined with liraglutide, 3.0 mg, or placebo,10 observed mean losses of 7.5% and 4.0% of baseline body weight, respectively, at 56 weeks. Direct comparison of effect sizes in these 2 studies is not possible because they were obtained in separate trials. A head-to-head comparison of the 2 medicines is being conducted (STEP 8, ClinicalTrials.gov identifier: NCT04074161).
The present findings suggest that the addition of semaglutide to intensive behavioral therapy may help patients achieve more than the average 5% to 10% reduction in body weight typically produced by behavioral interventions at 6 to 12 months.1,21 Weight loss with behavioral therapy often plateaus at this level, despite patients’ continuing to have obesity.22 Larger-than-expected reductions in resting and nonresting energy expenditure that occur with weight loss (ie, metabolic adaptation), compensatory changes in other homeostatic regulators of body weight, and patients’ behavioral fatigue in adhering to diet and activity recommendations may contribute to the 5% to 10% weight reduction plateau observed with behavioral therapy.23,24,25
Preclinical studies suggest that weight loss with semaglutide results from its effects on glucagon-like peptide 1 receptors that mediate direct and indirect effects on the brain areas involved in regulation of appetite, including in the hypothalamus and hindbrain, ultimately leading to reduced energy intake.26 A 20-week clinical study of participants with obesity found that treatment with once-weekly semaglutide, 2.4 mg, compared with placebo reduced self-reported hunger and food cravings and decreased energy intake during an ad libitum lunch by 35%.27
Weight losses of 5% or more of baseline weight are associated linearly with improvements in several obesity-related cardiometabolic risk factors and diseases.4,5,6,7,8 The larger proportions of participants treated with semaglutide compared with placebo who achieved categorical weight losses of at least 10%, 15%, or 20% translated into greater improvements in waist circumference, blood pressure, glycated hemoglobin level, C-reactive protein level, and several lipid parameters. Observed benefits of weight loss might have been larger if participants had been selected because of having elevated risk factors (eg, hypertension, hyperlipidemia), which they were not in the present study.
The adverse event and tolerability profile of semaglutide in this trial was consistent with that of the glucagon-like peptide 1 receptor agonist class28,29; gastrointestinal disorders were the most commonly reported adverse events. The proportion of participants in the semaglutide group who reported serious adverse events was greater than in the placebo group, in part because of a higher incidence of hepatobiliary disorders (mainly cholelithiasis). The incidence of hepatobiliary disorders could be attributed, at least partly, to rapid weight loss, which is a known risk factor for gallstones.30 The remaining events that contributed to the imbalance between the semaglutide and placebo groups were distributed across several system organ classes, without apparent biological relationship to semaglutide.
A question unanswered by the present study is whether intensive behavioral therapy and an initial low-calorie, meal-replacement diet were necessary to achieve the long-term reduction in baseline weight seen with semaglutide. The STEP 1 trial examined semaglutide, 2.4 mg, combined with a less-intensive lifestyle intervention program that provided behavioral counseling visits every 4 weeks (ie, 18 sessions in 68 weeks) and no initial low-calorie, meal-replacement diet.15,31 Participants in STEP 1 lost 14.9% of baseline weight with semaglutide at 68 weeks, compared with 2.4% for placebo plus the same lifestyle intervention.31 These findings suggest that the inclusion of intensive behavioral therapy plus an 8-week low-calorie diet ultimately may not contribute significant additional weight loss beyond that achieved by semaglutide and less-intensive lifestyle intervention. Further study is needed of the optimal program of lifestyle modification required with semaglutide, 2.4 mg.
Limitations
This study has several limitations. First, it could not identify the separate contributions to weight loss of intensive behavioral therapy and the initial low-calorie diet in the placebo group or, as previously indicated, determine the relative benefit of combining either of these enhanced interventions with semaglutide. Second, this was a relatively brief trial, which did not address whether semaglutide-treated participants would sustain their 16% weight reduction if they continued to receive the medication past 68 weeks. A 2-year trial of semaglutide, 2.4 mg, in participants with overweight or obesity is currently underway (STEP 5, ClinicalTrials.gov Identifier: NCT03693430). Third, further study is needed of the acceptability to patients of an injectable medication for obesity compared with traditional oral delivery.
Conclusions
Among adults with overweight or obesity, once-weekly subcutaneous semaglutide compared with placebo, used as an adjunct to intensive behavioral therapy and initial low-calorie diet, resulted in significantly greater weight loss during 68 weeks. Further research is needed to assess the durability of these findings.
eAppendix 1. List of Investigators in the Semaglutide Treatment Effect in People with obesity (STEP) 3 Trial
eAppendix 2. Participant Enrollment and Exclusions by Study Site
eAppendix 3. Inclusion and Exclusion Criteria
eAppendix 4. Intensive Behavioral Therapy Methodology
eAppendix 5. Summary of Assessments
eAppendix 6. Statistical Analysis
eAppendix 7. Patient-Reported Outcome Assessments
eAppendix 8. Supportive Secondary End Points
eTable 1. Changes in Secondary End Points from Baseline to Week 68 a
eTable 2. Supportive Secondary Safety End Points
eFigure 1. Trial Design
eFigure 2. Time to Permanent Discontinuation of Trial Product (Weeks)
eFigure 3. Variability in Body Weight Change from Baseline by Week
eFigure 4. Body Weight-Related Efficacy End Points
eFigure 5. Cumulative Distribution Plots of Change from Baseline to Week 68 in Body Weight
eFigure 6. Change from Baseline by Week in SF-36 Physical Functioning Score (Treatment Policy Estimand)
eFigure 7. SF-36 Change from Baseline to Week 68
eFigure 8. Prevalence, Duration, and Severity of Selected Gastrointestinal Events
eReferences
Protocol
Statistical Analysis Plan
Data Sharing Statement
References
- 1.Jensen MD, Ryan DH, Apovian CM, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society . 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation. 2014;129(25)(suppl 2):S102-S138. doi: 10.1161/01.cir.0000437739.71477.ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heymsfield SB, van Mierlo CA, van der Knaap HC, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab Disord. 2003;27(5):537-549. doi: 10.1038/sj.ijo.0802258 [DOI] [PubMed] [Google Scholar]
- 3.Wadden TA, Foster GD, Sarwer DB, et al. Dieting and the development of eating disorders in obese women: results of a randomized controlled trial. Am J Clin Nutr. 2004;80(3):560-568. doi: 10.1093/ajcn/80.3.560 [DOI] [PubMed] [Google Scholar]
- 4.Wing RR, Lang W, Wadden TA, et al. ; Look AHEAD Research Group . Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481-1486. doi: 10.2337/dc10-2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garvey WT, Mechanick JI, Brett EM, et al. ; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines . American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(suppl 3):1-203. doi: 10.4158/EP161365.GL [DOI] [PubMed] [Google Scholar]
- 6.Ryan DH, Yockey SR. Weight loss and improvements in comorbidity: differences at 5%, 10%, 15%, and over. Curr Obes Rep. 2017;6(2):187-194. doi: 10.1007/s13679-017-0262-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregg EW, Chen H, Wagenknecht LE, et al. ; Look AHEAD Research Group . Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012;308(23):2489-2496. doi: 10.1001/jama.2012.67929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuna ST, Reboussin DM, Strotmeyer ES, et al. ; Sleep AHEAD Research Subgroup of the Look AHEAD Research Group . Effects of weight loss on obstructive sleep apnea severity: ten-year results of the Sleep AHEAD study. Am J Respir Crit Care Med. 2021;203(2):221-229. doi: 10.1164/rccm.201912-2511OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wadden TA, Walsh OA, Berkowitz RI, et al. Intensive behavioral therapy for obesity combined with liraglutide 3.0 mg: a randomized controlled trial. Obesity (Silver Spring). 2019;27(1):75-86. doi: 10.1002/oby.22359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wadden TA, Tronieri JS, Sugimoto D, et al. Liraglutide 3.0 mg and intensive behavioral therapy (IBT) for obesity in primary care: the SCALE IBT randomized controlled trial. Obesity (Silver Spring). 2020;28(3):529-536. doi: 10.1002/oby.22726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Food and Drug Administration. Ozempic (semaglutide) injection, for subcutaneous use [prescribing information]. Accessed September 25, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209637s003lbl.pdf
- 12.Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41(2):258-266. doi: 10.2337/dc17-0417 [DOI] [PubMed] [Google Scholar]
- 13.Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341-354. doi: 10.1016/S2213-8587(17)30092-X [DOI] [PubMed] [Google Scholar]
- 14.O’Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392(10148):637-649. doi: 10.1016/S0140-6736(18)31773-2 [DOI] [PubMed] [Google Scholar]
- 15.Kushner RF, Calanna S, Davies M, et al. Semaglutide 2.4 mg for the treatment of obesity: key elements of the STEP trials 1 to 5. Obesity (Silver Spring). 2020;28(6):1050-1061. doi: 10.1002/oby.22794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Food and Drug Administration. Guidance for industry: developing products for weight management. Accessed January 20, 2021. https://www.fda.gov/media/71252/download
- 17.Food and Drug Administration. E9(R1) statistical principles for clinical trials: addendum: estimands and sensitivity analysis in clinical trials. Accessed October 22, 2020. https://www.fda.gov/media/108698/download
- 18.Wharton S, Astrup A, Endahl L, et al. Estimating and reporting treatment effects in clinical trials for weight management: using estimands to interpret effects of intercurrent events and missing data. Int J Obes (Lond). Published online January 18, 2021. doi: 10.1038/s41366-020-00733-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McEvoy BW. Missing data in clinical trials for weight management. J Biopharm Stat. 2016;26(1):30-36. doi: 10.1080/10543406.2015.1094814 [DOI] [PubMed] [Google Scholar]
- 20.Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons; 1987. doi: 10.1002/9780470316696 [DOI] [Google Scholar]
- 21.Webb VL, Wadden TA. Intensive lifestyle intervention for obesity: principles, practices, and results. Gastroenterology. 2017;152(7):1752-1764. doi: 10.1053/j.gastro.2017.01.045 [DOI] [PubMed] [Google Scholar]
- 22.Hall KD, Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin North Am. 2018;102(1):183-197. doi: 10.1016/j.mcna.2017.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leibel RL, Seeley RJ, Darsow T, Berg EG, Smith SR, Ratner R. Biologic responses to weight loss and weight regain: report from an American Diabetes Association research symposium. Diabetes. 2015;64(7):2299-2309. doi: 10.2337/db15-0004 [DOI] [PubMed] [Google Scholar]
- 24.Heckman BW, Mathew AR, Carpenter MJ. Treatment burden and treatment fatigue as barriers to health. Curr Opin Psychol. 2015;5:31-36. doi: 10.1016/j.copsyc.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597-1604. doi: 10.1056/NEJMoa1105816 [DOI] [PubMed] [Google Scholar]
- 26.Gabery S, Salinas CG, Paulsen SJ, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6):e133429. doi: 10.1172/jci.insight.133429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedrichsen M, Breitschaft A, Tadayon S, Wizert A, Skovgaard D. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes Metab. 2021;23(3):754-762. doi: 10.1111/dom.14280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bettge K, Kahle M, Abd El Aziz MS, Meier JJ, Nauck MA. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: a systematic analysis of published clinical trials. Diabetes Obes Metab. 2017;19(3):336-347. doi: 10.1111/dom.12824 [DOI] [PubMed] [Google Scholar]
- 29.Nauck MA, Meier JJ. Management of endocrine disease: are all GLP-1 agonists equal in the treatment of type 2 diabetes? Eur J Endocrinol. 2019;181(6):R211-R234. doi: 10.1530/EJE-19-0566 [DOI] [PubMed] [Google Scholar]
- 30.Weinsier RL, Wilson LJ, Lee J. Medically safe rate of weight loss for the treatment of obesity: a guideline based on risk of gallstone formation. Am J Med. 1995;98(2):115-117. doi: 10.1016/S0002-9343(99)80394-5 [DOI] [PubMed] [Google Scholar]
- 31.Wilding JPH, Batterham RL, Calanna S, et al. ; the STEP 1 Study Group . Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. Published February 10, 2021. doi: 10.1056/NEJMoa2032183 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. List of Investigators in the Semaglutide Treatment Effect in People with obesity (STEP) 3 Trial
eAppendix 2. Participant Enrollment and Exclusions by Study Site
eAppendix 3. Inclusion and Exclusion Criteria
eAppendix 4. Intensive Behavioral Therapy Methodology
eAppendix 5. Summary of Assessments
eAppendix 6. Statistical Analysis
eAppendix 7. Patient-Reported Outcome Assessments
eAppendix 8. Supportive Secondary End Points
eTable 1. Changes in Secondary End Points from Baseline to Week 68 a
eTable 2. Supportive Secondary Safety End Points
eFigure 1. Trial Design
eFigure 2. Time to Permanent Discontinuation of Trial Product (Weeks)
eFigure 3. Variability in Body Weight Change from Baseline by Week
eFigure 4. Body Weight-Related Efficacy End Points
eFigure 5. Cumulative Distribution Plots of Change from Baseline to Week 68 in Body Weight
eFigure 6. Change from Baseline by Week in SF-36 Physical Functioning Score (Treatment Policy Estimand)
eFigure 7. SF-36 Change from Baseline to Week 68
eFigure 8. Prevalence, Duration, and Severity of Selected Gastrointestinal Events
eReferences
Protocol
Statistical Analysis Plan
Data Sharing Statement


