Key Points
Question
What Medicare costs are attributable to delirium after elective surgery each year?
Findings
In this cohort study of 497 older adults undergoing major elective surgery, the cumulative costs attributable to delirium were $44 291 per patient over 1 year. The adjusted mean cumulative costs attributable to severe delirium were $56 474 per patient over 1 year.
Meaning
Results suggest that incident delirium and severe delirium after elective surgery are associated with a high cost to the health care system, with substantial public health implications that warrant renewed efforts to bolster prevention, early detection, and management of delirium.
This cohort study uses data from the Successful Aging after Elective Surgery (SAGES) study, an ongoing cohort study of older adults undergoing major elective surgery at 2 acute care hospitals, to assess the Medicare costs associated with delirium in older adults 1 year after major elective surgery.
Abstract
Importance
Delirium is a common, serious, and potentially preventable problem for older adults, associated with adverse outcomes. Coupled with its preventable nature, these adverse sequelae make delirium a significant public health concern; understanding its economic costs is important for policy makers and health care leaders to prioritize care.
Objective
To evaluate current 1-year health care costs attributable to postoperative delirium in older patients undergoing elective surgery.
Design, Setting, and Participants
This prospective cohort study included 497 patients from the Successful Aging after Elective Surgery (SAGES) study, an ongoing cohort study of older adults undergoing major elective surgery. Patients were enrolled from June 18, 2010, to August 8, 2013. Eligible patients were 70 years or older, English-speaking, able to communicate verbally, and scheduled to undergo major surgery at 1 of 2 Harvard-affiliated hospitals with an anticipated length of stay of at least 3 days. Eligible surgical procedures included total hip or knee replacement; lumbar, cervical, or sacral laminectomy; lower extremity arterial bypass surgery; open abdominal aortic aneurysm repair; and open or laparoscopic colectomy. Data were analyzed from October 15, 2019, to September 15, 2020.
Exposures
Major elective surgery and hospitalization.
Main Outcomes and Measures
Cumulative and period-specific costs (index hospitalization, 30-day, 90-day, and 1-year follow-up) were examined using Medicare claims and extensive clinical data. Total inflation-adjusted health care costs were determined using data from Medicare administrative claims files for the 2010 to 2014 period. Delirium was rated using the Confusion Assessment Method. We also examined whether increasing delirium severity was associated with higher cumulative and period-specific costs. Delirium severity was measured with the Confusion Assessment Method–Severity long form. Regression models were used to determine costs associated with delirium after adjusting for patient demographic and clinical characteristics.
Results
Of the 566 patients who were eligible for the study, a total of 497 patients (mean [SD] age, 76.8 [5.1] years; 281 women [57%]; 461 White participants [93%]) were enrolled after exclusion criteria were applied. During the index hospitalization, 122 patients (25%) developed postoperative delirium, whereas 375 (75%) did not. Patients with delirium had significantly higher unadjusted health care costs than patients without delirium (mean [SD] cost, $146 358 [$140 469] vs $94 609 [$80 648]). After adjusting for relevant confounders, the cumulative health care costs attributable to delirium were $44 291 (95% CI, $34 554-$56 673) per patient per year, with the majority of costs coming from the first 90 days: index hospitalization ($20 327), subsequent rehospitalizations ($27 797), and postacute rehabilitation stays ($2803). Health care costs increased directly and significantly with level of delirium severity (none-mild, $83 534; moderate, $99 756; severe, $140 008), suggesting an exposure-response relationship. The adjusted mean cumulative costs attributable to severe delirium were $56 474 (95% CI, $40 927-$77 440) per patient per year. Extrapolating nationally, the health care costs attributable to postoperative delirium were estimated at $32.9 billion (95% CI, $25.7 billion-$42.2 billion) per year.
Conclusions and Relevance
These findings suggest that the economic outcomes of delirium and severe delirium after elective surgery are substantial, rivaling costs associated with cardiovascular disease and diabetes. These results highlight the need for policy imperatives to address delirium as a large-scale public health issue.
Introduction
Delirium is a common, serious clinical problem for older adults, often complicating major surgery.1 Delirium has been associated with poor hospital outcomes, including prolonged hospital stay, functional and cognitive decline, institutionalization, and death.2,3 With robust evidence documenting the effectiveness of prevention by multicomponent nonpharmacologic strategies,4,5 incident delirium is currently used as a quality marker for hospital care. Accumulating evidence also links postoperative delirium to adverse long-term outcomes, including accelerated cognitive decline and dementia.6,7 With these many adverse sequelae, delirium has emerged as a significant public health concern.8,9,10
There is a substantial gap in knowledge about the current costs of delirium for our health care system. Such knowledge is essential to guide policy makers and health care leaders in decision-making and prioritization surrounding delirium care.11 The most widely cited cost estimates for delirium of $16 303 to $64 421 per patient per year were derived from a controlled clinical trial of a delirium prevention model using Medicare claims data from 1995 to 1999.12 Three more recent prospective studies have examined health care costs associated with delirium. One study of 479 patients in the intensive care unit (ICU) estimated cumulative 30-day costs attributable to ICU delirium at $17 838 per patient; however, this likely represents an underestimate given the substantial rate of early mortality in these patients.13 A second study of 66 patients who had cardiac surgery analyzed the differences in median length of hospital stay, leading to a nonsignificant $10 000 adjusted cost difference between patients with and without delirium.14 A third prospective study in 500 patients who had elective surgery found differences of approximately $2000 per patient in health care costs related to delirium.15 All other prior studies examining costs of delirium have involved retrospective analyses or simulation studies.16
Updated cost estimates are urgently needed to assist leaders of health care organizations to motivate investing scarce resources into programs to address delirium. Accurate cost estimates are needed by funders and health systems to develop incentives and strategies to improve processes and quality of care for older adults and to track improvements over time. Thus, we undertook this study to examine 1-year cost estimates attributable to postoperative delirium derived from an ongoing prospective cohort of older patients having elective surgery, using Medicare claims and clinical data. Our specific aims were to evaluate total health care costs associated with incident postoperative delirium during the index hospitalization and at 30-day, 90-day, and 1-year follow-up in a prospective cohort of 497 patients undergoing elective major surgery, with careful adjustment for relevant confounders. We examined both cumulative and period-specific costs and hypothesized that incident delirium and increasing delirium severity would be associated with higher costs.
Methods
Study Sample
The Successful Aging after Elective Surgery (SAGES) study is an ongoing prospective cohort study of older adults undergoing major elective surgery. The study design and methods have been described previously.17,18 In brief, eligible participants were 70 years or older, English-speaking, able to communicate verbally, and scheduled to undergo major surgery at 1 of 2 Harvard-affiliated hospitals with an anticipated length of stay of at least 3 days. Eligible surgical procedures were total hip or knee replacement; lumbar, cervical, or sacral laminectomy; lower extremity arterial bypass surgery; open abdominal aortic aneurysm repair; and open or laparoscopic colectomy. Exclusion criteria included evidence of dementia, delirium, prior hospitalization within 3 months, legal blindness, severe deafness, terminal condition, history of schizophrenia or psychosis, and history of alcohol abuse or withdrawal. A total of 566 patients met eligibility criteria and were enrolled between June 18, 2010, and August 8, 2013. Six patients were excluded after enrollment for suspected dementia after neuropsychological testing and clinical adjudication. An additional 63 (11%) were excluded from the present analyses due to missing data: 33 (6%) because their Social Security numbers were unavailable to provide linkage to the Medicare database, and 30 (5%) because cost data were not available owing to enrollment in a Medicare managed care plan. Thus, the final sample was 497 participants (eFigure 1 in the Supplement). Written informed consent for study participation was obtained from all participants according to procedures approved by the institutional review boards of the 2 Harvard hospitals and Hebrew SeniorLife, the study coordinating center in Boston, Massachusetts. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Data Source
Participants were interviewed at baseline in their homes within 30 days (median [interquartile range], 9 [5-17] days) before their scheduled surgery for detailed demographic characteristics, cognitive functioning using the Modified Mini-Mental State Examination,19 and assessments of basic and instrumental activities of daily living20,21 before surgery. During hospitalization, participants were interviewed daily with cognitive and delirium assessments from the first postoperative day through discharge. A family caregiver was interviewed at baseline about the participant’s functional status using the proxy Informant Questionnaire on Cognitive Decline in the Elderly,22,23 a validated assessment for dementia based on proxy-reported cognitive changes over 10 years. Medical record review for the index hospitalization was conducted by a trained research physician to extract information on medical diagnoses, comorbidities, and surgical type.
Our total cost calculations were derived from Medicare Part A and B administrative claims, including Medicare Provider Analysis and Review (MEDPAR), Outpatient (fee-for-service), and Home Health Agency (fee-for-service) files, acquired for the 2010 to 2014 period. MEDPAR data detail inpatient hospital and skilled postacute rehabilitation stays covered by Medicare. Outpatient data comprise fee-for-service claims billed by institutional outpatient health care workers. Home Health Agency data include services covered by Medicare Home Health Agency health care workers. We derived further costs from the Cost and Utilization segment of the Master Beneficiary Summary File, which included summarized annual payments for services such as professional fees, hospice, and durable medical equipment.24 These latter costs were added separately in sensitivity analyses (eMethods in the Supplement).
Assessment of Delirium and Delirium Severity
Daily delirium assessment included brief cognitive testing,17,25 a Delirium Symptom Interview,26 and interviews with family and nurses. Delirium was rated using the Confusion Assessment Method (CAM),27 a standardized approach with high sensitivity (94%-100%) and specificity (90%-95%).28,29 Combined with a validated chart review method,30,31 patients were classified as having developed delirium if either CAM or chart criteria were met on a given day. Delirium severity was measured with the CAM-Severity (CAM-S) score long form (scored 0-19; 19 = most severe).32 The highest value of CAM-S during hospitalization, peak CAM-S, was used as the measure of delirium severity for these analyses, categorized into 3 severity groups: none-mild (0-2 points), moderate (3-7 points), and severe (8-19 points), based on prior work.6
Outcome Measures
Costs were measured from the perspective of the health care system. Cumulative total health care costs were calculated for the index hospitalization and for 30 days, 90 days, and 1 year after discharge. Total costs incurred during the index hospitalization were determined from MEDPAR data. The 30-day, 90-day, and 1-year costs were derived from MEDPAR (inpatient and postacute rehabilitation stays), outpatient, and home health services files. Period-specific costs were computed for intervals between discharge and 30 days, 30 days and 90 days, and 90 days and 1 year. Cumulative and period-specific costs were calculated for individual health care cost categories, including inpatient, postacute rehabilitation facility, outpatient, and home health care.12 Costs were calculated using Medicare reimbursed amounts rather than charges, because reimbursed amounts represent the actual payments received by hospitals and clinicians and are considered better measures of transaction prices than billed charges.12 All costs are reported in 2019 US dollars, adjusting for inflation using the medical care component of the Consumer Price Index.33
Statistical Analysis
Statistical analyses were conducted from October 15, 2019, to September 15, 2020. For baseline characteristics, standard statistics were used to compare delirium and nondelirium groups, including analysis of variance test for continuous variables and χ2 test for categorical variables. We also used standard statistics to compare the delirium and nondelirium groups on hospital factors, including length of stay, and rates and duration of ICU stay. For the cost analyses, we compared unadjusted mean total costs between delirium and nondelirium groups using the Wilcoxon test. For the 3 delirium severity groups, we compared unadjusted mean costs using the Kruskal-Wallis test. We then used generalized linear models to examine our cost data, which displayed substantial skewness in their distributions with error terms exhibiting heteroscedasticity.34 To account for skewness, adjusted mean total costs were estimated with generalized linear models assuming γ-distributed costs and a log link function.35 All covariables were mean-centered. Model estimated cumulative adjusted mean total costs from index hospitalization through discharge were first computed cumulatively, then similarly computed for each time period. The excess cumulative costs attributable to incident delirium and delirium severity were calculated as mean cost differences from the adjusted models with 95% CIs derived from standard error estimates.
With complete data collection and follow-up, there were no missing data on any of the outcomes or covariables used in the models. We used SAS, version 9.4 (SAS Institute) for processing the Medicare data files and Stata, version 15 (StataCorp LLC) for all statistical analyses. All P values were 2 sided, and P < .05 was used to indicate statistical significance. Detailed description of the selection of covariables, 2-part models for analysis health care cost categories, and details of sensitivity analyses are provided in the eMethods in the Supplement.
Results
Of the 566 patients who were eligible for the study, a total of 497 patients (mean [SD] age, 76.8 [5.1] years; 281 women [57%]; 461 White participants [93%]) were enrolled after exclusion criteria were applied. Baseline characteristics are presented in Table 1. We compared the excluded sample (n = 63) to the final sample (n = 497) and found them to be similar (eTable 1 in the Supplement). Among the 497 SAGES participants, delirium developed in 122 (25%) during hospitalization. The patients who developed delirium were significantly more medically complex (55 of 122 [45%] vs 95 of 375 [25%] with a Charlson score37 ≥2; P < .001), were more cognitively impaired at baseline (12 of 122 [10%] vs 15 of 375 [4%] with proxy Informant Questionnaire for Cognitive Decline in the Elderly score >3.5; P = .01), and were higher health care users in the prior 6 months (mean [SD] total costs, $8880 [$25 168] vs $3959 [$10 181]; P = .002). Only 1 patient with and 6 patients without delirium died during 1-year follow-up, with no significant differences in survival rate or time.
Table 1. Baseline Characteristics of Patients.
| Characteristic | No. (%)a | ||
|---|---|---|---|
| Total cohort (N = 497) | Delirium | ||
| Yes (n = 122) | No (n = 375) | ||
| Age, mean (SD), y | 76.8 (5.1) | 77.5 (5.0) | 76.6 (5.1) |
| Male sex, self-reported | 216 (43) | 52 (43) | 164 (44) |
| Race/ethnicity | |||
| White | 461 (93) | 110 (90) | 351 (94) |
| Black or African American | 26 (72) | 7 (58) | 19 (80) |
| Asian | 2 (6) | 0 (0) | 2 (8) |
| Native Hawaiian or Pacific Islander | 1(3) | 0 (0) | 1 (4) |
| Hispanic | 4 (11) | 3 (25) | 1 (4) |
| Multiple races/ethnicities | 3 (8) | 2 (17) | 1 (4) |
| Education, mean (SD), y | 14.9 (2.9) | 14.7 (3.0) | 14.9 (2.9) |
| Married (vs unmarried) | 299 (60) | 74 (61) | 225 (60) |
| Lives alone (vs with others) | 146 (29) | 36 (30) | 110 (29) |
| ADL score at baseline, mean (SD) | 0.2 (0.7) | 0.2 (0.6) | 0.2 (0.8) |
| Any ADL impairment at baselineb | 45 (9) | 12 (10) | 33 (9) |
| Orthopedic surgery | |||
| Total knee replacement | 175 (35) | 35 (29) | 140 (37) |
| Total hip replacement | 105 (21) | 14 (11) | 91 (24) |
| Laminectomy (cervical and lumbar) | 123 (25) | 45 (37) | 78 (21) |
| Nonorthopedic surgery (general and vascular surgery)c | 94 (19) | 28 (23) | 66 (18) |
| Charlson Comorbidity Scoreb | 1.1 (1.3) | 1.3 (1.4) | 1.0 (1.2) |
| ASA class ≥3b | 322 (65) | 94 (77) | 228 (61) |
| IQCODE >3.5 or 3MS ≤77b | 30 (6) | 13 (11) | 17 (5) |
| Total health care costs, 6 mo before index hospitalization, $ (SD)b | 5167 (15 401) | 8880 (25 168) | 3959 (10 181) |
Abbreviations: ADL, activities of daily living; ASA, American Society of Anesthesiologists Physical Status Classification System; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly; 3MS, Modified Mini-Mental State Examination.
Values are listed as No. (%) unless otherwise specified.
ADL scored 0-14 by patient self-report, with higher scores indicating impairment. Charlson Comorbidity Score was calculated based on diagnoses abstracted from medical record review, scored from 0 to 35 with higher scores indicating more comorbidity. ASA class36 was abstracted from medical record review, scored from 0 to 4. Costs are in 2019 dollars.
Nonorthopedic surgeries include general surgeries (laparoscopic and open colectomy) and vascular surgeries (lower extremity revascularization and abdominal aortic aneurysm repair). To observe our federal confidentiality agreement, these surgical types must be combined to avoid cell sizes <10.
Adjusted cumulative health care costs per patient were significantly higher for those who developed incident delirium compared with those who did not at all time points (mean costs at 30 days after discharge, $99 676 [95% CI, $85 577-$116 097] vs $71 599 [95% CI, $62 223-$82 387]; at 90 days after discharge, $108 978 [95% CI, $90 596-$131 091] vs $74 644 [95% CI, $63 096-$88 306]; and at 1 year after discharge, $130 816 [95% CI, $105 676-$161 937] vs $86 525 [95% CI, $71 122-$105 264]) (Table 2). At 1-year follow-up, patients who developed delirium accumulated an adjusted mean $44 291 (P < .001) more health care costs compared with those without delirium. Period-specific costs were also significantly higher in all periods for patients with delirium vs those without (mean cost for discharge to 30 days, $13 526 [95% CI, $8767-$20 870] vs $7600 [95% CI, $5007-$11 537]; for 30-90 days, $7351 [95% CI, $2262-$23 887] vs $2467 [95% CI, $904-$6737]; and for 90 days to 1 year, $22 636 [95% CI, $11 240-$45 589] vs $11 780 [95% CI, $6032-$23 005]). The adjusted mean differences were highest for the index hospitalization costs (adjusted mean difference = $20 327; P < .001) and relatively lower for the discharge to 30-day costs ($5926; P = .001), 30- to 90-day costs ($4884; P = .01), and 90-day to 1-year costs ($10 856; P = .01). Thus, patients with delirium consistently cost more to the health care system with significantly higher costs in all time periods. The unadjusted analyses for costs of incident delirium appear in eTable 2 in the Supplement.
Table 2. Adjusted Health Care Costs per Patient Associated With Incident Deliriuma.
| Measure | Delirium, mean (95% CI) | Mean difference | P value | |
|---|---|---|---|---|
| Yes (n = 122) | No (n = 375) | |||
| Cumulative total costs, $ | ||||
| Index hospitalization | 82 431 (70 848-95 908) | 62 104 (54 132-71 250) | 20 327 | <.001 |
| Time after discharge | ||||
| 30 d | 99 676 (85 577-116 097) | 71 599 (62 223-82 387) | 28 077 | <.001 |
| 90 d | 108 978 (90 596-131 091) | 74 644 (63 096-88 306) | 34 334 | <.001 |
| 1 y | 130 816 (105 676-161 937) | 86 525 (71 122-105 264) | 44 291 | <.001 |
| Period-specific costs, $ | ||||
| Index hospitalization | 82 431 (70 848-95 908) | 62 104 (54 132-71 250) | 20 327 | <.001 |
| Discharge to 30 d | 13 526 (8767-20 870) | 7600 (5007-11 537) | 5926 | .001 |
| 30 to 90 d | 7351 (2262-23 887) | 2467 (904-6737) | 4884 | .01 |
| 90 d to 1 y | 22 636 (11 240-45 589) | 11 780 (6032-23 005) | 10 856 | .01 |
Results are reported in 2019 dollars, adjusted for age, male sex, education, non-White race/ethnicity, Charlson Comorbidity Score, Informant Questionnaire on Cognitive Decline in the Elderly score >3.5 or Modified Mini-Mental State Examination score ≤77, surgical site, surgery type (total knee replacement, total hip replacement, lumbar laminectomy, cervical laminectomy, open colectomy, lower extremity revascularization, abdominal aortic aneurysm repair, laparoscopic colectomy), American Society of Anesthesiologists Physical Status Classification System score ≥3, and total costs 6 months before index hospitalization; using generalized linear models.
When stratified by delirium severity, mean adjusted health care costs increased directly with degree of severity (Table 3) from none to mild delirium (peak CAM-S score 0-2) to moderate delirium (peak CAM-S score 3-7) and to severe delirium (peak CAM-S score 8-19). Cumulative total costs demonstrated significant increases across the severity groups, with the largest cost differences at 1 year following surgery (adjusted mean costs for groups with none to mild delirium were $83 534 [95% CI, $67 300-$103 683]; for moderate delirium, $99 756 [95% CI, $81 683-$121 829]; and for severe delirium, $140 008 [95% CI, $108 227-$181 123]; P < .001). For period-specific costs, significant increasing mean costs were demonstrated across delirium severity groupings for index hospitalization (none-mild, $62 608 [95% CI, $54 003-$72 584]; moderate, $66 255 [95% CI, $57 746-$76 017]; and severe, $90 700 [95% CI, $75 772-$108 570]; P < .001). Although values increased across delirium severity groupings for other time periods, results were generally not statistically significant. The unadjusted analyses for costs of delirium stratified by severity appear in eTable 3 in the Supplement.
Table 3. Adjusted Health Care Costs per Patient Associated With Delirium Severitya.
| Measure | Delirium severity groups, peak CAM-S, mean (95% CI)b | P valuec | ||
|---|---|---|---|---|
| None-mild: 0-2 (n = 212) | Moderate: 3-7 (n = 223) | Severe: 8-19 (n = 62) | ||
| Cumulative total costs, $ | ||||
| Index hospitalization | 62 608 (54 003-72 584) | 66 255 (57 746-76 017) | 90 700 (75 772-108 570) | <.001 |
| Time after discharge | ||||
| 30 d | 69 802 (59 923-81 310) | 80 286 (69 681-92 505) | 105 926 (88 205-127 208) | <.001 |
| 90 d | 73 107 (60 883-87 787) | 83 519 (70 475-98 978) | 117 646 (94 474-146 500) | <.001 |
| 1 y | 83 534 (67 300-103 683) | 99 756 (81 683-121 829) | 140 008 (108 227-181 123) | <.001 |
| Period-specific costs, $ | ||||
| Index hospitalization | 62 608 (54 003-72 584) | 66 255 (57 746-76 017) | 90 700 (75 772-108 570) | <.001 |
| Discharge to 30 d | 6384 (4079-9989) | 10 271 (6821-15 466) | 13 200 (7940-21 945) | .003 |
| 30 to 90 d | 2236 (700-7138) | 2480 (871-7062) | 8267 (2312-29 560) | .06 |
| 90 d to 1 y | 10 330 (4956-21 529) | 16 199 (8335-31 482) | 23 776 (10 383-54 444) | .02 |
Abbreviations: AAA, abdominal aortic aneurysm; ASA, American Society of Anesthesiologists Physical Status Classification System; CAM-S, Confusion Assessment Method–Severity; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly; 3MS, Modified Mini-Mental State Examination.
Results are reported in 2019 dollars, adjusted for age, male sex, education, non-White race/ethnicity, Charlson Comorbidity Score, IQCODE score >3.5 or 3MS score ≤77, surgical site, surgery type (total knee replacement, total hip replacement, lumbar laminectomy, cervical laminectomy, open colectomy, lower extremity revascularization, AAA repair, laparoscopic colectomy), ASA score ≥3, and total costs 6 months before index hospitalization; using generalized linear models.
Peak CAM-S groups derived from CAM-S long form.
P values for trend.
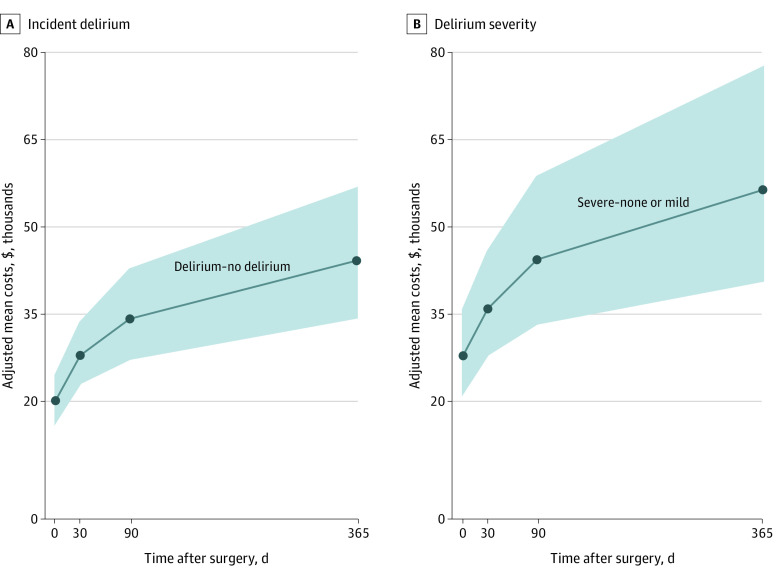
Attributable costs (adjusted mean differences) are presented in the Figure for incident delirium and for delirium severity (severe vs none-mild severity groups) for 1 year after surgery. The excess cumulative costs attributable to delirium were $44 291 (95% CI, $34 554-$56 673) for incident delirium and $56 474 (95% CI, $40 927-$77 440) for severe delirium over 1 year. Extrapolating nationally, the health care costs attributable to postoperative delirium were estimated at $32.9 billion (95% CI, $25.7 billion-$42.2 billion) per year.38,39,40
Figure. Attributable Costs Due to Incident Delirium and Delirium Severity (Adjusted Mean Differences, 95% Confidence Intervals).
Attributable costs (adjusted mean differences) are presented for incident delirium (A) (delirium vs no delirium) and for delirium severity (B) (severe vs none-mild severity groups) through 1 year after surgery. The excess cumulative costs attributable to incident delirium are $44 291 (95% CI, $34 554-$56 673). The excess cumulative costs attributable to severe delirium are $56 474 (95% CI, $40 927-$77 440). The shaded area represents 95% CI.
We examined categories of health care costs to determine their relative contributions to the increased total health care costs in patients with delirium (eTable 4 in the Supplement). For patients who developed delirium, adjusted mean differences in cumulative costs at 1 year were highest for inpatient costs ($36 404; P < .001) with approximately half of these costs due to the initial index hospitalization, where patients with delirium had longer lengths of stay (mean [SD] days, 6.9 [5.6] vs 4.8 [2.2]; P < .001), higher rates of ICU stay (19 of 122 [16%] vs 15 of 375 [4%]; P < .001), and longer ICU duration (1.3 [5.5] vs 0.1 [0.9] days; P < .001) compared with patients without delirium. The next highest cost category at 1 year after discharge was for postacute rehabilitation facility stays (mean difference, $4986; P = .02). Adjusted mean differences in outpatient and home health care costs were small and not statistically significant. During 1-year follow-up, patients in the delirium group (n = 122) compared with the nondelirium group (n = 375) had significantly more rehospitalizations (65 of 122 [53%] vs 116 of 375 [31%]; P < .001) and postacute rehabilitation stays (64 of 122 [52%] vs 135 of 375 [36%]; P = .001). Moreover, in the period from 90 days to 1 year, the rates of rehospitalization (34 of 122 [28%] vs 67 of 375 [18%]; P = .02) and postacute rehabilitation stays (15 of 122 [12%] vs 18 of 375 [5%]; P = .04) remained significantly higher in the delirium group. To adjust for survival time, we performed sensitivity analysis using Cox regression models (eTable 5 in the Supplement). We also conducted sensitivity analyses adding in prorated Part B hospice and durable medical equipment costs (eTable 6 in the Supplement). The results and conclusions remained unchanged across these sensitivity analyses. The details of the generalized linear regression models for all analyses appear in eTable 7 in the Supplement (incident delirium), eTable 8 in the Supplement (delirium severity), and eTables 9 to 12 in the Supplement (individual models for cost categories).
Discussion
Results of this study suggest that delirium after elective surgery was associated with significant increases in cumulative health care costs from hospital admission through 1 year follow-up. The adjusted cumulative costs attributable to delirium originated from the index hospitalization, subsequent rehospitalizations, and postacute rehabilitation stays. Increases in health care costs were directly and significantly associated with delirium severity, supporting an exposure-response relationship. Because the presence and severity of delirium have been directly associated with adverse clinical outcomes in our prior work,32,41 it is not surprising that they may be contributing to increased health care costs.
Following Leslie et al,12 and assuming that incident delirium complicates hospital stays for 25% of the 3 million older adults hospitalized for elective surgery each year in the US, our results suggest that total direct 1-year health care costs attributable to postoperative delirium were approximately $32.9 billion (95% CI, $25.7-$42.2 billion) per year extrapolating nationally (eFigure 2 in the Supplement).38,39,40 By comparison, estimates of US health care costs for other conditions range from $6 billion for hip fracture42 to $34 billion for nonfatal falls,43 $248 billion for diabetes,44 and $357 to $363 billion for cardiovascular disease.45 Thus, postoperative delirium appears to be a major contributor to health care costs, rivaling other well-recognized conditions for which more resources are routinely directed. Given the documented effectiveness of delirium prevention strategies,5 some of these costs may be avoidable.
Because delirium is associated with many adverse short-term and long-term outcomes, the substantial contributions to health care costs are not surprising. However, the exact mechanisms leading to these increased costs warrant further investigation. Our results suggest that delirium was associated with an increase in the acuity and duration of hospitalization, resulting in increased costs from greater daily expenditures, ICU stays, longer lengths of stay, and higher rates of rehospitalization and institutionalization.
Strengths and Limitations
This study has a number of noteworthy strengths, including rigorous longitudinal follow-up over 1 year with detailed clinical information and comprehensive service use and cost data. We used standardized, validated delirium assessments daily throughout hospitalization. The study examined costs associated with delirium across a wide range of services (inpatient, emergency department, outpatient, postacute rehabilitation stay, home health care, etc) over a full year after hospital discharge. With complete follow-up, there were no missing data on any key study outcomes or covariables.
This study has some limitations. First, our costs reflected those incurred by an older elective surgical population with hospitalization and may not reflect the entire older surgical population. Second, medications and polypharmacy are well-known risk factors for delirium in the hospital, but we did not have medication cost data. Exploring mediating factors leading to the association of delirium with high costs will be an important area for future research. Third, cost estimates were derived from 2 medical centers in a single city, and thus the results may not generalize to other sites. This particular caveat may be less of a concern, because our findings were based on mean differences in costs between patients with and without delirium rather than absolute costs. Similarly, our cohort was made up of mostly White patients who were English-speaking, well-educated, and residing close to the urban Boston area. As such, additional studies are needed in the future with more heterogeneous health care systems and diverse participants to further corroborate our findings. Comparable with other studies based on Medicare data, approximately 11% of our cohort participated in Medicare managed care plans, and their costs were not available. The group of excluded patients was similar in baseline characteristics to the included group. In addition, the cost estimates include direct health care costs only and do not consider important indirect costs which may be substantial, such as out-of-pocket expenses, job or productivity losses for the patient or family members, informal caregiving costs, caregiver burden, and reduced quality of life. This study was a prospective cohort and not a randomized clinical trial; thus, we were unable to calculate the cost savings per case of delirium prevented. We examined an elective surgery cohort that does not include patients with coronavirus disease 2019 or those receiving emergency surgical, general medical, or palliative care, all of whom would have had higher illness severity and more costly care. Our elective surgery population also underwent careful evaluation and documentation of delirium, which is not routinely done across all hospitals and thus likely underestimates the true costs of delirium and severe delirium actually present throughout the US. For these reasons, our estimates are likely to be conservative, and the overall health care costs per year attributable to delirium are likely to be higher than reflected in our results.
Conclusions
This cohort study found that the economic outcomes of delirium and severe delirium after elective surgery were substantial, rivaling costs associated with those of cardiovascular disease and diabetes. These results suggest the need for policy imperatives to address delirium as a large-scale public health issue. It is our hope that these results on the high cost of postoperative delirium will drive much-needed attention to address this problem and, ultimately, to reduce its long-term clinical and economic implications. We hope to encourage health care leaders and policy makers to invest resources to address this critically important area, especially in this era of bundled health insurance payments. Future research is needed to determine whether delirium prevention programs, such as the cost-effective Hospital Elder Life Program,4 would be successful in reducing these delirium-related costs on a wide scale. Given that the condition is increasing in magnitude with the aging population worldwide, renewed efforts to prevent, detect, and manage delirium are urgently needed.
eMethods
eFigure 1. Flow Diagram for Study Enrollment
eFigure 2. Cost Calculations: One-Year Health Care Costs Attributable to Postoperative Delirium in Older Elective Surgery Patients (2019 USD)
eTable 1. Characteristics of Patients Included vs Excluded From the Analysis
eTable 2. Unadjusted Health Care Costs Per Patient Associated With Incident Delirium
eTable 3. Unadjusted Health Care Costs Per Patient Associated With Delirium Severity
eTable 4. Adjusted Health Care Costs Per Patient by Category Associated With Incident Delirium
eTable 5. Adjusted Health Care Costs Per Patient Associated With Incident Delirium, Cox Regression Analysis
eTable 6. Adjusted Health Care Costs (Including Part B, Hospice, and DME Costs) Per Patient
eTable 7. Generalized Linear Model (GLM) Estimates for Health Care Cost Outcomes Associated With Incident Delirium (N = 497)
eTable 8. Generalized Linear Model (GLM) Estimates for Health Care Cost Outcomes Associated With Delirium Severity (N = 497)
eTable 9. Generalized Linear Model (GLM) Estimates for Inpatient Cost Outcomes Associated With Incident Delirium (N = 497)
eTable 10. Two-Part Model Estimates for Postacute Rehabilitation Facility Cost Outcomes Associated With Incident Delirium (N = 497)
eTable 11. Two-Part Model Estimates for Outpatient Cost Outcomes Associated With Incident Delirium (N = 497)
eTable 12. Two-Part Model Estimates for Home Health Cost Outcomes
References
- 1.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. doi: 10.1016/S0140-6736(13)60688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. JAMA. 2017;318(12):1161-1174. doi: 10.1001/jama.2017.12067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hshieh TT, Saczynski J, Gou RY, et al. ; SAGES Study Group . Trajectory of functional recovery after postoperative delirium in elective surgery. Ann Surg. 2017;265(4):647-653. doi: 10.1097/SLA.0000000000001952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital elder life program: systematic review and meta-analysis of effectiveness. Am J Geriatr Psychiatry . 2018;26(10):1015-1033. doi: 10.1016/j.jagp.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520. doi: 10.1001/jamainternmed.2014.7779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasunilashorn SM, Fong TG, Albuquerque A, et al. Delirium severity post-surgery and its relationship with long-term cognitive decline in a cohort of patients without dementia. J Alzheimers Dis. 2018;61(1):347-358. doi: 10.3233/JAD-170288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morandi A, Davis D, Fick DM, et al. Delirium superimposed on dementia strongly predicts worse outcomes in older rehabilitation inpatients. J Am Med Dir Assoc. 2014;15(5):349-354. doi: 10.1016/j.jamda.2013.12.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong TG, Davis D, Growdon ME, Albuquerque A, Inouye SK. The interface between delirium and dementia in elderly adults. Lancet Neurol. 2015;14(8):823-832. doi: 10.1016/S1474-4422(15)00101-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hshieh TT, Fong TG, Schmitt EM, et al. ; BASIL Study Group . Does Alzheimer’s disease and related dementias modify delirium severity and hospital outcomes? J Am Geriatr Soc. 2020;68(8):1722-1730. doi: 10.1111/jgs.16420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inouye SK, Marcantonio ER, Kosar CM, et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12(7):766-775. doi: 10.1016/j.jalz.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leslie DL, Inouye SK. The importance of delirium: economic and societal costs. J Am Geriatr Soc. 2011;59(suppl 2):S241-S243. doi: 10.1111/j.1532-5415.2011.03671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32. doi: 10.1001/archinternmed.2007.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasilevskis EE, Meltzer D, Schnipper J, et al. Quality of care for decompensated heart failure: comparable performance between academic hospitalists and non-hospitalists. J Gen Intern Med. 2008;23(9):1399-1406. doi: 10.1007/s11606-008-0680-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown CH IV, Laflam A, Max L, et al. The impact of delirium after cardiac surgical procedures on postoperative resource use. Ann Thorac Surg. 2016;101(5):1663-1669. doi: 10.1016/j.athoracsur.2015.12.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franco K, Litaker D, Locala J, Bronson D. The cost of delirium in the surgical patient. Psychosomatics. 2001;42(1):68-73. doi: 10.1176/appi.psy.42.1.68 [DOI] [PubMed] [Google Scholar]
- 16.Caplan GA, Teodorczuk A, Streatfeild J, Agar MR. The financial and social costs of delirium. Eur Geriatr Med. 2020;11(1):105-112. doi: 10.1007/s41999-019-00257-2 [DOI] [PubMed] [Google Scholar]
- 17.Schmitt EM, Marcantonio ER, Alsop DC, et al. Novel risk markers and long-term outcomes of delirium: the Successful Aging after Elective Surgery (SAGES) study design and methods. J Am Med Dir Assoc. 2012;13(9):818.e1-818.e10. doi: 10.1016/j.jamda.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitt EM, Saczynski JS, Kosar CM, et al. ; SAGES Study Group . The Successful Aging after Elective Surgery (SAGES) study: cohort description and data quality procedures. J Am Geriatr Soc. 2015;63(12):2463-2471. doi: 10.1111/jgs.13793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teng ELCH, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314-318. [PubMed] [Google Scholar]
- 20.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. the index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. doi: 10.1001/jama.1963.03060120024016 [DOI] [PubMed] [Google Scholar]
- 21.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179-186. doi: 10.1093/geront/9.3_Part_1.179 [DOI] [PubMed] [Google Scholar]
- 22.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24(1):145-153. doi: 10.1017/S003329170002691X [DOI] [PubMed] [Google Scholar]
- 23.Jorm AF. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): a review. Int Psychogeriatr. 2004;16(3):275-293. doi: 10.1017/S1041610204000390 [DOI] [PubMed] [Google Scholar]
- 24.Research Data Assistance Center . About us. Accessed May 17, 2020. https://www.resdac.org/about-resdac
- 25.Simon SE, Bergmann MA, Jones RN, Murphy KM, Orav EJ, Marcantonio ER. Reliability of a structured assessment for nonclinicians to detect delirium among new admissions to postacute care. J Am Med Dir Assoc. 2006;7(7):412-415. doi: 10.1016/j.jamda.2006.02.006 [DOI] [PubMed] [Google Scholar]
- 26.Albert MS, Levkoff SE, Reilly C, et al. The delirium symptom interview: an interview for the detection of delirium symptoms in hospitalized patients. J Geriatr Psychiatry Neurol. 1992;5(1):14-21. doi: 10.1177/002383099200500103 [DOI] [PubMed] [Google Scholar]
- 27.Wong CL, Holroyd-Leduc J, Simel DL, Straus SE. Does this patient have delirium?: value of bedside instruments. JAMA. 2010;304(7):779-786. doi: 10.1001/jama.2010.1182 [DOI] [PubMed] [Google Scholar]
- 28.Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830. doi: 10.1111/j.1532-5415.2008.01674.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST Jr, Leslie DL, Agostini JV. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53(2):312-318. doi: 10.1111/j.1532-5415.2005.53120.x [DOI] [PubMed] [Google Scholar]
- 30.Saczynski JS, Kosar CM, Xu G, et al. A tale of two methods: chart and interview methods for identifying delirium. J Am Geriatr Soc. 2014;62(3):518-524. doi: 10.1111/jgs.12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gross AL, Jones RN, Fong TG, Tommet D, Inouye SK. Calibration and validation of an innovative approach for estimating general cognitive performance. Neuroepidemiology. 2014;42(3):144-153. doi: 10.1159/000357647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inouye SK, Kosar CM, Tommet D, et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. 2014;160(8):526-533. doi: 10.7326/M13-1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Bureau of Labor Statistics. Consumer price index databases. Accessed November 13, 2019. https://www.bls.gov/cpi/data.htm
- 34.Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ. 2005;24(3):465-488. doi: 10.1016/j.jhealeco.2004.09.011 [DOI] [PubMed] [Google Scholar]
- 35.Deb P, Norton EC. Modeling health care expenditures and use. Annu Rev Public Health. 2018;39:489-505. doi: 10.1146/annurev-publhealth-040617-013517 [DOI] [PubMed] [Google Scholar]
- 36.Mayhew D, Mendonca V, Murthy BVS. A review of ASA physical status - historical perspectives and modern developments. Anaesthesia. 2019;74(3):373-379. doi: 10.1111/anae.14569 [DOI] [PubMed] [Google Scholar]
- 37.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 38.United States Census Bureau. US and world population clock . Accessed May 18, 2020. https://www.census.gov/popclock/
- 39.United States Census Bureau. Facts for features: older Americans month: May 2017. Accessed May 18, 2020. https://www.census.gov/newsroom/facts-for-features/2017/cb17-ff08.html
- 40.Sun R, Karaca Z, Wong H. Statistical brief #235: trends in hospital inpatient stays by age and payer, 2000-2015. Accessed May 18, 2020. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb235-Inpatient-Stays-Age-Payer-Trends.jsp
- 41.Vasunilashorn SM, Marcantonio ER, Gou Y, et al. Quantifying the severity of a delirium episode throughout hospitalization: the combined importance of intensity and duration. J Gen Intern Med. 2016;31(10):1164-1171. doi: 10.1007/s11606-016-3671-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu Q, Koenig L, Mather RC III, Tongue J. Surgery for hip fracture yields societal benefits that exceed the direct medical costs. Clin Orthop Relat Res. 2014;472(11):3536-3546. doi: 10.1007/s11999-014-3820-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burns ER, Stevens JA, Lee R. The direct costs of fatal and non-fatal falls among older adults—United States. J Safety Res. 2016;58:99-103. doi: 10.1016/j.jsr.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.American Diabetes Association . Economic costs of diabetes in the US in 2017. Diabetes Care. 2018;41(5):917-928.doi: 10.2337/dci18-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benjamin EJ, Virani SS, Callaway CW, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67-e492. doi: 10.1161/CIR.0000000000000558 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eFigure 1. Flow Diagram for Study Enrollment
eFigure 2. Cost Calculations: One-Year Health Care Costs Attributable to Postoperative Delirium in Older Elective Surgery Patients (2019 USD)
eTable 1. Characteristics of Patients Included vs Excluded From the Analysis
eTable 2. Unadjusted Health Care Costs Per Patient Associated With Incident Delirium
eTable 3. Unadjusted Health Care Costs Per Patient Associated With Delirium Severity
eTable 4. Adjusted Health Care Costs Per Patient by Category Associated With Incident Delirium
eTable 5. Adjusted Health Care Costs Per Patient Associated With Incident Delirium, Cox Regression Analysis
eTable 6. Adjusted Health Care Costs (Including Part B, Hospice, and DME Costs) Per Patient
eTable 7. Generalized Linear Model (GLM) Estimates for Health Care Cost Outcomes Associated With Incident Delirium (N = 497)
eTable 8. Generalized Linear Model (GLM) Estimates for Health Care Cost Outcomes Associated With Delirium Severity (N = 497)
eTable 9. Generalized Linear Model (GLM) Estimates for Inpatient Cost Outcomes Associated With Incident Delirium (N = 497)
eTable 10. Two-Part Model Estimates for Postacute Rehabilitation Facility Cost Outcomes Associated With Incident Delirium (N = 497)
eTable 11. Two-Part Model Estimates for Outpatient Cost Outcomes Associated With Incident Delirium (N = 497)
eTable 12. Two-Part Model Estimates for Home Health Cost Outcomes