Key Points
Question
Will screening older individuals for atrial fibrillation with a wearable electrocardiographic monitor be feasible, detect a high rate of atrial fibrillation, and lead to anticoagulation for most patients?
Findings
In a randomized clinical trial of 856 participants aged 75 years or older with hypertension from outpatient primary care practices, new atrial fibrillation was detected in 5.3% of the screening group vs 0.5% of the control group. Median atrial fibrillation duration on continuous electrocardiographic monitoring was 6.3 hours, and anticoagulation was prescribed to 75.0% of the participants with screen-detected atrial fibrillation.
Meaning
In this trial, a wearable electrocardiogram-based screening intervention increased atrial fibrillation detection 10-fold and prompted anticoagulation in most cases; this strategy warrants evaluation to prevent stroke.
Abstract
Importance
Atrial fibrillation (AF) is a major cause of preventable strokes. Screening asymptomatic individuals for AF may increase anticoagulant use for stroke prevention.
Objective
To evaluate 2 home-based AF screening interventions.
Design, Setting, and Participants
This multicenter randomized clinical trial recruited individuals from primary care practices aged 75 years or older with hypertension and without known AF. From April 5, 2015, to March 26, 2019, 856 participants were enrolled from 48 practices.
Interventions
The control group received standard care (routine clinical follow-up plus a pulse check and heart auscultation at baseline and 6 months). The screening group received a 2-week continuous electrocardiographic (cECG) patch monitor to wear at baseline and at 3 months, in addition to standard care. The screening group also received automated home blood pressure (BP) machines with oscillometric AF screening capability to use twice-daily during the cECG monitoring periods.
Main Outcomes and Measures
With intention-to-screen analysis, the primary outcome was AF detected by cECG monitoring or clinically within 6 months. Secondary outcomes included anticoagulant use, device adherence, and AF detection by BP monitors.
Results
Of the 856 participants, 487 were women (56.9%); mean (SD) age was 80.0 (4.0) years. Median cECG wear time was 27.4 of 28 days (interquartile range [IQR], 18.4-28.0 days). In the primary analysis, AF was detected in 23 of 434 participants (5.3%) in the screening group vs 2 of 422 (0.5%) in the control group (relative risk, 11.2; 95% CI, 2.7-47.1; P = .001; absolute difference, 4.8%; 95% CI, 2.6%-7.0%; P < .001; number needed to screen, 21). Of those with cECG-detected AF, median total time spent in AF was 6.3 hours (IQR, 4.2-14.0 hours; range 1.3 hours-28 days), and median duration of the longest AF episode was 5.7 hours (IQR, 2.9-12.9 hours). Anticoagulation was initiated in 15 of 20 patients (75.0%) with cECG-detected AF. By 6 months, anticoagulant therapy had been prescribed for 18 of 434 participants (4.1%) in the screening group vs 4 of 422 (0.9%) in the control group (relative risk, 4.4; 95% CI, 1.5-12.8; P = .007; absolute difference, 3.2%; 95% CI, 1.1%-5.3%; P = .003). Twice-daily AF screening using the home BP monitor had a sensitivity of 35.0% (95% CI, 15.4%-59.2%), specificity of 81.0% (95% CI, 76.7%-84.8%), positive predictive value of 8.9% (95% CI, 4.9%-15.5%), and negative predictive value of 95.9% (95% CI, 94.5%-97.0%). Adverse skin reactions requiring premature discontinuation of cECG monitoring occurred in 5 of 434 participants (1.2%).
Conclusions and Relevance
In this randomized clinical trial, among older community-dwelling individuals with hypertension, AF screening with a wearable cECG monitor was well tolerated, increased AF detection 10-fold, and prompted initiation of anticoagulant therapy in most cases. Compared with continuous ECG, intermittent oscillometric screening with a BP monitor was an inferior strategy for detecting paroxysmal AF. Large trials with hard clinical outcomes are now needed to evaluate the potential benefits and harms of AF screening.
Trial Registration
ClinicalTrials.gov Identifier: NCT02392754
This randomized clinical trial evaluates the use of continuous electrographic monitoring with a patch in older patients with hypertension to detect atrial fibrillation.
Introduction
Atrial fibrillation (AF) is one of the most common treatable risk factors for stroke, and its prevalence is increasing.1,2 By age 55 years, the lifetime risk of developing AF in the US is 1 in 3.3 When AF is identified, initiation of oral anticoagulant therapy (OAC) can prevent two-thirds of strokes. However, AF often goes undetected and untreated because it is frequently short-lasting and asymptomatic, and stroke can be its first manifestation.4,5,6 Approximately 10% to 20% of ischemic strokes are attributed to previously undiagnosed AF,7 and AF-associated strokes tend to be more disabling and more often fatal compared with other types of ischemic strokes.8 If screening can effectively detect sufficient numbers of individuals with AF and trigger initiation of OAC, many strokes could potentially be prevented.
Interest in AF screening has increased with advances in wearable technologies for arrhythmia detection9,10 and the availability of safer and highly effective OAC medications for stroke prevention.11 In the secondary prevention context, among patients with recent ischemic stroke, ambulatory electrocardiographic (ECG) monitoring with wearable or implanted devices detects AF in 15% to 30% of patients.12,13,14,15 However, the concept of primary prevention screening for AF in asymptomatic individuals remains controversial.16,17,18 Randomized clinical trials are lacking to determine which patients merit screening, how best to screen, and whether screening will prevent stroke. In primary care, routine screening for AF is either not performed or is limited to a random pulse check or single opportunistic ECG. According to European guidelines, “systematic ECG screening should be considered to detect AF in individuals aged 75 years or older or those at high risk of stroke.”19(p3) However, the US Preventive Services Task Force recommended against routine ECG screening for AF, citing insufficient evidence.20
We conducted a randomized clinical trial of AF screening in older community-dwelling individuals without known AF using a wearable continuous ECG (cECG) patch monitor vs standard care. The secondary objective assessed whether AF detection led to OAC treatment. Within the screening group, we also evaluated an automated home blood pressure (BP) device for oscillometric AF screening.
Methods
Trial Design, Setting, and Oversight
SCREEN-AF was an investigator-initiated, multicenter, open-label, randomized clinical trial investigating noninvasive home-based AF screening interventions. Participants were recruited from primary care clinics in Canada and Germany. Participants provided written informed consent and were not paid to participate. The study protocol and prespecified statistical analysis plan are available in Supplement 1. The study was funded by peer-reviewed national government grants, coordinated at the Population Health Research Institute, Hamilton, Canada, and approved by the national regulatory authorities and research ethics committees representing all sites. The list of site principal investigators and individuals who provided statistical assistance and study coordination is available in the eAppendix in Supplement 2. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized clinical trials.
Participants
We recruited community-dwelling individuals aged 75 years or older without known AF who were not receiving OAC but were potential OAC candidates if AF was diagnosed (CHADS2 [congestive heart failure, hypertension, age ≥75 years, diabetes, stroke]) score ≥2, indicating moderate or high risk for stroke, and no OAC contraindications). All participants had a history of hypertension requiring antihypertensive medication and were in sinus rhythm at enrollment as assessed by 30-second pulse palpation and heart auscultation by enrolling physicians (eTable 1 in Supplement 2). Key exclusion criteria were previously documented AF or atrial flutter, pacemaker, defibrillator, or implanted loop recorder.
At Canadian sites, patients attending regularly scheduled outpatient visits were screened for eligibility and offered study participation. Study visits were conducted by local investigators and coordinators. German sites screened electronic records for potentially eligible patients and sent invitation letters. A mobile study team (physician, nurse, and medical student) performed study procedures; 6-month visits were conducted by the patients’ family physician.
Randomization
Eligible participants were randomly allocated (1:1) to the screening group or control group via web-based randomization using computer-generated random block sizes of 4 and 6, stratified by center. The control group received standard clinical care and follow-up for 6 months, including pulse check and heart auscultation by a physician at baseline and 6 months. The screening group received a 2-week ambulatory cECG patch monitor, one at baseline and another at 3 months, in addition to standard care. The screening group also received an automated home BP monitor with an AF screening algorithm to be used twice daily during each of the 2-week cECG monitoring periods.
Study Interventions
We studied an adhesive patch cECG (Zio XT; iRhythm Technologies), a miniature, single-lead Holter-type device worn on the chest that provides up to 2 weeks of continuous ECG recording.21,22,23,24,25 It was applied by study personnel immediately after randomization, with instructions to wear it for 2 weeks, including sleep and showering. At 3 months, participants returned to receive another 2-week cECG. Devices were mailed back for central interpretation. Results were sent to each participant’s primary care physician who was responsible for clinical treatment decisions.
The home BP monitor (WatchBP-Home A; Microlife Corp) has been endorsed as an AF screening tool.26,27,28,29 Participants in the screening group were instructed to record their home BP twice daily (morning and evening) during each of the cECG monitoring periods. Each assessment consisted of 3 sequential BP measurements; a positive AF screen was indicated if 2 or more of the 3 consecutive measurements were positive for AF. Participants and clinicians were advised not to act upon the home BP monitor AF screening results.
Assessments
Study visits were conducted in the clinic at baseline, 3 months, and 6 months. Baseline assessments collected data via patient interview and medical records review on demographics, medical history, and medications, and measured pulse and BP. Follow-up assessments recorded medications and any interim outcome events; specifically, any new clinical diagnosis of AF, ischemic or hemorrhagic stroke, transient ischemic attack, systemic embolism, major bleeding, death, and physician/hospital visits. For all outcome events, original source documents were requested for central adjudication (ECG tracings, hospital records).
Outcomes
The primary outcome was detection of AF within 6 months postrandomization, either by study cECG monitors or as part of routine clinical care. We defined AF for the primary outcome as 1 or more episode of continuous AF or atrial flutter lasting more than 5 minutes on cECG or diagnosed clinically (12-lead ECG or other source documentation). All AF outcome events (from cECG and site-reported clinical AF diagnoses) underwent central adjudication by 2 arrhythmia physicians (F.R.Q., W.F.M., A.P.B., and J.A.W.) blinded to randomization group.
The secondary outcome was OAC use at 6 months. Additional outcomes included device adherence, tolerability, detection of other prespecified arrhythmias, health care use, and AF screening performance of the home BP monitor. Site-reported clinical events (stroke, transient ischemic attack, and systemic embolism) underwent central blinded adjudication by 2 neurologists.
Statistical Analysis
Sample size calculations are reported in the eMethods in Supplement 2. All analyses were performed using SAS, version 9.4 (SAS Institute Inc), using an intention-to-screen principle of all randomized patients, regardless of device use, adherence, or duration of participation. Categorical variables are presented as numbers and percentages, and groups were compared using χ2 tests or Fisher exact tests for small samples. Continuous variables are presented as means (SDs) or medians and interquartile ranges (IQRs), and groups were compared using the t tests or Wilcoxon rank sum tests if normality was questionable. Missing values were treated as missing and no imputation was done for any analyses.
The primary analysis compared the proportion of participants in each group achieving the primary outcome of AF detection using the χ2 test and is presented with relative risk and corresponding 95% CI from a modified Poisson regression model with robust error variances. The absolute risk difference is also reported along with 95% CI. A 2-sided significance level of P < .05 was used for all analyses. A planned per-protocol analysis evaluated the primary outcome among patients who wore 2 cECG monitors for 12 or more days each.
For patients with the primary outcome of AF detected by cECG, we summarized the time to first AF detection, number and longest duration of AF episodes, total time in AF, and AF burden (percentage of analyzable time spent in AF per cECG monitor). Adherence was measured by cECG wear times (automatically time-stamped by the device). Tolerability was measured by patient satisfaction surveys and incidence of adverse skin reactions.
For the BP monitor analysis, we estimated sensitivity, specificity, and positive and negative predictive values at the patient level using the simultaneously acquired cECG results as the standard for AF detection. Post hoc, we separately considered AF duration of more than 24 hours and AF duration of less than 24 hours. Details are available in the eMethods in Supplement 2. We compared the number of physician visits, hospitalizations, and emergency department visits at 6 months between groups using the Poisson regression model with log as the link function.
Results
Patient Characteristics
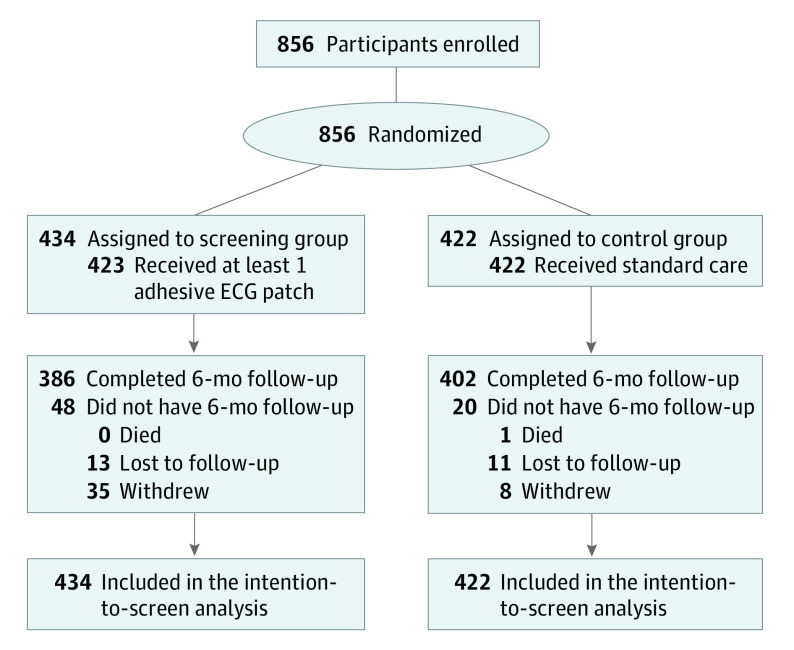
From April 5, 2015, to March 26, 2019, 856 participants (555 from Canada, 301 from Germany) underwent randomization: 434 were assigned to the screening group and 422 to the control group. Follow-up was complete in 95.6% of the participants at 3 months and 92.6% at 6 months (Figure 1). There were no interim analyses. Baseline patient characteristics were balanced between groups (Table 1). Mean (SD) age was 80.0 (4.0) years, 487 participants (56.9%) were women, 369 were men (43.1%), with a median score of 4 points (IQR, 4-5 points) on the CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes, stroke or transient ischemic attack, vascular disease, age 65-74 years, sex category) scale.
Figure 1. Flow Diagram.
ECG indicates electrocardiograhic.
Table 1. Patient Characteristics.
| Characteristic | No. (%) | |
|---|---|---|
| Screening group (n = 434) | Control group (n = 422) | |
| Age, mean (SD), y | 79.8 (3.8) | 80.1 (4.1) |
| Sex | ||
| Female | 255 (58.8) | 232 (55.0) |
| Male | 179 (41.2) | 190 (45.0) |
| Location | ||
| Canada | 283 (65.2) | 272 (64.4) |
| Germany | 151 (34.8) | 150 (35.5) |
| Race/ethnicity | ||
| White | 409 (94.2) | 397 (94.1) |
| Black | 7 (1.6) | 6 (1.4) |
| Asian | 14 (3.2) | 13 (3.1) |
| Othera | 4 (0.9) | 6 (1.4) |
| Baseline blood pressure, mean (SD), mm Hg | ||
| Systolic | 140 (17.1) | 141 (17.9) |
| Diastolic | 75.1 (9.5) | 74.6 (9.9) |
| Height, mean (SD), cm | 165 (10.6) | 165 (9.7) |
| Weight, mean (SD), kg | 76.9 (16.4) | 76.1 (16.2) |
| BMI, mean (SD) | 28.1 (5.4) | 27.8 (5.4) |
| CHA2DS2-VASc score, median (IQR) | 4.0 (4.0-5.0) | 4.0 (4.0-5.0) |
| Medical history | ||
| Diabetes | 102 (23.7) | 103 (24.4) |
| Congestive heart failure | 16 (3.7) | 19 (4.5) |
| Ischemic stroke, TIA, or systemic embolism | 40 (9.3) | 43 (10.2) |
| Coronary artery disease | 72 (16.7) | 80 (19.0) |
| Coronary angioplasty/coronary stent | 47 (10.9) | 46 (10.9) |
| Myocardial infarction | 42 (9.7) | 38 (9.0) |
| Hyperlipidemia | 241 (56.0) | 234 (55.5) |
| Severe aortic or mitral valve disease | 4 (0.9) | 4 (0.9) |
| Rheumatic valve disease | 3 (0.7) | 1 (0.2) |
| Prosthetic heart valve | 3 (0.7) | 1 (0.2) |
| Sleep apnea | 31 (7.2) | 23 (5.5) |
| Dialysis | 0 | 0 |
| Cardiac surgery | ||
| CABG | 20 (4.6) | 29 (6.9) |
| Valve surgery | 4 (0.9) | 3 (0.7) |
| Peripheral artery disease (aortic plaque) | 26 (6.0) | 24 (5.7) |
| Dementia | 6 (1.4) | 2 (0.5) |
| Smoker | ||
| Current | 26 (6.0) | 20 (4.7) |
| Past | 153 (38.0) | 154 (38.3) |
| History of any palpitations in past year (patient self-report) | 73 (16.9) | 75 (17.8) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CABG, coronary artery bypass graft; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years, diabetes, stroke or transient ischemic attack, vascular disease, age 65-74 years, sex category; TIA, transient ischemic attack.
Other includes the following categories: Hispanic/Latino, Middle Eastern, Aboriginal, and Other.
Adherence to cECG Monitoring
The first cECG monitor was worn by 423 participants (97.5%), and a second monitor was worn by 344 individuals (79.3%). Overall, 93.8% of the participants completed at least 1 week of monitoring, 85.9% completed at least 2 weeks, and 74.2% completed at least 3 weeks. Median wear times were 14.0 days (IQR, 13.4-14.0 days) for the first monitoring period, 13.9 days (IQR, 9.8-14.0 days) for the second monitoring period, and 27.5 days (IQR, 20.4-28.0 days) in total. The ECG quality (signal-to-noise) was high, yielding a median analyzable time of 13.7 days (IQR, 12.9-14.0 days) for the first monitoring period, 13.5 days (IQR, 8.0-13.9 days) for the second monitoring period, and 26.8 days (IQR, 15.6-27.7 days) in total.
AF Detection
In the intention-to-screen analysis, the primary outcome of AF detection at 6 months occurred in 23 of 434 participants (5.3%) in the screening group vs 2 of 422 (0.5%) in the control group (relative risk, 11.2; 95% CI, 2.7-47.1; P = .001; absolute difference, 4.8%; 95% CI, 2.6%-7.0%; P < .001; number-needed-to-screen, 21). Sensitivity analysis using a different AF definition yielded similar results (eResults in Supplement 2). Results did not differ substantially by country. In the screening group, 20 of 23 of the AF cases (87.0%) were detected by the cECG monitors and 3 of 23 (13.0%) were diagnosed clinically (these 3 patients presented to the hospital with symptomatic ECG-documented AF).
In a per-protocol analysis restricted to patients with the highest level of adherence (wearing both cECG monitors for at least 12 days each, n = 294), the primary outcome was detected in 17 of 294 individuals (5.8%) in the screening group vs 2 of 422 (0.5%) in the control group (relative risk, 12.2; 95% CI, 2.8-52.4; P < .001; absolute difference, 5.3%; 95% CI, 2.6%-8.1%; P < .001, number-needed-to-screen, 19). The 3-month rate of AF detection (a secondary outcome, based on only a single 2-week cECG) was significantly higher in the screening group (4.6% vs 0.2%; relative risk, 19.5; 95% CI, 2.6-144.3; P = .004; absolute difference, 4.4%; 95% CI, 2.3% to 6.4%; P < .001; number-needed-to-screen, 23).
Anticoagulant Therapy
Oral anticoagulant therapy for any indication increased from 0 at baseline in both groups to 18 of 434 (4.1%) in the screening group vs 4 of 422 (0.9%) in the control group at 6 months (relative risk, 4.4; 95% CI, 1.5-12.8; P = .007; absolute difference, 3.2%; 95% CI, 1.1%-5.3%; P = .003). Atrial fibrillation was the only indication for OAC in the screening group. In the control group, reasons for OAC were AF (2 patients), venous thromboembolism (1 patient), and lower limb angioplasty/stenting (1 patient). The 6-month rate of OAC use for AF was 18 of 434 (4.1%) in the screening group vs 2 of 422 (0.5%) in the control group (relative risk, 8.8; 95% CI, 2.0-37.5; P = .004; absolute difference, 3.7%; 95% CI, 1.7%-5.7%; P < .001).
In the screening group, OAC was prescribed by 6 months for 15 of 20 (75.0%) patients who had cECG-detected AF and for all 3 patients who had AF diagnosed clinically. There were no major bleeding events within 6 months among patients with AF or those prescribed OAC.
Details of AF Detected by cECG Monitors
Of the 20 patients with cECG-detected AF duration of more than 5 minutes, 19 had AF and 1 had atrial flutter. Atrial fibrillation was paroxysmal in 18 of 20 patients (90.0%) and continuous throughout the recording in 2 of 20 (10.0%). Atrial fibrillation was asymptomatic in 17 of 20 patients (85.0%); only 1 of 20 patients (5.0%) presented to the hospital with symptomatic AF during the 6-month follow-up.
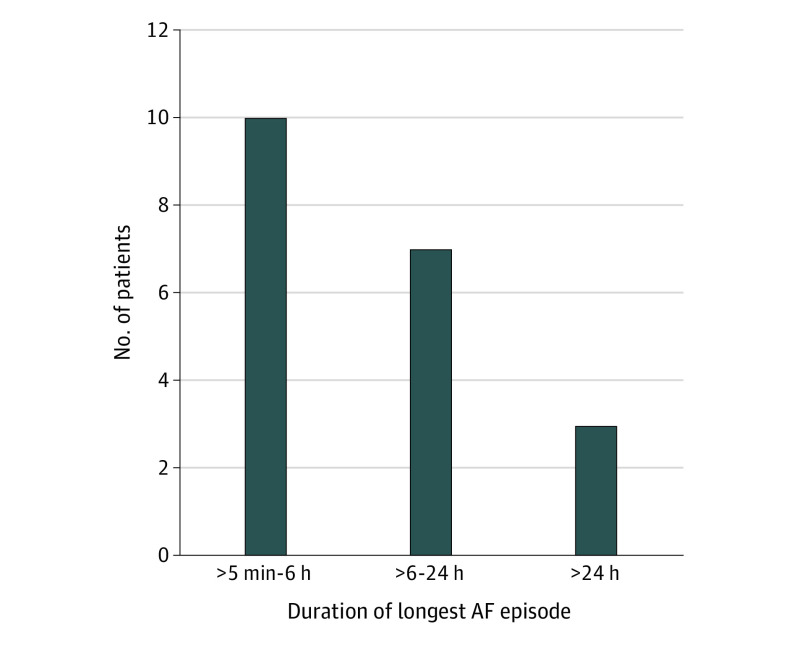
The median time in AF per patient was 6.3 hours (IQR, 4.2-14.0 hours; range, 1.3 hours-28 days). Atrial fibrillation episodes lasted more than 1 hour in 95% of the participants, more than 4 hours in 70%, more than 6 hours in 50%, more than 9 hours in 30%, more than 12 hours in 25%, and more than 24 hours in 15%. The median duration of the longest AF episode was 5.7 hours (IQR, 2.9-12.9 hours) (Figure 2 and Table 2).
Figure 2. Duration of Longest Episode of Atrial Fibrillation (AF) Detected by Continuous Electrocardiographic Monitoring.
Table 2. Details of the AF Cases Detected by Continuous Electrocardiographic Patch Monitoring in the Screening Group.
| Characteristic | Median (IQR) [range] |
|---|---|
| Total time in AF per patient | 6.3 h (4.2-14.0 h) [1.3 h-28 d] |
| AF per patient | |
| Maximum burden per 2-wk patch, % | 1.9 (1.3-17.1) [0.4-17.1] |
| No. of episodes | 3 (2-13) [1-110] |
| Duration of longest episode | 5.7 h (2.9-12.9 h) [50 min-14 d] |
| Time to first detected episode, d | 10.2 (2.8-15.2) [0-24.3] |
Abbreviations: AF, atrial fibrillation; IQR, interquartile range.
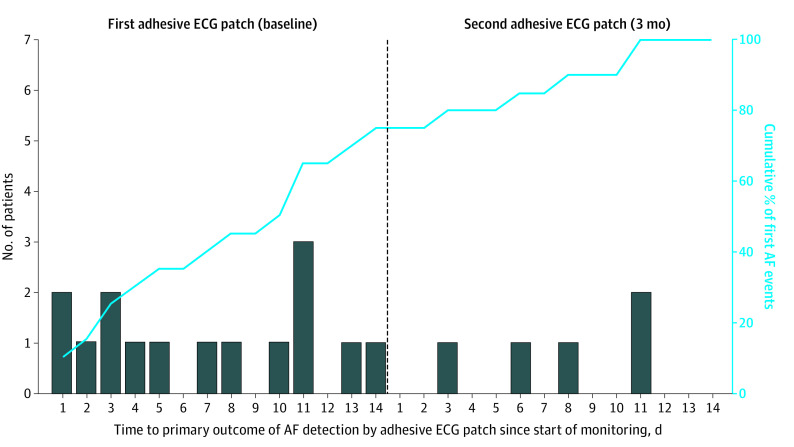
The time course of AF detection is shown in Figure 3. Of the 20 AF cases, 8 (40%) were detected within the first week of cECG monitoring, 15 (75%) were detected within 2 weeks, 17 (85%) were detected within 3 weeks, and 3 (15%) were first detected only during the fourth week; 18 AF cases (90%) were first detected after the first 24 hours of monitoring. Among patients who wore 2 cECG monitors (baseline and 3 months), if the first cECG was negative for AF, the probability of the second cECG finding AF was 5 of 329 (1.5%). Detection of other arrhythmias was rare (eTable 2 in Supplement 2).
Figure 3. Time Course of Atrial Fibrillation (AF) Detection by Continuous Electrocardiographic (cECG) Monitoring in the Screening Group.
The bars show when AF was first detected for each of the 20 patients with cECG-detected AF during the first (A) and second (B) monitoring periods. The curve shows the cumulative incidence of AF. Of the 20 AF cases, 8 (40%) were detected within the first week of cECG monitoring, 15 (75%) were detected within 2 weeks, 17 (85%) were detected within 3 weeks, and 3 (15%) were first detected only during the fourth week; 18 (90%) of AF cases were first detected after the first 24 hours of monitoring.
cECG Tolerability
Adverse skin reactions requiring premature discontinuation of cECG monitoring occurred in 5 of 434 participants (1.2%). On a 5-point scale, most participants agreed or strongly agreed that the cECG monitor was comfortable (daytime: 289 of 356 [81.2%]; sleeping: 285 of 357 [79.8%]); 33 of 355 participants (9.3%) reported that the monitor hindered daily activities and 134 of 354 (37.9%) reported pruritus.
Clinical Outcomes
During the 6-month study period, 1 participant died (control group; cardiovascular death) and 2 participants had an ischemic stroke (both in the screening group) (eMethods in Supplement 2). One patient had a transient ischemic attack (screening group); this patient’s cECG was negative for AF, but AF was subsequently diagnosed in the hospital at the time of presentation with transient ischemic attack. There were no cases of intracranial hemorrhage or systemic embolism. There were no significant differences between the screening and control groups in total physician visits (1233 vs 1251; P = .83), emergency department visits (5 vs 2; P > .99), hospitalizations (5 vs 3; P = .48), or pacemaker implantations (3 vs 2; P > .99).
AF Screening by Home BP Monitors
In the screening group, 412 of 434 participants (94.9%) used the BP monitor for a median of 27.4 days (IQR, 14-28 days), with a median of 55 measurements (IQR, 28-56 measurements) recorded per patient. Among 399 patients with concurrent BP monitor and cECG recordings, the BP monitor had a sensitivity of 35.0% (95% CI, 15.4%-59.2%), specificity of 81.0% (95% CI, 76.7%-84.8%), positive predictive value of 8.9% (95% CI, 4.9%-15.5%), and negative predictive value of 95.9% (95% CI, 94.5%-97.0%) (eTable 3 in Supplement 2). Sensitivity increased to 66.7% for detecting AF episodes lasting more than 24 hours. Specificity increased to 93.4% when BP monitoring was positive for AF at both the morning and evening measurement times in a given day (additional details in the eMethods in Supplement 2).
The BP monitors had at least 1 positive screen for AF in 79 of 434 patients (18.2%), of which 72 (91.1%) were false-positives. When BP monitoring was positive for AF on both the morning and evening measurements in a single day (28 of 434 patients [6.5%]), 3 of 28 (10.7%) were true-positives and 25 (89.3%) were false-positives. In total, BP monitoring yielded 497 false-positive screens, of which 39.2% were associated with frequent atrial or ventricular premature complexes (>60/h) on cECG monitoring. The median AF duration among patients with a true-positive AF screen by BP monitoring was 15.8 hours; the median AF duration among episodes missed by BP monitoring was 4.9 hours.
Discussion
SCREEN-AF investigated 2 technologies for home-based AF screening in older primary care patients with hypertension. A wearable cECG strategy for up to 4 weeks (1) detected a substantial prevalence (5%) of subclinical AF, (2) was superior to 6 months of standard clinical care for AF detection (number-needed-to-screen, 21), and (3) resulted in more patients prescribed OAC for stroke prevention. Most AF cases were paroxysmal, with episodes lasting many hours. Patient adherence to cECG was high, three-quarters of AF cases were detected within the first 2 weeks of ECG monitoring, and 90% of cases would have been missed using a 24-hour Holter monitor.
Our trial strengthens the evidence supporting the effectiveness of screening for early detection of AF. Most previous AF screening studies have been single-group observational studies. Only 1 other completed randomized clinical trial (mSTOPS) investigated screening interventions beyond pulse palpation or random 30-second ECGs in asymptomatic individuals30; our results are consistent with that trial, which found a 3.9% AF detection rate within 4 months among patients randomized to two 2-week cECG patch monitors vs 0.9% in controls (mean age, 74 years; median CHA2DS2-VASc score, 3).29 Our AF detection rate with 2- or 4-week noninvasive cECG monitoring was similar to the yield of implanted cardiac loop recorders after 2 weeks (3.1%) and 1 month (6.2%)31,32 and it was lower, as expected, than in patients with a recent stroke undergoing a similar duration of ECG monitoring.12,13 Our results in an older population complement the Apple Heart Study33 and Huawei Heart Study,34 which both focused on younger patients, lending support for further testing of wearables for screening of older individuals who are at risk for AF.
Our findings highlight several advantages of ambulatory cECG monitoring over other types of AF screening interventions. First, cECG monitoring yields higher AF detection rates than either single time point screening (eg, pulse palpation, BP monitoring, or handheld ECG device) or brief intermittent screening.35,36,37,38,39 Single random screening primarily detects nonparoxysmal AF, whereas cECG monitoring detects both paroxysmal and nonparoxysmal AF. Thus, it is not surprising that 2 recent trials found that single time point AF screening was not superior to usual care in primary care clinics in the Netherlands.40,41 Second, unlike handheld ECGs, watches, and BP monitors in which false-positive AF detection is an issue, wearable cECG devices serve as both the screening tool and the diagnostic test, essentially eliminating the need for confirmatory testing. Third, compared with implanted cardiac monitors, wearable ECG devices are noninvasive, less costly, more accessible, can be self-applied by patients at home, and have fewer false-positive results.42 Although 2- or 4-week cECG monitoring will miss infrequent paroxysmal AF compared with long-term implanted devices,43 AF detected within only 2 weeks by a wearable monitor is of higher burden and likely more clinically significant than the same amount of AF that is detectable only after longer monitoring durations (months to years) with implanted devices.
Twice-daily AF screening by a home BP monitor had a high false-positive rate and missed most AF episodes lasting less than 24 hours. The low sensitivity and specificity of BP monitoring in this study, as compared with previous reports,27,44,45 may reflect patient adherence to the BP monitor, a different standard compared with other studies,38,39,40 the patient population, and our detection of relatively brief episodes of paroxysmal AF rather than persistent AF.
Strengths of our study include the randomized multicenter trial design and broad eligibility criteria to maximize generalizability. We selected a population at risk for both AF and stroke based on older age and hypertension. Our findings should not be generalized to younger individuals who will have a lower prevalence of AF. In our patients, with a median CHA2DS2-VASc score of 4, the finding of many hours of AF is potentially clinically important. The minimum clinically significant amount of subclinical device-detected AF is currently debated.46 An AF burden of greater than 11% during 2-week cECG monitoring has been associated with a 3-fold increase in stroke risk without OAC.47 In patients with a pacemaker or cardioverter-defibrillator, AF durations associated with significantly increased stroke risk range from greater than 6 minutes48 to greater than 1 hour,49 greater than 5.5 hours,50,51,52 and greater than 24 hours.53,54 A subset of patients with brief AF progress to longer AF episodes over time.55,56,57,58
This work has implications for primary stroke prevention, as the prevalence of AF and AF-associated strokes is increasing with an aging population. The primary goal of screening is to identify patients with a sufficiently high burden of AF (eg, >24 continuous hours) for whom OAC is likely to provide net benefit.59 For subclinical AF of less than 24 hours, clinical equipoise for OAC exists.60 Trials underway should help define the role of OAC for brief subclinical AF.61,62 Until such results are available, use of OAC is empirical and treatment decisions must be individualized taking into account the CHA2DS2-VASc score and likelihood of recurrent AF (eg, left atrial enlargement). If the efficacy of OAC for patients with low-burden, device-detected AF is similar to that of clinically detected AF, then future AF screening programs could have major public health benefits for improving stroke prevention at a population level.63
Limitations
The main limitation of our trial is that it was underpowered to detect differences in clinical outcomes. Follow-up duration was short and we cannot exclude a lead-time bias effect. Extended follow-up is ongoing to explore these questions. Larger trials and pooled analyses will be necessary to determine the effect of AF screening on stroke prevention, and several such trials are underway.64,65,66,67,68 Screening for AF, like screening for other conditions, has potential harms.69 The main concerns are overdiagnosis and overtreatment of low-risk patients for whom OAC may be unwarranted (eg, AF lasting seconds only), increasing bleeding risk without benefit. Screening may also cause patient anxiety, increase health care use, or affect health insurance eligibility.70 We found no signal of increased bleeding, although our follow-up duration was short, and we have not assessed cost-effectiveness. Preliminary estimates suggest wearable cECG strategies are potentially cost-effective,71,72 but further work is needed. Given the rapid proliferation of wearable technologies, we are entering an uncharted new era of consumer-driven screening and direct-to-consumer marketing, and consumers must be informed of the potential benefits and risks.73,74,75 We caution against premature or inappropriate uptake of screening until its effect on hard clinical outcomes and cost-effectiveness have been established.
Conclusions
This randomized clinical trial provides evidence that a wearable continuous ECG strategy is well tolerated and effective for early detection of AF in older primary care patients, often leading to OAC treatment with the potential to avert future strokes. Intermittent oscillometric screening with a BP monitor is an inferior strategy for detecting paroxysmal AF. Future studies need to determine the effect of AF screening on clinical outcomes.
Trial Protocol and Statistical Analysis Plan
eAppendix. Acknowledgments
eMethods. Methods – Additional Details
eResults. Results – Additional Details
eTable 1. Eligibility Criteria
eTable 2. Incidence of Other Prespecified Device-Detected Arrhythmias in the Screening Group (n=434)
eTable 3. Performance of Home BP Monitor for AF Detection
Data Sharing Statement
References
- 1.Freedman B, Potpara TS, Lip GY. Stroke prevention in atrial fibrillation. Lancet. 2016;388(10046):806-817. doi: 10.1016/S0140-6736(16)31257-0 [DOI] [PubMed] [Google Scholar]
- 2.Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34(35):2746-2751. doi: 10.1093/eurheartj/eht280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weng L-C, Preis SR, Hulme OL, et al. Genetic predisposition, clinical risk factor burden, and lifetime risk of atrial fibrillation. Circulation. 2018;137(10):1027-1038. doi: 10.1161/CIRCULATIONAHA.117.031431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turakhia MP, Shafrin J, Bognar K, et al. Estimated prevalence of undiagnosed atrial fibrillation in the United States. PLoS One. 2018;13(4):e0195088. doi: 10.1371/journal.pone.0195088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perera KS, Vanassche T, Bosch J, et al. ; ESUS Global Registry Investigators . Global survey of the frequency of atrial fibrillation–associated stroke: Embolic Stroke of Undetermined Source Global Registry. Stroke. 2016;47(9):2197-2202. doi: 10.1161/STROKEAHA.116.013378 [DOI] [PubMed] [Google Scholar]
- 6.Sposato LA, Cipriano LE, Saposnik G, Ruíz Vargas E, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2015;14(4):377-387. doi: 10.1016/S1474-4422(15)70027-X [DOI] [PubMed] [Google Scholar]
- 7.Borowsky LH, Regan S, Chang Y, Ayres A, Greenberg SM, Singer DE. First diagnosis of atrial fibrillation at the time of stroke. Cerebrovasc Dis. 2017;43(3-4):192-199. doi: 10.1159/000457809 [DOI] [PubMed] [Google Scholar]
- 8.Saposnik G, Gladstone D, Raptis R, Zhou L, Hart RG; Investigators of the Registry of the Canadian Stroke Network (RCSN) and the Stroke Outcomes Research Canada (SORCan) Working Group . Atrial fibrillation in ischemic stroke: predicting response to thrombolysis and clinical outcomes. Stroke. 2013;44(1):99-104. doi: 10.1161/STROKEAHA.112.676551 [DOI] [PubMed] [Google Scholar]
- 9.Freedman B, Camm J, Calkins H, et al. ; AF-Screen Collaborators . Screening for atrial fibrillation: a report of the AF-SCREEN International Collaboration. Circulation. 2017;135(19):1851-1867. doi: 10.1161/CIRCULATIONAHA.116.026693 [DOI] [PubMed] [Google Scholar]
- 10.Jones NR, Taylor CJ, Hobbs FDR, Bowman L, Casadei B. Screening for atrial fibrillation: a call for evidence. Eur Heart J. 2020;41(10):1075-1085. doi: 10.1093/eurheartj/ehz834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi: 10.1016/S0140-6736(13)62343-0 [DOI] [PubMed] [Google Scholar]
- 12.Gladstone DJ, Spring M, Dorian P, et al. ; EMBRACE Investigators and Coordinators . Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370(26):2467-2477. doi: 10.1056/NEJMoa1311376 [DOI] [PubMed] [Google Scholar]
- 13.Wachter R, Gröschel K, Gelbrich G, et al. ; Find-AF(randomised) Investigators and Coordinators . Holter-electrocardiogram-monitoring in patients with acute ischaemic stroke (Find-AFRANDOMISED): an open-label randomised controlled trial. Lancet Neurol. 2017;16(4):282-290. doi: 10.1016/S1474-4422(17)30002-9 [DOI] [PubMed] [Google Scholar]
- 14.Sanna T, Diener HC, Passman RS, et al. ; CRYSTAL AF Investigators . Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478-2486. doi: 10.1056/NEJMoa1313600 [DOI] [PubMed] [Google Scholar]
- 15.Schnabel RB, Haeusler KG, Healey JS, et al. Searching for atrial fibrillation poststroke: a white paper of the AF-SCREEN International Collaboration. Circulation. 2019;140(22):1834-1850. doi: 10.1161/CIRCULATIONAHA.119.040267 [DOI] [PubMed] [Google Scholar]
- 16.Burns RB, Zimetbaum P, Lubitz SA, Smetana GW. Should this patient be screened for atrial fibrillation? grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med. 2019;171(11):828-836. doi: 10.7326/M19-1126 [DOI] [PubMed] [Google Scholar]
- 17.Neubeck L, Orchard J, Lowres N, Freedman SB. To screen or not to screen? examining the arguments against screening for atrial fibrillation. Heart Lung Circ. 2017;26(9):880-886. doi: 10.1016/j.hlc.2017.05.118 [DOI] [PubMed] [Google Scholar]
- 18.Khurshid S, Healey JS, McIntyre WF, Lubitz SA. Population-based screening for atrial fibrillation. Circ Res. 2020;127(1):143-154. doi: 10.1161/CIRCRESAHA.120.316341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hindricks G, Potpara T, Dagres N, et al. ; ESC Scientific Document Group . 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). [published online August 29, 2020]. Eur Heart J. 2020;ehaa612. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 20.Curry SJ, Krist AH, Owens DK, et al. ; US Preventive Services Task Force . Screening for atrial fibrillation with electrocardiography: US Preventive Services Task Force recommendation statement. JAMA. 2018;320(5):478-484. doi: 10.1001/jama.2018.10321 [DOI] [PubMed] [Google Scholar]
- 21.Turakhia MP, Ullal AJ, Hoang DD, et al. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high-risk patients: the Screening Study for Undiagnosed Atrial Fibrillation (STUDY-AF). Clin Cardiol. 2015;38(5):285-292. doi: 10.1002/clc.22387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med. 2014;127(1):95.e11-95.e17. doi: 10.1016/j.amjmed.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turakhia MP, Hoang DD, Zimetbaum P, et al. Diagnostic utility of a novel leadless arrhythmia monitoring device. Am J Cardiol. 2013;112(4):520-524. doi: 10.1016/j.amjcard.2013.04.017 [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg MA, Samuel M, Thosani A, Zimetbaum PJ. Use of a noninvasive continuous monitoring device in the management of atrial fibrillation: a pilot study. Pacing Clin Electrophysiol. 2013;36(3):328-333. doi: 10.1111/pace.12053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ackermans PA, Solosko TA, Spencer EC, et al. A user-friendly integrated monitor-adhesive patch for long-term ambulatory electrocardiogram monitoring. J Electrocardiol. 2012;45(2):148-153. doi: 10.1016/j.jelectrocard.2011.10.007 [DOI] [PubMed] [Google Scholar]
- 26.Verberk WJ, Omboni S, Kollias A, Stergiou GS. Screening for atrial fibrillation with automated blood pressure measurement: research evidence and practice recommendations. Int J Cardiol. 2016;203:465-473. doi: 10.1016/j.ijcard.2015.10.182 [DOI] [PubMed] [Google Scholar]
- 27.Chan PH, Wong CK, Pun L, et al. Diagnostic performance of an automatic blood pressure measurement device, Microlife WatchBP Home A, for atrial fibrillation screening in a real-world primary care setting. BMJ Open. 2017;7(6):e013685. doi: 10.1136/bmjopen-2016-013685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quinn FR, Gladstone DJ, Ivers NM, et al. Diagnostic accuracy and yield of screening tests for atrial fibrillation in the family practice setting: a multicentre cohort study. CMAJ Open. 2018;6(3):E308-E315. doi: 10.9778/cmajo.20180001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willits I, Keltie K, Craig J, Sims A. WatchBP Home A for opportunistically detecting atrial fibrillation during diagnosis and monitoring of hypertension: a NICE Medical Technology Guidance. Appl Health Econ Health Policy. 2014;12(3):255-265. doi: 10.1007/s40258-014-0096-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinhubl SR, Waalen J, Edwards AM, et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSTOPS randomized clinical trial. JAMA. 2018;320(2):146-155. doi: 10.1001/jama.2018.8102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiffel JA, Verma A, Kowey PR, et al. ; REVEAL AF Investigators . Incidence of previously undiagnosed atrial fibrillation using insertable cardiac monitors in a high-risk population: the REVEAL AF Study. JAMA Cardiol. 2017;2(10):1120-1127. doi: 10.1001/jamacardio.2017.3180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reiffel JA, Verma A, Kowey PR, et al. ; REVEAL AF Investigators . Rhythm monitoring strategies in patients at high risk for atrial fibrillation and stroke: a comparative analysis from the REVEAL AF study. Am Heart J. 2020;219:128-136. doi: 10.1016/j.ahj.2019.07.016 [DOI] [PubMed] [Google Scholar]
- 33.Perez MV, Mahaffey KW, Hedlin H, et al. ; Apple Heart Study Investigators . Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381(20):1909-1917. doi: 10.1056/NEJMoa1901183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo Y, Wang H, Zhang H, et al. ; MAFA II Investigators . Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol. 2019;74(19):2365-2375. doi: 10.1016/j.jacc.2019.08.019 [DOI] [PubMed] [Google Scholar]
- 35.Lowres N, Olivier J, Chao T-F, et al. Estimated stroke risk, yield, and number-needed-to-screen for atrial fibrillation detected through single time screening: a multicountry patient-level meta-analysis of 141,220 screened individuals. PLoS Med. 2019;16(9):e1002903. doi: 10.1371/journal.pmed.1002903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petryszyn P, Niewinski P, Staniak A, et al. Effectiveness of screening for atrial fibrillation and its determinants: a meta-analysis. PLoS One. 2019;14(3):e0213198. doi: 10.1371/journal.pone.0213198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benito L, Coll-Vinent B, Gómez E, et al. EARLY: a pilot study on early diagnosis of atrial fibrillation in a primary healthcare centre. Europace. 2015;17(11):1688-1693. doi: 10.1093/europace/euv146 [DOI] [PubMed] [Google Scholar]
- 38.Halcox JPJ, Wareham K, Cardew A, et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation. 2017;136(19):1784-1794. doi: 10.1161/CIRCULATIONAHA.117.030583 [DOI] [PubMed] [Google Scholar]
- 39.Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation. 2015;131(25):2176-2184. doi: 10.1161/CIRCULATIONAHA.114.014343 [DOI] [PubMed] [Google Scholar]
- 40.Uittenbogaart SB, Verbiest-van Gurp N, Lucassen WAM, et al. Opportunistic screening versus usual care for detection of atrial fibrillation in primary care: cluster randomised controlled trial. BMJ. 2020;370:m3208. doi: 10.1136/bmj.m3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaasenbrood F, Hollander M, de Bruijn SHM, et al. Opportunistic screening versus usual care for diagnosing atrial fibrillation in general practice: a cluster randomised controlled trial. Br J Gen Pract. 2020;70(695):e427-e433. doi: 10.3399/bjgp20X708161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hindricks G, Pokushalov E, Urban L, et al. ; XPECT Trial Investigators . Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: results of the XPECT trial. Circ Arrhythm Electrophysiol. 2010;3(2):141-147. doi: 10.1161/CIRCEP.109.877852 [DOI] [PubMed] [Google Scholar]
- 43.Diederichsen SZ, Haugan KJ, Kronborg C, et al. Comprehensive evaluation of rhythm monitoring strategies in screening for atrial fibrillation: insights from patients at risk monitored long term with an implantable loop recorder. Circulation. 2020;141(19):1510-1522. doi: 10.1161/CIRCULATIONAHA.119.044407 [DOI] [PubMed] [Google Scholar]
- 44.Kollias A, Destounis A, Kalogeropoulos P, Kyriakoulis KG, Ntineri A, Stergiou GS. Atrial fibrillation detection during 24-hour ambulatory blood pressure monitoring: comparison with 24-hour electrocardiography. Hypertension. 2018;72(1):110-115. doi: 10.1161/HYPERTENSIONAHA.117.10797 [DOI] [PubMed] [Google Scholar]
- 45.Wiesel J, Salomone TJ. Screening for atrial fibrillation in patients ≥65 years using an automatic blood pressure monitor in a skilled nursing facility. Am J Cardiol. 2017;120(8):1322-1324. doi: 10.1016/j.amjcard.2017.07.016 [DOI] [PubMed] [Google Scholar]
- 46.Chen LY, Chung MK, Allen LA, et al. ; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council . Atrial fibrillation burden: moving beyond atrial fibrillation as a binary entity—a scientific statement from the American Heart Association. Circulation. 2018;137(20):e623-e644. doi: 10.1161/CIR.0000000000000568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Go AS, Reynolds K, Yang J, et al. Association of burden of atrial fibrillation with risk of ischemic stroke in adults with paroxysmal atrial fibrillation: the KP-RHYTHM study. JAMA Cardiol. 2018;3(7):601-608. doi: 10.1001/jamacardio.2018.1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uittenbogaart SB, Lucassen WAM, van Etten-Jamaludin FS, de Groot JR, van Weert HCPM. Burden of atrial high-rate episodes and risk of stroke: a systematic review. Europace. 2018;20(9):1420-1427. doi: 10.1093/europace/eux356 [DOI] [PubMed] [Google Scholar]
- 49.Boriani G, Glotzer TV, Santini M, et al. Device-detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke Prevention Strategies Based on Atrial Fibrillation Information From Implanted Devices). Eur Heart J. 2014;35(8):508-516. doi: 10.1093/eurheartj/eht491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2(5):474-480. doi: 10.1161/CIRCEP.109.849638 [DOI] [PubMed] [Google Scholar]
- 51.Perino AC, Fan J, Askari M, et al. ; Insights from the Veterans Health Administration . Practice variation in anticoagulation prescription and outcomes after device-detected atrial fibrillation. Circulation. 2019;139(22):2502-2512. doi: 10.1161/CIRCULATIONAHA.118.038988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turakhia MP, Ziegler PD, Schmitt SK, et al. Atrial fibrillation burden and short-term risk of stroke: case-crossover analysis of continuously recorded heart rhythm from cardiac electronic implanted devices. Circ Arrhythm Electrophysiol. 2015;8(5):1040-1047. doi: 10.1161/CIRCEP.114.003057 [DOI] [PubMed] [Google Scholar]
- 53.Van Gelder IC, Healey JS, Crijns HJGM, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38(17):1339-1344. doi: 10.1093/eurheartj/ehx042 [DOI] [PubMed] [Google Scholar]
- 54.Witt CT, Kronborg MB, Nohr EA, Mortensen PT, Gerdes C, Nielsen JC. Early detection of atrial high rate episodes predicts atrial fibrillation and thromboembolic events in patients with cardiac resynchronization therapy. Heart Rhythm. 2015;12(12):2368-2375. doi: 10.1016/j.hrthm.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 55.Wong JA, Conen D, Van Gelder IC, et al. Progression of device-detected subclinical atrial fibrillation and the risk of heart failure. J Am Coll Cardiol. 2018;71(23):2603-2611. doi: 10.1016/j.jacc.2018.03.519 [DOI] [PubMed] [Google Scholar]
- 56.Diederichsen SZ, Haugan KJ, Brandes A, et al. Natural history of subclinical atrial fibrillation detected by implanted loop recorders. J Am Coll Cardiol. 2019;74(22):2771-2781. doi: 10.1016/j.jacc.2019.09.050 [DOI] [PubMed] [Google Scholar]
- 57.Glotzer TV, Hellkamp AS, Zimmerman J, et al. ; MOST Investigators . Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the Mode Selection Trial (MOST). Circulation. 2003;107(12):1614-1619. doi: 10.1161/01.CIR.0000057981.70380.45 [DOI] [PubMed] [Google Scholar]
- 58.Boriani G, Glotzer TV, Ziegler PD, et al. Detection of new atrial fibrillation in patients with cardiac implanted electronic devices and factors associated with transition to higher device-detected atrial fibrillation burden. Heart Rhythm. 2018;15(3):376-383. doi: 10.1016/j.hrthm.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 59.Andrade JG, Verma A, Mitchell LB, et al. ; CCS Atrial Fibrillation Guidelines Committee . 2018 Focused update on the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol. 2018;34(11):1371-1392. doi: 10.1016/j.cjca.2018.08.026 [DOI] [PubMed] [Google Scholar]
- 60.Gorenek B, Bax J, Boriani G, et al. ; ESC Scientific Document Group . Device-detected subclinical atrial tachyarrhythmias: definition, implications and management: an European Heart Rhythm Association (EHRA) consensus document, endorsed by Hearth Rhythm Society (HRS), Asia Pacific heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLEACE). Europace. 2017;19(9):1556-1578. doi: 10.1093/europace/eux163 [DOI] [PubMed] [Google Scholar]
- 61.Lopes RD, Alings M, Connolly SJ, et al. Rationale and design of the Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation (ARTESiA) trial. Am Heart J. 2017;189:137-145. doi: 10.1016/j.ahj.2017.04.008 [DOI] [PubMed] [Google Scholar]
- 62.Kirchhof P, Blank BF, Calvert M, et al. Probing oral anticoagulation in patients with atrial high rate episodes: Rationale and design of the Non-vitamin K Antagonist Oral Anticoagulants in Patients With Atrial High Rate Episodes (NOAH-AFNET 6) trial. Am Heart J. 2017;190:12-18. doi: 10.1016/j.ahj.2017.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Engdahl J, Holmén A, Rosenqvist M, Strömberg U. A prospective 5-year follow-up after population-based systematic screening for atrial fibrillation. Europace. 2018;20(FI_3):f306-f311. doi: 10.1093/europace/euy045 [DOI] [PubMed] [Google Scholar]
- 64.A study to determine if identification of undiagnosed atrial fibrillation in people at least 70 years of age reduces the risk of stroke (GUARD-AF). ClinicalTrials.gov identifier: NCT04126486. Updated November 19, 2020. Accessed October 20, 2020. https://clinicaltrials.gov/ct2/show/NCT04126486
- 65.Screening for Atrial Fibrillation Among Older Patients in Primary Care Clinics (VITAL-AF) . ClinicalTrials.gov identifier: NCT03515057. Updated January 31, 2020. Accessed October 20, 2020. https://clinicaltrials.gov/ct2/show/NCT03515057
- 66.AMALFI : Active Monitoring for Atrial Fibrillation. Updated January 29, 2020. Accessed October 20, 2020. http://www.isrctn.com/ISRCTN15544176
- 67.The SAFER Study: Screening for Atrial Fibrillation With ECG to Reduce Stroke. Published 2020. Accessed October 1, 2020. https://www.safer.phpc.cam.ac.uk/
- 68.A study to investigate if early atrial fibrillation (AF) diagnosis reduces risk of events like stroke in the real-world. ClinicalTrials.gov identifier: NCT04276441. Updated December 14, 2020. Accessed October 1, 2020. https://clinicaltrials.gov/ct2/show/NCT04276441
- 69.Mandrola J, Foy A, Naccarelli G. Screening for atrial fibrillation comes with many snags. JAMA Intern Med. 2018;178(10):1296-1298. doi: 10.1001/jamainternmed.2018.4038 [DOI] [PubMed] [Google Scholar]
- 70.Orchard JJ, Neubeck L, Orchard JW, et al. ECG-based cardiac screening programs: legal, ethical, and logistical considerations. Heart Rhythm. 2019;16(10):1584-1591. doi: 10.1016/j.hrthm.2019.03.025 [DOI] [PubMed] [Google Scholar]
- 71.McIntyre WF, Yong JHE, Sandhu RK, et al. Prevalence of undiagnosed atrial fibrillation in elderly individuals and potential cost-effectiveness of non-invasive ambulatory electrocardiographic screening: the ASSERT-III study. J Electrocardiol. 2020;58:56-60. doi: 10.1016/j.jelectrocard.2019.11.040 [DOI] [PubMed] [Google Scholar]
- 72.Yong JH, Thavorn K, Hoch JS, et al. ; EMBRACE Steering Committee . Potential cost-effectiveness of ambulatory cardiac rhythm monitoring after cryptogenic stroke. Stroke. 2016;47(9):2380-2385. doi: 10.1161/STROKEAHA.115.011979 [DOI] [PubMed] [Google Scholar]
- 73.Boriani G, Schnabel RB, Healey JS, et al. Consumer-led screening for atrial fibrillation using consumer-facing wearables, devices and apps: a survey of health care professionals by AF-SCREEN international collaboration. Eur J Intern Med. 2020;82:97-104. doi: 10.1016/j.ejim.2020.09.005 [DOI] [PubMed] [Google Scholar]
- 74.Ding EY, Marcus GM, McManus DD. Emerging technologies for identifying atrial fibrillation. Circ Res. 2020;127(1):128-142. doi: 10.1161/CIRCRESAHA.119.316342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lown M, Wilcox CR, Hughes S, et al. Patients’ views about screening for atrial fibrillation (AF): a qualitative study in primary care. BMJ Open. 2020;10(3):e033061. doi: 10.1136/bmjopen-2019-033061 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eAppendix. Acknowledgments
eMethods. Methods – Additional Details
eResults. Results – Additional Details
eTable 1. Eligibility Criteria
eTable 2. Incidence of Other Prespecified Device-Detected Arrhythmias in the Screening Group (n=434)
eTable 3. Performance of Home BP Monitor for AF Detection
Data Sharing Statement