Abstract
Introduction:
Though 85% of children and young adults with acute lymphoblastic leukemia (ALL) are cured, the prognosis of relapsed or refractory disease is poor, with little progress made until recently. The advent of chimeric antigen receptor (CAR) T cell therapy has transformed treatment of relapsed/refractory ALL. The most well-studied, successful CARs are autologous, murine-based anti-CD19 CARs, but new CAR constructs are currently under clinical investigation.
Areas covered:
This review describes the history and design of CAR T cells, clinical trial outcomes of anti-CD19 and newer CARs, treatment-related toxicities including cytokine release syndrome and neurotoxicity, and issues with resistance and relapse. A search of PubMed and clinicaltrials.gov spanning from 2012 to present was used to select original reports investigating use of CAR T in pediatric patients.
Expert opinion:
CD19-targeted CARs have demonstrated remarkable response rates and produced durable remissions in very high-risk pediatric patient populations. The therapies, however, are limited by unique treatment-related toxicities and considerable rates of antigen-positive and antigen-negative relapses. Current research efforts focused on elucidating mechanisms of resistance/relapse and on developing strategies to prevent and treat relapse are critical to optimizing the use of CAR T cells. In addition, ongoing trials testing CARs earlier in therapy and for new indications are key to informing their widespread usage.
Keywords: acute lymphoblastic leukemia, adoptive T cell therapy, CAR T cell, CD19, chimeric antigen receptor, cytokine release syndrome, immunotherapy, tisagenlecleucel
1. Introduction
Acute lymphoblastic leukemia (ALL) is the most common cancer in children and adolescents. Over 3000 individuals less than age 20 years are newly diagnosed in the United States each year [1]. Of those, approximately 85% are expected to be cured with frontline, multi-agent chemotherapy administered over the course of 2.5 to 3.5 years [2,3]. For the 10–20% of patients with relapsed or refractory disease, however, the prognosis is much less favorable [4]. Children with late relapses (>36 months after diagnosis) have about a 50% chance of achieving long-term remission [5]. Those with early relapses have less than a 30% chance of long-term survival [5,6]. Similarly, for children with primary refractory disease, long-term survival rates are approximately 30% [7]. Outcomes are even more dismal for children with multiply relapsed disease, with each subsequent relapse becoming more difficult to treat [8]. Therefore, despite excellent cure rates in the upfront settings, due to poor outcomes of relapsed/refractory disease, ALL remains a leading cause of cancer death in children [9,10].
Until recently, little progress had been made in the treatment of relapsed/refractory ALL [5,8]. Over the past 5–10 years, the advent of targeted immunotherapy has transformed the landscape of relapsed disease therapy. Novel immunotherapies have been most successful for B-precursor cell ALL (B-ALL), which accounts for 85% of pediatric ALL [2]. These immunotherapeutic approaches include bi-specific T cell engaging antibodies such as blinatumomab (BLINCYTO®, Amgen Inc.), conjugated monoclonal antibodies such as inotuzumab ozogamicin (Besponsa®, Pfizer Inc.), and adoptive T cell therapy [11,12]. Autologous chimeric antigen receptor (CAR)-modified T cells targeting CD19, in particular, have demonstrated impressive antitumor efficacy in relapsed/refractory B-ALL [13,14,15].
We conducted a literature search using PubMed and clinicaltrials.gov to identify original studies using CAR T cells in pediatric patients. Studies from 2012 to present were included. Data from phase 1 and phase 2 trials were included as were updates from longitudinal reporting. Information on outcomes and toxicity were taken directly from original reporting. A separate PubMed search was conducted on the history and development of CAR T using the following keywords in different combinations: “chimeric antigen receptor” “cytokine release syndrome” “acute lymphoblastic leukemia” “neurotoxicity” “CD19” “CD22” and “tumor infiltrating lymphocyte”. Original reporting and review articles were considered. Preference was given to original reporting, but review articles were considered based on thoroughness of the topic covered.
CAR T cells are patient-derived T cells that have been engineered ex vivo to produce antigen receptors coupled with intracellular T cell signaling domains that target specific tumor antigens. CD19 is the most well-studied target, and anti-CD19 CARs have demonstrated striking response rates with many children achieving durable remissions [16,17,18,19]. In August 2017, clinical trial results led to tisagenlecleucel (formerly CTL019; Kymriah®, Novartis Pharmaceuticals Corporation) becoming the first gene therapy or CAR T cell product to receive approval by the United States Food and Drug administration (FDA) [20]. Subsequently, tisagenlecleucel was also approved by the European Medicines Agency (EMA) [21]. The approvals include treatment of children up to age 25 years with B-ALL that is refractory or in second or later relapse. Since tisagenlecleucel became commercially available, its use has expanded to increasing numbers of patients at a wider variety of centers.
Notwithstanding the successes of CAR T cell therapy, substantial challenges remain. The therapy is associated with potentially severe or life-threatening toxicities, including cytokine release syndrome (CRS) and neurologic toxicity [22,23]. In addition, CAR T cell persistence is variable across products and trials. Both CD19-positive and CD19-negative relapses are frequently reported and are challenging to prevent and treat. In this review, we describe the history and design of CAR T cells, clinical trial outcomes of anti-CD19 CARs and other constructs, treatment-related toxicities, and challenges with relapse and resistance. In addition, we discuss gaps in the literature and future directions to optimize the use of this transformative therapy.
2. History and design of CAR T cells
Treatment of tumors using directed T cells began in the 1980s with the application of autologous tumor-infiltrating lymphocytes (TILs) infused to treat melanoma and allogenic T cells to treat relapsed leukemia. Early experiments attempted to identify and select T cells with maximal anti-tumor effect. Rosenberg and colleagues used a selection process to collect TILs with improved in vitro anti-tumor effect [24]. In conjunction with cyclophosphamide and Interleukin-2 (IL-2), TILs were able to demonstrate anti-tumor effect in 50% of patients with melanoma [25]. In the 1990s, donor lymphocyte infusion in the absence of additional chemotherapy was shown to have an anti-tumor effect in patients who had received a bone marrow transplant for CML [26]. Donor lymphocyte infusion has since been described by numerous studies and is commonly utilized in bone marrow transplant. These early studies demonstrated proof of concept that immune surveillance could be improved using select allogeneic lymphocytes.
In the late 1980s into the 1990s, gene transfer techniques began to highlight the importance of the T cell receptor (TCR) for anti-tumor effect. In 1986 the Steinmetz group demonstrated alpha and beta T cell receptor genes could undergo cellular transfer and retain similar specificity and restriction in the host cell [27]. This technique was adapted to humans and tumor antigen-reactive TCRs were successfully transferred and shown to have anti-tumor effect against melanoma [28]. Parallel research in the 1990s showed T cell signaling via the CD3 zeta chain was sufficient for activation [29,30].
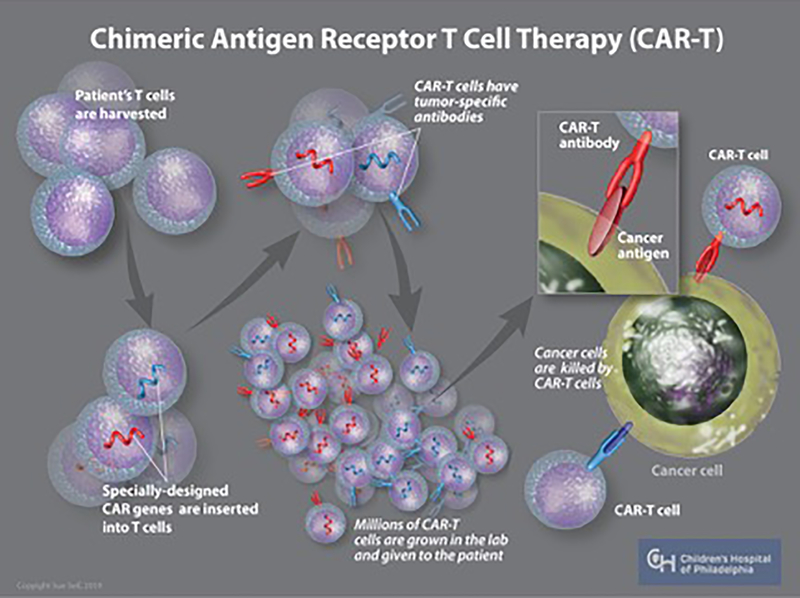
Moving the TCR between lymphocytes and understanding intracellular signaling would serve as the platform for development of novel TCRs in the early 2000s. Early constructs linked the TCR/CD3 domain or novel chimeric antigens to a CD3 domain and demonstrated active signaling [31,32]. However, early chimeric receptors also demonstrated that active signaling was not sufficient to produce an immunogenic response [33]. The addition of a CD28 costimulatory domain significantly increased the in vitro immune response [34,35]. The addition of co-stimulatory molecules allowed chimeric antigen T cell receptors to both produce IL-2 and proliferate as an immune response to ligand bindings [36,37]. The combination of a chimeric transmembrane TCR attached to a co-stimulatory cell signaling domain subsequently became the defining feature of second generation CARs (Figure 1).
Figure 1: Autologous chimeric antigen receptor T-cell generation.
For autologous CAR-T cell generation, peripheral blood mononuclear cells are collected from a patient by leukapheresis. T-cells are then transduced with a viral vector containing genetic information that encodes the receptor of interest. The CAR-Ts are expanded ex-vivo and then infused into the patient. Once infused, the CAR-Ts survey for the antigen of interest and are activated upon engagement with the target.
Intracellular signal augmentation has primarily been studied with the CD28 or 4–1BB co-stimulatory signals but other co-stimulatory molecules have been investigated [38]. CD28 and 4–1BB are also the most common co-stimulatory domains for CD19-targeted CARs. Use of different co-stimulatory domains has revealed cellular proliferation, persistence and immune response are likely influenced by cell signaling (see Section 5.2 below).
Equally important to the function and potency of an engineered T cell is the antigen specificity of the CAR. Autologous TCRs undergo a process of positive and negative selection to ensure self-tolerance. In contrast, CARs are synthetically constructed and the affinity of the receptor generally comes from empiric testing. Most CARs use the single-chain variable fragment (scFv) of a monoclonal antibody (mAb) that recognizes a particular tumor antigen. Features such as the extracellular spacer domain of the CAR can impact function [39]. Several studies also elucidate that receptor affinity may play a role in the potency and side effects of CARs designed to target the same tumor marker [40,41,42]. In these studies, higher affinity of the scFv domain was usually associated with increased tumor lysis. However, other data from class 1 restricted TCRs suggest that if a certain affinity threshold is exceeded, T cells lose therapeutic activity and undergo cell death [43].
Conditioning prior to infusion of CAR T cells has also proven to be an essential part of therapy. Early studies showed that lymphodepleting chemotherapy prior to infusion led to dramatically increased proliferation [44]. The mechanism for increased proliferation is likely multifactorial but involves reduction of immunosuppressive cells such as T-regulatory cells and other myeloid suppressor cells, downregulation of homeostatic cytokines such as IL-2, IL-7 and IL-15 and downregulation of small molecules produced by tumors for a survival advantage [45,46]. Early studies using anti-CD19 CARs to treat leukemia used a combination of fludarabine and cyclophosphamide prior to infusion. The same combination was associated with improved persistence and improved outcomes in patients with non-Hodgkin lymphoma [47].
3. Anti-CD19 CAR T cell clinical trials and outcomes
Clinical trials of anti-CD19 CAR T cells in pediatric and young adult patients have demonstrated remarkable response rates. Early-phase, single-center trials were fundamental to describing the efficacy and safety of the therapy. Several early clinical trials were followed by multicenter trials, which further established outcomes in larger patient samples. The various CD19 CARs tested across clinical trial programs do have substantial differences, including with their CAR designs (scFv and costimulatory domains), type of vector used to transduce the T cells, and selection of CAR T cells for infusion. In addition, clinical trials have differed with regard to their patient inclusion criteria, lymphodepletion regimens, toxicity grading systems, and decisions about consolidative allogeneic hematopoietic cell transplants (alloHCT). Therefore, direct comparisons between trials or CD19 CAR products have not been feasible. Despite these differences, however, similarly high complete remission rates of 70–90% have been reported across trials [16,17,19,48,49]. A summary of the major clinical trials is presented in Table 1.
Table 1.
Summary of outcomes from major CAR T cells trials of children and young adults with B-ALL
| Reference | Program and CAR construct | Phase | Lymphodepletion regimen | Population | Response | Long-term efficacy | Consolidative alloHCT |
|---|---|---|---|---|---|---|---|
| Tisagenlecleucel | |||||||
| Maude et al.[17] NCT01624695 NCT01029366 |
CHOP/Penn 4–1BB |
1–2a | Varied | N = 30 25 children, 5 adults |
CR: 90% | 6-mos EFS: 67% 6-mos OS: 78% |
3 of 27 in CR |
| Maude et al.[51]#
(updated analysis of pediatric patients) |
Varied | N = 59 Ages 1–24y |
CR: 93% MRDneg: 88% |
12-mos RFS: 76% 12-mos OS: 79% |
5 of 55 in CR | ||
| Maude et al.[16] NCT02435849 |
Global study 4–1BB |
2 | Fludarabine/ Cyclophosphamide | N = 75 Ages 3–23y |
CR: 81% MRDneg: 81% |
12-mos RFS: 59% 12-mos EFS: 50% 12-mos OS: 76% |
8 of 61 in CR |
| Other murine anti-CD19 CARs | |||||||
| Lee et al.[19] NCT01593696 |
NCI CD28 |
1 | Fludarabine/ Cyclophosphamide | N = 21 Ages 1–30y |
CR: 70% (of 20 with B-ALL) MRDneg: 60% |
OS with median follow-up of 10 mos: 52% | 10 of 12 in MRDneg CR |
| Lee et al.[54]#
(updated analysis) |
Varied | N = 53 Ages 1–30y |
CR: 70% (of 51 with B-ALL) MRDneg: 55% |
18-mos RFS: 50% | 21 of 28 in MRDneg CR | ||
| Gardner et al.[49] NCT02028455 |
Seattle 4–1BB |
1/2 | Fludarabine/ Cyclophosphamide or Cyclosphamide alone | N = 43 Ages 1–26y |
MRDneg CR: 93% | 12-mos EFS: 51% 12-mos OS: 70% |
11 of 40 in MRDneg CR |
| Curran et al.[48] NCT01860937 |
MSKCC CD28 |
1 | Cyclophosphamide +/- Fludarabine | N = 25 Ages 1–25y |
CR: 75% MRDneg CR: 67% |
Median OS varied by cohort | 15 of 18 in CR |
| Humanized anti-CD19 CARs | |||||||
| Maude et al.[59]#
NCT02374333 |
CHOP 4–1BB |
1 | Fludarabine/ Cyclophosphamide | N = 38 Ages 2–24y |
CAR naïve CR: 100% MRDneg CR: 100% Retreatment CR: 56% MRDneg CR: 44% |
CAR naïve 12-mos RFS: 86% Retreatment 12-mos RFS: 56% |
3 of 22 in CR (CAR naïve) |
| Anti-CD22 CARs | |||||||
| Shah et al.[63] NCT02315612 |
NCI 4–1BB |
1 | N = 58 Ages 3–30y |
CR: 70% MRDneg CR: 61% |
Median RFS: 6mos Median OS: 13mos |
12 of 35 in MRDneg CR | |
Abbreviations: B-ALL, B-precursor acute lymphoblastic leukemia; y, years; mos, months; CR, complete remission; MRD neg, minimal residual disease negative by multiparameter flow cytometry; RFS, relapse-free survival; EFS, event-free survival; OS, overall survival; alloHCT, allogeneic hematopoietic cell transplant; CHOP, Children’s Hospital of Philadelphia; Penn, University of Pennsylvania; NCI, The National Cancer Institute, Seattle, Seattle Children’s Research Institute; MSKCC, Memorial Sloan Kettering Cancer Center
Results presented in abstract form; not yet peer-reviewed
3.1. Murine-based CD19 CARs
3.1.1. Tisagenlecleucel
Tisagenlecleucel, formerly CTL019, is a second generation CD19 CAR developed by Novartis Pharmaceuticals in collaboration with Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania (Penn). It utilizes a lentiviral vector containing a DNA sequence encoding a murine anti-CD19 scFv receptor, which is linked to the CD3-zeta signaling domain of the TCR and a 4–1BB co-stimulatory domain [15,50].
The seminal trial of tisagenlecleucel, CART19, was a phase 1–2a single-arm, open-label study at CHOP and Penn (ClinicalTrials.gov NCT01626495 and NCT01029366). The initial manuscript reported outcomes of the first 30 patients infused, including 25 pediatric and 5 adult patients with relapsed/refractory CD19+ ALL [17]. Of those, 18 had relapsed disease after a prior alloHCT and 3 had disease refractory to blinatumomab. The complete remission (CR) rate, with or without complete hematologic recovery, at one month after infusion was 90%. Durable remissions were observed in many patients; the 6-month event-free survival (EFS) rate was 67% (95% CI, 51 to 88) and the 6-month overall survival (OS) rate was 78% (95% CI, 65 to 95). The probability of relapse-free B cell aplasia, an indication of tisagenlecleucel persistence, was 73% (95% CI, 57 to 94) at 6 months. During the follow-up period (median, 7 months), 7 of 27 patients who achieved a CR had a subsequent relapse; 4 were CD19-positive and 3 were CD19-negative. Seven patients died during the follow-up period, all due to disease progression or relapsed disease.
Updated results of the CART19 trial, which were presented in 2016, focused on 59 pediatric patients with a median follow-up of 12 months [51]. At one month after infusion, the CR rate was 93%, with 88% achieving a minimal residual disease (MRD)-negative state, defined as <0.01% bone marrow blasts by multiparameter flow cytometry. At 12-months, the relapse-free survival (RFS) rate was 76% (95% CI, 65 to 89) and the OS rate was 79% (95% CI, 69 to 91). Of the 20 relapses, 13 were associated with CD19-negative escape variants.
CART19 was followed by the ELIANA trial, a phase 2, single-cohort, 25-center, global study of tisagenlecleucel in pediatric and young adult patients (ages 3–23 years) with CD19+ ALL (ClinicalTrials.gov NCT02435849) [16]. The results of ELIANA were pivotal to the FDA approval of tisagenlecleucel [52]. The primary analysis included 75 infused patients with a median follow-up of 13.1 months after infusion. Forty-six (61%) patients had a prior alloHCT; the median bone marrow blasts at enrollment was 74%. Almost all patients (96%) received lymphodepleting therapy with fludarabine/ cyclophosphamide prior to infusion. By 3 months after infusion, the CR rate was 81% (95% CI, 71 to 89). All responders were negative for MRD. At 12-months, the RFS rate was 59% (95% CI, 41 to 73), the EFS rate was 50% (95% CI, 35 to 64) and the OS rate was 76% (95% CI, 63 to 86). The probability of maintenance of B cell aplasia at 6 months after infusion was 83% (95% CI, 69 to 91). Among the 22 patients who relapsed after achieving CR, 1 had a CD19-positive recurrence, 15 had CD19-negative recurrences, and 6 had unknown CD19 statuses. A total of 8 patients proceeded to alloHCT while in CR, including 2 with MRD-positive bone marrow and 2 with early B cell recovery.
3.1.2. The national cancer institute’s anti-CD19 CAR
The National Cancer Institute (NCI) conducted a phase 1, dose-escalation trial of a CD19 CAR in children and young adults (ages 1–30 years) with CD19+ B-ALL or non-Hodgkin lymphoma (ClinicalTrials.gov NCT01593696) [19]. Their CAR utilized a retroviral vector encoding an anti-CD19 scFv receptor linked to the CD3-zeta TCR and a CD28 costimulatory domain [53]. The initial manuscript reported outcomes of the first 21 enrolled and infused patients [19]. Eight (38%) had received a prior alloHCT; median bone marrow blasts were 26%. Among the 20 patients with B-ALL, the CR rate at one-month was 70% (95% CI, 46 to 88) and the MRD-negative CR rate was 60% (95% CI, 36 to 81). With a median follow-up of 10 months, the OS rate was 52%.
Updated results of the NCI trial were presented in 2016 and included 53 patients with a median follow up of 19 months [54]. All patients received lymphodepleting therapy prior to infusion, but multiple different regimens were used. Of the 51 patients with B-ALL, the CR rate was 70% and the MRD-negative CR rate was 55%. The RFS rate at 18 months was 50%. Of the 28 MRD-negative responders, 21 received a consolidative alloHCT and 8 relapsed during the follow-up period. The CD19 phenotype at relapse was positive in 2 patients, negative or dim in 5 patients, and unknown in 1 patient. The updated analyses also found that efficacy outcomes were most favorable among patients who received a fludarabine/cyclophosphamide lymphodepletion regimen, had low tumor burden, and underwent consolidative alloHCT.
3.1.3. Seattle children’s research institute’s anti-CD19 CAR
Seattle Children’s Research Institute conducted a phase 1/2 trial of a murine-based, anti-CD19 CAR, the PLAT-02 trial, in children and young adults (ages 1–26 years) with CD19+ B-ALL (ClinicalTrials.gov NCT02028455) [49]. Their product was engineered with a defined ratio of CD4+/CD8+ CAR T cells and contained a 4–1BB costimulatory domain. The primary analysis reported outcomes of 45 enrolled patients, of which 28 (62%) had a prior alloHCT, 6 (13%) received prior blinatumomab, and 22 (49%) had an M3 bone marrow. The rate of MRD-negative CR at day 21 was 89% in all 45 enrolled subjects and 93% among the 43 infused subjects. With a median follow-up time of 9.6 months, the estimated 12-month EFS rate was 51% (95% CI, 37 to 70), the estimated 12-month OS rate was 70% (95% CI, 56 to 87), and the median expected duration of B cell aplasia was 3 months (95% CI, 2.1 to 6.4). Of the 40 responders, 18 relapsed; 7 were CD19-negative and 11 were CD19-positive. Eleven patients underwent a consolidative alloHCT. CR rates were most favorable in the subset of patients who received fludararabine/cyclophosphamide lymphodepletion. Durability of remission was associated with longer duration of B cell aplasia, which correlated with higher CD19 antigen load.
3.1.4. Memorial Sloan Kettering cancer center’s anti-CD19 CAR
Memorial Sloan Kettering Cancer Center (MSKCC) recently published outcomes of their anti-CD19 CAR, which contains a CD28 costimulatory domain, in a phase 1 trial of children and young adults with CD19+ B-ALL (ClinicalTrials.gov NCT01860937) [48]. The product was previously tested in adult patients, but the recent report described its first use in a pediatric cohort [55,56]. A total of 25 patients (ages 1–25 years) were treated at MSKCC or the Dana-Farber Cancer Institute/Boston Children’s Hospital. All patients had detectable bone marrow disease before conditioning and received lymphodepletion with high- or low-dose cyclophosphamide with or without fludarabine. Five (20%) patients had a prior alloHCT. Of the 24 patients evaluable for response, the CR rate was 75% and the MRD-negative CR rate was 67%. Of patients who received high-dose cyclophosphamide, the CR rate was 94% (15 of 16) compared with 38% (3 of 8) among patients who received low-dose cyclophosphamide. Of the 18 responders, 83% (15 of 18) proceeded to alloHCT in remission. During the follow-up period (median, 29 months for responders), there were 6 relapses, including 4 after alloHCT. All relapses were CD19-positive. OS rates for the full cohort were not reported, but median OS ranged from 4.3 months to not reached in the different sub-cohorts. In addition to low-dose cyclophosphamide containing lymphodepletion regimens, higher disease burden was associated with worse outcomes.
3.2. Humanized CD19 CAR T cells
All the previously described clinical trials evaluated CD19 CARs that incorporated a murine scFv. A primary limitation of these CARs is poor persistence in subsets of patients, which can be the result of induction of transgene immunogenicity [57,58]. It has been hypothesized that using a humanized CAR design would prevent the risk of developing anti-murine CAR antibodies, thereby potentially improving persistence. In collaboration with Novartis Pharmaceuticals, the CHOP/Penn group developed a humanized anti-CD19 scFv domain attached to the CD3-zeta TCR and a 4–1BB costimulatory domain to produce CTL119.
CTL119 was evaluated in a phase 1, two-cohort trial of children and young adults (ages 2–24 years) with relapsed/refractory B-ALL or B-lymphoblastic lymphoma (ClinicalTrials.gov NCT02374333) [59]. The CAR retreatment and CAR-naïve cohorts were comprised of patients with or without prior exposure to a CD19 CAR T cell product, respectively. Preliminary results, which were presented in 2017, reported on 38 infused subjects, of which 22 (58%) had a prior alloHCT. All patients received lymphodepletion with fludarabine/cyclophosphamide. Among the 22 CAR naïve patients, the 1-month CR rate was 100% with all responders achieving an MRD-negative CR, and 14% proceeding to alloHCT in remission. With a median follow-up of 14 months, the 12-month RFS rate was 82% (95% CI, 58 to 93). There were 4 relapses during the follow-up period, of which 2 were CD19-positive and 2 were CD19-negative. Among the 16 CAR retreatment patients, the 1-month CR response rate, defined as morphologic remission with B cell aplasia, was 56%, with 44% also achieving an MRD-negative CR. With a median follow-up of 13 months, the 12-month RFS rate was 56% (95% CI, 20 to 80). Of the 26 patients with adequate follow-up, 15 had at least 6 months of B cell aplasia: 13 of 17 CAR naïve and 2 of 9 CAR retreatment patients.
Several groups from China have also reported results of early phase trials of humanized CD19 CARs. Southwest Hospital of Third Military Medical University (Chongqing, China) reported outcomes of 10 patients ages 5 to 40 years treated with a humanized CD19 CAR for B-ALL (ClinicalTrials.gov NCT02349698) [60]. None had received a prior CAR T cell product. The day 28 CR rate was 100%, with all responders achieving an MRD-negative CR and 2 (20%) proceeding to alloHCT in remission. Seven patients remained in remission for at least 12-months. Of the 2 relapses, 1 was CD19-positive and 1 was CD19-negative.
Xuzhou Medical University (Jiangsu, China) conducted a phase 1 trial of a humanized CD19 CAR in children and adults with B-ALL. Of the 18 enrolled patients, 3 had received a prior murine CD19 CAR [61]. Among the 14 evaluable CAR naïve patients, the day 28 CR rate was 93% and the MRD-negative CR rate was 79%. The 6-month RFS rate was 71% (95% CI, 41 to 88) and the OS rate was 66% (95% CI, 39 to 83). Of the 3 retreatment patients, one achieved an MRD-negative CR.
4. Clinical trials and outcomes of other CAR T cell products
4.1. CD22 CAR T cells
The most experience and success has been achieved with autologous CD19 CARs, but several additional products have recently been developed and tested. Since CD19 antigen loss is a frequent cause of resistance to CD19 CARs, but most patients retain CD22 expression, CD22 has emerged as an attractive antigen target. The NCI developed a CD22-targeted/4–1BB CAR T cell product and tested it in a phase 1 dose-escalation trial in children and young adults (ages 3–30 years) with CD22+ relapsed/refractory hematologic malignancies (ClinicalTrials.gov NCT02315612) [62,63]. The most updated published results reported on 58 infused patients, 56 of whom had B-ALL. Prior therapy exposures included CD19 CARs in 36 (62%), CD22 CARs in 5 (9%), inotuzumab in 14 (24%), and alloHCT in 39 (67%) patients. Of the 57 patients evaluable for response, the CR rate was 70% and the MRD-negative CR rate was 61%. Twelve patients proceeded to alloHCT while in MRD-negative CR. With a median follow-up of 24 months, the median RFS was 6.0 months (95% CI, 4.1 to 6.5) and the median OS was 13.4 months (95% CI, 7.7 to 20.3). Of the 40 responders, 30 (75%) relapsed, the majority with CD22-negative/dim disease. Exposure to prior CD22-targeted therapy was associated with worse efficacy. Receipt of a consolidative alloHCT was associated with more favorable outcomes.
The group at Beijing Boren Hospital (Beijing, China) also reported outcomes of their CD22-targeted/4–1BB CAR, which enrolled 34 children and adults (ages 1–55 years) with relapsed/refractory B-ALL, 31 (91%) of whom relapsed after a prior CD19 CAR (chictr.org.cn ChiCTR-OIC-17013523) [64]. Among the 30 patients evaluable for response, the day 30 CR rate was 80% and 37% of responders proceeded to alloHCT. The 1-year RFS rate was 58% (95% CI, 35 to 81) among all responders and 72% (95% CI, 44 to 99) among those who had a consolidative alloHCT. Unlike with the NCI’s experience, most relapsed patients were found to have intact CD22 expression.
4.2. Multi-antigen targeted CARs
Given high rates of relapse due to CD19 antigen escape with CD19 CARs and decreased CD22 site density with CD22 CARs, there is interest in developing multi-antigen targeted CARs. Several groups have reported early experiences with these products. Seattle Children’s reported preliminary outcomes of 7 children and young adults (ages 1–26 years) treated on PLAT-05, a phase 1 trial of a dual specific CD19 x CD22 CAR (ClinicalTrials.gov NCT03330691) for CD19+/CD22+ B-ALL [65]. Peak engraftment was predominantly composed of the CD19 CAR population; 5 of 7 patients (71%) engrafted, all of whom achieved a CR. Long-term efficacy data is not yet available. Beijing Boren Hospital reported outcomes of 20 pediatric patients (ages 1–16 years) with B-ALL enrolled on a phase 1 trial of sequential CD19/CD22 CAR T cell treatments (chictr.org.cn ChiCTR-OIB-17013670) [66]. Patients were first infused with a CD19 CAR, then with a CD22 CAR when CD19 CAR levels became undetectable in peripheral blood. The day 30 MRD-negative CR rate after the CD19 CAR was 100%; all remained in CR through the CD22 CAR infusion. The 1-year RFS and OS rates were 80% and 92%, respectively. Of the 3 relapses, 2 had CD19 antigen loss and 1 had CD22 downregulation. Updated results of ongoing trials will be needed to establish the impact of these approaches on long-term outcomes.
4.3. Universal anti-CD19 CARs
In addition to the risk of relapse, currently available CARs are limited by the need for sufficient numbers and quality of autologous T cells, long manufacturing times and occasional manufacturing failures. Thus, there is considerable interest in developing off-the-shelf, allogeneic CAR T cell products. Servier Pharmaceuticals is currently testing UCART19, a lentiviral-transduced anti-CD19 scFv/4–1BB/CD3-zeta CAR manufactured from non-HLA matched healthy donors, in phase 1 studies of pediatric and adult patients with CD19+ B-ALL (ClinicalTrials.gov NCT02808442 and NCT02746952) [67]. The CAR is modified to disrupt the T cell receptor alpha constant (TRAC) and CD52 genes in order to allow infusion into non-HLA matched patients. In addition, it contains an RQR8 safety switch to allow for elimination of RQR8+ cells by rituximab. Preliminary results were presented in 2017 and 2018 [68,69]. The most recent results pooled data from the pediatric (n=7) and adult (n=13) studies. Of the 16 patients evaluable for response, the CR rate was 88%, the MRD-negative CR rate was 75%, and 69% proceeded to alloHCT in remission. With a follow-up of up to 16 months, 5 of 12 patients who achieved an MRD-negative CR remained in remission. These early data show promising response rates, but further follow-up in a larger cohort is needed to determine durability of remissions.
5. Toxicity
5.1. Overview and grading systems
Side-effects of CAR T cells can be varied, but some features are distinct among cancer therapies. Two of the most defining side effects from CD19-targeted CAR T cells are CRS and neurotoxicity. CRS was described in the first pediatric patients treated with anti-CD19 CARs [18]. CRS is not unique to CAR T cells as it has been described in other targeted therapies that engage T cells such as blinatumomab [70]. However, the prevalence and severity after CAR administration is often greater.
Current studies report between 54–90% of patients develop CRS in the first two weeks following infusion. Severe CRS is reported between 8.3–43% in the same cohorts [16,19,49,71]. Reporting of toxicity on early phase studies of CAR T cells used Common Terminology Criteria for Adverse Events (CTCAE) grading of individual organ systems. As CRS and neurotoxicity were recognized as unique entities, several tools were developed to better capture toxicity related to CRS. The Lee criteria and Penn Criteria were two early tools used to capture toxicity specifically related to CRS [23,72]. More recently, the American Society for Transplantation and Cellular Therapy (ASTCT) made consensus recommendations for grading CRS based on the presence of fever and the degree of hypotension and hypoxia [22]. A comparison of these grading systems is presented in Table 2. The ASTCT guidelines also provide recommendations for grading neurotoxicity. The proposed scoring system for neurotoxicity involves evaluating encephalopathy using orientation, naming, ability to follow commands, writing and attention. The encephalopathy score is then combined with separate evaluations of the level of conscious, presence of seizures, motor abnormalities and elevations in ICP or imaging concerning for cerebral edema [22].
Table 2.
Cytokine Release Syndrome Grading Scales
| Grading System | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| CTCAE v5 | Fever, with or without constitutional symptoms | Hypotension: fluid responsive Hypoxia requiring <40% FiO2 |
Hypotension: requiring one vasoactive Hypoxia requiring >40% FiO2 |
Life-threatening consequences requiring urgent intervention |
| CHOP/Penn | Mild symptoms: treated with supportive care measures | Moderate symptoms with some signs of organ dysfunction not attributable to other causes. (Grade 2 creatinine or Grade 3 ALT/AST) Hospitalization for management of CRS symptoms and IV therapy (not including fluid resuscitation) |
Severe symptoms with increased organ dysfunction not attributable to other causes. (Grade 3 creatinine or grade 4 ALT/AST, Coagulopathy requiring fresh frozen plasma or cryoprecipitate) Hypotension requiring multiple fluid boluses or low dose vasoactives Hypoxia requiring supplemental oxygen (including high-flow, CPAP or BiPAP) |
Life threatening complications requiring high dose vasoactives Hypoxia that requires mechanical ventilation |
| Lee Criteria | Symptoms that require only supportive care measures | Symptoms that respond to moderate interventions: Grade 2 organ dysfunction, Hypotension responsive to fluids or a single vasoactive, Hypoxia responsive to <40% supplemental O2 |
Increasing symptomatology: Grade 3 organ toxicity (or Grade 4 ALT/AST), Hypotension necessitating multiple vasoactives, Hypoxia necessitating O2 supplementation (>40%) |
Life-threatening symptoms: Grade 4 organ toxicity (excluding elevated transaminases), Hypoxia requiring mechanical respiratory support |
| ASTCT | Temp ≥ 38C with: No hypotension No hypoxia |
Temp ≥ 38C with: Hypotension not requiring vasoactives and/or: Hypoxia requiring low-flow nasal canula or blow-by |
Temp ≥ 38C with: Hypotension requiring 1 vasoactive (+/- vasopressin) and/or: Hypoxia requiring high flow nasal canula, facemask, nonrebreather mask or Venturi mask |
Temp ≥ 38C with: Hypotension requiring multiple vasoactives and/or: Hypoxia requiring positive pressure (including CPAP, BiPAP and mechanical ventilation) |
Abbreviations: CTCAEv5, Common Terminology Criteria for Adverse Events v5.0; CHOP, Children’s Hospital of Philadelphia; Penn, University of Pennsylvania; ASTCT, American Society for Transplantation and Cellular Therapy; Temp, temperature
5.2. Cytokine release syndrome
Physiologically, CRS parallels hemophagocytic lymphohistiocytosis (HLH) or macrophage activation syndrome (MAS). CRS is characterized by markedly elevated ferritin levels often over 10,000 mg/dL which has been shown to be sensitive and specific for MAS/HLH [73]. Other markers of generalized inflammations such as alanine aminotransferase, aspartate aminotransferase, blood urea nitrogen, creatinine and CRP are also elevated [74]. There is disagreement if a rapidly rising CRP is predictive of the severity of CRS, but patients who develop severe CRS tend to have higher ferritin levels along with several other cytokines that form a unique signature [55,74].
Analysis of cytokine profiles in patients with severe CRS (defined as grade 4–5) following tisagenlecleucel infusion at CHOP showed elevation in numerous cytokines including IL6, IL8, IFN gamma, sIL2r alpha, and sgp130 [74]. Patients who develop severe CRS had higher levels of IL6, ferritin, CRP, IFN gamma and soluble IL-2 receptor compared to patients who did not develop severe CRS [17]. Gardner et al. published a similar cytokine profile with elevation in GMCSF, TNF-a, IFN gamma, IL-6, IL-2, IL-10, IL-5, soluble FAS and Granzyme B in patients who developed severe CRS following SCRI-CAR19v1 infusion [75]. These studies represent 4–1BB based CARs but other groups show similar cytokine profiles. Lee et al. also reported elevations in IL-6, IL-10, IFN gamma, GMCSF using a CD28 CAR and Neelapu et al. showed elevations in IL-6, IL-15 and soluble IL-2 in patients with Grade 3 or higher CRS following axicabtagene in adults with large B cell lymphoma [19,76].
In addition, to predicting severity of CRS, cytokines have also been targeted for therapeutic interventions. Blockade of IL6 using tocilizumab has been shown to be effective in management of patients with severe CRS [18,55]. Tocilizumab has a historic precedent being used to treat the first pediatric patient infused with tisagenlecleucel [18]. The success saw an increased clinical use to treat patients with CRS following CTL019 and KTE-C19 and led to FDA label indication [52]. However, its use was generally restricted to patients with severe CRS due to concerns that using an immunomodulatory medicine may impair proliferation leading to an adverse impact on remission and/or persistence. A recent publication from Seattle Children’s demonstrated that administration of tocilizumab and corticosteroids for persistent, mild symptoms after receipt of an anti-CD19 CAR reduced the progression to severe CRS without increasing rates of resistance or relapse [75]. Similarly, preliminary data from CHOP reported that preemptive administration of tocilizumab after tisagenlecleucel to patients with high-tumor burden decreased the incidence of grade 4 CRS without compromising antitumor efficacy [77,78].
5.3. Hematologic, infectious and specific organ toxicities
Clinically, the release of cytokines triggers systemic inflammation that typically manifests as fever and tachycardia. In cases of severe CRS, patients can go on to develop signs of organ related toxicity.
Hematologic toxicity includes the expected on-target effect of producing B cell aplasia [17,18,75]. B cell aplasia is expected in all patients who respond to CAR therapy directed against CD19 or CD22. Longer term follow up shows that duration of B cell aplasia can be variable depending on the construct used to produce the CAR, but ranges from 3–6 months for about 50% of patients and can persist for years in some patients [16,75]. Grade 3–4 pancytopenia is seen in virtually all patients receiving a CD19-targeted CAR T. Initial cytopenia is generally indistinguishable from hematologic toxicity seen from lymphodepleting chemotherapy. However, patients with severe CRS can have cytopenias that persist beyond the duration from chemotherapy alone [16,17,79]. Persistent cytopenias may be related to marrow suppression due to inflammation during CRS.
In addition to cytopenias, patients who receive anti-CD19 CARs can develop coagulopathy. The prothrombin and partial prothrombin time can lengthen and d-dimer elevation is common [17–19]. During initial CRS, there is usually an increase in fibrinogen followed by a period of hypofibrinogenemia [17,18]. The hypofibrinogenemia is more pronounced than PT/PTT rise, similar to HLH.
Infectious complications are frequently seen and likely associated with the hematologic toxicity of neutropenia. The study of CTLO19 leading to FDA approval reported Grade 3 infections in 13% of patients with bacterial sources infections, 18% with viral sources and 16% with an unspecified source of infection [20]. Grade 3–4 infections were seen in 10% of patients using a CD22 directed CAR [62]. Early reports suggest that patients receiving a universal CAR had higher rates of infection at 44% [68]. This may be due to the heavily pretreated nature of patients on this trial and/or the addition of alemtuzumab to lymphodepleting chemotherapy. All authors note increased morbidity or mortality was seen in patients that had prolonged neutropenia.
There is theoretical concern that persistent B-cell aplasia may lead to a long-term increase in infectious complications. However, there is little longitudinal data to understand the relationship between persistent B-cell aplasia and susceptibility to infections. Chronic intravenous immunoglobulin replacement following CAR T infusion is common is pediatric practice where it is not standardly used by adult centers.
Cardiovascular toxicity commonly includes tachycardia that is concurrent with fever. In severe CRS, Grade 3–4 hypotension was reported in 22–38% of patients with leukemia or lymphoma [17,19,55,80]. Severe CRS has also been associated with a decrease in left ventricular function that is typically reversible [19,79,81].
Pulmonary toxicity caused by CRS generally manifests as hypoxia and edema thought to be due to capillary leak. Respiratory support can range from supplemental nasal cannula to mechanical ventilation [19,55,74,82]. Rates of grade 3–4 hypoxia have been reported in around 10% of patients who received CAR T cells, [19,55,79,80] but is likely higher in patients with higher levels of disease immediately prior to infusion of CAR cells.
Renal injury with elevated creatinine and electrolyte disturbances can be seen after CAR administration [19,55,81]. The mechanism of acute renal injury likely stems from hypoperfusion due to vasodilation and/or impaired cardiac output. Acute kidney injury is thought to be reversible in most cases.
Liver and GI toxicity is generally self-limited. Elevation in transaminases often accompanies CRS [18,19,83]. No specific complains of nausea and GI discomfort have also been regularly reported, but it is unclear if these symptoms are related to the CAR T cells vs the systemic inflammation produced during CRS.
5.4. Neurotoxicity
Neurotoxicity related to anti-CD19 CARs exists on a spectrum. Symptoms are generally global and do not localize to a specific region of the brain. The constellation of symptoms related to CAR administration are now being recognized as Immune Effector Cell-associated Neurotoxicity Syndrome (ICANS). The pathogenesis of ICANS following CAR T cells is unknown but it is thought to be related to elevation of cytokines and T cell infiltration in the CNS. It may be similar but more severe than the global encephalopathy that has been seen with exogenous IL-2 administration and blinatumomab, which can also cause symptoms of CRS, encephalopathy, aphasia and tremor [84,85].
Clinically, symptoms of ICANS appear to correlate with the pre-treatment disease burden as well as the severity of CRS [86,87]. The most common findings are delirium with transient impairment of cognition and attention [16,76,80]. Headaches are also common and may accompany delirium but there is movement away from grouping them with neurotoxicity as they are often non-specific. An expressive or global aphasia can also be seen and, in an adult study, was the most common feature of severe neurotoxicity [86]. Other symptoms can include tremor and somnolence. Seizures are relatively rare but reported in 3–4% of pediatric patients who received a CD19 directed CAR and 8% in the largest studied adult cohort [16,17,49,87]. No seizures were reported in the anti-CD22 directed studies from the NCI but have been described in other CD22-directed CAR studies [63,64,88]. Several groups report using prophylactic anti-epileptics in patients thought to be at high risk for neurotoxicity but no formal studies have compared use of prophylactic anti-epileptics. In most cases, seizures are effectively managed with benzodiazepine rescue. Cerebral edema is the most serious side effect of ICANS and can be fatal. The majority of reported deaths related to cerebral edema have been in adult literature.
Biologically, the cytokine profile reported with severe ICANS is similar to that of CRS with elevations seen in IL-6, IFN gamma, IL-2, IL-10, IL-15 and MCP1 [87]. This group also reported an earlier peak in serum IL-6 was associated with higher risk of severe ICANS. Additional elevations seen with endothelial activation were seen in severe ICANS suggesting that endothelial activation may play a role in the pathogenesis related to vascular leak and disseminated intravascular coagulation in patients with severe CRS [87].
5.5. Unintended on-target toxicity
It is important to note that the on-target toxicity from CAR T cells can be expected. However, if the antigen recognized by CART cells is expressed on non-tumor tissue there can be considerable off-target toxicity. Examples include grade 3–4 elevation in liver transaminases following infusion with a carboxy-anhydrase-IX directed CAR in patients with metastatic renal cell carcinoma where bile duct epithelial cells also express carboxy-anhydrase-IX [89,90]. In another study, a patient who was infused with ERBB2 CAR T cells for metastatic colorectal cancer developed pulmonary edema and it was hypothesized related to ERBB2 expression in lung tissue [91]. These are important lessons as new CARs are developed.
6. Resistance and relapse
As highlighted previously in this review, remission rates across different CD19 CAR T cell trials and products are strikingly high. However, 25–50% of patients who achieve a CR subsequently relapse in the first 12–24 months after infusion [16,17,49,54]. Disease recurrences can be CD19-positive or CD19-negative, indicating different mechanisms of relapse, but both are difficult to treat. In addition, there is still a small proportion of patients who fail to achieve an initial remission after infusion.
6.1. Resistance
Approximately 10–20% of patients infused with CD19 CARs do not achieve a CR [16,17,49,54]. There are multiple potential etiologies of poor responses. A poor-quality product can be manufactured if the quantity or quality of T cells collected from the patient is insufficient. This is often due to lymphopenia from prior cytotoxic therapy [92]. Additionally, previous treatment with certain chemotherapies such as clofarabine or doxorubicin has been associated with generation of suboptimal products [19,93]. Other factors that may impact product quality include methods of T cell expansion, the starting T cell phenotype, and purity of the apheresis product [88,92,94]. Both CD4+ and CD8+ T cells are used to manufacture CARs. Efficacy of the product may be impacted by the ratio of CD4+ to CD8+ T cells. Several trials have, thus, used a defined ratio to manufacture their product [49,71]. While the products have yielded high response rates, the extent to which this technique influences outcomes has not been determined.
In addition to the quality of the manufactured product, patient-related factors contribute to CAR T cell activation and expansion, and in turn, the likelihood of achieving a response. Disease and antigen burden can impact cell expansion [49]. The results of several clinical trials highlight the potential contribution of the lymphodepletion regimen to cell expansion, but the most efficacious regimen and dosages are not yet clear [48].
For patients who only have a poor-quality CAR T cell product generated or do not respond to the product, an attractive alternative may be a universal, allogeneic product. The early clinical experience with UCART19 in pediatric patients was discussed in section 4.3.
6.2. Antigen-positive relapse
Antigen-positive relapses are generally associated with limited CAR T cell persistence, indicated by loss of B cell aplasia, which suggest loss of active leukemia surveillance by the CAR T cells [92]. Factors that influence persistence have not been fully elucidated, but include T cell quality and CAR design. Specifically, the co-stimulatory domain has been associated with duration of persistence. Both pre-clinical and clinical trial data have demonstrated that CAR products containing a 4–1BB costimulatory domain persistent longer than those containing a CD28 costimulatory domain [16,56,95]. Additionally, patient-related factors such as tumor or antigen burden likely impact persistence. Research efforts are currently underway to better understand mechanisms of persistence and, in turn, to develop CAR T cell designs that optimize persistence.
Multiple strategies to improve persistence or treat antigen-positive relapse are currently under clinical investigation. Humanized CD19 CARs are being tested by several groups, based on the hypothesis that a humanized anti-CD19 scFv domain may be able to overcome anti-mouse reactivity that can contribute to immune-mediated rejection with murine-based products [59,60,61]. Early results from a phase 1 trial of CTL119, a humanized CD19 CAR, were reviewed in section 3.3. In CAR naïve patients, high rates of RFS were reported and most patients had at least 6 months of relapse-free B cell aplasia. In addition, complete responses were observed in many patients who lost persistence or relapsed after receiving a CD19 CAR. A phase 2 trial is currently underway (ClinicalTrials.gov NCT03792633).
Other strategies being investigated include re-infusion of CAR T cells, combining CAR T cell infusion with immune-checkpoint inhibitors, and administration of T cell-antigen-presenting cells (T-APCs). Re-infusions have been administered to some patients with loss of B cell aplasia or antigen positive relapse. Only limited success has been reported in the literature, [19,49] but our large CAR T cell program has observed that many patients can re-establish B cell aplasia or remission (data not yet published). There has also been interest in augmenting CAR T infusions with immune-checkpoint inhibitors to potentially decrease T cell exhaustion and improve persistence. Early data from 14 patients at CHOP who received pembrolizumab, a PD1 inhibitor, after CD19 CAR infusion due to repeated early CAR T cell loss or lack of response to CAR T cells were presented in 2018 [96]. The data showed promising results; 3 of 6 patients with early B cell recovery re-established B cell aplasia for 5 to 15 months, 4 of 4 patients with bulky extramedullary disease who were unresponsive or relapsed after CAR T cells alone had partial or complete responses, but 0 of 4 patients who failed initial CAR infusion alone achieved CRs with the addition of pembrolizumab. Finally, Seattle Children’s is conducting a trial of repeated administration of T-APCs after remission induction to determine if recurrent stimulation can prevent antigen-positive relapses (ClinicalTrials.gov NCT03186118) [97,98].
6.3. Antigen-negative relapse
Target antigen downregulation or escape is a common mechanism of relapse after CAR T cell therapy, and has also been described after other targeted immunotherapies, such as bispecific T cell-engager antibodies [99,100]. In trials of tisagenlecleucel, for example, CD19-negative variants were observed 13 of 20 relapses on the phase 1 study and 15 of 22 relapses on the global, phase 2 study [16,51]. Risk factors for antigen-negative relapses have not been well-elucidated, but this is an active area of investigation. It has been found that some patients have pre-existent antigen-negative subclones, which likely predispose to antigen-escape [101]. Patients with high-tumor burden prior to CAR infusion may have larger clonal heterogeneity, which could increase the risk of having a CD19-negative relapse [102]. Indeed, some trials have found higher tumor burden to be associated with higher relapse rates [48,56]. Prior treatment with other antigen-targeted therapies may also increase the risk of antigen-negative relapses after CAR T cells. For example, prior blinatumomab exposure has been associated with higher risk of antigen escape after tisagenlecleucel infusion [103].
Another mechanism of antigen escape is lineage switching. Most reports of lineage switch after CD19 CARs have described infants and older patients with KMT2A-rearranged B-ALL, who relapsed with acute myeloid leukemia [104]. However, lineage switch has also been reported in other leukemia subtypes and the risk of lineage switch with KMT2A-rearranged B-ALL has not been quantified [105].
The primary strategy under investigation to prevent CD19-negative relapses is the development of multi-antigen targeted CAR constructs. There are pre-clinical data to support multi-targeted approaches, and multiple trials are now underway to assess their clinical efficacy [106]. A number of these trials incorporate CD19 and CD22, either by using a single CAR construct targeting both antigens or two unique CARs targeting each antigen individually [92]. Early data have been presented by a few groups (see section 4.2) with some promising results, but follow-up data is needed to determine the effectiveness of these approaches [65,66]. Treatment of CD19-negative relapses is challenging, but there may be a role for multi-antigen targeted CARs. In addition, single-antigen targeted CD22 CARs have produced clinical responses in patients with CD19-negative recurrences after anti-CD19 CARs, but their long-term efficacy has been suboptimal thus far (see section 4.1) [62,63,64].
7. Conclusion
CAR T cell therapy has transformed the landscape of treatment for relapsed/refractory B-ALL. CD19-targeted CARs, in particular, have led to CR rates of up to 90% in children and young adults who previously would have had extremely limited curative options. Many patients have also subsequently achieved durable remissions without further therapies. With the FDA and EMA approvals of tisagenlecleucel, there is now greatly expanded access to this therapy. Notwithstanding the remarkable successes of CAR T cells, significant challenges and opportunities remain. Treatment-related toxicities can be severe, some patients develop resistance, and up to 25–50% of patients relapse after achieving a CR. To address these limitations, there are multiple new CAR constructs under clinical investigation as well as other research efforts aimed at improving toxicity and understanding, preventing and treating relapse. As the field continues to rapidly advance, the full potential of CAR T cell therapy will be able to be optimized.
8. Expert opinion
Adoptive T cell therapy, most notably CD19-targeted CAR T cell therapy, has demonstrated remarkable antitumor efficacy in pediatric and young adult relapsed/refractory B-ALL. For high-risk patients who previously had very limited treatment options with dismal outcomes, anti-CD19 CARs have been transformative. Multiple products tested across multiple trials have shown similarly high complete response rates. Furthermore, many children and young adults have also achieved durable remissions. Despite its successes, however, significant challenges and opportunities remain to harness the full potential of this therapy.
Expanding access to CAR T cells represents both a limitation and an opportunity. With the FDA and EMA approvals of tisagenlecleucel, the therapy became more widely available. There are now over 50 pediatric centers in the United States and additional centers internationally that have tisagenlecleucel available. However, there are many patients who still need to travel long distances to receive tisagenlecleucel or another CAR product. In addition, tisagenlecleucel is a complex therapy that requires teams to have high levels of expertise and resources in order deliver it safely. Appropriate supports and collaborations are needed to facilitate expansion to a broader group of centers.
Given the success of CAR T cells in the relapsed/refractory disease setting, testing its efficacy earlier in therapy and for new indications is clearly of interest. The Children’s Oncology Group and Novartis are currently conducting a collaborative phase 2 trial, AALL1721/Cassiopeia, that incorporates tisagenlecleucel into frontline treatment for B-ALL. (ClinicalTrials.gov NCT03876769) [107]. Specifically, the trial is testing tisagenlecleucel as definitive therapy in a subset of very high risk pediatric patients, who historically had poor disease free survival with standard chemotherapy. A phase 2 trial at CHOP is testing tisagenlecleucel in children and young adults with central nervous system (CNS) relapsed B-ALL (ClinicalTrials.gov NCT04276870). Although patients with active CNS disease have typically been excluded from CAR T cell clinical trials, there are data demonstrating that CD19 CARs are effective at clearing CNS disease [108,109]. The current trial will evaluate if tisagenlecleucel can achieve durable remissions in children with CNS-relapsed ALL, thereby also avoiding cranial irradiation in a population particularly vulnerable to toxicities.
As previously discussed, CAR T cells are associated with unique and potentially severe toxicities. Evaluation and management of CRS and neurotoxicity continue to be significant challenges. Emerging data from multiple groups has shown that tocilizumab reduces the rate of progression to severe CRS [75,77]. However, direct comparisons of toxicities between trials remain challenging because of the heterogeneous nature of reporting and grading systems. Implementation of the ASTCT consensus guidelines will be helpful to compare trial outcomes in subsequent studies [22]. Similarly, understanding the rates and severity of neurotoxicity will potentially become more uniform with the adaptation of the ASTCT guidelines for ICANS grading. More work is also being done to understand the pathogenesis for neurotoxicity. This may lead to an expansion of treatment options beyond corticosteroids.
Undoubtedly, prevention and management of relapsed disease after CD19 CARs are the main, ongoing challenges of this therapy. Multiple strategies are currently under clinical investigation, many of which were discussed earlier in this review. One strategy for relapse prevention that has been questioned since the earliest CAR T cell trials is consolidation with an alloHCT. However, this question remains unanswered. No trials have randomly allocated patients to transplant versus no transplant. Furthermore, the extant literature has reported mixed results about the role of transplant. In CART19 and ELIANA, the tisagenlecleucel trials, less than 15% of patients underwent a consolidative alloHCT, but many achieved durable remissions and years of CAR T cell persistence [16,51]. Conversely, the NCI reported that consolidative alloHCT was associated with improved outcomes in their anti-CD19 CAR trial [54]. The relapse rates of patients who did and did not receive consolidative transplants were 85.7% and 9.5%, respectively (p<.001). Further complicating the decision is the fact that many patients have received an alloHCT prior to CAR therapy, and second or greater transplants are very high risk procedures. Ultimately, there will not be one answer to the question about consolidative transplants. Rather, the decision will need to be dependent on CAR product, patient-specific factors, and length of CAR T cell persistence.
A very active area of research aimed at relapse prevention/treatment is the development of multi-antigen targeted CARs. These approaches are especially geared towards antigen-negative relapses, with preclinical data to support them [106]. Early clinical experiences have been presented by several groups with some promising results, but follow-up data are needed to assess the effectiveness of multi-antigen targeted products [65,66]. To prevent and treat antigen-positive relapses, strategies under investigation include new CAR constructs such as humanized CD19 CARs, treatment with immune-checkpoint inhibitors in combination with CARs, and administration of T-APCs. Again, though early data has shown some promising results, additional data is needed to determine the effectiveness of these approaches.
In summary, CAR T cell therapy has had a profound impact on the landscape of treatment for pediatric and young adult relapsed/refractory B-ALL. Though challenges remain, the abundance of ongoing clinical investigations will be crucial to both optimizing the full potential of this therapy and informing its widespread usage.
Article highlights.
CD19-targeted chimeric antigen receptor (CAR) T cell therapy has demonstrated remarkable anti-tumor efficacy in pediatric and young adult relapsed/refractory B cell acute lymphoblastic leukemia (B-ALL).
Tisagenlecleucel, an anti-CD19 CAR, was the first cellular therapy product to receive FDA approval. Its commercialization has led to more widespread usage internationally.
Thus far, the most success has been achieved with murine-based, anti-CD19 CARs, but many new CAR constructs are currently under development and/or under clinical investigation.
Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) are the most common severe toxicities associated with CAR T cells. Tocilizumab, an antihuman IL-6 receptor antibody, is the cornerstone of severe CRS management, but optimal timing of administration has not been fully determined. Less is understood about mechanisms and optimal management of ICANS.
In order to optimize our understanding and management of treatment-related toxicities, it is critical that consistent grading systems are used across future clinical trials. The American Society for Transplantation and Cellular Therapy (ASTCT) consensus guidelines, which were recently published, will be very helpful in this regard.
Though anti-CD19 CARs achieve striking response rates, 25–50% of patients relapse within two years after infusion. Preclinical and clinical investigations focused on improving CAR T cell persistence, understanding mechanisms of relapse, and developing strategies for antigen-positive and antigen-negative relapse prevention/treatment are crucial to addressing this challenge.
Current and future research efforts aimed at testing anti-CD19 CARs earlier in therapy and for new indications are key to informing broader usage of these therapies.
Acknowledgments
Funding
This paper was not funded
Declaration of interest
RM Myers is supported by NIH K12-CA-076931-21 and Alex’s Lemonade Stand Foundation. DT Teachey is supported by the CHOP Frontiers Program Immune Dysregulation Team. NIH/NCI R01CA193776 and X01HD1007, Hematologic Malignancy ITSC (5UG1CA233249), Leukemia and Lymphoma Society TRP and SCOR, Cookies for Kids Cancer, Alex’s Lemonade Stand Foundation, Children’s Oncology Group Foundation, and Stand UP 2 Cancer. D Teachey serves on advisory boards for Amgen, LaRoche, Janssen, and Sobi. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers:
- [1].Ward E, DeSantis C, Robbins A, et al. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83–103. [DOI] [PubMed] [Google Scholar]
- [2].Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N. Engl. J. Med. Massachussetts Medical Society; 2015. p. 1541–1552. [DOI] [PubMed] [Google Scholar]
- [3].Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children’s oncology group. J Clin Oncol. 2012;30:1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: A Children’s Oncology Group study. Leukemia. 2008;22:2142–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bhojwani D, Pui CH. Relapsed childhood acute lymphoblastic leukaemia. Lancet Oncol. Lancet Publishing Group; 2013. p. e205–e217. [DOI] [PubMed] [Google Scholar]
- [6].Bailey LC, Lange BJ, Rheingold SR, et al. Bone-marrow relapse in paediatric acute lymphoblastic leukaemia. Lancet Oncol. Lancet Oncol; 2008. p. 873–883. [DOI] [PubMed] [Google Scholar]
- [7].Schrappe M, Hunger SP, Pui CH, et al. Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med. 2012;366:1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Raetz EA, Bhatla T. Where Do We Stand in the Treatment of Relapsed Acute Lymphoblastic Leukemia? Hematol Am Soc Hematol Educ Progr. 2012;2012. [DOI] [PubMed] [Google Scholar]
- [9].Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: Challenges for the twenty-first century. J Clin Oncol. 2010;28:2625–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Smith MA, Altekruse SF, Adamson PC, et al. Declining childhood and adolescent cancer mortality. Cancer. 2014;120:2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pulte ED, Vallejo J, Przepiorka D, et al. FDA Supplemental Approval: Blinatumomab for Treatment of Relapsed and Refractory Precursor B-Cell Acute Lymphoblastic Leukemia. Oncologist. 2018;23:1366–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lamb YN. Inotuzumab Ozogamicin: First Global Approval. Drugs. 2017;77:1603–1610. [DOI] [PubMed] [Google Scholar]
- [13].Frey NV Chimeric antigen receptor T cells for acute lymphoblastic leukemia. Am. J. Hematol. Wiley-Liss Inc.; 2019. p. S24–S27. [DOI] [PubMed] [Google Scholar]
- [14].Maude SL. Future directions in chimeric antigen receptor T cell therapy. Curr Opin Pediatr. 2017;29:27–33. [DOI] [PubMed] [Google Scholar]
- [15].Leahy AB, Elgarten CW, Grupp SA, et al. Tisagenlecleucel for the treatment of B-cell acute lymphoblastic leukemia. Expert Rev Anticancer Ther. 2018;18:959–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378:439–448.**The phase 2, 25-center, global registration trial of tisagenlecleucel in children and young adults with relapsed/refractroy CD19+ ALL that led to FDA approval of tisagenlecleucel
- [17].Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517.*Initial results from the seminal phase 1–2a trial of CTL019/tisagenlecleucel in 25 children and 5 adults at CHOP/Penn with CD19+ relapsed/refractory B-ALL
- [18].Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet. 2015;385:517–528.*Initial results from NCI’s phase 1 trial of an anti-CD19/CD28 CAR for children and young adults with relapsed/refractory CD19+ B-ALL or non-Hodgkin lymphoma
- [20].FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell-Induced Severe or Life-Threatening Cytokine Release Syndrome. - PubMed - NCBI [Internet]. [cited 2020 Mar 8]. Available from: https://www-ncbi-nlm-nih-gov.proxy.library.upenn.edu/pubmed/29622697. [DOI] [PMC free article] [PubMed]
- [21].Ali S, Kjeken R, Niederlaender C, et al. The European Medicines Agency Review of Kymriah (Tisagenlecleucel) for the Treatment of Acute Lymphoblastic Leukemia and Diffuse Large B-Cell Lymphoma. Oncologist. 2020;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. Elsevier Inc.; 2019. p. 625–638.**Includes a review of historic grading systems for CRS and ICANS as well as a proposal of new consensus guidelines to promote consistent reporting and allow for comparisons of toxicities across future CAR trials
- [23].Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yron I, Wood TA, Spiess PJ, et al. In vitro growth of murine T cells. V. The isolation and growth of lymphoid cells infiltrating syngeneic solid tumors. J Immunol. 1980;125:238–245. [PubMed] [Google Scholar]
- [25].Rosenberg SA, Packard BS, Aebersold PM, et al. Use of Tumor-Infiltrating Lymphocytes and Interleukin-2 in the Immunotherapy of Patients with Metastatic Melanoma. N Engl J Med. 1988;319:1676–1680. [DOI] [PubMed] [Google Scholar]
- [26].Kolb H, Mittermuller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–2465. [PubMed] [Google Scholar]
- [27].Dembić Z, Haas W, Weiss S, et al. Transfer of specificity by murine α and β T-cell receptor genes. Nature. 1986;320:232–238. [DOI] [PubMed] [Google Scholar]
- [28].Clay TM, Custer MC, Sachs J, et al. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol. 1999;163:507–513. [PubMed] [Google Scholar]
- [29].Letourneur F, Klausner RD. T-cell and basophil activation through the cytoplasmic tail of T-cell-receptor ζ family proteins. Proc Natl Acad Sci U S A. 1991;88:8905–8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor ζ chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64:891–901. [DOI] [PubMed] [Google Scholar]
- [31].Eshhar Z, Waks T, Gross G, et al. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the γ or ζ subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brocker T, Peter A, Traunecker A, et al. New simplified molecular design for functional T cell receptor. Eur J Immunol. 1993;23:1435–1439. [DOI] [PubMed] [Google Scholar]
- [33].Brocker T, Karjalainen K. Signals through T cell receptor-ζ chain alone are insufficient to prime resting T lymphocytes. J Exp Med. 1995;181:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gong MC, Latouche JB, Krause A, et al. Cancer Patient T Cells Genetically Targeted to Prostate-Specific Membrane Antigen Specifically Lyse Prostate Cancer Cells and Release Cytokines in Response to Prostate-Specific Membrane Antigen. Neoplasia. 1999;1:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Krause A, Guo HF, Latouche JB, et al. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. J Exp Med. 1998;188:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hombach A, Wieczarkowiecz A, Marquardt T, et al. Tumor-Specific T Cell Activation by Recombinant Immunoreceptors: CD3ζ Signaling and CD28 Costimulation Are Simultaneously Required for Efficient IL-2 Secretion and Can Be Integrated Into One Combined CD28/CD3ζ Signaling Receptor Molecule. J Immunol. 2001;167:6123–6131. [DOI] [PubMed] [Google Scholar]
- [37].Maher J, Brentjens RJ, Gunset G, et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nat Biotechnol. 2002;20:70–75. [DOI] [PubMed] [Google Scholar]
- [38].Van Der Stegen SJC, Hamieh M, Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat. Rev. Drug Discov. Nature Publishing Group; 2015. p. 499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hudecek M, Sommermeyer D, Kosasih PL, et al. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res. 2015;3:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hudecek M, Kosasih PL, Sommermeyer D, et al. Receptor Affinity and Extracellular Domain Modifications Affect Tumor Recognition by ROR1-Specific Chimeric Antigen Receptor T Cells. 2013;1:3153–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Liu X, Jiang S, Fang C, et al. Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res. 2015;75:3596–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Drent E, Poels R, Ruiter R, et al. Combined CD28 and 4–1BB costimulation potentiates affinity-tuned chimeric antigen receptor-engineered t cells. Clin Cancer Res. 2019;25:4014–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Engels B, Chervin AS, Sant AJ, et al. Long-term persistence of CD4+ but rapid disappearance of CD8+ T cells expressing an MHC class I-restricted TCR of nanomolar affinity. Mol Ther. 2012;20:652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Muranski P, Boni A, Wrzesinski C, et al. Increased intensity lymphodepletion and adoptive immunotherapy - How far can we go? Nat. Clin. Pract. Oncol. Nature Publishing Group; 2006. p. 668–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ninomiya S, Narala N, Huye L, et al. Tumor indoleamine 2,3-dioxygenase (IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood. 2015;125:3905–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Turtle CJ, Hanafi LA, Berger C, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Curran KJ, Margossian SP, Kernan NA, et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood. 2019;134:2361–2368.*MSKCC’s phase 1 trial of an anti-CD19/CD28 CAR in children and young adults at both MSKCC and Boston Children’s with CD19+ B-ALL
- [49].Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129:3322–3331.*Seattle Children’s phase 1 trial of an anti-CD19/4–1BB CAR that is engineered with a defined ratio of CD4+/CD8+ CAR T cells in children and young adults with relapsed/refractory CD19+ B-ALL
- [50].Porter DL, Hwang WT, Frey N V., et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Maude SL, Teachey DT, Rheingold SR, et al. Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL. J Clin Oncol. 2016;34:3011–3011. [Google Scholar]
- [52].O’Leary MC, Lu X, Huang Y, et al. FDA Approval summary: Tisagenlecleucel for treatment of patients with relapsed or refractory b-cell precursor acute lymphoblastic leukemia. Clin. Cancer Res. American Association for Cancer Research Inc.; 2019. p. 1142–1146. [DOI] [PubMed] [Google Scholar]
- [53].Tumaini B, Lee DW, Lin T, et al. Simplified process for the production of anti-CD19-CAR-engineered Tcells. Cytotherapy. 2013;15:1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lee DW, Stetler-Stevenson M, Yuan CM, et al. Long-Term Outcomes Following CD19 CAR T Cell Therapy for B-ALL Are Superior in Patients Receiving a Fludarabine/Cyclophosphamide Preparative Regimen and Post-CAR Hematopoietic Stem Cell Transplantation. Blood. 2016;128:218–218. [Google Scholar]
- [55].Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Park JH, Rivière I, Gonen M, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018;378:449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lamers CHJ, Willemsen R, Van Elzakker P, et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011;117:72–82. [DOI] [PubMed] [Google Scholar]
- [58].Kalos M, June CH. Adoptive T Cell Transfer for Cancer Immunotherapy in the Era of Synthetic Biology. Immunity. NIH Public Access; 2013. p. 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Maude SL, Hucks GE, Callahan C, et al. Durable Remissions with Humanized CD19-Targeted Chimeric Antigen Receptor (CAR)-Modified T Cells in CAR-Naive and CAR-Exposed Children and Young Adults with Relapsed/Refractory Acute Lymphoblastic Leukemia. Blood. 2017;130:1319–1319. [Google Scholar]
- [60].Heng G, Jia J, Li S, et al. Sustained therapeutic efficacy of humanized anti-CD19 chimeric antigen receptor T cells in relapsed/ refractory acute lymphoblastic leukemia. Clin Cancer Res. 2020;26:1606–1615. [DOI] [PubMed] [Google Scholar]
- [61].Cao J, Wang G, Cheng H, et al. Potent anti-leukemia activities of humanized CD19-targeted Chimeric antigen receptor T (CAR-T) cells in patients with relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. 2018;93:851–858. [DOI] [PubMed] [Google Scholar]
- [62].Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shah NN, Highfill SL, Shalabi H, et al. CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: Updated Results From a Phase I Anti-CD22 CAR T-Cell Trial. J Clin Oncol. 2020;JCO.19.03279.**The most updated analysis from NCI’s phase 1 trial of an anti-CD22/4–1BB CAR in children and young adults with relapsed/refractory CD22+ hematologic malignancies
- [64].Pan J, Niu Q, Deng B, et al. CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia. 2019;33:2854–2866. [DOI] [PubMed] [Google Scholar]
- [65].Gardner R, Annesley C, Finney O, et al. Early Clinical Experience of CD19 x CD22 Dual Specific CAR T Cells for Enhanced Anti-Leukemic Targeting of Acute Lymphoblastic Leukemia. Blood. 2018;132:278–278. [Google Scholar]
- [66].Pan J, Zuo S, Deng B, et al. Sequential CD19–22 CAR T therapy induces sustained remission in children with r/r B-ALL. Blood. American Society of Hematology; 2020. p. 387–391. [DOI] [PubMed] [Google Scholar]
- [67].Qasim W, Zhan H, Samarasinghe S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017;9. [DOI] [PubMed] [Google Scholar]
- [68].Qasim W, Ciocarlie O, Adams S, et al. Preliminary Results of UCART19, an Allogeneic Anti-CD19 CAR T-Cell Product in a First-in-Human Trial (PALL) in Pediatric Patients with CD19+ Relapsed/Refractory B-Cell Acute Lymphoblastic Leukemia. Blood. 2017;130:1271–1271. [Google Scholar]
- [69].Benjamin R, Graham C, Yallop D, et al. Preliminary Data on Safety, Cellular Kinetics and Anti-Leukemic Activity of UCART19, an Allogeneic Anti-CD19 CAR T-Cell Product, in a Pool of Adult and Pediatric Patients with High-Risk CD19+ Relapsed/Refractory B-Cell Acute Lymphoblastic Leukemia. Blood. 2018;132:896–896. [Google Scholar]
- [70].Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121:5154–5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Porter D, Frey N, Wood PA, et al. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J. Hematol. Oncol. BioMed Central Ltd.; 2018. p. 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Allen CE, Yu X, Kozinetz CA, et al. Highly elevated ferritin levels and the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2008;50:1227–1235. [DOI] [PubMed] [Google Scholar]
- [74].Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Gardner RA, Ceppi F, Rivers J, et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood. 2019;134:2149–2158.*Reports results of an early intervention for a subset of patients treated on a phase 1, anti-CD19 CAR study, demonstrating that preemptive use of tocilizumab and/or corticosteroids may reduce progression from mild to severe CRS, without adversely impacting CAR T cell efficacy.
- [76].Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377:2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Myers RM, Kadauke S, Li Y, et al. Risk-Adapted Preemptive Tocilizumab Decreases Severe Cytokine Release Syndrome (CRS) after CTL019 CD19-Targeted Chimeric Antigen Receptor (CAR) T-Cell Therapy for Pediatric B-Cell Acute Lymphoblastic Leukemia (B-ALL). Biol Blood Marrow Transplant. 2020;26:S39. [Google Scholar]
- [78].Kadauke S, Maude S, Gladney W, et al. Early administration of tocilizumab (Toci) for the prevention of grade 4 cytokine release syndrome (CRS) after CD19-directed CAR T-cell therapy (CTL019). Cytotherapy. 2019;21:e2–e3. [Google Scholar]
- [79].Brudno JN, Somerville RPT, Shi V, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34:1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kochenderfer JN, Dudley ME, Carpenter RO, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38–177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine- associated toxicity in a clinical trial of anti-CD19 chimeric-antigen- receptor–transduced T cells. Blood. 2012;119:2709–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Goebeler M-E, Knop S, Viardot A, et al. Bispecific T-Cell Engager (BiTE) Antibody Construct Blinatumomab for the Treatment of Patients With Relapsed/Refractory Non-Hodgkin Lymphoma: Final Results From a Phase I Study. J Clin Oncol. 2016;34:1104–1111. [DOI] [PubMed] [Google Scholar]
- [85].Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: A multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66. [DOI] [PubMed] [Google Scholar]
- [86].Santomasso BD, Park JH, Salloum D, et al. Clinical and biological correlates of neurotoxicity associated with car t-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8:958–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Gust J, Hay KA, Hanafi LA, et al. Endothelial activation and blood–brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7:1404–1419.*Describes features of neurotoxicity in a large cohort of adult patients as well as an analysis of cytokines and endothelial factors that may explain the pathogenesis of ICANS
- [88].Shah NN, Highfill SL, Shalabi H, et al. CD4/CD8 T-Cell Selection Enhances CD22 CAR-T Cell Transduction and in-Vivo CAR-T Expansion: Updated Results on Phase I Anti-CD22 CAR Dose Expansion Cohort. Blood. 2017;130:809–809. [Google Scholar]
- [89].Lamers CHJ, Sleijfer S, Vulto AG, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–2. [DOI] [PubMed] [Google Scholar]
- [90].Lamers CHJ, Sleijfer S, Van Steenbergen S, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: Clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21:904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of t cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. Nature Publishing Group; 2019. p. 372–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Das RK, Storm J, Barrett DM. Abstract 1631: T cell dysfunction in pediatric cancer patients at diagnosis and after chemotherapy can limit chimeric antigen receptor potential. Cancer Res. American Association for Cancer Research (AACR); 2018. p. 1631–1631. [Google Scholar]
- [94].Gargett T, Brown MP. Different cytokine and stimulation conditions influence the expansion and immune phenotype of third-generation chimeric antigen receptor Tcells specific for tumor antigen GD2. Cytotherapy. 2015;17:487–495. [DOI] [PubMed] [Google Scholar]
- [95].Zhao Z, Condomines M, van der Stegen SJC, et al. Structural Design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T Cells. Cancer Cell. 2015;28:415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Li AM, Hucks GE, Dinofia AM, et al. Checkpoint Inhibitors Augment CD19-Directed Chimeric Antigen Receptor (CAR) T Cell Therapy in Relapsed B-Cell Acute Lymphoblastic Leukemia. Blood. 2018;132:556 LP–556. [Google Scholar]
- [97].Hasan A, Selvakumar A, O’Reilly RJ. Artificial Antigen Presenting Cells: An Off the Shelf Approach for Generation of Desirable T-Cell Populations for Broad Application of Adoptive Immunotherapy. Adv Genet Eng. 2015;04. [PMC free article] [PubMed] [Google Scholar]
- [98].Turtle CJ, Riddell SR. Artificial antigen-presenting cells for use in adoptive immunotherapy. Cancer J. 2010. p. 374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Von Stackelberg A, Locatelli F, Zugmaier G, et al. Phase I/Phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34:4381–4389. [DOI] [PubMed] [Google Scholar]
- [100].Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell - Engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–2498. [DOI] [PubMed] [Google Scholar]
- [101].Fischer J, Paret C, El Malki K, et al. CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother. 2017;40:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ruella M, Maus M V. Catch me if you can: Leukemia Escape after CD19-Directed T Cell Immunotherapies. Comput. Struct. Biotechnol. J Elsevier B.V.; 2016. p. 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Pillai V, Muralidharan K, Meng W, et al. CAR T-cell therapy is effective for CD19-dim B-lymphoblastic leukemia but is impacted by prior blinatumomab therapy. Blood Adv. 2019;3:3539–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Gardner R, Wu D, Cherian S, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127:2406–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Jacoby E, Nguyen SM, Fountaine TJ, et al. CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nat Commun. 2016;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Xu X, Sun Q, Liang X, et al. Mechanisms of Relapse After CD19 CAR T-Cell Therapy for Acute Lymphoblastic Leukemia and Its Prevention and Treatment Strategies. Front. Immunol. Frontiers Media S.A.; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].McNeer JL, Rau RE, Gupta S, et al. Cutting to the Front of the Line: Immunotherapy for Childhood Acute Lymphoblastic Leukemia. Am Soc Clin Oncol Educ B. 2020;1–12. [DOI] [PubMed] [Google Scholar]
- [108].Maus MV, Grupp SA, Porter DL, et al. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123:2625–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Newman H, Barz Leahy AE, Li Y, et al. CD19-targeted chimeric antigen receptor (CAR) T cells in CNS relapsed acute lymphoblastic leukemia (ALL). J Clin Oncol. 2020;38:10511–10511. [Google Scholar]