Abstract
Objective
Since the last epidemiologic review of neuromyelitis optica/neuromyelitis optica spectrum disorder (NMO/NMOSD), 22 additional studies have been conducted. We systematically review the worldwide prevalence, incidence, and basic demographic characteristics of NMOSD and provide a critical overview of studies.
Methods
PubMed, Ovid MEDLINE, and Embase using Medical Subject Headings and keyword search terms and reference lists of retrieved articles were searched from 1999 until August 2019. We collected data on the country; region; methods of case assessment and aquaporin-4 antibody (AQP4-Ab) test; study period; limitations; incidence (per 100,000 person-years); prevalence (per 100,000 persons); and age-, sex-, and ethnic group–specific incidence or prevalence.
Results
We identified 33 relevant articles. The results indicated the highest estimates of incidence and prevalence of NMOSD in Afro-Caribbean region (0.73/100 000 person-years [95% CI: 0.45–1.01] and 10/100 000 persons [95% CI: 6.8–13.2]). The lowest incidence and prevalence of NMOSD were found in Australia and New Zealand (0.037/100 000 person-years [95% CI: 0.036–0.038] and 0.7/100,000 persons [95% CI: 0.66–0.74]). There was prominent female predominance in adults and the AQP4-Ab–seropositive subpopulation. The incidence and prevalence peaked in middle-aged adults. African ethnicity had the highest incidence and prevalence of NMOSD, whereas White ethnicity had the lowest. No remarkable trend of incidence was described over time.
Conclusion
NMOSD is a rare disease worldwide. Variations in prevalence and incidence have been described among different geographic areas and ethnicities. These are only partially explained by different study methods and NMO/NMOSD definitions, highlighting the need for specifically designed epidemiologic studies to identify genetic effects and etiologic factors.
Neuromyelitis optica (NMO) is an antibody-mediated inflammatory disease of the CNS. The understanding of the disease has evolved extensively through the last 100–125 years.1 In 2005, the discovery of specific antibodies against the aquaporin-4 (AQP4) water channel in patients with NMO enabled the distinction of NMO from MS and resulted in the 2006 Wingerchuk criteria.2,3 The introduction of the cell-based assay (CBA) to search for AQP4 antibody (AQP4-Ab) and improvements of test sensitivity allowed the recognition of additional syndromes associated with AQP4-Ab named as neuromyelitis optica spectrum disorders (NMOSDs).4 The 2015 International Panel for NMO Diagnosis (IPND) criteria united the NMO and NMOSD definitions, proposed a categorization by stratifying for AQP4-Ab serostatus, and recommend the use of CBA owing to highest sensitivity (76.7%) and specificity (99.8%).1
The cause of the disease is not fully understood.5,6 Well-established epidemiologic studies can generate hypotheses and thereby contribute to the identification of potential risk factors and essential features of disease etiology.
The last review of the incidence and prevalence of NMO/NMOSD was published in 2015, when 7 population-based studies were available.7 Since then, 22 additional epidemiologic studies have been published using also the 2015 criteria, and these may improve our understanding of the NMOSD epidemiology. Here, we provide a critical overview of studies reporting the incidence and prevalence of NMO/NMOSD from around the world and discuss the challenges of such epidemiologic studies.
Methods
We performed a systematic search in PubMed, Ovid MEDLINE, and Embase databases for epidemiologic studies in NMO/NMOSD published from 1999 to August 2019 (figure, supplementary file, doi.org/10.5061/dryad.prr4xgxh9). Two authors (V.P. and Z.I.) independently assessed whether the identified abstracts met the inclusion criteria: (1) NMO/NMOSD; (2) original data; (3) incidence or prevalence of NMO/NMOSD; and (4) available English abstract. Furthermore, we searched the reference lists of review articles and the relevant publications for additional studies. If either reviewer selected an abstract, it underwent independent full-text review by the 2 reviewers. Disagreements were resolved by consensus. We excluded review articles, conference proceedings, and abstracts due to limited data on study design for critical review.
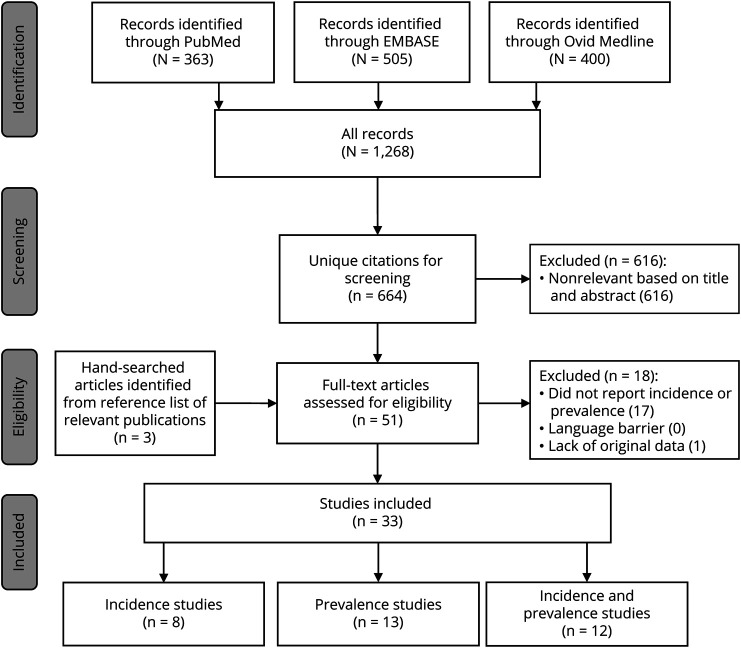
Figure. The Flowchart Demonstrates the Steps of the Literature Search.
We collected data on the country, region, size of source population, case ascertainment, applied diagnostic criteria and antibody test method, observation period of incidence, definition of incident event, limitations, incidence (100,000 person-years), prevalence (100,000 persons) and age-, sex-, or ethnic group–specific incidence or prevalence, and basic demographic data (tables 1–6, supplementary tables 1–3, doi.org/10.5061/dryad.prr4xgxh9). The review follows Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.
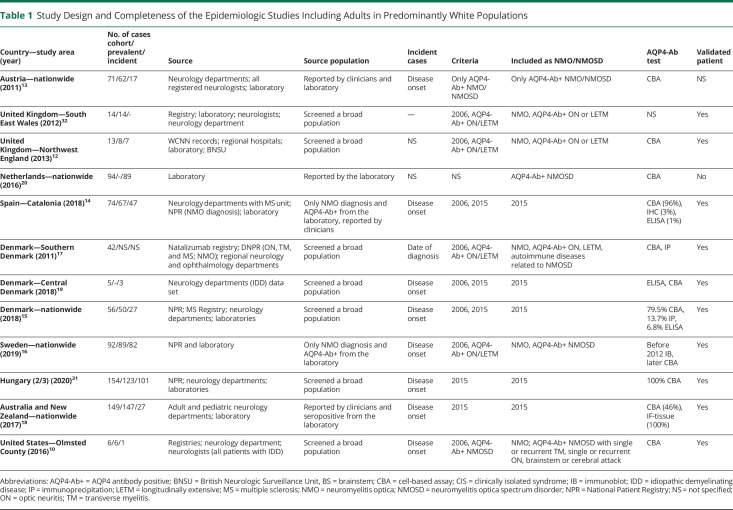
Table 1.
Study Design and Completeness of the Epidemiologic Studies Including Adults in Predominantly White Populations
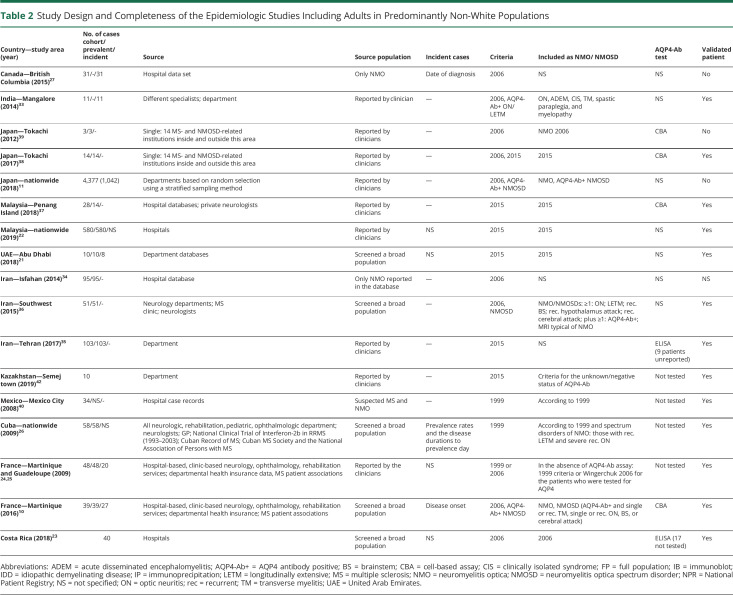
Table 2.
Study Design and Completeness of the Epidemiologic Studies Including Adults in Predominantly Non-White Populations
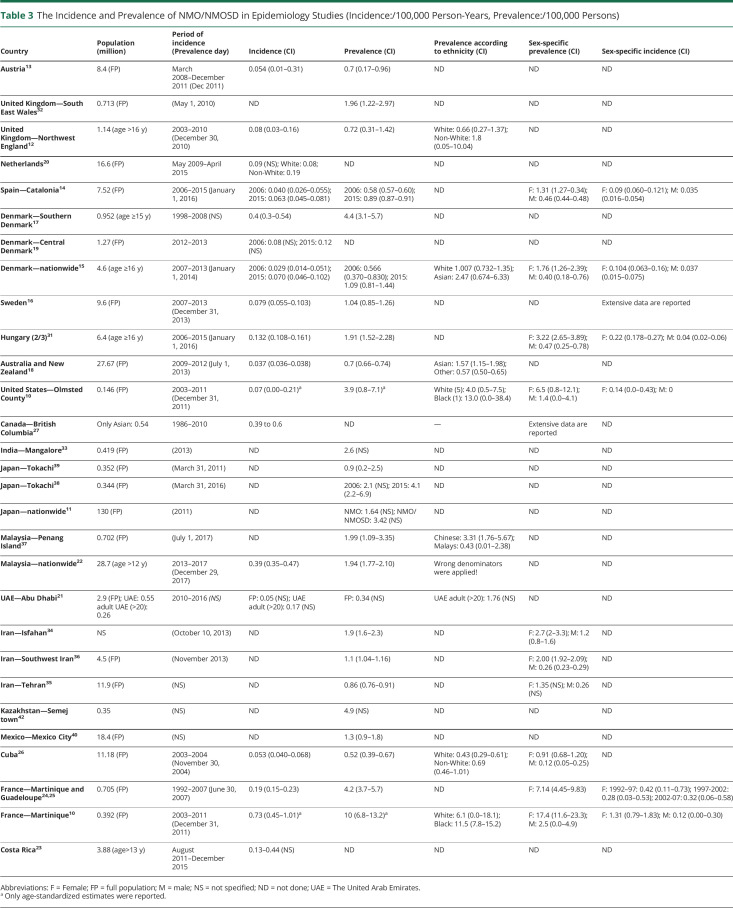
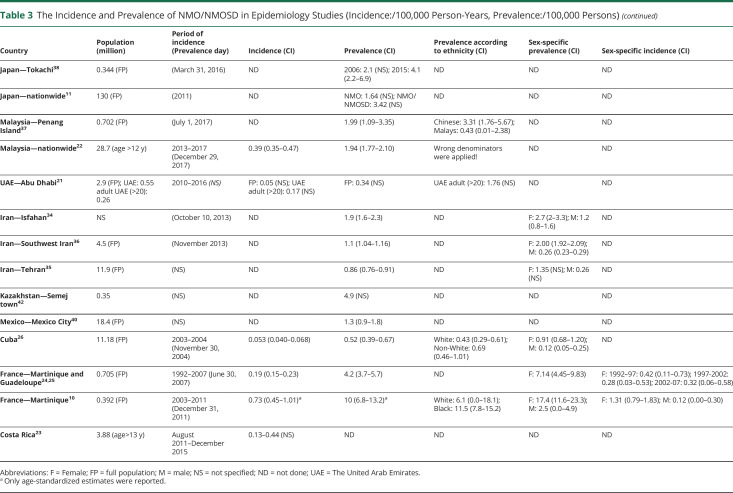
Table 3.
The Incidence and Prevalence of NMO/NMOSD in Epidemiology Studies (Incidence:/100,000 Person-Years, Prevalence:/100,000 Persons)
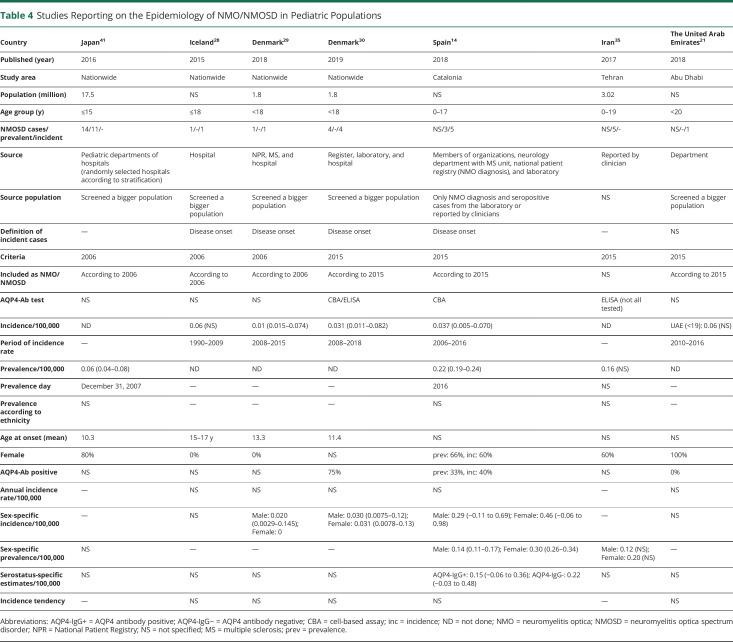
Table 4.
Studies Reporting on the Epidemiology of NMO/NMOSD in Pediatric Populations
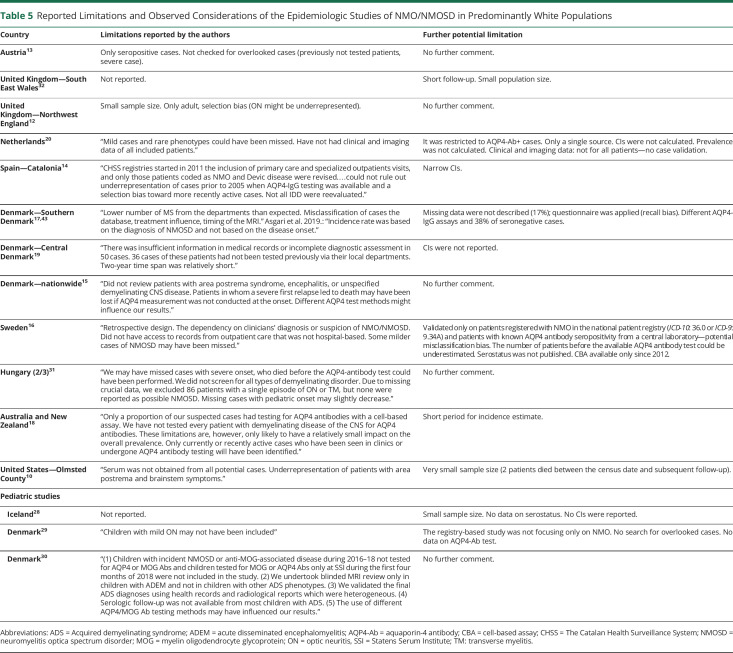
Table 5.
Reported Limitations and Observed Considerations of the Epidemiologic Studies of NMO/NMOSD in Predominantly White Populations
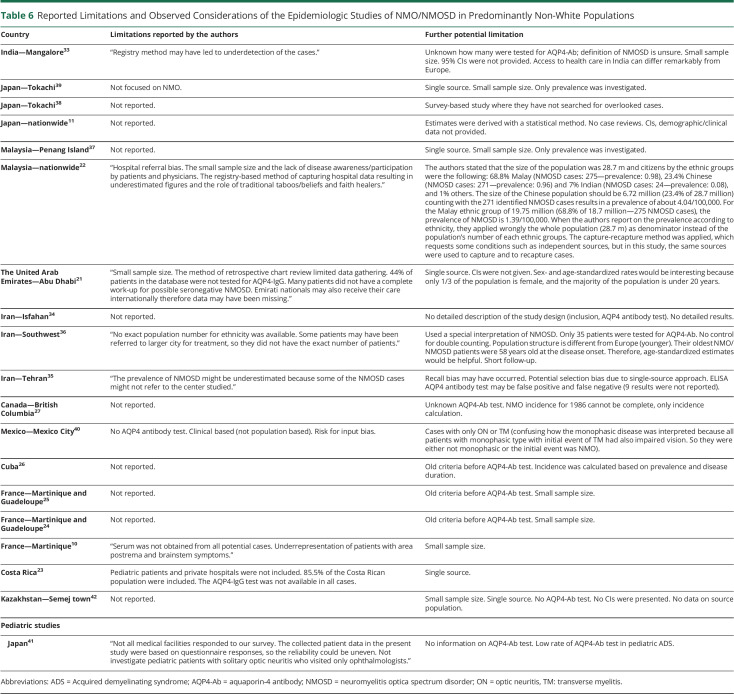
Table 6.
Reported Limitations and Observed Considerations of the Epidemiologic Studies of NMO/NMOSD in Predominantly Non-White Populations
Results
Search Strategy
From PubMed, Ovid MEDLINE, and Embase, we identified 664 unique references (figure). We selected 51 articles for full-text review based on relevant abstracts. Of these, we excluded 1 for not including original data and 17 for not providing the incidence and/or prevalence estimates of NMO/NMOSD. Ultimately, 33 articles met the inclusion criteria.
Study Characteristics
The number of epidemiologic data of NMO/NMOSD has remained low. All studies are retrospective (tables 1, 2, and 4). The timing of the study may influence the prevalence and incidence estimates due to several changes in the definition of NMO and NMOSD diagnostic criteria and antibody test method in the last decades.1,3,4,8,9 Diversity can also be seen in population sample size (varying from 145,97910 to 130 million11), study design (cohort study10,12–31 or cross-sectional study11,32–41; clinical-based10,12–16,18,19,31,32,37–39,42 or survey study11,17,22,35,41), case ascertainment (accepting the reported cases20,27,34 or validating each NMO/NMOSD case10,12–17,19,21,23,24,26,28–33,35–38,40,42), final number of cases calculate by statistical analysis,11,18,22,41 and AQP4-Ab test (IF-tissue assay, immunoprecipitation, ELISA, and CBA (tables 1, 2, and 4). The overall number of samples tested for AQP4-Ab was reported in 5 studies (supplementary table 3, doi.org/10.5061/dryad.prr4xgxh9).
Four nationwide studies involved solely pediatric populations,28–30,41 and 3 of these investigated the epidemiology of acquired demyelinating syndromes, not specifically NMO/NMOSD.28,29,41 Three studies applied the 2006 criteria to calculate the prevalence41 or incidence28,29 of NMO, and 1 used the 2015 IPND criteria for incidence assessment.30 Five studies selectively evaluated the adult population.12,15,17,22,23,31 The majority of the reports encompassed all ages,10,11,13,14,16,18–21,24,26,27,32,33,35–38,40 but some did not recognize any patients with pediatric disease onset (tables 1–4).19,33,37
Thirteen of the 33 included studies provided inadequate descriptions of study design, case assessment, or type of AQP4-Ab test.7,11,20–22,27–29,32,34–36,41 Many studies used multiple sources for case detection (clinical departments, registries, and/or laboratory databases),10,12–18,24,26,29–32 whereas others used data directly from clinical departments, physicians,11,19,21–23,27,28,33–42 or laboratory units.20 Confidence intervals were not always presented.11,19–21,28,33,42 Standardized estimates are essential when comparing findings in populations with different age and sex distributions, but these were rarely provided. One study applied the US standard population,10 1 used the Japanese standard population,38 and 4 others the WHO standard population.14,16,18,31
Some prevalence and incidence estimates were obtained before the discovery of the AQP4-Ab and were solely based on the diagnostic criteria from 1999 restricted to optic neuritis (ON) and transverse myelitis (TM).26,40 Two other studies applied the 1999 criteria if the AQP4-Ab test was not available, but the 2006 criteria when the AQP4-Ab test was performed.24,25 Eleven studies purely used the 2006 criteria,14,15,19,23,27–29,34,38,39,41 8 other studies reported estimates based on the 2006 criteria combined with other AQP4-Ab–seropositive (AQP4-Ab+) cases,10–12,16,17,32,33,36 and 10 other studies calculated estimates by applying the 2015 IPND criteria.14,15,18,19,21,22,30,31,35,37,38 Two studies included only AQP4-Ab+ patients with NMO/NMOSD.13,20 Another used the 2015 IPND criteria for unknown or AQP4-Ab–seronegative (AQP4-Ab−) NMOSD due to lack of access to AQP4-Ab test.42
Incidence
Considerable variation was observed in the definition of the incident event and the duration of the observation period for incidence. Most studies considered incident cases based on the disease onset,10,13–16,18,19,28,30,31 but a few calculated incidence by the diagnosis date,17,27,43 date of AQP4-Ab test,20 or calculated by using the point prevalence and the average disease duration to prevalence day.26 Other studies did not define incident event.21–24,29,41 We identified 20 reports regarding the incidence of NMO/NMOSD (tables 1–4, supplementary tables 1 and 2, doi.org/10.5061/dryad.prr4xgxh9). The observation period for the incidence rate ranged from 2 to 27 years.16,19 These may lead to remarkable differences in the incidence estimates. Incidence rates were reported between 0.039 and 0.73/100,000 person-years for adults10,18 and between 0.01 and 0.06/100,000 person-years for children21,28,29 (age <18 years).
Geography and Ethnicity: Incidence
The geographic distribution of the studies is demonstrated in tables 1–4. Most studies were conducted in Europe with predominantly White populations.
In smaller, predominantly White cohorts, the incidence of NMO/NMOSD was between 0.07 and 0.4/100,000 person-years.10,12,17,19 In larger White studies applying the 2006 criteria, the incidence was between 0.029 and 0.04/100,000 person-years.14,15 When including only AQP4-Ab+ NMOSD, the incidence ranged from 0.054 to 0.08/100,000 person-years.13,20 NMO and AQP4-Ab+ NMOSD together gave an incidence of 0.079/100,000 person-years,16 whereas based on the last NMOSD 2015 criteria, it ranged from 0.037 to 0.132/100,000 person-years.14,15,18,31
Only a few incidence estimates are available from other ethnicities: in Caribbean-African 0.22–0.4/100,000 person-years (1999/2006 criteria)24 and 0.7/100,000 person-years (2006 criteria plus AQP4-Ab+ NMOSD),10 in selected Asian populations 0.39–0.6/100,000 person-years (2015 IPND criteria),22,27 and in Arab populations 0.17/100,000 person-years (2015 IPND criteria).21 The Dutch study described a higher incidence among non-White compared with White populations living in the same geographic area.20
Four studies from White14,28–30 (0.01–0.06/100,000 person-years) pediatric populations and 1 from an Arab21 (0.06/100,000 person-years) pediatric population reported incidence estimates of NMO/NMOSD. Three of these were based on a single incident case observed in a period of 7–19 years (table 4).21,28,29
Sex- and Age-Related Incidence
The sex-specified incidence rates were higher in females than in males, although the difference was less remarkable or disappeared at extremes of age (supplementary tables 1 and 2, doi.org/10.5061/dryad.prr4xgxh9).10,14,15,24,28–31 Of interest, in Sweden, over the period 1987–2006, the incidence of NMOSD in males (0.151/100,000 person-years [95% CI: 0.085–0.261]) was more than 2 times higher compared with females (0.067/100,000 person-years [95% CI: 0.044–0.098]), whereas between 2007 and 2013, the incidence of NMOSD in females (0.150/100,000 person-years [95% CI: 0.108–0.203]) was more than 2 times greater compared with males (0.066/100,000 person-years [95% CI: 0.032–0.123]).16 The highest incidence rates in both sexes were found in the 40–59 year age group.14,31 Incidence studies showed that 20%–28% of the incidence cohorts had a late-onset NMOSD (age ≥ 60 years).14,31
The pediatric cohort of Iceland28 and Denmark29 (age ≤18 years) identified a single male case in each country, whereas the very recent Danish study30 (age ≤18 years) found 2 male and 2 female cases. The Catalonia study14 reported 3 female and 2 male pediatric patients.
Serostatus and Incidence
Only 4 publications described the serostatus-specific incidence.10,14,15,31 These found a higher incidence of AQP4-Ab+ NMOSD compared with AQP4-Ab− patients. In the United States, the incidence of AQP4-Ab+ NMOSD was 0.07/100,000 person-years (95% CI: 0.0–0.21) based on a single case, whereas no AQP4-Ab− incident cases were identified. The same study determined the estimates in Martinique and showed a 5 times higher incidence of AQP4-Ab+NMOSD (0.65/100,000 person-years [95% CI: 0.39–0.92]) in comparison with AQP4-Ab-NMOSD.10 In Catalonia, AQP4-Ab+ incidence increased from 0.015/100,000 person-years (95% CI: −0.002 to 0.004) in the pediatric population to 0.062/100,000 person-years (95% CI: 0.028–0.096) in adults, whereas the incidence of AQP4-Ab-NMOSD was low in all age groups (ranging from 0.006 to 0.029/100,000 person-years) but similar to seropositive incidence in children.14 In Denmark, the incidence of AQP4-Ab+ patients was 2-fold higher compared with AQP4-Ab− NMOSD,15 whereas the Hungarian study revealed 5-fold greater incidence estimates of the AQP4-Ab+ NMOSD.31
Temporal Changes in Incidence
Some studies reported the annualized incident rate over longer time periods, where temporal changes could be evaluated (supplementary table 1, doi.org/10.5061/dryad.prr4xgxh9). Studies from the French West Indies covering time periods 1992–1997, 1997–2002, and 2002–2007 and from British Columbia covering the period 1986–2010 showed stable incidence.25,27 Studies with shorter observational periods published from the Netherlands (6 years),20 Abu Dhabi (7 years),21 Denmark (7 years; not published data),15 Catalonia (10 years),14 and Hungary (10 years; not published data)31 revealed no temporal changes in the annualized incidence rates. In contrast to this, the Swedish study found an increasing incidence in females from 0.07/100,000 person-years (95% CI: 0.04–0.09) between 1987 and 2006 to 0.15/100,000 person-years (95% CI: 0.11–0.20) between 2007 and 2013.16 In Costa Rica, an increase from 0.13 to 0.44 was reported between 2011 and 2015.23
Prevalence
We recognized 25 articles presenting prevalence estimates ranging from 0.34 to 10/100,000 in adults and from 0.06 to 0.22/100,000 in children (tables 1–3, supplementary tables 1 and 2, doi.org/10.5061/dryad.prr4xgxh9). The number of prevalent cases ranged from 6 to 4,377 in adults10,11 and from 3 to 11 in children.14,41 The census date for prevalence was not reported in 7 publications.11,17,21,33,35,40,42
Geography and Ethnicity: Prevalence
The geographic distribution of prevalent studies is presented in tables 1–4. In smaller studies with predominantly White populations, the prevalence varied from 0.72 to 4.4 to/100,000, including patients with NMO based on the 2006 criteria and AQP4-Ab–positive NMOSD.10,12,17,32 In larger populations, it spanned from 0.57 to 0.58/100,000 based on the 2006 criteria14,15; 1.04/100,000 counting NMO and AQP4-Ab+ cases16; 0.7/100,000 involving only AQP4-Ab+ patients13; and from 0.7 to 1.91 using the 2015 IPND criteria.14,15,18,31,34–36
Seven reports from predominantly Asian populations revealed a prevalence between 1.57 and 4.9/100,000.22,33,37–39,41,42 Similar to incidence data, the highest prevalence was found in African populations: 10/100,00010 and 4.2/100,00024 before the era of AQP4-Ab detection. The prevalence estimate of a single study from Arab countries demonstrated a prevalence of 1.76/100,000 in adults.21
A few studies provided head-to-head comparison of the prevalence of NMOSD in different ethnicities living in the same geographic area under similar environmental White ancestry.10,12,15,18,22,26,37 They found that Asian and African ancestries have a higher risk to develop NMO/NMOSD than White ancestry.10,12,15,18 Furthermore, within Asian ethnicities, a higher prevalence was found among Chinese compared with Malays.37 When recalculating the prevalence rates of the nationwide Malaysian study based on their presented row data, similar difference can be seen between Chinese and Malay ethnicities.22
Sex- and Age-Related Prevalence
All studies that calculated the sex-specific estimates found the highest prevalence among females, which was 2.3–7.6-times greater than for males, both in Whites and Africans (table 3).10,14,15,26,31,34–36 Prevalence curves by age had a peak from 30 to 39 in India and between 40 and 59 years for both sexes in Catalonia and Hungary.14,31 For females in Australia and New Zealand,18 in Tehran and Cuba,26,35 the peak prevalence age was between 40 and 49 years and between 45 and 55 years in the French Indies (supplementary table 1, doi.org/10.5061/dryad.prr4xgxh9).24
Data are scarce in pediatric populations (table 4). The only 2 studies providing sex-specific prevalence in children reported 1.5 to 2 times higher prevalence for females.14,35
Serostatus and Prevalence
The prevalence of AQP4-Ab+ NMOSD was greater in the 5 studies reporting serostatus-specific data and indicated a seropositive to seronegative ratio of 2.3:1 in Denmark,15 3.4:1 in Japan,38 3.8:1 in Martinique,10 5:1 in the United States,10 and 5.2:1 in Hungary.31 However, this ratio depends also on applied criteria and definition of AQP4-Ab− cases. The serostatus-specific prevalence by age showed higher frequency of AQP4-Ab− NMOSD in children.14
Temporal Changes in Prevalence
Two studies from Japan evaluated the same geographic area of Tokachi province over approximately 5-year intervals and showed an increasing prevalence, but different methodological approaches and NMO/NMOSD criteria may also account for this change.38,39 In 2011, the research group primarily aimed to evaluate MS epidemiology, but also identified patients with NMO using 2006 criteria and calculated NMO prevalence showing 0.9/100,000 (95% CI: 0.2–2.5).39 In contrast, the surveillance in 2016 was dedicated to NMO/NMOSD and used both 2006 and 2015 criteria: a considerably higher prevalence was found (2006 criteria: 2.1/100,000; 2015 IPND criteria: 4.1/100,000 [95% CI: 2.2–6.9]).38 An increased prevalence was observed in the French Indies from 1.88/100,000 (95% CI: 0.82–2.94) in 1992 to 4.20/100,000 (95% CI: 2.70–5.70) in 2007, but this was explained by a decrease in mortality.24 To date, no study evaluated at the population level over a long period of time mortality in NMOSD.
Demographic Data
The basic demographic data are summarized in table 4 and supplementary table 2, doi.org/10.5061/dryad.prr4xgxh9. The mean age at onset varied from 29.236 to 45.7 years,13 whereas the median was between 30.521 and 4311,26 years. The youngest age at onset was reported from Iran (mean age: 29.2–31.54 years).34–36 The percentage of females in different epidemiologic cohorts ranged from 55% to 100%.33,37,39 However, highest percentages of females ranging from 86% to 100% have been consistently found in populations from West Indies and Far East.11,24,26,37–39,44 The percentage of AQP4-Ab seropositivity was between 27% and 100% (excluding studies only on AQP4-Ab+ cases and where AQP4-Ab was not tested).33,37,39 The lowest percentage of AQP4-Ab seropositivity was reported in India,33 followed by the 3 Iranian studies,34–36 and the study from Southern Denmark.17
The mean follow-up was between 5.85 and 9.9 years,26,40 and the median from 3.5 to 12.5 years.10,32 The duration of follow-up was not mentioned in 11 publications.12,13,17–21,23,24,27,37,39
The majority of the patients had recurrent disease (71%–97.5%); only 1 study in Iran described monophasic disease in 60% of the cases, but they did not provide data about serostatus of these patients.34 Progressive disease course was reported in 2 studies and was found between 0.2% and 0.6%.22,31 The frequency of patients with NMO/NMOSD, who did not survive until the census day, ranged between 0% and 38.5% when reported.10,12
Discussion
This systematic review provides several observations: (1) NMO/NMOSD is a rare disease; (2) is reported worldwide in a variety of ethnic ancestry populations; (3) studies investigating the incidence and prevalence of NMOSD are diverse; (4) the number of well-designed epidemiologic studies of NMOSD is increasing; (5) there is a female and AQP4-Ab+ predominance; (6) incidence and prevalence estimates show a peak age in middle-aged adults; however, these are often distorted by incomplete capture (possibly premature death) of older cohorts, and (7) late-onset NMOSD (age ≥60 years) is responsible for one-fourth of the incidence cases. (8) Most of the results suggest that African ethnicity is associated with the highest incidence and prevalence, and White ethnicity may have the lowest. It seems that there has been no definite change in the incidence of NMO/NMOSD over time. Rare head-to-head comparative studies suggest a role of genetic factors in developing NMOSD. However, there is need for more well-designed (large source population with long observation period) studies to identify potentially concealed features of NMOSD epidemiology.
One factor that can contribute to the noticed variation could be the genetic mixture of the observed populations. This is supported by studies involving different ethnic groups living in the same geographic region, where environmental factors are considered comparable.10,12,15,18,20,37,45 Epidemiologic studies and large case series have reached the same conclusion that individuals from Asian15,18,33,37–39 and African10,12,46 ancestries have a disproportionately greater risk to develop NMOSD compared with White ethnic groups. This suggests that genetic factors may be important in disease susceptibility. This hypothesis is also supported by familial clustering recognized in approximately 3% of the cases47 and by the results of a whole-genome association study.6 Of interest, genetic susceptibility alleles for NMOSD were more closely linked to those of systemic lupus erythematosus than MS.6
Besides these population-based studies, several research groups explored the frequency of NMOSD among patients with idiopathic inflammatory diseases. These works found increased susceptibility for NMOSD in populations with Asian, African, Arab, and Latin American background paralleled with a lower frequency of MS.48–51
As an environmental trigger factor, latitudinal gradient was considered in a survey across Australia and New Zealand, where a slightly higher prevalence of NMOSD was noted at lower latitudes.18 The nationwide Japanese survey also found a greater prevalence of NMO/NMOSD in the Southern part of Japan.11 A review found no correlation between latitude and prevalence; however, the compared prevalences were calculated based on different diagnostic criteria, i.e., both NMO and NMOSD.52 The comparison of large population-based studies in Europe involving White populations in neighboring countries in Northern and Central Europe (Denmark, Sweden, Austria, and Hungary) suggests that genetic factors may be responsible for some of these latitudinal differences.13,15,16,31
Different results among the studies could also reflect variations in the study design; the size of the study population; the timing and length of the study; the applied diagnostic criteria; access to health care and antibody tests; the type of antibody assays used; whether the estimates were age standardized; the underlying population's structure (age and sex); and ethnic mixture. These factors may remarkably limit the generalizability and reliability of the results. Estimates can be affected by multiple biases and random errors, which could even mask the potential differences among the geographic areas, ethnicities, and environmental factors.
The variable performance of AQP4-Ab assays could lead to misdiagnosis of patients due to false-positive or false-negative results.53,54 CBA uses transfected live or fixed cells and either semiquantitative immunofluorescence microscopy or fluorescence-activated cell sorting to detect AQP4-Ab. By using a semiquantitative method, the results are dependent on the experience of the person assessing the staining pattern. Further methods such as ELISA, tissue-based immunofluorescence, and immunoprecipitation have particularly shown a tendency to false-positive detection.55,56
The recent studies from Catalonia, Sweden, Denmark, Australia/New Zealand, and Hungary showed similarities in study design: large White populations, multiple sources, large proportion tested with CBA, and access to free health care. The results from Catalonia, Denmark, Sweden, and Australia/New Zealand showed similar incidence (0.039–0.063/100,000 person-years) and prevalence (0.7–1.09/100,000), but the Danish nationwide study only investigated the adult population with regard to prevalence.15 The Hungarian crude and age-standardized prevalence and incidence were higher than the Catalonian, Danish, Australian/New Zealand, and Swedish estimates both according to the 2015 NMOSD and 2006 NMO criteria.14,18 The 2006 criteria for NMO are more strict because they do not allow NMO diagnosis with a single ON or LETM attack despite the presence of AQP4-Ab. Therefore, it is unlikely that the difference observed between the Hungarian and the other 4 studies would be caused by a difference in the threshold of AQP4-Ab assays. In addition, the population-based Hungarian and Danish studies were designed by overlapping research groups and used similar designs. The major laboratory for the Hungarian study participated in an international validation of CBA.53
There have been 4 studies conducted in Scandinavian adults, including 2 nationwide studies15,16 and 2 smaller regional Danish studies.17,19 The results of 3 of the 4 reports seem to be consistent with each other and with other large White population studies, but are contrary to the data published from the small study in Southern Denmark delineating the highest prevalence (4.4/100,000) and incidence (4.0/1,000,000 person-year) so far in preponderantly White ancestry.17 According to the estimates from the Southern Denmark, the nationwide Danish study should have recognized 202 prevalent Danish NMOSD cases, but only 50 prevalent cases were identified. It is unlikely that such an inconsistency would be caused by different study methods. More likely, the higher rates may be due to less strict adherence to criteria or alternative diagnoses in AQP4-Ab− patients and patients with low and transient AQP4-Ab seropositivity.15 The final estimates of the nationwide Danish study were similar to the nationwide study in Sweden16 and to another regional study in Denmark.19
The Swedish study showed that the incidence was higher in males in the period of 1987–2006. Because of the low number of NMO cases during that time and the overlapping confidence intervals, these estimates should be interpreted with caution. Overall, 74% of all diagnosed patients with NMO in Sweden were women.16
Another smaller study of White ethnicity10 in Olmsted County also revealed a higher prevalence than the larger studies. However, the incidence estimate was comparable with those of the larger studies. The comprehensive approach of the study from Olmsted County is a strong advantage, such as each individual with suspected demyelinating disease in the county's population was tested for AQP4 antibody with CBA providing a probably higher recognition rate of cases. However, this study investigated a small population of 145,979 residents, and there were only 6 prevalent cases (prevalence: 3.9/100,000 (95% CI: 0.8–7.1)) and a single incident case between 2003 and 2011 (incidence: 0.07/100,000 person-years (95% CI: 0.00–0.21)).10 They also reported that 2 prevalent patients died in the following year after the census date, which would have reduced the prevalence rate to 2.7/100,000, assuming that there were no additional new cases in the following year. Furthermore, because of the small population and few NMOSD cases, even missing or adding 1 or 2 cases might have a considerable impact on the estimates; the lower precision of the estimates is indicated by the broad confidence intervals. Considering these, interpreting the differences between the large studies and the Olmsted County study is difficult.
The recent Dutch study solely estimated the incidence of AQP4-Ab+ NMOSD to be 0.09 presumably based on the 2015 IPND AQP4-Ab+ criteria, but confidence intervals are not reported, and clinical and radiologic information were available only in 38% of the incident cases.20 The ethnicity of the cases may have influenced the results, as the incidence was 0.08/100,000 for Whites and 0.19/100,000 for non-Whites. It is not clear whether disease onset was defined by the onset of the clinical symptoms or the date of the positive AQP4-Ab. Altogether, the incidence of the Dutch AQP4-Ab+ White NMOSD population seems to be more similar to other White population reports.12–16,19
Two works from the United Kingdom used a combination of 2006 criteria3 and the 2007 NMOSD definition.4 These publications described the epidemiology of NMO/NMOSD in a small area of Wales and England.12,32 In Wales, a prevalence of 1.96/100,000 (95% CI: 1.22–2.97) was observed derived from a background population of 712,572 inhabitants.32 Surprisingly, 21% of the reported prevalent cases were under age 20 years, resulting in a higher prevalence in the age group from 0 to 19 years than in the age group from 20 to 49 years. No other epidemiologic study has shown similar findings. This may be an anomaly of the relatively small study population or some hidden capture bias. Opposite to this, the prevalence of NMO/NMOSD was 0.72/100,000 (95% CI: 0.31–1.42) among the 1.14 million adult residents of England, and the incidence was 0.08/100,000 (95% CI: 0.03–0.16) similar to the other large White population studies.
Three separate investigations were published from different regions of Iran.34–36 The demographic and clinical data were different from other studies around the world, especially in Isfahan.38 All 3 Iranian studies found a lower percentage of AQP4-Ab–positive cases (46.8%–66.3%), lower mean age at onset (29.2–31.5 years), and lower last Expanded Disability Status Scale score compared with most of the other studies. This inconsistency may be due to different ethnicities or population structure, but limitations should be also considered (tables 5 and 6).
The first recent study from an Arab region, Abu Dhabi, showed a greater prevalence and incidence of NMOSD compared with larger White populations.21 The authors provided subgroup analysis for Arab residents and the adult population, but no sex-adjusted and sex- and age-standardized calculations. These would have given more informative estimates, as the percentage of women was only 30% among the residents, and half of the Emirati citizens in Abu Dhabi were younger than 19 years.21
According to the latest studies conducted in Asian populations (Japanese,11,38 Indian,33 Malaysian-Chinese,22,27,37 and Kazakhstan42), the prevalence ranged from 2.6 to 4.9/100,000, and the incidence was between 0.39 and 0.6/100,000 person-years, which are remarkably higher than in Whites. However, only 2 studies computed the incidence in Asian ancestries.22,27 The results from India and Kazakhstan may be an underestimation of the true prevalence due to differently organized health care systems and limited or no access to AQP4-Ab assays.33 It should be noted that there is a lack of prevalence and incidence data from continental China.
APQ4-Ab was found in a high proportion of the patients with NMOSD with a few exceptions from India and Iran,33,35,36 where low sensitivity assays or other autoantibodies may be suspected. Whereas AQP4-Ab+ patients are likely to be a more consistent cohort to cross compare, AQP4-Ab− cohorts will vary depending on criteria used, access to supportive investigations, and even on the clinician's subjective evaluation.57 In addition, antibody against myelin oligodendrocyte glycoprotein (MOG) has been recognized in the AQP4-Ab− NMOSD subgroup. Since the increasing knowledge about MOG-Ab and related diseases, there has been increasing concern to separate MOG-Ab–positive patients with NMOSD phenotype from the AQP4-Ab+ and double-seronegative NMOSD.58 Only epidemiologic studies from Catalonia and Japan had the possibility to test all included AQP4-Ab− patients for anti-MOG.14,38 They showed that 0% (0/3) of the AQP4-Ab− prevalent NMOSD cases in the Asian population38 vs 47% (9/19)14 in the White population were positive for MOG-Ab. More studies are needed to confirm such population differences, especially in cases with low or borderline/uncertain AQP4-Ab positivity. Less standardized methods for MOG-Ab may also contribute to differences.
Epidemiologic studies were conducted in Martinique and Guadeloupe before the era of the AQP4-Ab test and again in Martinique a few years ago.10,24,25 Both population-based evaluations presented a high prevalence and incidence in African ancestry regardless of diagnostic criteria. The results without AQP4-Ab measurement were 4.2/100,000 (95% CI: 3.7–5.7) for crude prevalence and 0.19/100,000 person-years (95% CI: 0.15–0.23) for crude incidence, whereas the recent study including AQP4-Ab–positive patients based on the 2006 criteria gave a prevalence of 10/100,000 (95% CI: 6.8–13.2) and incidence of 0.73/100,000 person-years (95% CI: 0.45–1.01).
Further epidemiologic studies from Cuba, Mexico, and French West Indies showed lower estimates, which can be expected since the 1999 criteria were applied and access to AQP4-Ab testing was not available.26,40,59 Overall, studies reported relatively stable annualized incidence without any trend for significant changes over 10–25 years.14,15,20,24,27 Only 2 studies showed increasing incidence,16,23 whereas a single study showed an increasing prevalence over time that could be explained by reduced mortality.24 The increasing awareness of the disease and easier access to AQP4-Ab CBA may explain the rise of incidence.16 This together with the earlier introduction of adequate treatments and promising new therapies may result in longer survival and slight increase in the prevalence of NMOSD.
Data regarding pediatric populations are sparse, but indicate that NMOSD rarely develops in childhood.14,21,28–30,35,41 In Australia/New Zealand, onset before age 20 years was seen in 12% (Prof. Simon Broadley, MD, PhD, written personal communication, December 12, 2019).
In common with other systemic autoimmune diseases, studies universally demonstrated a female predominance. This may relate to female sex hormones and hormonal changes. Female genetic susceptibility to autoimmune disorders due to silenced X chromosome via epigenetic mechanisms has also been suggested.60–62 A recent multicenter study found potential association between exogenous hormonal exposure and NMOSD onset, whereas it was not associated with endogenous hormonal exposure.44 The average and/or median age at disease onset were similar in the majority of the studies, but all 3 studies from Iran34–36 and the studies from the Caribbeans10,24–26 reported a younger age at onset, which could be caused by environmental factors, ethnic influence, or study design. Finally, highest incidence rates reported in West Indians could be due to accumulation of genes of susceptibility coming from White, Indian, and African ancestry in this population.
Limitation
The major limitation of this work is that we did not use inclusion criteria for study design and quality. Therefore, the included studies have variable study designs that may affect the quality of their results and influence our conclusions. However, we critically reviewed all studies as shown in tables 5 and 6 and report the limitations and point out crucial weaknesses in each publication.
Conclusion
The incidence and prevalence of NMOSD vary among the studies; this could be due to the effects of genetic and environmental factors, but may also be related to differences in study design, study population, and quality of data. NMOSD has been described worldwide, but it seems to be less frequent in White populations compared with Asian and African ones.
Future studies should be carefully planned to obtain reliable results considering the rarity of the disease and to address epidemiology in more specifically designed settings: (1) by systematic methods of ascertainment with AQP4-Ab testing in all suspected demyelinating disease cases in a given population as gold standard or targeting of high-risk cases as secondary standard; (2) use of CBA for AQP4-Ab testing as recommended; (3) strict adherence to clinical and paraclinical criteria for AQP4-Ab− cases, as epidemiology data within specific serologic groups may be useful in identifying genetic effects and etiologic factors; (4) exclusion of MOG-Ab–positive cases; (5) if (1) is not possible, multiple sources and capture/recapture methodology should be used to minimize risks of missed cases; (6) prolonged period of case ascertainment should be considered to avoid missing inactive disease; and (7) inclusion of pediatric sources of cases.
Glossary
- AQP4-Ab
aquaporin-4 antibody
- CBA
cell-based assay
- IPND
International Panel for NMO Diagnosis
- NMO
neuromyelitis optica
- NMOSD
neuromyelitis optica spectrum disorder
- MOG
myelin oligodendrocyte glycoprotein
- ON
optic neuritis
- TM
transverse myelitis
Appendix. Authors
Footnotes
Podcast: NPub.org/e52wrv
Study Funding
V. Papp and Z. Illes are supported by Scleroseforeningen (R561-A39086), Odense University Hospital (72-A3846), Forskningspulje mellem RH og OUH (R58-A2888-B347), and Ministry of Human Capacities Hungary (FIKP 2. theme 20765/3/2018/FEKUTSTRAT). O. Aktas has received support by the German Ministry for Education and Research (BMBF) for the Neuromyelitis Optica Study Group (NEMOS) as part of the “German Competence Network Multiple Sclerosis” (KKNMS; FKZ 01GI1602B). S.A. Broadley is supported by GINOP 2.3.2-15-2016-00050 PEPSYS, Multiple Sclerosis Research Australia (11–038). J.-i. Kira is supported by grants from JSPS KAKENHI (Grant No. 16H02657), Health and Labour Sciences Research Grants on Intractable Diseases (H29-Nanchitou (Nan)-Ippan-043), and grants from the Japan Agency for Medical Research and Development (AMED) under Grant Numbers JP17ek0109115 and JP18ek0109376. M. Sepulveda and A. Saiz are supported in part by Red Española de Esclerosis Múltiple (REEM) (RD16/0015/0002) integrated in the Plan Estatal I+D+I and cofunded by ISCIII-Subdirección General de Evaluación and Fondo Europeo de Desarrollo Regional (FEDER, “Otra manera de hacer Europa”).
Disclosure
V. Papp has received honoraria for lecturing from Alexion and support for congress participation from Merck. M. Magyari has served on scientific advisory boards for Biogen, Sanofi, Teva, Roche, Novartis, and Merck and has received honoraria for lecturing from Biogen, Merck, Novartis, Sanofi, and Genzyme and support for congress participation from Biogen, Genzyme, Teva, and Roche. O. Aktas has received fees from Almirall, Bayer HealthCare, Biogen, MedImmune, Merck, Roche, Sanofi Genzyme, and Teva for speaker services and advisory boards and research grants from Biogen, Merck, and Sanofi Genzyme. T. Berger has participated in the last 2 years in meetings sponsored by and received honoraria (lectures, advisory boards, and consultations) from pharmaceutical companies marketing treatments for multiple sclerosis: Almirall, Biogen, Biologix, Bionorica, Celgene, MedDay, Merck, Novartis, Roche, Sanofi Aventis/Genzyme, TG Therapeutics, and Teva. His institution has received financial support in the last 2 years by unrestricted research grants (Biogen, Novartis, Sanofi Aventis/Genzyme, Roche, and Teva) and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Merck, Novartis, Roche, Sanofi Aventis/Genzyme, and Teva. S.A. Broadley has been a principal investigator for clinical trials sponsored by Biogen Idec, Sanofi Genzyme, and Alexion and has received honoraria for lecturing and participation in advisory boards from Bayer Schering, Biogen Idec, Merck, Novartis, Roche, and Sanofi Genzyme. S.A. Broadley has received sponsorship to attend international scientific meetings from Bayer Schering, Biogen Idec, Merck, Novartis, Roche, and Sanofi Genzyme and has been a recipient of unencumbered grants from Biogen Idec and Merck. P. Cabre received support from Novartis for congress participation. A. Jacob has no relevant disclosures. The United Kingdom NMO service is funded by a highly specialized commissioning of the NHS. J.-i. Kira received consultant fees, speaking fees, and/or honoraria from Novartis Pharma, Mitsubishi Tanabe Pharma, Boehringer Ingelheim, Teijin Pharma, Takeda Pharmaceutical Company, Otsuka Pharmaceutical, Astellas Pharma, Pfizer Japan, and Eisai. M.I. Leite is partly supported by NHS England highly specialized commissioning group for a neuromyelitis service. She has received support for scientific meetings and honorariums for presentations from Biogen Idec and Novartis, and for advisory work from Viela Bio. R. Marignier served on the scientific advisory board of MedImmune and received travel funding and speaker honoraria from Biogen, Genzyme, Novartis, Merck Serono, Roche, Sanofi Aventis, and Teva. K. Miyamoto received consultant fees, speaking fees, and/or honoraria from Biogen Japan, Novartis Pharma, Mitsubishi Tanabe Pharma, Asahi Kasei Medical, Alexion Pharma, Sumitomo Dainippon Pharma, and Ono Pharma. J. Palace is partly funded by highly specialized services to run a national congenital myasthenia service and a neuromyelitis service. She has received support for scientific meetings and honorariums for advisory work from Merck Serono, Biogen Idec, Novartis, Teva, Chugai Pharma, Bayer Schering, Alexion, Roche, Genzyme, MedImmune, Euroimmun, MedDay, Abide ARGENX, UCB, and Viela Bio and grants from Merck Serono, Novartis, Biogen Idec, Teva, Abide, MedImmune, Bayer Schering, Genzyme, Chugai, and Alexion. She has received grants from the MS society, Guthy-Jackson Foundation, NIHR, Oxford Health Services Research Committee, EDEN, MRC, GMSI, John Fell, and Myaware for research studies. A. Saiz received compensation for consulting services and speaker honoraria from Bayer Schering, Merck Serono, Biogen Idec, Sanofi Aventis, Teva, Roche, and Novartis. M. Sepulveda received speaking honoraria from Sanofi, Novartis, and Biogen. O. Sveinsson has received grants from GSK and Biogen and personal fees from Biogen and UCB, all outside the submitted work. Z. Illes has served on scientific advisory boards for Biogen, Sanofi, Teva, Roche, Novartis, and Merck and has received honoraria for lecturing from Biogen, Merck, Novartis and Sanofi and for clinical endpoint committees in clinical trials of NMOSD and support for congress participation from Biogen, Genzyme, Merck, Teva, and Roche. Go to Neurology.org/N for full disclosures.
References
- 1.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lennon VA, Kryzer TJ, Pittock SJ, et al. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 2005;202:473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wingerchuk DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–1489. [DOI] [PubMed] [Google Scholar]
- 4.Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–815. [DOI] [PubMed] [Google Scholar]
- 5.Borisow N, Kleiter I, Gahlen A, et al. Influence of female sex and fertile age on neuromyelitis optica spectrum disorders. Mult Scler 2017;23:1092–1103. [DOI] [PubMed] [Google Scholar]
- 6.Estrada K, Whelan CW, Zhao F, et al. A whole-genome sequence study identifies genetic risk factors for neuromyelitis optica. Nat Commun 2018;9:1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandit L, Asgari N, Apiwattanakul M, et al. Demographic and clinical features of neuromyelitis optica: a review. Mult Scler 2015;21:845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wingerchuk DM, Hogancamp WF, O'Brien PC, et al. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology 1999;53:1107. [DOI] [PubMed] [Google Scholar]
- 9.Miller D, Weinshenker B, Filippi M, et al. Differential diagnosis of suspected multiple sclerosis: a consensus approach. Mult Scler 2008;14:1157–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flanagan EP, Cabre P, Weinshenker BG, et al. Epidemiology of aquaporin-4 autoimmunity and neuromyelitis optica spectrum. Ann Neurol 2016;79:775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyamoto K, Fujihara K, Kira J, et al. Nationwide epidemiological study of neuromyelitis optica in Japan. J Neurol Neurosurg Psychiatry 2018;89:667–668. [DOI] [PubMed] [Google Scholar]
- 12.Jacob A, Panicker J, Lythgoe D, et al. The epidemiology of neuromyelitis optica amongst adults in the Merseyside county of United Kingdom. J Neurol 2013;260:2134–2137. [DOI] [PubMed] [Google Scholar]
- 13.Aboul-Enein F, Seifert-Held T, Mader S, et al. Neuromyelitis optica in Austria in 2011: to bridge the gap between neuroepidemiological research and practice in a study population of 8.4 million people. PLoS One 2013;8:e79649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sepúlveda M, Aldea M, Escudero D, et al. Epidemiology of NMOSD in Catalonia: influence of the new 2015 criteria in incidence and prevalence estimates. Mult Scler 2018;24:1843–1851. [DOI] [PubMed] [Google Scholar]
- 15.Papp V, Illes Z, Magyari M, et al. Nationwide prevalence and incidence study of neuromyelitis optica spectrum disorder in Denmark. Neurology 2018;91:e2265–e2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonsson DI, Sveinsson O, Hakim R, et al. Epidemiology of NMOSD in Sweden from 1987 to 2013. Neurology 2019;93:e181–e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asgari N, Lillevang ST, Skejoe HPB, et al. A population-based study of neuromyelitis optica in Caucasians. Neurology 2011;76:1589–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bukhari W, Prain KM, Waters P, et al. Incidence and prevalence of NMOSD in Australia and New Zealand. J Neurol Neurosurg Psychiatry 2017;88:632–638. [DOI] [PubMed] [Google Scholar]
- 19.Dale GH, Svendsen KB, Gjelstrup MC, et al. Incidence of neuromyelitis optica spectrum disorder in the Central Denmark Region. Acta Neurol Scand 2018;137:582–588. [DOI] [PubMed] [Google Scholar]
- 20.Daniëlle van Pelt E, Wong YYM, Ketelslegers IA, et al. Incidence of AQP4-IgG seropositive neuromyelitis optica spectrum disorders in The Netherlands: about one in a million. Mult Scler J Exp Transl Clin 2016;2:2055217315625652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holroyd KB, Aziz F, Szolics M, et al. Prevalence and characteristics of transverse myelitis and neuromyelitis optica spectrum disorders in the United Arab Emirates: a multicenter, retrospective study. Clin Exp Neuroimmunol 2018;9:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viswanathan S, Wah LM. A nationwide epidemiological study on the prevalence of multiple sclerosis and neuromyelitis optica spectrum disorder with important multi-ethnic differences in Malaysia. Mult Scler 2019;25:1452–1461. [DOI] [PubMed] [Google Scholar]
- 23.Vásquez Céspedes J. Epidemiología de la neuromielitis óptica en Costa Rica: un análisis multicéntrico. Neurol Argentina 2018;10:185–193. [Google Scholar]
- 24.Cabre P, Gonzalez-Quevedo A, Lannuzel A, et al. Épidémiologie descriptive de la neuromyélite optique dans le bassin caraïbéen. Rev Neurol (Paris) 2009;165:676–683. [DOI] [PubMed] [Google Scholar]
- 25.Cabre P. Environmental changes and epidemiology of multiple sclerosis in the French West Indies. J Neurol Sci 2009;286:58–61. [DOI] [PubMed] [Google Scholar]
- 26.Cabrera-Gómez Ja, Kurtzke JF, González-Quevedo A, et al. An epidemiological study of neuromyelitis optica in Cuba. J Neurol 2009;256:35–44. [DOI] [PubMed] [Google Scholar]
- 27.Lee JD, Guimond C, Yee IM, et al. Incidence of multiple sclerosis and related disorders in Asian populations of British Columbia. Can J Neurol Sci 2015;42:235–241. [DOI] [PubMed] [Google Scholar]
- 28.Gudbjornsson BT, Haraldsson Á, Einarsdóttir H, et al. Nationwide incidence of acquired central nervous system demyelination in Icelandic children. Pediatr Neurol 2015;53:503–507. [DOI] [PubMed] [Google Scholar]
- 29.Boesen MS, Magyari M, Koch-Henriksen N, et al. Pediatric-onset multiple sclerosis and other acquired demyelinating syndromes of the central nervous system in Denmark during 1977–2015: a nationwide population-based incidence study. Mult Scler 2018;24:1077–1086. [DOI] [PubMed] [Google Scholar]
- 30.Boesen MS, Jensen PEH, Born AP, et al. Incidence of pediatric neuromyelitis optica spectrum disorder and myelin oligodendrocyte glycoprotein antibody-associated disease in Denmark 2008‒2018: a nationwide, population-based cohort study. Mult Scler Relat Disord 2019;33:162–167. [DOI] [PubMed] [Google Scholar]
- 31.Papp V, Iljicsov A, Rajda C, et al. A population-based epidemiological study of neuromyelitis optica spectrum disorder in Hungary. Eur J Neurol 2020;27:308–317. [DOI] [PubMed] [Google Scholar]
- 32.Cossburn M, Tackley G, Baker K, et al. The prevalence of neuromyelitis optica in South East Wales. Eur J Neurol 2012;19:655–659. [DOI] [PubMed] [Google Scholar]
- 33.Pandit L, Kundapur R. Prevalence and patterns of demyelinating central nervous system disorders in urban Mangalore, South India. Mult Scler 2014;20:1651–1653. [DOI] [PubMed] [Google Scholar]
- 34.Etemadifar M, Dashti M, Vosoughi R, et al. An epidemiological study of neuromyelitis optica in Isfahan. Mult Scler 2014;20:1920–1922. [DOI] [PubMed] [Google Scholar]
- 35.Eskandarieh S, Nedjat S, Azimi AR, et al. Neuromyelitis optica spectrum disorders in Iran. Mult Scler Relat Disord 2017;18:209–212. [DOI] [PubMed] [Google Scholar]
- 36.Kashipazha D, Mohammadianinejad SE, Majdinasab N, et al. A descriptive study of prevalence, clinical features and other findings of neuromyelitis optica and neuromyelitis optica spectrum disorder in Khuzestan Province, Iran. Iran J Neurol 2015;14:204–210. [PMC free article] [PubMed] [Google Scholar]
- 37.Hor JY, Lim TT, Chia YK, et al. Prevalence of neuromyelitis optica spectrum disorder in the multi-ethnic Penang Island, Malaysia, and a review of worldwide prevalence. Mult Scler Relat Disord 2018;19:20–24. [DOI] [PubMed] [Google Scholar]
- 38.Houzen H, Kondo K, Niino M, et al. Prevalence and clinical features of neuromyelitis optica spectrum disorders in northern Japan. Neurology 2017;89:1995–2001. [DOI] [PubMed] [Google Scholar]
- 39.Houzen H, Niino M, Hirotani M, et al. Increased prevalence, incidence, and female predominance of multiple sclerosis in northern Japan. J Neurol Sci 2012;323:117–122. [DOI] [PubMed] [Google Scholar]
- 40.Rivera JF, Kurtzke JF, Booth VJA, et al. Characteristics of Devic's disease (neuromyelitis optica) in Mexico. J Neurol 2008;255:710–715. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi Y, Torisu H, Kira R, et al. A nationwide survey of pediatric acquired demyelinating syndromes in Japan. Neurology 2016;87:2006–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khaibullin T, Kirillova E, Bikbaev R, et al. Clinical-epidemiological characteristics of multiple sclerosis and neuroopticomyelitis in the Central Asia. Zh Nevrol Psikhiatr Im S S Korsakova 2019;119:12–17. [DOI] [PubMed] [Google Scholar]
- 43.Asgari N, Lillevang ST, Skejoe HPB, et al. Epidemiology of neuromyelitis optica spectrum disorder in Denmark (1998–2008, 2007–2014). Brain Behav 2019;9:e01338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bove R, Elsone L, Alvarez E, et al. Female hormonal exposures and neuromyelitis optica symptom onset in a multicenter study. Neurol Neuroimmunol Neuroinflamm 2017;4:e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hor JY, Asgari N, Nakashima I, et al. Epidemiology of neuromyelitis optica spectrum disorder and its prevalence and incidence worldwide. Front Neurol 2020;11:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alvarenga M, Schimidt S, Alvarenga RP. Epidemiology of neuromyelitis optica in Latin America. Mult Scler J Exp Transl Clin 2017;3:2055217317730098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matiello M, Kim HJ, Kim W, et al. Familial neuromyelitis optica. Neurology 2010;75:310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Apiwattanakul M, Kasemsuk C. NMO spectrum disorders comprise the major portion of CNS inflammatory diseases in Thai patients: a cross sectional study. Mult Scler Relat Disord 2014;3:61–66. [DOI] [PubMed] [Google Scholar]
- 49.Howlett WP. Inflammatory neurologic disease in sub-Saharan Africa. Neurology 2014;83:656–658. [DOI] [PubMed] [Google Scholar]
- 50.Papais-Alvarenga RM, Vasconcelos CCF, Carra A, et al. Central nervous system idiopathic inflammatory demyelinating disorders in South Americans: a descriptive, multicenter, cross-sectional study. PLoS One 2015;10:e0127757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brill L, Mandel M, Karussis D, et al. Increased occurrence of anti-AQP4 seropositivity and unique HLA Class II associations with neuromyelitis optica (NMO), among Muslim Arabs in Israel. J Neuroimmunol 2016;293:65–70. [DOI] [PubMed] [Google Scholar]
- 52.Mori M, Kuwabara S, Paul F. Worldwide prevalence of neuromyelitis optica spectrum disorders. J Neurol Neurosurg Psychiatry 2018;89:555–556. [DOI] [PubMed] [Google Scholar]
- 53.Waters P, Reindl M, Saiz A, et al. Multicentre comparison of a diagnostic assay: aquaporin-4 antibodies in neuromyelitis optica. J Neurol Neurosurg Psychiatry 2016;87:1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marignier R, Bernard-Valnet R, Giraudon P, et al. Aquaporin-4 antibody-negative neuromyelitis optica: distinct assay sensitivity-dependent entity. Neurology 2013;80:2194–2200. [DOI] [PubMed] [Google Scholar]
- 55.Waters PJ, McKeon A, Leite MI, et al. Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assays. Neurology 2012;78:665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fryer JP, Lennon VA, Pittock SJ, et al. AQP4 autoantibody assay performance in clinical laboratory service. Neurol Neuroimmunol Neuroinflamm 2014;1:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Juryńczyk M, Weinshenker B, Akman-Demir G, et al. Status of diagnostic approaches to AQP4-IgG seronegative NMO and NMO/MS overlap syndromes. J Neurol 2016;263:140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zamvil SS, Slavin AJ. Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol Neuroimmunol Neuroinflamm 2015;2:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cabre P, Heinzlef O, Merle H, et al. MS and neuromyelitis optica in Martinique (French West Indies). Neurology 2001;56:507–514. [DOI] [PubMed] [Google Scholar]
- 60.Strickland FM, Hewagama A, Lu Q, et al. Environmental exposure, estrogen and two X chromosomes are required for disease development in an epigenetic model of lupus. J Autoimmun 2012;38:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yin X, Latif R, Tomer Y, et al. Thyroid epigenetics: X chromosome inactivation in patients with autoimmune thyroid disease. Ann N Y Acad Sci 2007;1110:193–200. [DOI] [PubMed] [Google Scholar]
- 62.Selmi C, Gershwin ME. Sex and autoimmunity: proposed mechanisms of disease onset and severity. Expert Rev Clin Immunol 2019;15:607–615. [DOI] [PubMed] [Google Scholar]