Abstract
Linear polymers for many materials applications are popularly produced via step-growth polymerizations of different pairs of A2 and B2 monomers. However, achieving high molecular weights during the synthesis is dramatically limited by the required stoichiometric balance of A and B reactive groups when reactivity is considered unchanged during the polymerization. This short review summarizes the recent progress on using Friedel–Crafts polycondensation reactions to produce high-molecular-weight linear polymers via the reaction-enhanced reactivity of intermediate (RERI) mechanism, in which the reaction of one functional group in the bifunctional monomer spontaneously increases the reactivity of the other functional group on the monoreacted intermediate for faster consumption and connection into polymer chains. Thus, using an excess amount of this monomer produces linear polymers in high molecular weights. Both Friedel–Crafts acylation and hydroxyalkylation reactions have been reported for syntheses of long polymer chains under nonstoichiometric conditions, although the focus is to illustrate the significant progress of applying Friedel–Crafts hydroxyalkylation reactions to produce linear polymers with high molecular weights and varied compositions.
1. Introduction
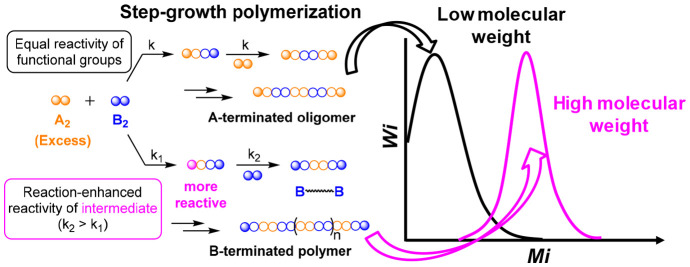
Step-growth polymerization, as one of the two fundamental mechanisms, is governing the production of various high-performance polymer materials, such as polyamides, polyketones, and various backbone-conjugated polymers. Classic kinetics theory in the step-growth polymerization has been well-established, in which a strictly balanced stoichiometry of bifunctional A2 and B2 monomers (i.e., A and B react with each other selectively) is required to produce linear polymers in high molecular weights.1 When all reactive groups are considered to share the same reactivity, the terminal groups of oligomers and polymer species in the system are determined by the molar ratio of A2 and B2 monomers (Scheme 1A). Slight imbalance of the stoichiometry of A and B groups, due to either physical loss of monomers or imperfect monomer synthesis, causes a significant decrease in the number-average degree of polymerization: DPn = (1 + r)/(1 – r) at 100% conversion of deficient reactive group, where r (≤1) is the molar ratio of the two bifunctional monomers. Meanwhile, impurity of multifunctional An or Bm (m, n > 2) compounds in feed monomers could produce branched and even cross-linked structures in the polymer chains.
Scheme 1. Two Scenarios in Step-Growth Polymerizations of A2 and B2 Monomers: (A) Equal Reactivity of All A and B Reactive Groups and (B) Reaction-Enhanced Reactivity of Intermediate (RERI) of the Bifunctional Monomer (e.g., A2).
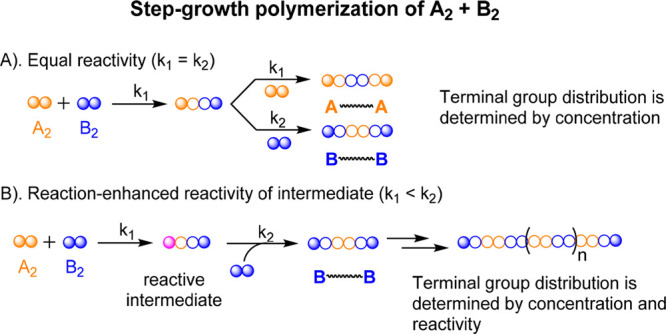
Mechanical properties of polymer materials are critically determined by the molecular weights. Meanwhile, polymerization systems with imbalanced A and B ratios are more readily obtained than strictly balanced ones. It becomes compelling that several polymerization systems have been developed that could achieve high molecular weight under nonstoichiometric conditions, and all of them are based on the same designing principle: reaction-enhanced reactivity of intermediate (RERI) of at least one participating monomer, such as A2. In this principle, the two A reactive groups in the original A2 monomer have the same reactivity, while the reaction of any A group with a B2 monomer increases the reactivity of the remaining A group in the formed intermediate for a faster reaction or instantaneous reaction in an ideal scenario when k2 ≫ k1 (Scheme 1B). Thus, there is no half-reacted A2 monomer unit in the system as all produced oligomers and polymers, regardless of the polymer DPs, are terminated with B groups, which share the same reactivity as the B2 monomer for further reacting with more A2 monomers when available. Based on this design, it is expected that the polymerization with a higher molar ratio of A2 to B2 monomers in the feed favors obtaining higher molecular weights than systems in a lower ratio as the A2 monomer functions as linker to connect all B-terminated monomers, oligomers, and polymers into higher-molecular-weight entities until all B groups are reacted.
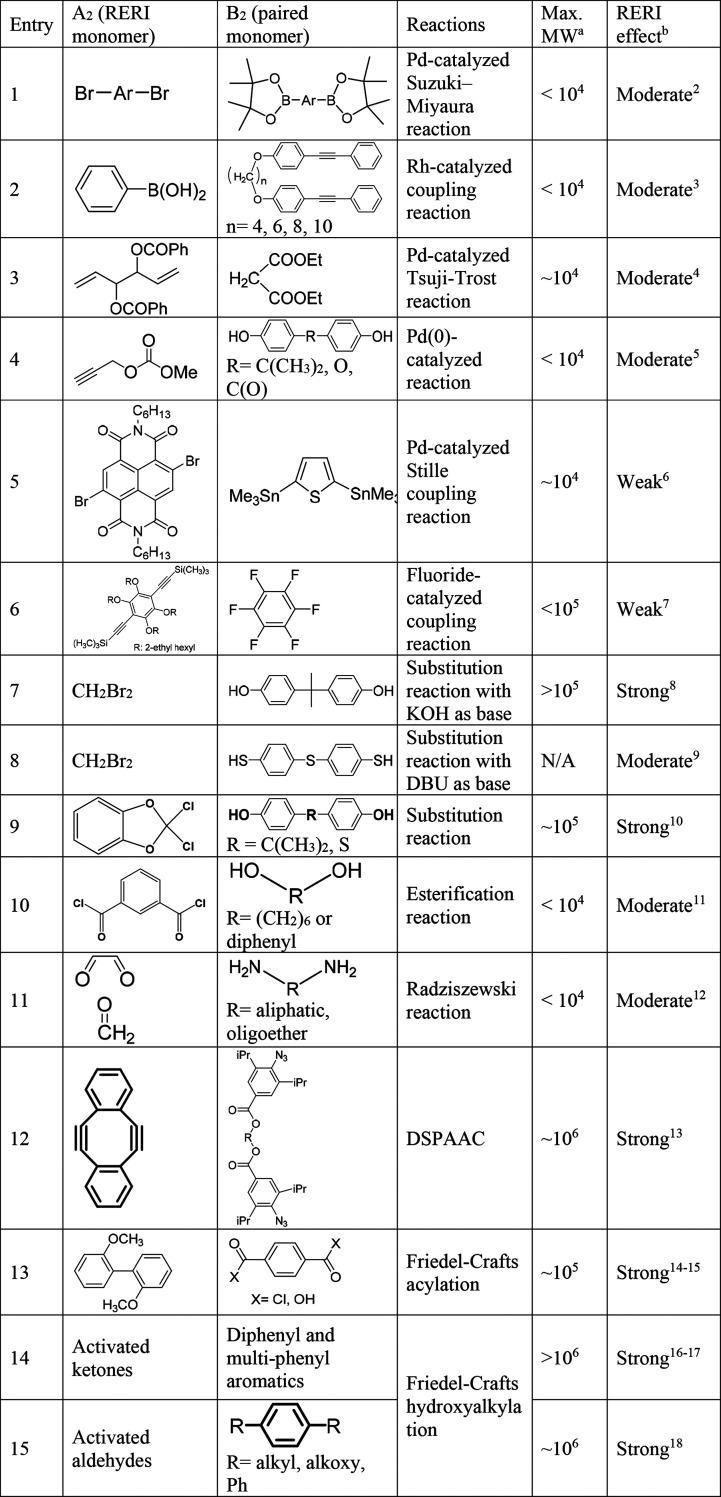
Several types of organic reactions that demonstrate such a RERI feature have been successfully applied in constructing polymers with high molecular weight when using a nonstoichiometric ratio of monomers, including transition-metal-catalyzed coupling reactions (entries 1–5, Table 1),3−6 fluoride-catalyzed coupling reaction (entry 6, Table 1),7 nucleophilic substitution reactions (entries 7–9, Table 1),8−10 esterification reaction (entry 10, Table 1),11 multicomponent Radziszewski reaction (entry 11, Table 1),12 double-strain-promoted azide–alkyne cycloaddition reaction (DSPAAC, entry 12, Table 1),13 and electrophilic aromatic substitution reactions (entries 13–15, Table 1). All of these reactions showed a two-stage kinetics of the A2 monomer, in which the kinetic constant k2 in the reaction of the second A group was always higher than the k1 in the first A group reaction (Scheme 1B). Excess A2 monomers in the polymerizations significantly increased the molecular weight of the polymers. For example, Pd-catalyzed Suzuki–Miyaura polycondensation reactions of dibromoarene and arylenediboronic acid ester produced oligomers and polymers with exclusive boronic acid ester (i.e., boronate) terminal groups as intramolecular catalyst transfer quickly consumed the two bromo groups within one monomer (entry 1, Table 1). The Pd catalyst could then release from one chain end and had an intermolecular transfer to another dibromoarene monomer for linking two new arylenediboronic acid ester monomers or boronate-terminated chains.2 Yokozawa’s group demonstrated that varying the monomer ratio of p-dibromophenylene to p-phenylenedibronic acid pinacol ester from 1:1.3 to 1:1 and 1.3:1 effectively increased the number-average molecular weight (Mn) of purified polymers from Mn = 3,520 to 19,600. In another example, Tang and his group applied Rh-catalyzed oxidation of 4,4′-(α,ω-alkylenedioxy)bis(diphenylacetylene)s with phenylboronic acid to form linear polymers containing highly substituted naphthalene units in backbones with high molecular weight (entry 2, Table 1).3 The key step in this reaction was the different rates of alkyne insertion (k1 < k2), which ensured all polymer chains were terminated with alkyne groups and promoted the formation of high-molecular-weight polymers.
Table 1. Summary of Various Step-Growth Polymerizations with RERI Mechanism.
Maximum molecular weight (MW) referred to the highest number-average molecular weight or weight-average molecular weight reported in the literature.
The categories of RERI effects were defined as (1) “strong” when the use of excess RERI monomer achieved a polymer with a molecular weight >105, (2) “moderate” when the use of more RERI monomer increased the polymer molecular weight, and (3) “weak” when the use of more RERI monomer showed decreased molecular weight although the reported k2 was still larger than k1.
As the Cu-catalyzed azide–alkyne cycloaddition reaction has been popularly applied in polymer synthesis and functionalization, RERI-featured diazide or dialkyne monomers were designed for the synthesis of linear polymers,13 densely grafted polymers,19 and branched polymers20 with high molecular weights. Zhang and co-workers recently reported the synthesis of ultrahigh-molecular-weight linear polymers via DSPAAC of sym-dibenzo-1,5-cyclooctadiene-3,7-diyne with a bis-azide compound, in which the cyclic diyne functions as the A2 monomer, showing a reaction-induced higher reactivity of the intermediate with the rate constant ratio (k2/k1) at about 185 in the model reaction (entry 12, Table 1).13
Acid-catalyzed Friedel–Crafts reactions represent one important type of carbon–carbon bond forming reactions to incorporate alkyl, acyl, and hydroxyalkyl groups onto aryl rings. Due to the presence of aromatic rings in the substrates, the polar effect shows significant influence in Friedel–Crafts reactions on reaction kinetics, which has been intensively studied regarding the polarity of precedent substituted groups on the kinetics (i.e., acceleration or deceleration) of the subsequent substitution reactions. In addition, the substrates, reagents, and reaction conditions in Friedel–Crafts reactions are commonly available and inexpensive, which facilitates the application of Friedel–Crafts reactions in RERI-featured polycondensation reactions to produce high-molecular-weight linear polymers.
There are three categories of Friedel–Crafts reactions: Friedel–Crafts alkylation, acylation, and hydroxyalkylation reactions that use different electrophiles, acid catalysts, and conditions. So far, all three reactions have been applied in polycondensation reactions to produce linear polymers containing aromatic rings in backbones, although not all of them have demonstrated significant reactivity enhancement of the monoreacted intermediates. For instance, Friedel–Crafts alkylation reactions have been reported for polymerization of an AB bifunctional monomer,21 such as benzyl chloride or benzyl alcohol, to synthesize linear poly(phenylene methylene)s or polybenzyl, although the syntheses did not exhibit a dramatic RERI feature. Nevertheless, the produced linear polybenzyls demonstrated an interesting feature of homoconjugation fluorescence in both solutions and thin films due to the proximity of aryl rings in the backbone.22,23
This mini-review article plans to summarize the recent employment of Friedel–Crafts acylation and hydroxyalkylation polycondensation reactions for synthesis of linear polymers using an excess amount of the RERI monomers. Depending on the reaction mechanism, these RERI monomers in the polymerizations could be nucleophilic monomers, such as biphenyls, in the acylation reaction or electrophilic monomers, such as benzaldehydes and activated ketones, in the hydroxyalkylation reaction. The reported reactions took advantage of the RERI feature to produce linear polymer chains with exclusive B terminal groups but varied primary chain sequences, ranging from statistical to block and multisegmented copolymers. These linear polymers with aromatic-rich backbones and varied substituent groups exhibit interesting properties and are expected to trigger further exploration of their applications in sensors, gas separation membranes, and gas storage materials.
2. Friedel–Crafts Acylation Reaction
Friedel–Crafts acylation reactions between diphenyl ether and aromatic dicarboxylic acid chlorides using Lewis acid catalysts, such as AlCl3, have been reported in the synthesis of aromatic poly(ether ketone ketone) polymers,24 as the polymers represent a type of semicrystalline plastic with excellent thermal, mechanical, and solvent resistance properties. However, the polymerization system was not used to demonstrate the RERI feature and produce high molecular weights until Matsumoto et al. recently reported the polycondensation between aromatic dicarboxylic acid or diacyl chloride and 2,2′-dimethoxybiphenyl for producing aromatic polyketones.14,15 High-molecular-weight polymers with weight-average molecular weight Mw ∼ 105 were successfully obtained when excess 2,2′-dimethoxybiphenyl was used in the presence of several equivalents of AlCl3 catalysts (entry 13, Table 1). Mechanistic studies found that the AlCl3 could selectively coordinate with the methoxy groups in the monomer to deactivate its reaction with acyl chloride. Meanwhile, there was no such deactivation on the monoacylated intermediates. Therefore, a first-step acylation of 2,2′-dimethoxybiphenyl slower than that of the second-step reaction (k1 < k2) resulted in an overall accelerating reaction of the monomers to achieve high molecular weight when using excess 2,2′-dimethoxybiphenyl monomers.
3. Friedel–Crafts Hydroxyalkylation Reaction
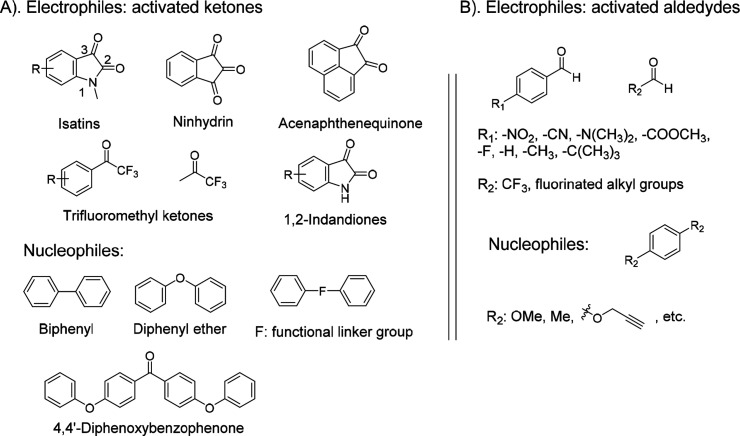
Acid-catalyzed condensation reactions between arenes and activated carbonyls (e.g., ketones and aldehydes), so-called the Friedel–Crafts hydroxyalkylation reactions, produce monosubstituted hydroxyalkylated compounds (entries 14 and 15, Table 1).25 When using superacids, such as trifluoromethanesulfonic acid (TFSA), as catalysts, some systems reported that only disubstituted products were observed since the generated hydroxyalkylated intermediate could be further activated into a much more reactive benzyl cation for further reaction with another arene ring, coined by Olah in the proposed superelectrophilic activation.26 For instance, the reactions of isatin, an activated ketone, with aromatics showed high regioselectivity, in which the para positions of two aromatics attacked the 3-carbonyl group of one isatin with no monoarylated product observed (Scheme 2A). So far, various activated ketones, including isatins, ninhydrin, acenaphthenequinone, trifluoromethyl ketones, or 1,2-indandiones, have demonstrated this RERI feature in the reaction with 2 equivalents of arenes to produce clean diarylated products. Many substrates have been used or modified as monomers in polymerizations with various biphenyls, diphenyl ethers, and multiphenyl aromatics for producing high-molecular-weight linear polymers,16,18 dendrimers, hyperbranched polymers, and microporous polymer networks.
Scheme 2. Friedel–Crafts Hydroxyalkylation Polycondensation Using RERI Monomers: (A) Activated Ketones and (B) Activated Aldehydes.
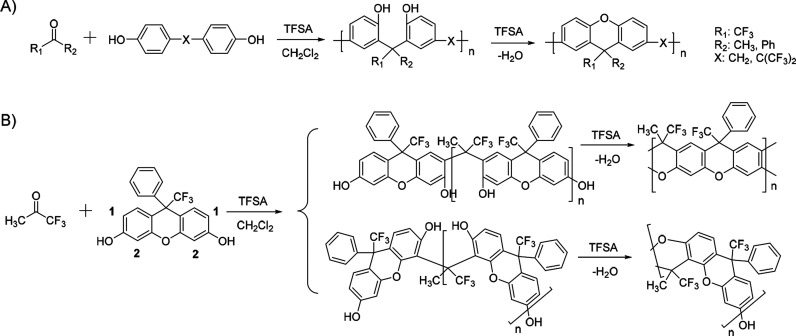
Zolotukhin and co-workers first applied Friedel–Crafts polycondensation of isatins with 4,4′-diphenoxybenzophenone (Scheme 2A) using TFSA as the catalyst to produce high-molecular-weight linear polymers.27 Although Olah demonstrated high regioselectivity in the reaction of monosubstituted benzene with isatin, the synthesis of linear polymers placed more demanding requirements on the regioselectivity of aromatics. The authors discovered that monomers, such as diphenyl ether, biphenyl, and p-terphenyl, could not achieve clean linear polymers as the aromatic rings in polymers also participated in ortho-substitution reactions to introduce branched structures, as observed in the proton nuclear magnetic resonance (1H NMR) spectroscopy. Meanwhile, 4,4′-diphenoxybenzophenone, due to the inductive deactivation of the carbonyl group, showed much higher regioselectivity only at the two terminal para positions, ensuring a linear polymer structure. Zolotukhin and co-workers subsequently expanded the electrophilic monomer family using acenaphthenequinone, trifluoroacetone, and trifluoroacetophenone for polycondensation with diphenyl ether, biphenyl, and substituted derivatives to produce linear polymers with high molecular weights under nonstoichiometric conditions.16,17 In all of these polymerizations, the activated carbonyl groups could quickly react with two nucleophilic arenes consecutively to produce oligomers and polymers exclusively terminated by the nucleophilic aromatic monomer units. Functional moieties,28 such as fluoroaromatics, thiadiazoles, and fluorenone groups, could be incorporated into the aromatic nucleophilic monomers to produce high-molecular-weight polymers with functional backbone compositions. One interesting example was the polycondensation reaction between trifluoroacetophenone and bisphenol monomers, which occurred exclusively at ortho positions to the hydroxy phenol group. Followed by an efficient cyclodehydration reaction of hydroxyl-containing intermediates, the polymerization produced substituted 9H-xanthene-2,7-diyl polymers, as shown in Scheme 3A.17 When a bisphenol monomer carrying a fused xanthene structure was used to react with trifluoroacetone for polycondensation, a fully fused-ring backbone polymer chain (ladder polymer) became possible, in which the two possible reaction sites (1 and 2 positions, Scheme 3B) on the xanthene rings resulted in two structural isomers of the ladder polymers.29 These polymers with rigid and contorted backbones have been applied as intrinsic porous membrane materials for gas separation (e.g., O2 vs N2). Meanwhile, further sulfonation of the aromatic backbones could generate confined ion channels to achieve selective transport of proton and potassium cations.
Scheme 3. (A) Synthesis of Linear Substituted 9H-Xanthene-2,7-diyl Polymers and (B) Ladder Polymers Using Friedel–Crafts Hydroxyalkylation Polycondensation.
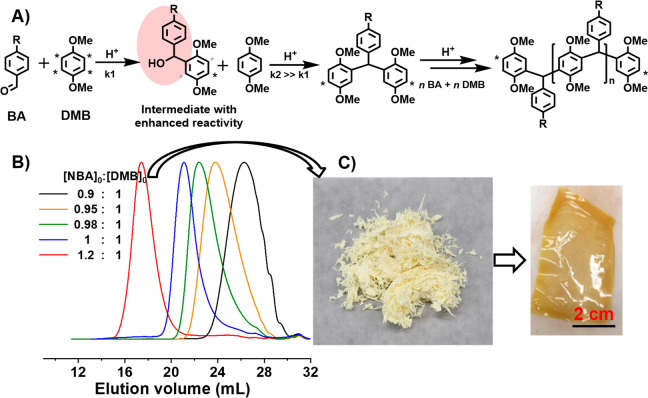
In addition to the use of activated ketones as RERI monomers, activated aldehydes with either aromatic or aliphatic substituent groups have also been explored for Friedel–Crafts polycondensations with a nucleophilic monomer (Scheme 2B). In particular, 4-substituted benzaldehydes (BA) are very available at an inexpensive price, which provides many compositional variations to produce functional linear polymers. Our group recently developed a polymerization system that used 1,4-disubstituted benzenes with four identical reactive sites, such as dimethoxybenzene (DMB) and xylene, as nucleophilic monomers to react with various 4-substituted BAs as electrophilic monomers for the synthesis of regioselective linear polymers carrying a triarylmethane-based backbone (Figure 1A).18 The polycondensation reactions showed a distinct RERI feature, in which any diaryl carbinol intermediates exhibited reactivity much higher than that of the BA monomers for reaction with either DMB monomers or DMB-terminated oligomers and, therefore, did not accumulate in the reaction system. The use of excess BA monomers, such as 4-nitrobenzaldedhye (NBA), produced linear polymers with a molecular weight >500 × 103 (Figure 1B), which was critical to produce purified polymer in fibrous textiles and to prepare freestanding membranes from facile solvent casting (Figure 1C).
Figure 1.
Friedel–Crafts hydroxyalkylation polycondensation of benzaldehydes (BAs) and 1,4-dimethoxybenzene (DMB) to produce high-molecular-weight linear polymers: (A) proposed mechanism with reactive intermediate, (B) increased polymer molecular weight when using excess amounts of 4-nitrobenzaldehyde (NBA), (C) digital picture of produced polymer in fibrous appearance and easy cast into freestanding membrane. Reprinted from ref (18). Copyright 2018 American Chemical Society.
The polymers and oligomers formed in this polymerization were exclusively terminated by DMB monomer units at any chain length, which share similar reactivity as free DMB monomers (Figure 1A). Thus, possibility arises that different oligomers could be mixed together being used as macromonomers for reaction with additional BA monomers to construct a multisegmented block copolymer (MSBCP) that carries various functional substituent groups along the polymer backbone. For instance, polymerizations of DMB with various BA monomers afforded a library of triarylmethane-backboned oligomers with nitro, fluoro, alkyl ester, dimethylamino, and tert-butyl pendant functional groups (Scheme 2B).30 A mixture of several oligomers was used as macromonomers in a subsequent polymerization to produce MSBCPs with tunable compositions and properties, such as tunable glass transition temperature and surface contact angle. The features identified during the synthesis of these MSBCPs, including the facile reaction setup, tunable segment lengths, and substituent groups in each block, demonstrate promising applicability of these triarylmethane-backboned polymers and copolymers for use in specialty membrane applications.
4. Conclusion and Outlook
Polymers synthesized via step-growth polycondensation reactions are limited by strictly required stoichiometric balance of monomers to achieve high molecular weights. To avoid this equal-mole requirement and obtain long linear polymer chains more easily, this paper summarizes recent progress on the synthesis of high-molecular-weight linear polymers via nonstoichiometry of A2 and B2 monomers in step-growth polymerizations that all follow the principle of the reaction-enhanced reactivity of intermediate mechanism. Various polymerization systems were discussed, although the focus was on the application of Friedel–Crafts hydroxyalkylation reactions to produce linear polymers with aromatic backbone units and high molecular weights. Both activated ketones and aldehydes (aromatic and fluorinated compounds) were selected as bifunctional RERI monomers to react with many choices of arene compounds, in which the intermediates demonstrated a reactivity much higher than that of the monomers, exclusively producing polymers and oligomers terminated by the arene monomer units.
As the facile synthesis has been demonstrated, future direction on exploring this compelling polymerization technique should focus on establishing the correlation from polymer structures and compositions to their physical properties, especially the dependence of mechanical properties on polymer molecular weights. Functional monomers by incorporating various reactive groups, electro-, opto-, and bioactive groups should be synthesized to explore their polymerizability and compatibility within the polymerization conditions. Furthermore, the rigid backbone structures of the polymers produced represent some ideal precursors for further aromatic ring fusing reactions, via dehydration, nucleophilic substitution, or electrophilic substitution reactions, to produce backbone-conjugated polymers and ladder-shaped polymers. These rigid polymers in high molecular weights are expected to demonstrate intrinsic microporosity properties in films and powders, which will create new opportunities to develop polymer porous materials for catalysis, gas separation, and capture.
Acknowledgments
The author thanks the financial support from the ACS Petroleum Research Fund (PRF #59601-ND7), the National Science Foundation (CBET-2006242), and the University of Notre Dame.
Biography

Haifeng Gao received a B.S. degree in Macromolecular Science (Fudan University, China) in 2000 and a Ph.D. degree in Chemistry (Carnegie Mellon University, USA) in 2008. He then worked as a postdoc at the University of California at Berkeley in 2009–2011 and is currently an Associate Professor at the University of Notre Dame. His current research focuses on the design and synthesis of functional polymers with controlled nanostructures by determining their fundamental structure–property relationships.
The author declares no competing financial interest.
References
- Odian G.Principles of Polymerization, 4h ed.; Wiley: Hoboken, NJ, 2004. [Google Scholar]
- Nojima M.; Kosaka K.; Kato M.; Ohta Y.; Yokozawa T. Alternating Intramolecular and Intermolecular Catalyst-Transfer Suzuki–Miyaura Condensation Polymerization: Synthesis of Boronate-Terminated π-Conjugated Polymers Using Excess Dibromo Monomers. Macromol. Rapid Commun. 2016, 37, 79–85. 10.1002/marc.201500587. [DOI] [PubMed] [Google Scholar]
- Gao M.; Lam J. W. Y.; Li J.; Chan C. Y. K.; Chen Y.; Zhao N.; Han T.; Tang B. Z. Stoichiometric imbalance-promoted synthesis of polymers containing highly substituted naphthalenes: rhodium-catalyzed oxidative polycoupling of arylboronic acids and internal diynes. Polym. Chem. 2013, 4, 1372–1380. 10.1039/C2PY20758C. [DOI] [Google Scholar]
- McCullagh J. V.; Daggett K. A. Synthesis of Triarylmethane and Xanthene Dyes Using Electrophilic Aromatic Substitution Reactions. J. Chem. Educ. 2007, 84, 1799. 10.1021/ed084p1799. [DOI] [Google Scholar]
- Takemura T.; Sugie K.; Nishino H.; Kawabata S.; Koizumi T. Pd(0)-catalyzed polycondensation of methyl propargyl carbonate and bisphenols under stoichiometrically imbalanced conditions. J. Polym. Sci., Part A: Polym. Chem. 2008, 46, 2250–2261. 10.1002/pola.22560. [DOI] [Google Scholar]
- Goto E.; Ando S.; Ueda M.; Higashihara T. Nonstoichiometric Stille Coupling Polycondensation for Synthesizing Naphthalene-Diimide-Based π-Conjugated Polymers. ACS Macro Lett. 2015, 4, 1004–1007. 10.1021/acsmacrolett.5b00532. [DOI] [PubMed] [Google Scholar]
- Dutta T.; Woody K. B.; Watson M. D. Transition-Metal-Free Synthesis of Poly(phenylene Ethynylene)s with Alternating Aryl-Perfluoroaryl Units. J. Am. Chem. Soc. 2008, 130, 452–453. 10.1021/ja710564b. [DOI] [PubMed] [Google Scholar]
- Miyatake K.; Hlil A. R.; Hay A. S. High Molecular Weight Aromatic Polyformals Free of Macrocyclic Oligomers. A Condensative Chain Polymerization Reaction. Macromolecules 2001, 34, 4288–4290. 10.1021/ma002020r. [DOI] [Google Scholar]
- Iimori H.; Shibasaki Y.; Ando S.; Ueda M. Nonstoichiometric polycondensation I. synthesis of polythioether from dibromomethane and 4,4′-thiobisbenzenethiol. Macromol. Symp. 2003, 199, 23–36. 10.1002/masy.200350903. [DOI] [Google Scholar]
- Kihara N.; Komatsu S.-i.; Takata T.; Endo T. Significance of Stoichiometric Imbalance in Step Polymerization via Reactive Intermediate. Macromolecules 1999, 32, 4776–4783. 10.1021/ma981835l. [DOI] [Google Scholar]
- Kricheldorf H. R.; Zolotukhin M. G.; Cárdenas J. Non-Stoichiometric Polycondensations and the Synthesis of High Molar Mass Polycondensates. Macromol. Rapid Commun. 2012, 33, 1814–1832. 10.1002/marc.201200345. [DOI] [PubMed] [Google Scholar]
- Lindner J.-P. Imidazolium-Based Polymers via the Poly-Radziszewski Reaction. Macromolecules 2016, 49, 2046–2053. 10.1021/acs.macromol.5b02417. [DOI] [Google Scholar]
- Zhang L.; Ren X.; Zhang Y.; Zhang K. Step-Growth Polymerization Method for Ultrahigh Molecular Weight Polymers. ACS Macro Lett. 2019, 8, 948–954. 10.1021/acsmacrolett.9b00475. [DOI] [PubMed] [Google Scholar]
- Matsumoto K.; Fukui C.; Shoji R.; Jikei M. Synthesis of aromatic polyketones by nonstoichiometric Friedel–Crafts polycondensation using AlCl3. Polym. Chem. 2020, 11, 4221–4227. 10.1039/D0PY00534G. [DOI] [Google Scholar]
- Matsumoto K.; Ogawa T.; Jikei M. Nonstoichiometric polycondensation based on Friedel-Crafts acylation in superacids for the syntheses of aromatic polyketones. Polym. Chem. 2017, 8, 7297–7300. 10.1039/C7PY01609C. [DOI] [Google Scholar]
- Cruz A. R.; Hernandez M. C. G.; Guzmán-Gutiérrez M. T.; Zolotukhin M. G.; Fomine S.; Morales S. L.; Kricheldorf H.; Wilks E. S.; Cárdenas J.; Salmón M. Precision Synthesis of Narrow Polydispersity, Ultrahigh Molecular Weight Linear Aromatic Polymers by A2 + B2 Nonstoichiometric Step-Selective Polymerization. Macromolecules 2012, 45, 6774–6780. 10.1021/ma301691f. [DOI] [Google Scholar]
- Olvera L. I.; Zolotukhin M. G.; Hernández-Cruz O.; Fomine S.; Cárdenas J.; Gavino-Ramirez R. L.; Ruiz-Trevino F. A. Linear, Single-Strand Heteroaromatic Polymers from Superacid-Catalyzed Step-Growth Polymerization of Ketones with Bisphenols. ACS Macro Lett. 2015, 4, 492–494. 10.1021/acsmacrolett.5b00164. [DOI] [PubMed] [Google Scholar]
- Zou L.; Cao X.; Zhang Q.; Dodds M.; Guo R.; Gao H. Friedel-Crafts A(2)+ B-4 Polycondensation toward Regioselective Linear Polymer with Rigid Triphenylmethane Backbone and Its Property as Gas Separation Membrane. Macromolecules 2018, 51, 6580–6586. 10.1021/acs.macromol.8b01394. [DOI] [Google Scholar]
- Gan W.; Shi Y.; Jing B.; Cao X.; Zhu Y.; Gao H. Produce Molecular Brushes with Ultrahigh Grafting Density Using Accelerated CuAAC Grafting-Onto Strategy. Macromolecules 2017, 50, 215–222. 10.1021/acs.macromol.6b02388. [DOI] [Google Scholar]
- Shi Y.; Graff R. W.; Cao X.; Wang X.; Gao H. Chain-Growth Click Polymerization of AB2Monomers for the Formation of Hyperbranched Polymers with Low Polydispersities in a One-Pot Process. Angew. Chem., Int. Ed. 2015, 54, 7631–7635. 10.1002/anie.201502578. [DOI] [PubMed] [Google Scholar]
- Shriner R. L.; Berger A. Condensation Products from Benzyl Alcohol. Polybenzyls. J. Org. Chem. 1941, 06, 305–318. 10.1021/jo01202a015. [DOI] [Google Scholar]
- Braendle A.; Perevedentsev A.; Cheetham N. J.; Stavrinou P. N.; Schachner J. A.; Mösch-Zanetti N. C.; Niederberger M.; Caseri W. R. Homoconjugation in poly(phenylene methylene)s: A case study of non-π-conjugated polymers with unexpected fluorescent properties. J. Polym. Sci., Part B: Polym. Phys. 2017, 55, 707–720. 10.1002/polb.24305. [DOI] [Google Scholar]
- Perevedentsev A.; Francisco-López A.; Shi X.; Braendle A.; Caseri W. R.; Goñi A. R.; Campoy-Quiles M. Homoconjugation in Light-Emitting Poly(phenylene methylene)s: Origin and Pressure-Enhanced Photoluminescence. Macromolecules 2020, 53, 7519–7527. 10.1021/acs.macromol.0c01153. [DOI] [Google Scholar]
- Evans A. G.; Holden D.; Plesch P.; Polanyi M.; Skinner H. A.; Weinberger M. A. Friedel – Crafts Catalysts and Polymerization. Nature 1946, 157, 102–102. 10.1038/157102a0. [DOI] [Google Scholar]
- Fuson R. C.; Weinstock H. H.; Ullyot G. E. A new synthesis of benzoins 2′,4′,6′-trimethylbenzoin. J. Am. Chem. Soc. 1935, 57, 1803–1804. 10.1021/ja01313a015. [DOI] [Google Scholar]
- Klumpp D. A.; Yeung K. Y.; Prakash G. K. S.; Olah G. A. Preparation of 3,3-diaryloxindoles by superacid-induced condensations of isatins and aromatics with a combinatorial approach. J. Org. Chem. 1998, 63, 4481–4484. 10.1021/jo980588g. [DOI] [Google Scholar]
- Colquhoun H. M.; Zolotukhin M. G.; Khalilov L. M.; Dzhemilev U. M. Superelectrophiles in Aromatic Polymer Chemistry. Macromolecules 2001, 34, 1122–1124. 10.1021/ma001579o. [DOI] [Google Scholar]
- Diaz A. M.; Zolotukhin M. G.; Fomine S.; Salcedo R.; Manero O.; Cedillo G.; Velasco V. M.; Guzman M. T.; Fritsch D.; Khalizov A. F. A novel, one-pot synthesis of novel 3F, 5F, and 8F aromatic polymers. Macromol. Rapid Commun. 2007, 28, 183–187. 10.1002/marc.200600656. [DOI] [Google Scholar]
- Olvera L. I.; Rodriguez-Molina M.; Ruiz-Trevino F. A.; Zolotukhin M. G.; Fomine S.; Cardenas J.; Gavino R.; Alexandrova L.; Toscano R. A.; Martinez-Mercado E. A Highly Soluble, Fully Aromatic Fluorinated 3D Nanostructured Ladder Polymer. Macromolecules 2017, 50, 8480–8486. 10.1021/acs.macromol.7b01413. [DOI] [Google Scholar]
- Cuneo T.; Cao X.; Zou L.; Gao H. Synthesis of multisegmented block copolymer by Friedel–Crafts hydroxyalkylation polymerization. Polym. Chem. 2020, 11, 2542–2549. 10.1039/D0PY00197J. [DOI] [Google Scholar]