Abstract
Water is critical for all lives to thrive. Access to potable and safe water has been argued to rank top among the prerequisites for defining the standard of living of a nation. However, there is a global decline in water quality due to human activities and other factors that severely impact freshwater resources such as saltwater intrusion and natural disasters. It has been pointed out that the millions of liters of industrial and domestic wastewater generated globally have the potential to help mitigate water scarcity if it is appropriately captured and remediated. Among the many initiatives to increase access to clean water, the scientific community has focused on wastewater remediation through the utilization of bioderived materials, such as nanocellulosics. Nanocellulosics, derived from cellulose, have the advantages of being ubiquitous, nontoxic, and excellent adsorbents. Furthermore, the surface properties of nanocellulosic materials can easily be modified. These advantages make them promising materials for water remediation applications. This perspective highlights the most important new developments in the application of nanocellulosics in water treatment technologies, such as membrane, adsorption, sensors, and flocculants/coagulants. We also identify where further work is urgently required for the widespread industrial application of nanocellulosics in wastewater treatment.
1. Introduction
Safe, potable, and usable water is an invaluable commodity and a necessity for ensuring the global pursuit of a sustainable future, for both the current and future generations.1 However, the rising inaccessibility and scarcity of safe, usable, and affordable water are becoming common threats worldwide. For example, a 2019 report stated that more than half a billion people worldwide do not have access to safe and potable water. This water distress cuts across urban and rural societal strata.2 The United Nations has raised the alarm that the water crisis is quickly becoming a global phenomenon, and no continent or nation is immune or excluded from this threat.3 The World Economic Forum has opined that the scarcity of potable and usable water tops among the challenges currently confronting humanity.4 There is no substitute for water. It is indispensable for life and all life entails.5 Therefore, a discourse on water, its scarcity, conservation, management, pollution, remediation, and purification could not be more important in light of the current environmental concerns. Studies have shown that rising water scarcity is a threat to global food security, especially impacting irrigated agricultural systems, and may result in the nonsustainability of certain food crops.6−8 Moreover, access to safe drinking water has been highlighted as one of the significant challenges that the United States Armed forces will be facing in the battle field in the near future.9
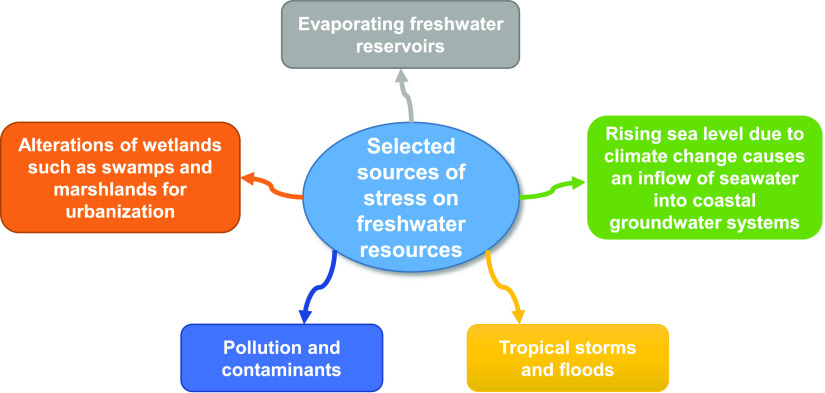
Rising water scarcity is a complex problem that impacts and cascades through almost every known human activity and endeavor. Even though the Food and Agriculture Organization of the United Nations has stated that the earth’s available freshwater resources are more than enough to satisfy the needs of humanity,10 this seems not to be the case. As shown in Figure 1, the alterations in the natural environment through various human activities (e.g., drainage and/or filling-up of natural water systems/ecosystems such as lakes and wetlands for urbanization),11,12 coupled with other irrepressible environmental interferences such as saltwater intrusions,13,14 disasters (e.g., flooding and hurricanes),15,16 evaporation from water reservoirs (estimated to exceed the combined consumption of both industrial and domestic usage yearly),17,18 poor water management, pollution, and contamination (chiefly from man-made inventions, e.g., plastic debris and toxic compounds),19,20 contribute significantly to the rising stress and degradation of earth’s freshwater resources (i.e., underground and surface freshwaters), which are the primary sources for the production and provision of potable, safe, and usable water for domestic, industrial, and agricultural activities.5,21
Figure 1.
Selected sources are contributing to the immense freshwater stress globally. Photography courtesy: S.S.R. who is the first author of this work.
Pollution has been recognized as one of the major culprits in global freshwater degradation.5,22−24 For example, Citarum River, in the west of Java (Indonesia) with more than 8 million people in its catchment zone, has been dubbed a “rotten river” and is considered one of the most polluted freshwater sources on earth. This river has an estimated coliform bacteria (stemming mainly from fecal matter) level exceeding 4000 times the acceptable limits and heavy metal pollution exceeding 1000 times the accepted international levels.25 One recent study reported that large amounts of mixed organic pollutants (e.g., polycyclic aromatic hydrocarbons, polychlorinated biphenyls, and bis-chlorophenyl-trichloroethane) stemming from industrial and municipal wastewaters generated in Jakarta, Indonesia, are discharged into open waters without being treated, thereby creating serious water pollution and degradation.26 A related report demonstrated that water pollution resulting from metals/metalloids in consumable waters and vegetables has become a health risk for South Africans and Mozambicans living along the Olifants river catchment area.27 It was shown that pesticides used in agricultural activities largely contributed to water quality degradation in many countries. Water quality significantly impacts the wellbeing of humans and other living components of the environment.28−31 These impacts cascade and permeate almost every microcosm of society—from dental clinics32 to the outbreak of microsporidial keratoconjunctivitis.33 Loss of water integrity places an overwhelming demand on health resources, facilities, and budgets.
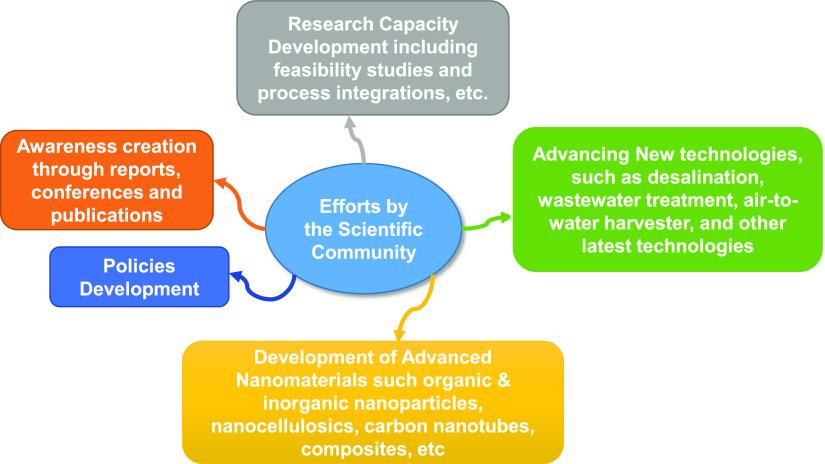
In the last century, the demand for safe and potable water has quickly exceeded its dwindling availability, even as humankind rigorously pursues economic advancement and industrialization.5,34 As the comity of nations seeks to fulfill Sustainable Development Goal 6, which aims to achieve universal safe water by the year 2030,35 the scientific community has made extensive efforts for the development of technology for “optimal” capturing and recycling of degraded water, such as domestic and industrial wastewaters (Figure 2).36−47
Figure 2.
Pictorial representation of selected efforts and contributions of the scientific community in mitigating the rising global water crisis. Photography courtesy: S.S.R. who is the first author of this work.
Recent advances in nanotechnology have proposed different methods to decontaminate polluted waters.48,49 Nanotechnology provides limitless opportunities for addressing global water challenges;50,51 the use of nanocellulosics or nanocellulose (NC)-based materials is one such application for remediating degraded water. In addition to being derived from cellulose (the most abundant polymeric system on earth), nanocellulosics are ubiquitous, are nontoxic, have easily modifiable surface properties, are exceptional adsorbents, and have good chirality, which makes them ideal materials for water remediation purposes.52,53
This perspective highlights the most important new developments in the application of nanocellulosic materials in water treatment technologies, giving a short overview on the key research challenges, as well as important references for future in-depth study. In addition, a brief description of current challenges in employing common nanomaterials in water remediation and treatment processes has been provided.
2. Challenges in Employing the Most Common Nanomaterials in Water Remediation and Treatment Processes
Applications of nanobased materials in the reclamation, treatment, and purification of polluted and degraded waters, such as wastewaters (domestic and industrial), saline waters, and storm waters, have increased recently.54−56 Recently, a number of nanomaterials such as titanium dioxide (TiO2), silver (Ag), zinc oxide (ZnO), carbon nanotubes, and ferrous oxide (FeO) have been used for these processes;57−62 however, there are growing concerns about their associated negative health and environmental impacts as they accumulate in living and nonliving systems, thereby triggering unpredictable environmental changes in natural systems.63 In this context, Table 1 summarizes the most important and recent studies associated with the negative impacts of nanomaterials as they accumulate in living and nonliving systems.
Table 1. Recent Reports Associated with the Negative Impacts of Nanomaterial Accumulation in Living and Nonliving Systems.
| title | highlights | refs |
|---|---|---|
| growth inhibition of aquatic plants caused by silver and titanium oxide nanoparticles | the authors established the propensity of Ag NPs to inhibit aquatic plant growth such as Lemna, even at a low concentration (parts per million, ppm) | (64) |
| TiO2 NPs were observed as having toxic consequences on the growth of the Lemna at a concentration of ≥250 ppm | ||
| evaluating nanoparticle breakthrough during drinking water treatment | the authors reported that nanobased materials/systems employed for drinking water treatment such as Ag, TiO2, and ZnO NPs have the high chance of contaminating drinking water resources | (65) |
| despite the extreme preventive measures employed to limit the entrainment of these particles into the final stream of drinking water, yet the finished waters still contained traces of these NPs that pose health hazards to humans | ||
| it was concluded that nanomaterials such as Ag, TiO2, and ZnO NPs are to be considered emerging contaminants and standard procedures for their effective removal from drinking water should be prioritized for the sake of public health. | ||
| titanium nanomaterial removal and release from wastewater treatment plants | this study demonstrated that titanium nanomaterials employed in wastewater treatment plants end up being discharged into the surface waters such as lakes, rivers, streams, andoceans, thus presenting a significant pathway for these NPs to enter the environment | (66) |
| according to the authors, the majority of the TiO2 NPs released from wastewater treatment effluents accumulated in the living components of the environment | ||
| it was established that these NPs were observed to accumulate in biosolids generally employed in agricultural purposes or human consumption. | ||
| this study established the urgency for the scientific community to investigate the transport, fate, and health implications of these NPs in the living and nonliving components of the environment and to find possible ways to mitigate their potential negative implications on the environment | ||
| toxic effects of different types of zinc oxide nanoparticles on algae, plants, invertebrates, vertebrates, and microorganisms | this work demonstrated that the use of NPs such as ZnO NPs and their entrainment into the environment could not be overemphasized | (67) |
| it is noted that the ZnO NP presented significant toxicity to biological systems such as algae | ||
| because the ZnO NP possesses a high number of oxygen vacancies on its surface, this presents the opportunity for the stimulation of electron pairs that may consequently initiate reactive oxygen species (ROS) with oxygen molecules and hydroxyl ions in living systems which may trigger toxicity and carcinogenesis and damage lipids and proteins | ||
| silver nanoparticles: toxicity in model organisms as an overview of its hazard for human health and the environment | the discharge of Ag NPs from wastewater treatment plants remains a significant source for these materials to enter the environment | (68) |
| bioaccumulation of Ag NPs have the potential to release silver ions and can promote reactive oxygen species which have attendant negative implications for living organisms |
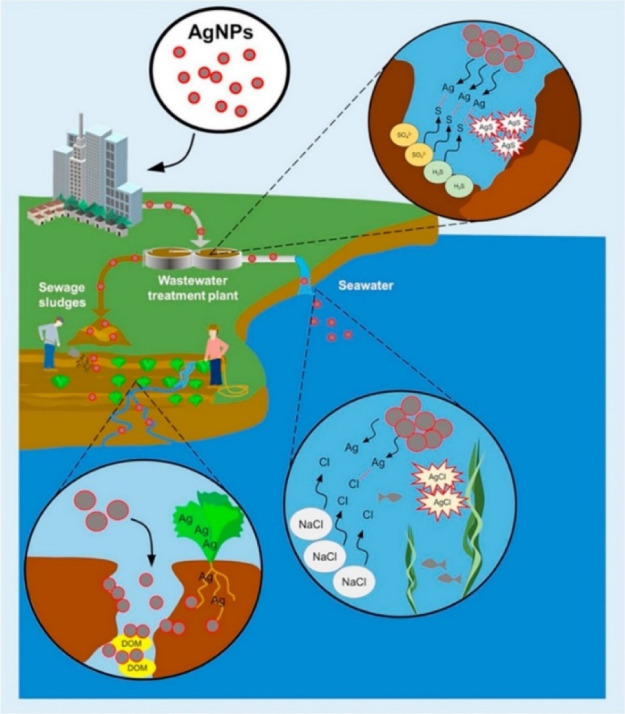
Over the years, concerns have been raised about the possibilities of Ag, TiO2, ZnO, and other NPs employed in water remediation processes becoming next-generation wastes, creating environmental problems.69−71 For example, it was reported that TiO2 NPs employed in water treatment processes may react with other metals (e.g., biogenic metals) that are ubiquitous in the environment, creating a toxic joint mechanism that can become unpredictable and catastrophic. It was further argued that copper ions (Cu2+) may react with exposed TiO2 and cause acute toxicity in aquatic organisms (e.g., Daphnia magna and Gammarus fossarum).72 Similarly, it has been shown that Ag NPs can be extremely toxic and hazardous to humans and the environment.68,73 As demonstrated in Figure 3, the report showed that the entrainment of Ag NPs into the environment resulted in multiplier effects that resulted from its dissolution, aggregation, oxidation, and sulfidation, which may limit or exacerbate the toxicity levels in the given natural system.68 It was argued that the dissolution and leaching of ZnO NPs employed in water remediation played a significant role in the toxicity level for aquatic organisms (e.g., Escherichia coli).74 Hence, these concerns about NP toxicity have resulted in the scientific community seeking eco-friendly alternatives that are efficient and sustainable. One such nanomaterial with these attributes is obtained from the most abundant and ubiquitous natural polymer–cellulose. In the quest to meet the sustainable development goals, nanocellulosics (NC-based materials) have gained considerable global interest. To the best of our knowledge, there is no known report(s) on the environmental impact associated with the use of nanocellulosics in water remediation and treatment processes.
Figure 3.
Entrainment of Ag NPs into the natural environment poses hazardous challenges as a result of their reactions with other chemical compounds, thereby impacting natural environments.73 Reprinted with permission from Tortella, G. R.; Rubilar, O.; Durán, N.; Diez, M. C.; Martínez, M.; Parada, J.; and Seabra, A. B. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater.2020,390, 121974. Copyright 2020, Elsevier Science Ltd.
3. Nanocellulosics: A Brief Background
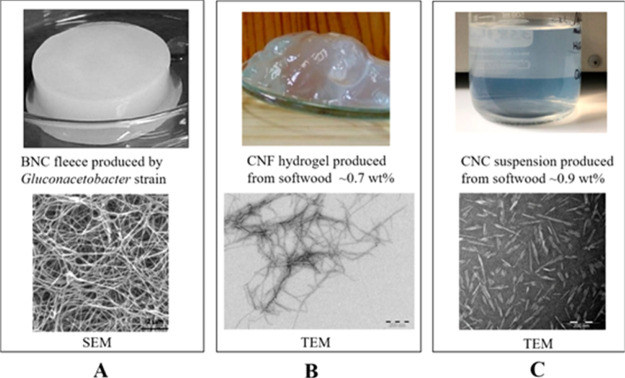
Even though the knowledge and application potentials of nanoscale cellulosic materials, generally referred to as nanocellulosics, have been known for more than a century,75,76 it was not until a decade and a half ago that study of nanoscale properties and characteristics became possible.53 Nanocellulosic materials include nanofibrillated cellulose (NFC), cellulose nanowhiskers, and cellulose nanocrystals (CNCs). Figure 4 shows the bulk and microscopic morphologies of various nanocellulosic materials. The fascinating properties of nanocellulosics include (i) biodegradability, (ii) biocompatibility, (iii) transparency, (iv) low thermal expansibility coefficient, (v) unlimited reactive sites for functionalization, (vi) ease of surface modification, (vii) renewability and sustainability, (ix) inherent electrical conductivity, (x) exceptional barrier properties for oxygen and mineral oils, and (xi) exceptional weight-to-strength ratio (eight times that of stainless steel and more unyielding than Kevlar).77−82 With an assortment of potential applications, nanocellulosics are proving to be the material of yesterday, today, and tomorrow.83,84
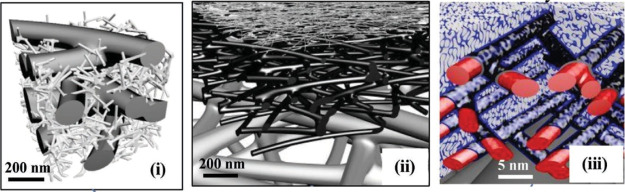
Figure 4.
Bulk and microscopic morphologies of various nanocellulosic materials. (A) Bacterial NC (BNC) and its morphology as observed under scanning electron microscopy; (B) cellulose nanofibril (CNF) hydrogel and its morphology as observed under TEM; (C) CNC suspension and its observed morphology under TEM.85 Photography courtesy: Wang, X.; Wang, Q.; and Xu, C. Reprinted with permission from Wang, X.; Wang, Q.; and Xu, C. Nanocellulose-based inks for 3d bioprinting: Key aspects in research development and challenging perspectives in applications—a mini review. Bioengineering2020,7, 40. Copyright 2020, the authors under the creative commons license 4.0.
In August 2012, the US Forest Products Laboratory under the Department of Agriculture unveiled the first of its kind NC facility for studying the potentials of biomass nanomaterials in the United States.86 In Canada, in 2011, a new facility for the development and applications of CNC was established to keep up with the emerging trends in bioderived nanomaterials from woody resources.87 More recently, the environment ministry of the Japanese government demonstrated a novel super automobile that was designed and fabricated entirely from nanocellulosics and dubbed “the NC vehicle project”, which demonstrated the matchless opportunities that biomass presents in the quest for sustainable development and reduction of CO2 emissions.88 A recent report described a pilot fabrication of a laminated nanocomposite material consisting of NC and Kevlar that could be used for various military applications.89 The NC material was locally derived from rice straw. The study concluded that reinforcement of Kevlar with NC optimized the mechanical properties of the developed material by offering higher strength with nearly no weight changes, thereby demonstrating the potential of the developed material for application in strong and lightweight military gears.89
Various resources, methodologies, techniques, and processes have been reported in the literature on the derivation, preparation, characterization, and properties of nanocellulosics, which are outside the scope of this perspective. Hence, we refer to cited literature for further reading.90−92 It is important to note that although nanocellulosics can be prepared from a variety of biomass resources such as woody biomass (e.g., coniferous, eucalyptus, cannabis trees, and even from the volumes of waste toilet papers used daily for sanitation), bacteria, and algae, different cellulosic sources offer different nanocellulosic functionalities and properties.90,93−96 We believe that the biomass utilization industries (e.g., paper industry/mills) will play a significant role in the global development of nanocellulosics in the future. Table 2 summarizes the commonly used synthesis/preparation routes for nanocellulosic materials.
Table 2. Overview of Current Preparation/Synthesis Methods for Nanocellulosic Materials.
| type of nanocellulosic | preparation/synthesis methods | refs |
|---|---|---|
| CNF/CNCs | acid hydrolysis of cellulose pulp in the presence of either mineral or organic acid | (97) and (98) |
| enzymatic hydrolysis employing the cellulose enzyme. Although an environmentally friendly route, it is very expensive | ||
| subcritical water extraction. Also, an environmentally friendly method but nanocellulosics obtained from this method demonstrate instability in suspension | ||
| transition metals catalysis such as Fe(III), Co(II), etc., have been used for effective hydrolysis for obtaining nanocellulosic materials from cellulose pulp, thereby reducing the use of acids | ||
| ionic liquid hydrolysis methodology. Notwithstanding the noteworthy efficiency in using this process for NC production, they, however, come with drawbacks such as toxicity, high-cost implications, and high chemical footprints | ||
| deep eutectic solvents hydrolysis. Similar to ionic liquids; however, it eliminates some noted drawbacks associated with the use of ionic liquids for cellulose pulp hydrolysis which includes lower-cost implications and simplicity | ||
| BNC | fermentation process. However, it comes with challenges such as the long cultivation period | (99−101) |
| bioreactor process |
4. Nanocellulosics: Efficient and Benign Water Remediation and Treatment Systems
Nanocellulosics have vast application potential in almost every field, including composite fabrications, display systems in electronics, energy storage systems, environmental remediation, and water treatment technologies and/or processes.102−104 In this section, we demonstrate the selected recent advancements in using nanocellulosics (micro/nanoscale) to remediate and/or treat degraded waters.
4.1. Membranes
It is well known that membrane technology ranks the top among the most energy-efficient technologies for the decontamination of degraded waters. The membrane technology is based on either pressure-driven concepts such as microfiltration techniques (e.g., for particulate matter elimination) or osmotic movement (e.g., for salinity treatment salt removal). Regardless, membrane treatment processes remain the most favored and dominant industrial methods owing to the ease in their scalability, low-cost operations, versatility, ease of integration into the existing technologies, low chemical footprint, efficiency, and performance.5 However, factors such as material configuration (e.g., pore size > 10 nm and bonding sites), hydrodynamics (e.g., mass flow rate and permeability), wettability and adhesive properties, mechanical properties, chemical and thermal stability during use, ease of large-scale processing, and cost implication are critical when selecting materials for membrane application in water treatment.105 Considering the fact that fibrillated nanocellulosics have a cross-sectional dimension of ∼2–10 nm, a length of a few micrometers, a high surface area of 750 m2/g, and a Young’s modulus of 100 GPa with excellent wettability and surface functionality, they are excellent candidates for the fabrication of separation membranes for water purification.106 As demonstrated in Figure 5, fibrillated nanocellulosics can be used for developing efficient pressure-driven and/or osmotic-based membranes for water treatment and/or decontamination.106
Figure 5.
Cross sections of potential design for various types of fibrillated nanocellulosic membrane systems for both pressure-driven membranes and reverse osmosis: conceptualized design suited for (i) microfiltration, (ii) ultra- and nanofiltration, and (iii) reverse osmosis.106 Reprinted with permission from Sharma, P. R.; Sharma, S. K.; Lindström, T.; and Hsiao, B. S. Nanocellulose-enabled membranes for water purification: Perspectives. Adv. Sustain. Syst.2020,4 (5), 1900114. Copyright 2020, Wiley-VCH Verlag.
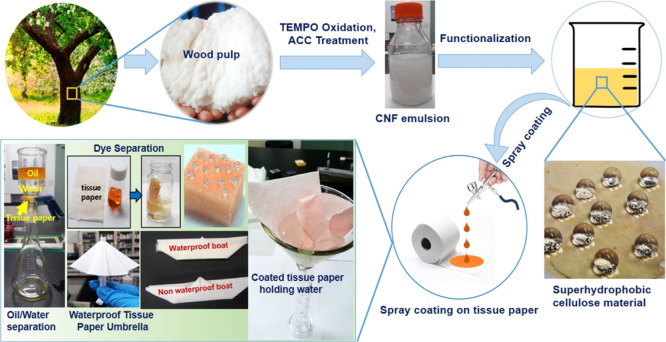
Derami et al.107 developed a facile and inexpensive polydopamine and bacterial-derived NC (BNC) hybrid membrane system for effective wastewater treatment. They argued that this membrane was not only versatile but also biocompatible, biodegradable, industrially scalable, and efficient for the removal of a variety of pollutants, such as heavy metallic ions (e.g., lead and cadmium ions) and organic dyes (e.g., methylene orange). Although the membrane had limitations, that is, it was inefficient in the removal of negatively charged pollutants, it demonstrated robustness in terms of recyclability and retention of its separation capabilities, with no degradation, even after 10 cycles of filtration and regeneration.107 Thus, this developed membrane was a very promising material for cheap water treatment processes using NC-based materials. Another paper described a layer deposition technique employing the vacuum drying method that has been used in the fabrication of NC/filter paper (NC/FP) as composite filtration membranes.108 The study showed that sources of NC had a significant influence on the overall performance of the NC/FP composites. The high length-to-diameter (100–400 and 3–15 nm, respectively) ratio affected the efficiency of the embedment of the NC fibrils into the FP substrate, thereby making it effective for the filtration (even reaching ultrafiltration capabilities) of particulate matter from contaminated water. The authors concluded that through minor adjustments, membranes with different properties and capabilities can be fabricated for particular applications, thereby exhibiting great potential in the design of efficient and simple water filtration systems.96 In another study, Roy et al.109 successfully converted tissue paper (TP) to an efficient separation membrane by applying a novel superhydrophobic coating prepared from NC. As claimed, a novel, eco-friendly, and benign superhydrophobic coating was developed using a simple one-step approach employing cellulose nanofibers (CNFs) and octadecylamine via a glutaraldehyde (GA) coupling mechanism in the presence of deionized water and alcohol. After the application of the developed coating to the TP, it was determined that the enhanced TP became a highly efficient separation membrane for oil and water mixtures.109 As shown in Figure 6, this novel approach provides opportunities for developing a variety of low-cost separation membranes, especially in low-income nations. The exceptional capability of this simple coating system was tested on other substrates such as a normal kitchen dishwashing sponge (made from polyurethane), and the sponge exhibited exceptional adsorbing capability.109
Figure 6.
Schematics demonstrating how a common TP can be used as an exceptional separation membrane for oil and water separation using NC-based coating.109 Photography courtesy: Roy, S.; Zhai, L.; Van Hai, L.; Kim, J. W.; Park, J. H.; Kim, H. C.; and Kim, J. Reprinted with permission from Roy, S.; Zhai, L.; Van Hai, L.; Kim, J. W.; Park, J. H.; Kim, H. C.; and Kim, J. One-step nanocellulose coating converts tissue paper into an efficient separation membrane. Cellulose2018,25 (9), 4871–4886. Copyright 2018, Springer.
Yang et al.110 reported that antifouling challenges, a well-known problem in membrane technology (costing 20–50% of the operational cost on average), can be mitigated through surface modification resulting from surface charges aiding in self-cleaning. The study is based on the argument that understanding the relationship between electrostatic repulsion and fibrillated nanocellulosics is the key to mitigating the antifouling of these membrane systems. Hence, from the degree of oxidation (DO), dimensions, and zeta potential values of the NC fibers, it was observed that at a DO of 1.80, these nanocellulosic fibers displayed the highest flux recovery (≈98 ± 2%) after a modest hydraulic flush. This was in contrast with that of commercially available separation membranes, such as polyvinylidene fluoride (PVDF), which displayed serious fouling with low flux recovery. The report concluded that due to the presence of surface charges, the use of nanocellulosic fibers has advantages such as reusability, ease of recyclability, longer lifespans, and higher cost efficiency compared to other conventional polymer-based membrane materials such as PVDF, polyethersulfone, polysulfone, and polyacrylonitrile.110
Researchers at Princeton University have developed an efficient and cost-efficient hydrophobic “nanowood” membrane with high porosity (≈89 ± 3%).111 The pore size distribution of the inherent crystalline nanofibrils combined with the xylem vessels and channels was responsible for the facilitation of water vapor transportation.111 Entirely fabricated from woody resources, this innovative material demonstrated superior characteristics and better potential for membrane distillation in water desalination compared with conventionally available membranes derived from fossil resources. With an excellent water flux and exceptional thermal efficiency exceeding 60%, the developed material is a promising alternative to petroleum-derived membranes. It was revealed that unlike the complex fabrication methods employed to create conventional membranes such as polypropylene and polytetrafluoroethylene, this “nanowood” membrane was manufactured using a top-down approach that is easily scalable for industrial deployment. However, pore size distribution caused issues; the report argued that this could be eliminated using microtomes and by carefully selecting a woody material source that meets the pore size criteria/demand. It was further suggested that future perspectives should consider re-engineering nanocellulosic fibers through electrospinning to optimize the capabilities of the material.111
4.2. Adsorbents
There is a growing trend in the application of nanobased materials as adsorbents for water treatment purposes.112,113 This has been attributed to the higher adsorption capacities, improved binding affinities, advanced interfacial phenomena, and large surface area of nanoscale materials compared to their macroscale counterparts. This is in addition to the capability to fine-tune and modify their surfaces.5,114 Due to the easy and simple approach, adsorption is considered the most significant process in water treatment; this is the reason for the popularity of nanoscale materials in the removal of a vast array of pollutants, ranging from heavy metals to organic matters to pesticides.115 Generally, the benchmark for considering a material for adsorbent applications includes economy, robustness during long usage, maintenance of adsorption capacity integrity even after heavy usage, good surface area, ease of recyclability, and low environmental footprint. Because of the limitations and challenges faced by conventional industrial adsorbents, enumerated by Mahfoudhi and Boufi114 and Hokkanen et al.,116 recent efforts are being directed toward more sustainable and effective alternatives such as NC for water treatment purposes.117Table 3 summarizes the most important studies demonstrating the increasing research on NC/NC-based materials/systems as adsorbents for the removal of various pollutants.
Table 3. Selected Studies Demonstrating the Increasing Research on Nanocellulosic Materials/Systems as Adsorbents for the Removal of Various Pollutants.
| title | highlights | year/refs |
|---|---|---|
| lead adsorption with sulfonated wheat pulp NCs | inexpensive nanocellulosic materials were developed as effective adsorbents for the removal of Pb(II) in aqueous systems | (119) |
| Pb(II) was adsorbed efficiently at 1.2 mmol/g | ||
| sulfonated NC possessed a large area of binding sites for adsorbing the pollutant | ||
| super light 3D hierarchical NC aerogel foam with superior oil adsorption | NC and sodium dodecylsulfate (SDS) were used to produce 3D NC aerogel foam (NAF/SDS) via a high speed mechanical foaming and solvent-free method | (120) |
| the optimal concentration of NC and SDS for this 3D NAF/SDS was 0.4 and 0.4 wt %, respectively | ||
| the adsorption capacity of the foam was 206.79, 194.75, and 145.20 g g–1 for cyclohexane, ethyl acetate, and vacuum pump oil, respectively. These values were higher compared to conventional NC aerogel with adsorption capacities of 52.07, 81.12, and 34.52 g g–1 for the same chemicals | ||
| magnetic NC-magnetite aerogel for easy oil adsorption | the optimal fabricating conditions for these magnetic NC aerogel NCA/OA/Fe3O4 were 0.4 wt % NC, 3 mg L–1 oleic acid (OA), and 0.5 wt % Fe3O4 | (121) |
| the density of the aerogel fabricated was ∼9.2 mg cm–3, and the aerogel demonstrated a high adsorption capacity of 68.06 g g–1 for cyclohexane. This is lower than that reported by Zhang et al.120 | ||
| ease of recyclability and good magnetic responsivity were key advantages of these materials | ||
| hydrophobic NC aerogels as floating, sustainable, reusable, and recyclable oil absorbents | highly porous nanocellulosic aerogels were prepared via vacuum freeze-drying from microfibrillated NC hydrogels | (122) |
| these aerogels were functionalized with hydrophobic oleophilic coating for selective oil adsorption and were capable of floating on water | ||
| the low density and potential to adsorb nonpolar liquids and oils up to nearly their own initial volume was demonstrated | ||
| modification of the aerogel’s surfaces further demonstrated the capability to collect organic pollutants from the water surface | ||
| ease of reusability and discarding (with adsorbed oil) makes these aerogels a versatile system for environmental remediation processes | ||
| synthesis and characterization of multi carboxyl-functionalized NC/nanobentonite composite for the adsorption of uranium(VI) from aqueous solutions: kinetic and equilibrium profiles | multicarboxylated functionalized polymer composite poly(itaconic acid)-poly(meth acrylic acid)-grafted-NC/nanobentonite (P(IA/MAA)-g-NC/NB) was synthesized and characterized | (123) |
| adsorption of U(VI) was pH-dependent, and an optimal pH of 5.5 promoted the removal in 120 min | ||
| no significant loss in performance was observed in the material even after six reuse/recycles | ||
| biohybrid hydrogel and aerogel from self-assembled NC and nanochitin as a high-efficiency adsorbent for water purification | a facial and novel self-assembled NC and nanochitin were developed as a highly efficient and versatile biohybrid hydrogel and aerogel for water purification | (124) |
| the self-assembly resulted from the electrostatic force between the one-dimensional charged TEMPO-oxidized cellulose nanofiber (TOCNF) and positively charged partly deacetylated chitin nanofiber (PDChNF) at room temperature with no addition of cross-linking agents | ||
| the resultant 3D system that physically cross-linked due to electrostatic interactions and hydrogen bonding exhibited adsorption capacities of 217 mg g–1 for arsenic(III) under neutral pH conditions and 531 mg g–1 for methylene blue under alkaline aqueous conditions, with rapid adsorption kinetics | ||
| these materials demonstrated good reusability; even after five successive reuse (adsorption–desorption cycles), the materials demonstrated an adsorption capacity of 505 mg g–1 for methylene blue |
Sharma et al.118 demonstrated the efficient removal of cadmium(II) ions from contaminated water using NC derived from spinifex (genus Triodia), an underutilized grass that is abundant and widely distributed across almost all continents. By employing a nitro-oxidation technique, an NC material with low crystallinity (∼50%), high surface charge (−68 mV), and good hydrophilicity was developed. The study established that the highest efficiency exhibited by the nanocellulosic suspension was approximately 2550 mg g–1, which is substantially higher than that by any adsorbents reported in the literature, thereby demonstrating the potential for harnessing spinifex for water remediation.118
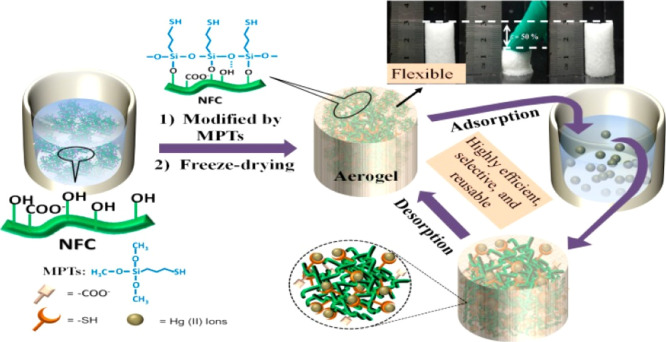
Another study reported the use of a simple, recyclable, and benign bioadsorbent for the removal of highly toxic mercury ions (Hg+) from contaminated water via the application of surface-tailored NC aerogels.125 As demonstrated in Figure 7, the superficially prepared NC aerogel was obtained by freeze-drying bamboo-derived 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), which was oxidized into NFC in the presence of mercaptopropysiloxane sols. As a result of the abundant thiol groups affixed on the surfaces, mercury ions were very efficiently removed (>90% efficiency). In addition, despite variations in pH values over a wide initial concentration range of 0.01–85 mg L–1, the decrease in the adsorption capacity of the fibrillated NC system was negligible. The report concluded that following an observed Langmuir isotherm and pseudo-second-order kinetics, a maximum adsorption capacity of 718.5 mg g–1 was achieved, thereby demonstrating the promising application potential of this nanocellulosic material. This is in addition to other added advantages such as flexibility and ease of recyclability.125
Figure 7.
Diagrammatic representation of a facile, robust, recyclable, and efficient NC aerogel for the efficient removal of hazardous mercury ions from contaminated water.125 Photography courtesy: Geng, B.; Wang, H.; Wu, S.; Ru, J.; Tong, C.; Chen, Y.; Liu, H.; Wu, S.; and Liu, X. Reprinted with permission from Geng, B.; Wang, H.; Wu, S.; Ru, J.; Tong, C.; Chen, Y.; Liu, H.; Wu, S.; and Liu, X. Surface-tailored nanocellulose aerogels with thiol-functional moieties for highly efficient and selective removal of Hg(II) ions from water. ACS Sustain. Chem. Eng.2017,5 (12), 11715–11726. Copyright 2017, the American Chemical Society.
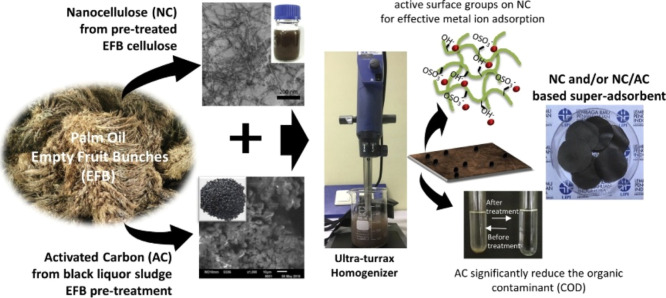
Pervasive empty fruit bunches (EFBs) discarded from processed palm-oil seeds in Indonesia have been used for the production of superadsorbent NC material for water treatment (Figure 8). Septevani et al.126 claimed that after preparing the fine-sized fibers of EFB, they were treated via chemical explosion to obtain high-content cellulose systems that were further hydrolyzed in acidic media and neutralized. Activated carbon, obtained from the lignin content of the EFB, was added to the NC to develop a superadsorbent material. The NC-based superadsorbent material obtained by the treatment with sulfuric acid was denoted as NCS and that obtained by phosphoric acid treatment was denoted as NCP. The study reported that NCS exhibited superior heavy metal ion adsorption, especially for lead ions (Pb2+), as compared to NCP at an initial metal concentration of 100 ppm.
Figure 8.
Preparation of superadsorbent NC materials from EFB, incorporated with activated carbon from black liquor obtained during the EFB pretreatment process. The presence of sulfonated active sites as a result of acid hydrolysis was momentous in the heavy metal adsorption capacity.126 Photography courtesy: Septevani, A. A.; Rifathin, A.; Sari, A. A.; Sampora, Y.; Ariani, G. N.; Sudiyarmanto; and Sondari, D. Reprinted with permission from Septevani, A. A.; Rifathin, A.; Sari, A. A.; Sampora, Y.; Ariani, G. N.; Sudiyarmanto; and Sondari, D. Oil palm empty fruit bunch-based nanocellulose as a superadsorbent for water remediation. Carbohydr. Polym.2020,229, 115433. Copyrright 2020, Elsevier Science Ltd.
It was concluded that these EFB-NCs functionalized by sulfuric and phosphoric acids enabled the modification of EFB-NC surfaces. The incorporation of the activated carbon obtained from the lignin liquor (obtained during the pretreatment of the EFB fibers) demonstrated the possibility of developing superadsorbent materials from almost any lignocellulosic material for water remediation.126 Kumar et al.127 demonstrated the possibility of fabricating a quick and recyclable polyaniline (PANI)-impregnated NC (PANI–NC) composite-based system with improved efficiency for chromium metal removal and the decontamination of wastewaters. The two-step process involved in the fabrication of the PANI–NC included the polymerization of monomeric aniline using ammonium persulfate, after which the NC was impregnated in the PANI matrix. The developed system was fabricated in various forms (i.e., powder and globular). This material exhibited dual advantages: efficient removal (almost 100%) of industrial dyes and ability to remove more than 95% of chromium metal ions from the industrial wastewater; hence, it acted as a multifunctional adsorbent.127
4.3. Flocculants and/or Coagulants
An important step in water treatment processes is the removal of particulate suspensions. This is generally achieved through the neutralization of charged particulate suspensions (coagulation) and the aggregation/agglomeration of suspended particulate matter (flocculation).5 In pursuit of sustainable development goals, more research is focused on finding new ways to “go green” through developing efficient alternatives that are benign, sustainable, and efficient in water treatment, while mitigating the limitations of unsustainable and conventional systems, such as synthetic polymers from fossil resources and inorganic coagulants such as aluminum and iron-based alum.5,19,128 In the last decade, cellulosic nanomaterials have found increasing use as efficient coagulation and flocculation systems. For example, an anionized NC system has been developed as an alternative coagulation–flocculation agent for municipal wastewater treatment.129 The bleached wood pulp was disintegrated in deionized water to produce the anionic cellulosic nanomaterial. This functionalized nanocellulosic system demonstrated good efficiency in the coagulation–flocculation treatment of municipal wastewater samples, although it fell short of ineffectual turbidity reduction when compared to the conventional systems currently in use. However, it exhibited a comparable performance in chemical oxygen demand (COD). In addition, the developed biosystem was very robust and shows high levels of stability under prolonged usage and changing pH values.129 Another paper reported the use of cationic NC as an efficient flocculant for municipal activated sludge.130 Kraft pulp was used as a source of the cellulose material, which was disintegrated using deionized water while fabricating cellulose nanofibers. The study claimed that the developed cationic NC demonstrated good flocculation performance and its efficiency was comparable to that of conventionally used polymeric systems from fossil resources; however, an increase in alkalinity of the municipal wastewater sludge decreased the performance of this system. This was attributed to the possible cleavage of C-5 and O-5 bonds of the NC, which degraded its efficiency. As the optimal operating pH for activated wastewater sludge falls between 6.5 and 7.5, this cationic NC flocculant can operate efficiently under these environmental conditions.130
Another study131 reported the synthesis of bamboo pulp, an efficient and hydrophobic cellulose-graft-sodium silicate-polyacrylamide (BPC-g-Si-PAM) flocculant and dewatering system, for the treatment of municipal wastewater sludge. The developed NC system exhibited good performance, especially for kaolin suspension and machining wastewater when compared to conventional polyacrylamide, especially in the removal of total suspended solids, COD, ammonia nitrogen, total iron, total phosphorus, and total zinc, with efficiencies exceeding 70%.131 In addition to demonstrating the promising features of biobased and ecological flocculation and coagulation system, Koshani et al.132 highlighted the important developments in NC-based flocculants and dispersants, with some still at the pilot scale and others already at the industrial level.
It remains evident from the applications of NC for flocculation–coagulation that there is a need to improve the efficiencies of these systems, especially in media with changing pH values. The nanocellulosic materials/systems for water remediation and treatment processes should be further optimized and improved before being used as effective alternatives to conventional materials and/or systems currently in use.
4.4. Sensors
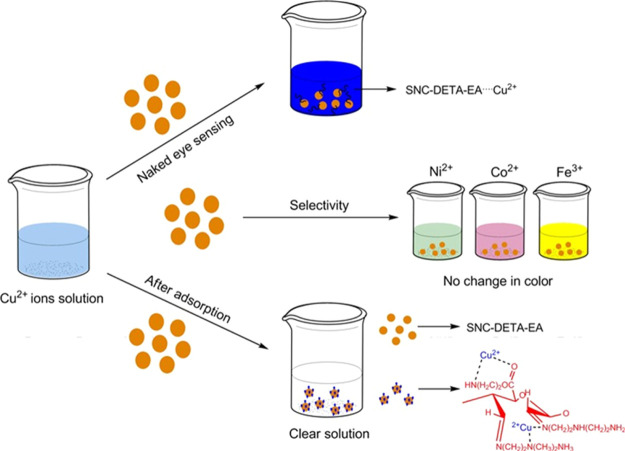
Because of their inherent electrical properties, good optical transparency, and capability to exhibit piezoelectric characteristic, the application of nanocellulosic-based materials as sensors for water and biological systems has been proposed. This is an emerging area, and this section highlights the most important new developments in the application of nanocellulosic-based materials as chemical sensors. A biocomposite plasmonic sensor for detecting cyanide ions (CN–) was fabricated via in situ embedding of stabilized Cu NPs within a nanocellulosic film without any surfactants and/or capping agents, thus presenting neat and well-ordered Cu NPs. The fixation of the Cu NPs into the NC film prevented any form of particle aggregation that may arise, thus enhancing good dispersion. This developed bionanocomposite demonstrated suitable optical sensing for CN– at low detection limits of about ∼0.015 μg mL–1 in water.133 It was further reported that spherical NC (SNC) was modified with diethylenetriamine (DETA) and/or ethanolamine (EA) (i.e., SNC–DETA and SNC–DETA–EA) to develop a highly selective and rapid sensor for Cu ions (Cu2+), visible with the naked eyes, via colorimetric sensing and fluorescence.134 The study demonstrated that the SNC–DETA–EA exhibited a distinctive structure–property relationship through these well-defined selective colorimetric and fluorometric sensing characteristics toward Cu2+. Therefore, it was possible to observe the fluorescent quenching behavior upon the addition of Cu2+ ions even at low concentrations with the naked eye (Figure 9). Moreover, this biocomposite sensor demonstrated good tolerance to pH variations, in addition to the added advantages of recyclability and reversibility.134
Figure 9.
Schematics representing the naked-eye observation of the fluorescent activity of SNC–DETA–EA in the presence of copper ions. Distinguishable selectivity for Cu2+ and reversibility of the activity are evident.134 Photography courtesy: Ram, B.; Jamwal, S.; Ranote, S.; Chauhan, G. S.; and Dharela, R. Reprinted with permission from Ram, B.; Jamwal, S.; Ranote, S.; Chauhan, G. S.; and Dharela, R. Highly selective and rapid naked-eye colorimetric sensing and fluorescent studies of Cu2+ ions derived from spherical nanocellulose. ACS Appl. Polym. Mater.2020,2, 5290–5299.
To detect GA in water, Wu et al.135 developed a fluorescent aerogel by chemically cross-linking NC and amino-modified carbon dots. The cross-linking reaction was conducted in the absence of organic solvents or toxic cross-linking reagents or fluorescent sources. The results showed the parts per million level of detection of GA in water. Furthermore, the developed fluorescent aerogel displayed a considerable selection of fluorescence quenching toward specific gaseous and liquid molecules such as nitric oxide and aldehyde species.
5. Limitations and Challenges in Advancements of Cellulose Nanomaterials in Global Water Remediation
Regardless of the potentials and possibilities cellulose nanomaterials present for the production of low-cost, cheap, and safe water, the lack of serious funding still hampers technological transfer and localization.136−138 In addition, most poor countries, where access to clean water is a large issue, do not have a national nanotechnology plan or strategy and the national yearly budgets of countries do not reflect any sort of commitment for harnessing these technological advancements.5,139 In addition, according to the study of Piccinno et al.,140 factors such as solvents and heat and electricity consumption are critical contributory components in developing nanotechnology optimally; this poses a serious challenge for countries, where electricity generation is a problem, in optimizing the technology and processes required for the industrial use of NC to meet local demands. There are also concerns that the modification of nanocellulosic materials may make them resistant to biodegradability,114 which is one of the primary disadvantages NC presents; we believe that any form of modification of cellulose-based nanomaterials and/or systems must take the environment into consideration.
Another possible challenge that may hamper the advancements in NC utilization in ameliorating the rising water crisis is religious and local belief systems. For example, wastewater recycling and reuse aid in producing potable and usable water; however, religious beliefs in certain countries make people hesitant to such technologies.141−144
6. Conclusions
The requirement for potable safe water will continue to increase in the coming years. The need to harvest degraded waters for mitigating global water scarcity cannot be overlooked. In this perspective, we have shown that the earth’s freshwater resources are under pressure, and no continent or country is safe from the raging water crisis. Recently, a number of nanomaterials have been extensively used in the reclamation, treatment, and purification of polluted and degraded waters; however, there are growing concerns about their associated negative health and environmental impacts as they accumulate in living and nonliving systems, thereby triggering unpredictable environmental changes in natural systems. Here, we have demonstrated that nanoscale cellulosic materials/systems are benign, sustainable, and ubiquitous biomaterials for global water remediation and that the scientific community has made efforts to resolve the challenges of the rising global water scarcity and degradation. However, there are still knowledge gaps that need to be investigated, for example, understanding the interfacial reactions of NC systems and materials in changing pH to optimize NC system resilience and robustness. We also highlighted the vast and untapped opportunity that the TP presents to be used as nanocellulosic materials for water remediation. Furthermore, we believe that as nanobased cellulosic materials/systems gain research interest, however, in the context of material properties, enhancements, such as surface modifications, of cellulose nanomaterials may result in the deterioration of its biodegradability. Therefore, there is an emerging need to assess and evaluate emerging data to understand the potential environmental risks of nanocellulose production, its ability to be used on an industrial scale, and its future use so as to ensure that the potential and efficient material will not become the source of our destruction.
Acknowledgments
The authors would like to thank the Council for Scientific and Industrial Research (HGER74p) and the Department of Science and Innovation (HGERA8x) for financial support.
Author Contributions
The manuscript was written through contributions of both authors.
The authors declare no competing financial interest.
References
- UNDP . Department of Economic and Social Affairs Disability. https://www.un.org/development/desa/disabilities/envision2030-goal16.html (accessed Oct 31, 2020).
- Seung-soo H.The Growing Global Challenge of Water Scarcity—We Must Seek Innovative Solutions That Ensure a Fair Distribution of Water Resources. Special to Gulf News: United Arab Emirates, Oct 10, 2019.
- United Nations . Water Scarcity. https://www.unwater.org/water-facts/scarcity/ (accessed Oct 5, 2020).
- World Economic Forum . Water Scarcity—One of the greatest challenges of our time. https://www.weforum.org/agenda/2019/03/water-scarcity-one-of-the-greatest-challenges-of-our-time/ (accessed Oct 28, 2020).
- Ray S. S.; Iroegbu A. O. C.; Bordado J. C. Polymer-Based Membranes and Composites for Safe, Potable, and Usable Water: A Survey of Recent Advances. Chem. Afr. 2020, 3, 593–608. 10.1007/s42250-020-00166-z. [DOI] [Google Scholar]
- Huang J.; Ridoutt B. G.; Thorp K. R.; Wang X.; Lan K.; Liao J.; Tao X.; Wu C.; Huang J.; Chen F.; Scherer L. Water-Scarcity Footprints and Water Productivities Indicate Unsustainable Wheat Production in China. Agric. Water Manag. 2019, 224, 105744. 10.1016/j.agwat.2019.105744. [DOI] [Google Scholar]
- Besada H.; Werner K. An Assessment of the Effects of Africa’s Water Crisis on Food Security and Management. Int. J. Water Resour. Dev. 2015, 31, 120–133. 10.1080/07900627.2014.905124. [DOI] [Google Scholar]
- Zhang L.; Chen F.; Lei Y. Climate Change and Shifts in Cropping Systems Together Exacerbate China’s Water Scarcity. Environ. Res. Lett. 2020, 15, 104060. 10.1088/1748-9326/abb1f2. [DOI] [Google Scholar]
- Thomsen M.US Army Warns That Military Is “precariously Unprepared” for Climate Change and Says Access to Drinking Water for Soldiers Will Be Major Concern in Future Conflicts. DailyMail UK: United Kingdom, Oct 24, 2019.
- FAO . Water : A finite resource. www.fao.org/3/u8480e/U8480E01.htm#Contents%0D (accessed Oct 12, 2020).
- Breen S.-P. W.; Loring P. A.; Baulch H. When a Water Problem Is More Than a Water Problem: Fragmentation, Framing, and the Case of Agricultural Wetland Drainage. Front. Environ. Sci. 2018, 6, 129. 10.3389/fenvs.2018.00129. [DOI] [Google Scholar]
- Carroll R.; Reynolds J. K.; Wright I. A. Geochemical Impact of Urban Development on Fragile Freshwater Wetlands. IOP Conf. Ser. Earth Environ. Sci. 2019, 344, 012004. 10.1088/1755-1315/344/1/012004. [DOI] [Google Scholar]
- White E.; Kaplan D. Restore or Retreat? Saltwater Intrusion and Water Management in Coastal Wetlands. Ecosyst. Heal. Sustain. 2017, 3, e01258 10.1002/ehs2.1258. [DOI] [Google Scholar]
- Tully K.; Gedan K.; Epanchin-Niell R.; Strong A.; Bernhardt E. S.; BenDor T.; Mitchell M.; Kominoski J.; Jordan T. E.; Neubauer S. C.; Weston N. B. The Invisible Flood: The Chemistry, Ecology, and Social Implications of Coastal Saltwater Intrusion. Bioscience 2019, 69, 368–378. 10.1093/biosci/biz027. [DOI] [Google Scholar]
- Gulbin S.; Kirilenko A. P.; Kharel G.; Zhang X. Wetland Loss Impact on Long Term Flood Risks in a Closed Watershed. Environ. Sci. Policy 2019, 94, 112–122. 10.1016/j.envsci.2018.12.032. [DOI] [Google Scholar]
- Steichen J. L.; Labonté J. M.; Windham R.; Hala D.; Kaiser K.; Setta S.; Faulkner P. C.; Bacosa H.; Yan G.; Kamalanathan M.; Quigg A. Microbial, Physical, and Chemical Changes in Galveston Bay Following an Extreme Flooding Event, Hurricane Harvey. Front. Mar. Sci. 2020, 7, 186. 10.3389/fmars.2020.00186. [DOI] [Google Scholar]
- Friedrich K.; Grossman R. L.; Huntington J.; Blanken P. D.; Lenters J.; Holman K. D.; Gochis D.; Livneh B.; Prairie J.; Skeie E.; Healey N. C.; Dahm K.; Pearson C.; Finnessey T.; Hook S. J.; Kowalski T. Reservoir Evaporation in the Western United States: Current Science, Challenges, and Future Needs. Bull. Am. Meteorol. Soc. 2018, 99, 167–187. 10.1175/BAMS-D-15-00224.1. [DOI] [Google Scholar]
- Zhao G.; Gao H. Estimating Reservoir Evaporation Losses for the United States: Fusing Remote Sensing and Modeling Approaches. Remote Sens. Environ. 2019, 226, 109–124. 10.1016/j.rse.2019.03.015. [DOI] [Google Scholar]
- Iroegbu A. O. C.; Sadiku R. E.; Ray S. S.; Hamam Y. Plastics in Municipal Drinking Water and Wastewater Treatment Plant Effluents: Challenges and Opportunities for South Africa—a Review. Environ. Sci. Pollut. Res. 2020, 27, 12953–12966. 10.1007/s11356-020-08194-5. [DOI] [PubMed] [Google Scholar]
- Fu L.; Lu X.; Niu K.; Tan J.; Chen J. Bioaccumulation and Human Health Implications of Essential and Toxic Metals in Freshwater Products of Northeast China. Sci. Total Environ. 2019, 673, 768–776. 10.1016/j.scitotenv.2019.04.099. [DOI] [PubMed] [Google Scholar]
- Albert J. S.; Destouni G.; Duke-Sylvester S. M.; Magurran A. E.; Oberdorff T.; Reis R. E.; Winemiller K. O.; Ripple W. J. Scientists’ Warning to Humanity on the Freshwater Biodiversity Crisis. Ambio 2020, 50, 85–94. 10.1007/s13280-020-01318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Yang Z. Industrial Water Pollution, Water Environment Treatment, and Health Risks in China. Environ. Pollut. 2016, 218, 358–365. 10.1016/j.envpol.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Chowdhary P.; Yadav A.; Kaithwas G.; Bharagava R. N.. Distillery Wastewater: A Major Source of Environmental Pollution and Its Biological Treatment for Environmental Safety. Green Technologies and Environmental Sustainability; Springer International Publishing: Cham, 2017; pp 409–435. [Google Scholar]
- Cosgrove W. J.; Loucks D. P. Water management: Current and future challenges and research directions. Water Resour. Res. 2015, 51, 4823–4839. 10.1002/2014WR016869. [DOI] [Google Scholar]
- Carrubba A.Rotten river: life on one of the world’s most polluted waterways—photo essay. https://www.theguardian.com/global-development/2020/nov/02/rotten-river-life-on-one-of-the-worlds-most-polluted-waterways-photo-essay%0D (accessed Nov 4, 2020).
- Dsikowitzky L.; Hagemann L.; Dwiyitno; Ariyani F.; Irianto H. E.; Schwarzbauer J. Complex Organic Pollutant Mixtures Originating from Industrial and Municipal Emissions in Surface Waters of the Megacity Jakarta—an Example of a Water Pollution Problem in Emerging Economies. Environ. Sci. Pollut. Res. 2017, 24, 27539–27552. 10.1007/s11356-017-0164-2. [DOI] [PubMed] [Google Scholar]
- Genthe B.; Kapwata T.; Le Roux W.; Chamier J.; Wright C. Y. The Reach of Human Health Risks Associated with Metals/Metalloids in Water and Vegetables along a Contaminated River Catchment: South Africa and Mozambique. Chemosphere 2018, 199, 1–9. 10.1016/j.chemosphere.2018.01.160. [DOI] [PubMed] [Google Scholar]
- Wijesiri B.; Deilami K.; McGree J.; Goonetilleke A. Use of Surrogate Indicators for the Evaluation of Potential Health Risks Due to Poor Urban Water Quality: A Bayesian Network Approach. Environ. Pollut. 2018, 233, 655–661. 10.1016/j.envpol.2017.10.076. [DOI] [PubMed] [Google Scholar]
- Mahboob S.; Al-Ghanim K. A.; Al-Misned F.; Shahid T.; Sultana S.; Sultan T.; Hussain B.; Ahmed Z. Impact of Water Pollution on Trophic Transfer of Fatty Acids in Fish, Microalgae, and Zoobenthos in the Food Web of a Freshwater Ecosystem. Biomolecules 2019, 9, 231. 10.3390/biom9060231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreen U.; Ahmed Z.; Khalid A.; Di Serafino A.; Habiba U.; Ali F.; Hussain M. Water Pollution and Occupational Health Hazards Caused by the Marble Industries in District Mardan, Pakistan. Environ. Technol. Innov. 2019, 16, 100470. 10.1016/j.eti.2019.100470. [DOI] [Google Scholar]
- Lai W. Pesticide Use and Health Outcomes: Evidence from Agricultural Water Pollution in China. J. Environ. Econ. Manag. 2017, 86, 93–120. 10.1016/j.jeem.2017.05.006. [DOI] [Google Scholar]
- Cicciù M. Water Contamination Risks at the Dental Clinic. Biology 2020, 9, 43. 10.3390/biology9030043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.-Y.; Chu H.-S.; Lin P.-C.; Lee T.-F.; Kuo K.-T.; Hsueh P.-R.; Hu F.-R.; Wang I.-J. Outbreak of Microsporidial Keratoconjunctivitis Associated With Water Contamination in Swimming Pools in Taiwan. Am. J. Ophthalmol. 2018, 194, 101–109. 10.1016/j.ajo.2018.07.019. [DOI] [PubMed] [Google Scholar]
- Guo M.; Lu X.; Nielsen C. P.; McElroy M. B.; Shi W.; Chen Y.; Xu Y. Prospects for Shale Gas Production in China: Implications for Water Demand. Renewable Sustainable Energy Rev. 2016, 66, 742–750. 10.1016/j.rser.2016.08.026. [DOI] [Google Scholar]
- United Nations . Water and Sanitation—United Nations Sustainable Development. http://www.un.org/sustainabledevelopment/water-and-sanitation/ (accessed Jan 5, 2020).
- Herold C. Des Midgley Memorial Lecture : The Water Crisis in South Africa. Civ. Eng. 2010, 2010, 6–15. 10.10520/EJC26346. [DOI] [Google Scholar]
- Weigner K. K.Water Crisis: It’s Almost Here. Forbes 1979, 124, 56−63. [Google Scholar]
- Waterbury J.Yes, California, There Is a Water Crisis: Observations on the UN Water Conference; South America Series; FieldsStaff Report: Argentina, 1977; Vol. 21. [Google Scholar]
- Groenfeldt D.Water Ethics—A Value Approach to Solving the Water Crisis, 2nd ed.; Taylor & Francis Books, 2019. [Google Scholar]
- Maryam B.; Büyükgüngör H. Wastewater Reclamation and Reuse Trends in Turkey: Opportunities and Challenges. J. Water Process Eng. 2019, 30, 100501. 10.1016/j.jwpe.2017.10.001. [DOI] [Google Scholar]
- Nagar A.; Pradeep T. Clean Water through Nanotechnology: Needs, Gaps, and Fulfillment. ACS Nano 2020, 14, 6420–6435. 10.1021/acsnano.9b01730. [DOI] [PubMed] [Google Scholar]
- Bekele E.; Page D.; Vanderzalm J.; Kaksonen A.; Gonzalez D. Water Recycling via Aquifers for Sustainable Urban Water Quality Management: Current Status, Challenges and Opportunities. Water 2018, 10, 457. 10.3390/w10040457. [DOI] [Google Scholar]
- Wanjiru E.; Xia X. Optimal Energy-Water Management in Urban Residential Buildings through Grey Water Recycling. Sustain. Cities Soc. 2017, 32, 654–668. 10.1016/j.scs.2017.05.009. [DOI] [Google Scholar]
- Xie B.; Zhu G.; Liu B.; Su Q.; Deng S.; Yang L.; Liu G.; Dong C.; Wang M.; Liu H. The Water Treatment and Recycling in 105-Day Bioregenerative Life Support Experiment in the Lunar Palace 1. Acta Astronaut. 2017, 140, 420–426. 10.1016/j.actaastro.2017.08.026. [DOI] [Google Scholar]
- Janani T.; Sudarsan J. S.; Prasanna K. Grey Water Recycling with Corn Cob as an Adsorbent. AIP Conf. Proc. 2019, 2112, 020181. 10.1063/1.5112366. [DOI] [Google Scholar]
- Geng H.; Xu Q.; Wu M.; Ma H.; Zhang P.; Gao T.; Qu L.; Ma T.; Li C. Plant Leaves Inspired Sunlight-Driven Purifier for High-Efficiency Clean Water Production. Nat. Commun. 2019, 10, 1512. 10.1038/s41467-019-09535-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson D.; Sevanthi R.; Morse A.; Jackson A. Assessment of Membrane-Aerated Biological Reactors (MABRs) for Integration into Space-Based Water Recycling System Architectures. Gravitational Sp. Res. 2020, 6, 12–27. 10.2478/gsr-2018-0007. [DOI] [Google Scholar]
- Ali Z.; Ahmad R.. Nanotechnology for Water Treatment. Environmental Nanotechnology; Springer: Cham, 2020; Vol. 3, pp 143–163. [Google Scholar]
- Jassby D.; Cath T. Y.; Buisson H. The Role of Nanotechnology in Industrial Water Treatment. Nat. Nanotechnol. 2018, 13, 670–672. 10.1038/s41565-018-0234-8. [DOI] [PubMed] [Google Scholar]
- Mauter M. S.; Zucker I.; Perreault F.; Werber J. R.; Kim J.-H.; Elimelech M. The Role of Nanotechnology in Tackling Global Water Challenges. Nat. Sustain. 2018, 1, 166–175. 10.1038/s41893-018-0046-8. [DOI] [Google Scholar]
- Alvarez P. J. J.; Chan C. K.; Elimelech M.; Halas N. J.; Villagrán D. Emerging Opportunities for Nanotechnology to Enhance Water Security. Nat. Nanotechnol. 2018, 13, 634–641. 10.1038/s41565-018-0203-2. [DOI] [PubMed] [Google Scholar]
- Mondal S. Preparation, Properties and Applications of Nanocellulosic Materials. Carbohydr. Polym. 2017, 163, 301–316. 10.1016/j.carbpol.2016.12.050. [DOI] [PubMed] [Google Scholar]
- Nanocelluloses: Their Preparation, Properties, and Applications; Agarwal U. P., Atalla R. H., Isogai A., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, United States of America, 2017; Vol. 1251. [Google Scholar]
- Seyednezhad M.; Sheikholeslami M.; Ali J. A.; Shafee A.; Nguyen T. K. Nanoparticles for Water Desalination in Solar Heat Exchanger. J. Therm. Anal. Calorim. 2020, 139, 1619–1636. 10.1007/s10973-019-08634-6. [DOI] [Google Scholar]
- Zhao C.; Wang Z.; Wang C.; Li X.; Wang C.-C. Photocatalytic Degradation of DOM in Urban Stormwater Runoff with TiO2 Nanoparticles under UV Light Irradiation: EEM-PARAFAC Analysis and Influence of Co-Existing Inorganic Ions. Environ. Pollut. 2018, 243, 177–188. 10.1016/j.envpol.2018.08.062. [DOI] [PubMed] [Google Scholar]
- Liu X.; Tian J.; Li Y.; Sun N.; Mi S.; Xie Y.; Chen Z. Enhanced Dyes Adsorption from Wastewater via Fe3O4 Nanoparticles Functionalized Activated Carbon. J. Hazard. Mater. 2019, 373, 397–407. 10.1016/j.jhazmat.2019.03.103. [DOI] [PubMed] [Google Scholar]
- Lazar M.; Varghese S.; Nair S. Photocatalytic Water Treatment by Titanium Dioxide: Recent Updates. Catalysts 2012, 2, 572–601. 10.3390/catal2040572. [DOI] [Google Scholar]
- Orha C.; Pode R.; Manea F.; Lazau C.; Bandas C. Titanium Dioxide-Modified Activated Carbon for Advanced Drinking Water Treatment. Process Saf. Environ. Prot. 2017, 108, 26–33. 10.1016/j.psep.2016.07.013. [DOI] [Google Scholar]
- Morsi R. E.; Alsabagh A. M.; Nasr S. A.; Zaki M. M. Multifunctional Nanocomposites of Chitosan, Silver Nanoparticles, Copper Nanoparticles and Carbon Nanotubes for Water Treatment: Antimicrobial Characteristics. Int. J. Biol. Macromol. 2017, 97, 264–269. 10.1016/j.ijbiomac.2017.01.032. [DOI] [PubMed] [Google Scholar]
- Dimapilis E. A. S.; Hsu C.-S.; Mendoza R. M. O.; Lu M.-C. Zinc Oxide Nanoparticles for Water Disinfection. Sustainable Environ. Res. 2018, 28, 47–56. 10.1016/j.serj.2017.10.001. [DOI] [Google Scholar]
- Ali Q.; Ahmed W.; Lal S.; Sen T. Novel Multifunctional Carbon Nanotube Containing Silver and Iron Oxide Nanoparticles for Antimicrobial Applications in Water Treatment. Mater. Today: Proc. 2017, 4, 57–64. 10.1016/j.matpr.2017.01.193. [DOI] [Google Scholar]
- Lakhotia S. R.; Mukhopadhyay M.; Kumari P. Iron Oxide (FeO) Nanoparticles Embedded Thin-Film Nanocomposite Nanofiltration (NF) Membrane for Water Treatment. Sep. Purif. Technol. 2019, 211, 98–107. 10.1016/j.seppur.2018.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystrzejewska-Piotrowska G.; Golimowski J.; Urban P. L. Nanoparticles: Their Potential Toxicity, Waste and Environmental Management. Waste Manag. 2009, 29, 2587–2595. 10.1016/j.wasman.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Kim E.; Kim S.-H.; Kim H.-C.; Lee S. G.; Lee S. J.; Jeong S. W. Growth Inhibition of Aquatic Plant Caused by Silver and Titanium Oxide Nanoparticles. Toxicol. Environ. Health Sci. 2011, 3, 1–6. 10.1007/s13530-011-0071-8. [DOI] [Google Scholar]
- Chalew T. E. A.; Ajmani G. S.; Huang H.; Schwab K. J. Evaluating Nanoparticle Breakthrough during Drinking Water Treatment. Environ. Health Perspect. 2013, 121, 1161–1166. 10.1289/ehp.1306574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser M. A.; Westerhoff P.; Benn T.; Wang Y.; Pérez-Rivera J.; Hristovski K. Titanium Nanomaterial Removal and Release from Wastewater Treatment Plants. Environ. Sci. Technol. 2009, 43, 6757–6763. 10.1021/es901102n. [DOI] [PubMed] [Google Scholar]
- Hou J.; Wu Y.; Li X.; Wei B.; Li S.; Wang X. Toxic Effects of Different Types of Zinc Oxide Nanoparticles on Algae, Plants, Invertebrates, Vertebrates and Microorganisms. Chemosphere 2018, 193, 852–860. 10.1016/j.chemosphere.2017.11.077. [DOI] [PubMed] [Google Scholar]
- Tortella G. R.; Rubilar O.; Durán N.; Diez M. C.; Martínez M.; Parada J.; Seabra A. B. Silver Nanoparticles: Toxicity in Model Organisms as an Overview of Its Hazard for Human Health and the Environment. J. Hazard. Mater. 2020, 390, 121974. 10.1016/j.jhazmat.2019.121974. [DOI] [PubMed] [Google Scholar]
- Handy R. D.; Shaw B. J. Toxic Effects of Nanoparticles and Nanomaterials: Implications for Public Health, Risk Assessment and the Public Perception of Nanotechnology. Health Risk Soc. 2007, 9, 125–144. 10.1080/13698570701306807. [DOI] [Google Scholar]
- Schrand A. M.; Rahman M. F.; Hussain S. M.; Schlager J. J.; Smith D. A.; Syed A. F. Metal-based Nanoparticles and Their Toxicity Assessment. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2010, 2, 544–568. 10.1002/wnan.103. [DOI] [PubMed] [Google Scholar]
- Pohanka M. Copper and Copper Nanoparticles Toxicity and Their Impact on Basic Functions in the Body. Bratislava Med. J. 2019, 120, 397–409. 10.4149/BLL_2019_065. [DOI] [PubMed] [Google Scholar]
- Liu S.; Cui M.; Li X.; Thuyet D. Q.; Fan W. Effects of Hydrophobicity of Titanium Dioxide Nanoparticles and Exposure Scenarios on Copper Uptake and Toxicity in Daphnia Magna. Water Res. 2019, 154, 162–170. 10.1016/j.watres.2019.01.055. [DOI] [PubMed] [Google Scholar]
- Panyala N. R.; Peña-Méndez E. M.; Havel J. Silver or silver nanoparticles: a hazardous threat to the environment and human health?. J. Appl. Biomed. 2008, 6, 117–129. 10.32725/jab.2008.015. [DOI] [Google Scholar]
- Li M.; Lin D.; Zhu L. Effects of Water Chemistry on the Dissolution of ZnO Nanoparticles and Their Toxicity to Escherichia Coli. Environ. Pollut. 2013, 173, 97–102. 10.1016/j.envpol.2012.10.026. [DOI] [PubMed] [Google Scholar]
- Brunswig H.Method of transforming fibrous cellulose into a dense material. U.S. Patent 622,325 A, April 4, 1899.
- Joseph B.; Maria H. J.; Thomas S.; Kalarikka N.. Nanocellulose: Health Care Applications. Encyclopedia of Polymer Applications; CRC Press, 2018; pp 1829–1852. [Google Scholar]
- Anthony S.Nanocellulose: A cheap, conductive, stronger-than-Kevlar wonder material made from wood pulp. www.extremetech.com/extreme/134910-nanocellulose-a-cheap-conductive-stronger-than-kevlar-wonder-material-made-from-wood-pulp%0D (accessed Nov 2, 2020).
- TACC . Perm scientists have invented cellulose stronger than steel. https://nauka.tass.ru/nauka/1891125 (accessed Nov 2, 2020).
- Dufresne A. Nanocellulose: A New Ageless Bionanomaterial. Mater. Today 2013, 16, 220–227. 10.1016/j.mattod.2013.06.004. [DOI] [Google Scholar]
- Håkansson K. M. O.; Fall A. B.; Lundell F.; Yu S.; Krywka C.; Roth S. V.; Santoro G.; Kvick M.; Prahl Wittberg L.; Wågberg L.; Söderberg L. D. Hydrodynamic Alignment and Assembly of Nanofibrils Resulting in Strong Cellulose Filaments. Nat. Commun. 2014, 5, 4018. 10.1038/ncomms5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bio-Based News . NanoCELL—Nanocellulose as great hope for environmentally friendly packaging. https://news.bio-based.eu/nanocell-nanocellulose-as-great-hope-for-environmentally-friendly-packaging/%0D (accessed Nov 8, 2020).
- Soutter W.What is Nanocellulose?. https://www.azonano.com/article.aspx?ArticleID=3139#:~:text=Nanocelluloseistransparent%2Celectrically,conductive%2Candstrongerthansteel (accessed Nov 8, 2020).
- Isogai A. Emerging Nanocellulose Technologies: Recent Developments. Adv. Mater. 2020, 2000630. 10.1002/adma.202000630. [DOI] [PubMed] [Google Scholar]
- Uetani K.; Kitaoka T. Nanocellulose: Beyond the Ordinary. BioResources 2021, 16, 1–4. [Google Scholar]
- Wang X.; Wang Q.; Xu C. Nanocellulose-Based Inks for 3d Bioprinting: Key Aspects in Research Development and Challenging Perspectives in Applications—a Mini Review. Bioengineering 2020, 7, 40. 10.3390/bioengineering7020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spartz J. T.Nanocellulose Pilot Plant is “A Game-Changer”. https://www.fpl.fs.fed.us/labnotes/?p=122%0D (accessed Nov 3, 2020).
- FPInnovations . New research facilities for nanocellulose. https://www.canadianbiomassmagazine.ca/new-research-facilities-for-nanocellulose-2530/%0D (accessed Nov 1, 2020).
- Go green to the extreme! Japan rolls out eco-friendly supercar made from WOOD at Tokyo Motor Show (VIDEO). https://www.rt.com/news/472580-japan-unveils-wooden-supercar/ (accessed Nov 8, 2020).
- Toha N.; Yaacob W. M. H. W.; Razali N. A. M.; Rusdi R. A. A.; Ismail A.; Ahmad K. Z. K.; Aziz F. A. Preliminary Development of Laminated Nanocomposite from Nanocellulose-Kevlar for Military Application. Int. J. Curr. Res. Sci. Eng. Technol. 2018, 1, 566. 10.30967/ijcrset.1.S1.2018.566-570. [DOI] [Google Scholar]
- a Maiti S.; Jayaramudu J.; Das K.; Reddy S. M.; Sadiku R.; Ray S. S.; Liu D. Preparation and Characterization of Nano-cellulose with New Shape from Different Precursor. Carbohydr. Polym. 2013, 98, 562–567. 10.1016/j.carbpol.2013.06.029. [DOI] [PubMed] [Google Scholar]; b Morán J. I.; Alvarez V. A.; Cyras V. P.; Vázquez A. Extraction of Cellulose and Preparation of Nanocellulose from Sisal Fibers. Cellulose 2008, 15, 149–159. 10.1007/s10570-007-9145-9. [DOI] [Google Scholar]
- Gray D. Nanocellulose: From Nature to High Performance Tailored Material. Holzforschung 2013, 67, 353. 10.1515/hf-2013-0027. [DOI] [Google Scholar]
- Saito T.; Nishiyama Y.; Putaux J.-L.; Vignon M.; Isogai A. Homogeneous Suspensions of Individualized Microfibrils from TEMPO-Catalyzed Oxidation of Native Cellulose. Biomacromolecules 2006, 7, 1687–1691. 10.1021/bm060154s. [DOI] [PubMed] [Google Scholar]
- Klemm D.; Schumann D.; Kramer F.; Heßler N.; Koth D.; Sultanova B. Nanocellulose Materials - Different Cellulose, Different Functionality. Macromol. Symp. 2009, 280, 60–71. 10.1002/masy.200950608. [DOI] [Google Scholar]
- Le Bras D.; Strømme M.; Mihranyan A. Characterization of Dielectric Properties of Nanocellulose from Wood and Algae for Electrical Insulator Applications. J. Phys. Chem. B 2015, 119, 5911–5917. 10.1021/acs.jpcb.5b00715. [DOI] [PubMed] [Google Scholar]
- Kumar V.; Pathak P.; Bhardwaj N. K. Waste Paper: An Underutilized but Promising Source for Nanocellulose Mining. Waste Manag. 2020, 102, 281–303. 10.1016/j.wasman.2019.10.041. [DOI] [PubMed] [Google Scholar]
- Toor A.Can we grow a stronger-than-steel “wonder material” to save the world?—The Verge. https://www.theverge.com/2013/4/8/4195982/nanocellulose-wonder-material-produced-from-algae-solar-energy (accessed Nov 9, 2020).
- Sirviö J. A.; Visanko M.; Liimatainen H. Acidic Deep Eutectic Solvents As Hydrolytic Media for Cellulose Nanocrystal Production. Biomacromolecules 2016, 17, 3025–3032. 10.1021/acs.biomac.6b00910. [DOI] [PubMed] [Google Scholar]
- Xie H.; Du H.; Yang X.; Si C. Recent Strategies in Preparation of Cellulose Nanocrystals and Cellulose Nanofibrils Derived from Raw Cellulose Materials. Int. J. Polym. Sci. 2018, 2018, 1–25. 10.1155/2018/7923068. [DOI] [Google Scholar]
- Hong F.; Wei B.; Chen L. Preliminary Study on Biosynthesis of Bacterial Nanocellulose Tubes in a Novel Double-Silicone-Tube Bioreactor for Potential Vascular Prosthesis. BioMed Res. Int. 2015, 2015, 1–9. 10.1155/2015/560365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S.; Ono E.; Watanabe K.. Hollow Microbial Cellulose, Process for Preparation Thereof, and Artificial Blood Vessel Formed of Said Cellulose. EP 0396344 A3, 1990.
- Abol-Fotouh D.; Hassan M. A.; Shokry H.; Roig A.; Azab M. S.; Kashyout A. E.-H. B. Bacterial Nanocellulose from Agro-Industrial Wastes: Low-Cost and Enhanced Production by Komagataeibacter Saccharivorans MD1. Sci. Rep. 2020, 10, 3491. 10.1038/s41598-020-60315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B.; Raj M. C.; Athira K. B.; Moores A.; Drisko G. L.; Sanchez C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. 10.1021/acs.chemrev.7b00627. [DOI] [PubMed] [Google Scholar]
- Nanocellulose Based Composites for Electronics; Thomas S., Pottathara Y. B., Eds.; Elsevier: India, 2021; Vol. 1. [Google Scholar]
- Niu Z.; Yuan W. Highly Efficient Thermo- and Sunlight-Driven Energy Storage for Thermo-Electric Energy Harvesting Using Sustainable Nanocellulose-Derived Carbon Aerogels Embedded Phase Change Materials. ACS Sustainable Chem. Eng. 2019, 7, 17523–17534. 10.1021/acssuschemeng.9b05015. [DOI] [Google Scholar]
- Lee A.; Elam J. W.; Darling S. B. Membrane Materials for Water Purification: Design, Development, and Application. Environ. Sci.: Water Res. Technol. 2016, 2, 17–42. 10.1039/C5EW00159E. [DOI] [Google Scholar]
- Sharma P. R.; Sharma S. K.; Lindström T.; Hsiao B. S. Nanocellulose-Enabled Membranes for Water Purification: Perspectives. Adv. Sustainable Syst. 2020, 4, 1900114. 10.1002/adsu.201900114. [DOI] [Google Scholar]
- Gholami Derami H.; Jiang Q.; Ghim D.; Cao S.; Chandar Y. J.; Morrissey J. J.; Jun Y.-S.; Singamaneni S. A Robust and Scalable Polydopamine/Bacterial Nanocellulose Hybrid Membrane for Efficient Wastewater Treatment. ACS Appl. Nano Mater. 2019, 2, 1092–1101. 10.1021/acsanm.9b00022. [DOI] [Google Scholar]
- Wang Z.; Zhang W.; Yu J.; Zhang L.; Liu L.; Zhou X.; Huang C.; Fan Y. Preparation of Nanocellulose/Filter Paper (NC/FP) Composite Membranes for High-Performance Filtration. Cellulose 2019, 26, 1183–1194. 10.1007/s10570-018-2121-8. [DOI] [Google Scholar]
- Roy S.; Zhai L.; Van Hai L.; Kim J. W.; Park J. H.; Kim H. C.; Kim J. One-Step Nanocellulose Coating Converts Tissue Paper into an Efficient Separation Membrane. Cellulose 2018, 25, 4871–4886. 10.1007/s10570-018-1945-6. [DOI] [Google Scholar]
- Yang M.; Hadi P.; Yin X.; Yu J.; Huang X.; Ma H.; Walker H.; Hsiao B. S. Antifouling Nanocellulose Membranes: How Subtle Adjustment of Surface Charge Lead to Self-Cleaning Property. J. Membr. Sci. 2021, 618, 118739. 10.1016/j.memsci.2020.118739. [DOI] [Google Scholar]
- Hou D.; Li T.; Chen X.; He S.; Dai J.; Mofid S. A.; Hou D.; Iddya A.; Jassby D.; Yang R.; Hu L.; Ren Z. J. Hydrophobic Nanostructured Wood Membrane for Thermally Efficient Distillation. Sci. Adv. 2019, 5, eaaw3203 10.1126/sciadv.aaw3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Gusain R.; Kumar N.; Ray S. S. Recent Advances in Carbon Nanomaterials-based Adsorbents for Water Purification. Coord. Chem. Rev. 2020, 405, 213111. 10.1016/j.ccr.2019.213111. [DOI] [Google Scholar]; b Nie C.; Peng Z.; Yang Y.; Cheng C.; Ma L.; Zhao C. Kevlar Based Nanofibrous Particles as Robust, Effective and Recyclable Absorbents for Water Purification. J. Hazard. Mater. 2016, 318, 255–265. 10.1016/j.jhazmat.2016.06.061. [DOI] [PubMed] [Google Scholar]
- a Kumar N.; Fosso-Kankeu E.; Ray S. S. Achieving Controllable MoS2 Nanostructures with Increased Spacing for Efficient Removal of Pb(II) from Aquatic Systems. ACS Appl. Mater. Interfaces 2019, 11, 19141–19155. 10.1021/acsami.9b03853. [DOI] [PubMed] [Google Scholar]; b Gusain R.; Kumar N.; Fosso-Kankeu E.; Ray S. S. Efficient Removal of Pb(II) and Cd(II) from Industrial Mine Water by a Hierarchical MoS2/SH-MWCNT Nanocomposite. ACS Omega 2019, 4, 13922–13935. 10.1021/acsomega.9b01603. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Yue X.; Zhang T.; Yang D.; Qiu F.; Rong J.; Xu J.; Fang J. The Synthesis of Hierarchical Porous Al2O 3 /Acrylic Resin Composites as Durable, Efficient and Recyclable Absorbents for Oil/Water Separation. Chem. Eng. J. 2017, 309, 522–531. 10.1016/j.cej.2016.10.049. [DOI] [Google Scholar]
- Mahfoudhi N.; Boufi S. Nanocellulose as a Novel Nanostructured Adsorbent for Environmental Remediation: A Review. Cellulose 2017, 24, 1171–1197. 10.1007/s10570-017-1194-0. [DOI] [Google Scholar]
- Ali I.; Gupta V. K. Advances in Water Treatment by Adsorption Technology. Nat. Protoc. 2006, 1, 2661–2667. 10.1038/nprot.2006.370. [DOI] [PubMed] [Google Scholar]
- Hokkanen S.; Repo E.; Sillanpää M. Removal of Heavy Metals from Aqueous Solutions by Succinic Anhydride Modified Mercerized Nanocellulose. Chem. Eng. J. 2013, 223, 40–47. 10.1016/j.cej.2013.02.054. [DOI] [Google Scholar]
- Xue Y.; Mou Z.; Xiao H. Nanocellulose as a Sustainable Biomass Material: Structure, Properties, Present Status and Future Prospects in Biomedical Applications. Nanoscale 2017, 9, 14758–14781. 10.1039/C7NR04994C. [DOI] [PubMed] [Google Scholar]
- Sharma P. R.; Chattopadhyay A.; Sharma S. K.; Geng L.; Amiralian N.; Martin D.; Hsiao B. S. Nanocellulose from Spinifex as an Effective Adsorbent to Remove Cadmium(II) from Water. ACS Sustainable Chem. Eng. 2018, 6, 3279–3290. 10.1021/acssuschemeng.7b03473. [DOI] [Google Scholar]
- Suopajärvi T.; Liimatainen H.; Karjalainen M.; Upola H.; Niinimäki J. Lead Adsorption with Sulfonated Wheat Pulp Nanocelluloses. J. Water Process Eng. 2015, 5, 136–142. 10.1016/j.jwpe.2014.06.003. [DOI] [Google Scholar]
- Zhang H.; Lyu S.; Zhou X.; Gu H.; Ma C.; Wang C.; Ding T.; Shao Q.; Liu H.; Guo Z. Super Light 3D Hierarchical Nanocellulose Aerogel Foam with Superior Oil Adsorption. J. Colloid Interface Sci. 2019, 536, 245–251. 10.1016/j.jcis.2018.10.038. [DOI] [PubMed] [Google Scholar]
- Gu H.; Zhou X.; Lyu S.; Pan D.; Dong M.; Wu S.; Ding T.; Wei X.; Seok I.; Wei S.; Guo Z. Magnetic Nanocellulose-Magnetite Aerogel for Easy Oil Adsorption. J. Colloid Interface Sci. 2020, 560, 849–856. 10.1016/j.jcis.2019.10.084. [DOI] [PubMed] [Google Scholar]
- Korhonen J. T.; Kettunen M.; Ras R. H. A.; Ikkala O. Hydrophobic Nanocellulose Aerogels as Floating, Sustainable, Reusable, and Recyclable Oil Absorbents. ACS Appl. Mater. Interfaces 2011, 3, 1813–1816. 10.1021/am200475b. [DOI] [PubMed] [Google Scholar]
- Anirudhan T. S.; Deepa J. R.; Binusreejayan Synthesis and Characterization of Multi-Carboxyl-Functionalized Nanocellulose/Nanobentonite Composite for the Adsorption of Uranium(VI) from Aqueous Solutions: Kinetic and Equilibrium Profiles. Chem. Eng. J. 2015, 273, 390–400. 10.1016/j.cej.2015.03.007. [DOI] [Google Scholar]
- Zhang X.; Elsayed I.; Navarathna C.; Schueneman G. T.; Hassan E. B. Biohybrid Hydrogel and Aerogel from Self-Assembled Nanocellulose and Nanochitin as a High-Efficiency Adsorbent for Water Purification. ACS Appl. Mater. Interfaces 2019, 11, 46714–46725. 10.1021/acsami.9b15139. [DOI] [PubMed] [Google Scholar]
- Geng B.; Wang H.; Wu S.; Ru J.; Tong C.; Chen Y.; Liu H.; Wu S.; Liu X. Surface-Tailored Nanocellulose Aerogels with Thiol-Functional Moieties for Highly Efficient and Selective Removal of Hg(II) Ions from Water. ACS Sustainable Chem. Eng. 2017, 5, 11715–11726. 10.1021/acssuschemeng.7b03188. [DOI] [Google Scholar]
- Septevani A. A.; Rifathin A.; Sari A. A.; Sampora Y.; Ariani G. N.; Sudiyarmanto; Sondari D. Oil Palm Empty Fruit Bunch-Based Nanocellulose as a Super-Adsorbent for Water Remediation. Carbohydr. Polym. 2020, 229, 115433. 10.1016/j.carbpol.2019.115433. [DOI] [PubMed] [Google Scholar]
- Kumar N.; Kardam A.; Jain V.; Nagpal S. A Rapid, Reusable Polyaniline-Impregnated Nanocellulose Composite-Based System for Enhanced Removal of Chromium and Cleaning of Waste Water. Sep. Sci. Technol. 2020, 55, 1436–1448. 10.1080/01496395.2019.1600552. [DOI] [Google Scholar]
- Abiola O. N.Polymers for Coagulation and Flocculation in Water Treatment. In Polymeric Materials for Clean Water; Das R., Ed.; Springer Nature Switzerland: AG, 2019; pp 77–92. [Google Scholar]
- Suopajärvi T.; Liimatainen H.; Hormi O.; Niinimäki J. Coagulation–Flocculation Treatment of Municipal Wastewater Based on Anionized Nanocelluloses. Chem. Eng. J. 2013, 231, 59–67. 10.1016/j.cej.2013.07.010. [DOI] [Google Scholar]
- Suopajärvi T.; Sirviö J. A.; Liimatainen H. Cationic Nanocelluloses in Dewatering of Municipal Activated Sludge. J. Environ. Chem. Eng. 2017, 5, 86–92. 10.1016/j.jece.2016.11.021. [DOI] [Google Scholar]
- Yang X.; Zhang L.; Jin X.; Liu L.; Zhang Y.; Ni Q.; Yao J. Synthesis of Hydrophobically Modified Cellulose-Based Flocculant and Its Application in Treatments of Kaolin Suspension and Machining Wastewater. Cellulose 2017, 24, 5639–5647. 10.1007/s10570-017-1525-1. [DOI] [Google Scholar]
- Koshani R.; Tavakolian M.; van de Ven T. G. M. Cellulose-Based Dispersants and Flocculants. J. Mater. Chem. B 2020, 8, 10502. 10.1039/D0TB02021D. [DOI] [PubMed] [Google Scholar]
- Pouzesh M.; Nekouei S.; Ferdosi Zadeh M. A.; Keshtpour F.; Wang S.; Nekouei F. Fabrication of Stable Copper Nanoparticles Embedded in Nanocellulose Film as a Bionanocomposite Plasmonic Sensor and Thereof for Optical Sensing of Cyanide Ion in Water Samples. Cellulose 2019, 26, 4945–4956. 10.1007/s10570-019-02405-0. [DOI] [Google Scholar]
- Ram B.; Jamwal S.; Ranote S.; Chauhan G. S.; Dharela R. Highly Selective and Rapid Naked-Eye Colorimetric Sensing and Fluorescent Studies of Cu 2+ Ions Derived from Spherical Nanocellulose. ACS Appl. Polym. Mater. 2020, 2, 5290–5299. 10.1021/acsapm.0c01025. [DOI] [Google Scholar]
- Wu B.; Zhu G.; Dufresne A.; Lin N. Fluorescent Aerogels Based on Chemical Crosslinking between Nanocellulose and Carbon Dots for Optical Sensor. ACS Appl. Mater. Interfaces 2019, 11, 16048–16058. 10.1021/acsami.9b02754. [DOI] [PubMed] [Google Scholar]
- Beegle K.; Christiaensen L.. Accelerating Poverty Reduction in Africa; World Bank: Washington, DC, 2019. [Google Scholar]
- Kwasi Fosu A. Economic Structure, Growth, and Evolution of Inequality and Poverty in Africa: An Overview. J. Afr. Econ. 2018, 27, 1–9. 10.1093/jae/ejx036. [DOI] [Google Scholar]
- Shenton R.; Rodney W. How Europe Underdeveloped Africa. Can. J. Afr. Stud. 1975, 9, 146. 10.2307/484037. [DOI] [Google Scholar]
- Languille S. The Politics of the Education Budget: Financing Mass Secondary Education in Tanzania (2004–2012). Int. J. Educ. Dev. 2019, 66, 96–104. 10.1016/j.ijedudev.2019.02.003. [DOI] [Google Scholar]
- Piccinno F.; Hischier R.; Seeger S.; Som C. Predicting the Environmental Impact of a Future Nanocellulose Production at Industrial Scale: Application of the Life Cycle Assessment Scale-up Framework. J. Cleaner Prod. 2018, 174, 283–295. 10.1016/j.jclepro.2017.10.226. [DOI] [Google Scholar]
- Baghapour M. A.; Shooshtarian M. R.; Djahed B. A Survey of Attitudes and Acceptance of Wastewater Reuse in Iran: Shiraz City as a Case Study. J. Water Reuse Desalin. 2017, 7, 511–519. 10.2166/wrd.2016.117. [DOI] [Google Scholar]
- Qureshi A. S. Challenges and Prospects of Using Treated Wastewater to Manage Water Scarcity Crises in the Gulf Cooperation Council (GCC) Countries. Water 2020, 12, 1971. 10.3390/w12071971. [DOI] [Google Scholar]
- Warner W. Influence of Religion on Wastewater Treatment: A Consideration for Sanitation Experts. Water 2000, 21, 11. [Google Scholar]
- Wilson Z.; Pfaff B. Religious, Philosophical and Environmentalist Perspectives on Potable Wastewater Reuse in Durban, South Africa. Desalination 2008, 228, 1–9. 10.1016/j.desal.2007.07.022. [DOI] [Google Scholar]