Abstract
More than 40 years after the 1978 Bethesda Conference on the Declining Mortality from Coronary Heart Disease (CHD) provided the scientific community with a blueprint for systematic analysis to understand declining rates of CHD, there are indications the decline has ended or even reversed despite advances in our knowledge about the condition and treatment. Recent data show a more complex situation, with mortality rates for overall cardiovascular disease (CVD), including CHD and stroke, decelerating while those for heart failure are increasing. To mark the 40th anniversary of the Bethesda Conference, the National Heart Lung and Blood Institute (NHLBI) and the American Heart Association (AHA) co-sponsored the “Bending the Curve in Cardiovascular Disease Mortality: Bethesda + 40” symposium. The objective was to examine the immediate and long-term outcomes of the 1978 conference and understand the current environment. Symposium themes included trends and future projections in CVD (in the US and internationally), the evolving obesity and diabetes epidemics, and harnessing emerging and innovative opportunities to preserve and promote cardiovascular health (CVH) and prevent CVD. In addition, participant-led discussion explored the challenges and barriers in promoting CVH across the lifespan and established a potential framework for observational research and interventions that would begin in early childhood (or ideally in utero). This report summarizes the relevant research, policy and practice opportunities discussed at the symposium.
Keywords: Cardiovascular Disease Prevention, Cardiovascular Health Promotion, Lifespan, Trends
Introduction
The 1978 Bethesda Conference on the Declining Mortality from Coronary Heart Disease1 brought together a panel of international experts from a wide range of disciplines, including epidemiology, clinical cardiology, and public health. The panel called attention to the previously unappreciated and unexplained abatement of the epidemic of coronary heart disease (CHD). A report stemming from the 1978 conference served in many ways as a model for a systematic and careful analysis for understanding the decline in CHD mortality. The panel concluded that the decline was almost certainly real and likely due to multiple factors, including changes in risk factor profiles and improved clinical management. However, this phenomenon appeared to be confined to the United States (US) with only equivocal evidence that similar declines were occurring in other countries. In the ensuing years, age-adjusted cardiovascular death rates have continued to decline sharply in many countries though less so in the US (since about 2010).2 Despite increased knowledge and advances in treatment made over the 40 years since the Bethesda Conference, several questions remain about the decline, including whether it has halted or reversed in the US, why that may be happening, and why disparities have persisted.
In 2018, the National Heart, Lung, and Blood Institute (NHLBI) and the American Heart Association (AHA) co-sponsored a symposium held during the AHA’s 2018 Scientific Sessions titled, “Bending the Curve in Cardiovascular Disease Mortality: Bethesda + 40.” The intent was to review the short-term outcomes following the 1978 Bethesda Conference, contemporary trends in and the current burden of total cardiovascular disease (CVD) in the US in comparison with global CVD mortality trends, potential determinants of these trends, and emerging opportunities to preserve and promote cardiovascular health (CVH) and prevent CVD. This report summarizes the symposium and identifies relevant research, policy, and practice opportunities.
The Aftermath of the 1978 Bethesda Conference
The goals of the 1978 conference included determining whether the decline was real and, if so, the potential factors underlying it and to suggest new studies to monitor and understand trends. The panel’s recommendations focused on improved surveillance systems, standardized data collection and endpoints, and opportunities for prevention and therapeutic trials. The NHLBI and the scientific community responded to those recommendations with a substantial investment in population studies, including cohort studies and surveillance projects (Supplemental Table). By substantially increasing the understanding of the causes of all common forms of CVD, the progression of subclinical disease, and the precipitators of clinical events, those cohort studies and surveillance projects provided insight into factors that underly recent trends.
The Current Pattern of CVD in the US
Vital Statistics for Patterns in CVD mortality
CVD—a broad category that includes CHD, stroke, heart failure (HF), and other heart diseases (HD) and CVD—has been the leading cause of mortality in the US since 1910, with the exception of the flu pandemic years 1918–1920. CHD mortality rates have decreased nearly 75% since 1968 (Figure 1). In recent years, from 1999 to 2011, the average annual percent change in the age-adjusted mortality rate was −3.8% for total CVD, −5.0%, for CHD, and −4.5% for cerebrovascular disease (Figure 2).3 However, the rate of decline slowed considerably thereafter beginning in 2011 to less than 1% annualized from 2011 to 2018 for total CVD with near stagnation of mortality rates from total CVD. However, there is some heterogeneity in trends by CVD subgroups. CHD mortality, which accounts for well over half of all HD deaths and is by far the most common underlying cause of cardiovascular death listed on death certificates, has continued to decline with a pattern similar to total CVD. In contrast, the age-adjusted mortality rate from other HD, in particular HF, which accounts for 12.4 % of all HD deaths as ascertained by the underlying cause, increased by 20.0% from 2011 to 2016.4, 5
Figure 1. Trends in unadjusted death rates per 100,000 population attributable to coronary heart disease and stroke as underlying causes of death in the United States, 1950–1998.

Declines in crude death rates per 100,00 population of coronary heart disease and stroke between 1950 to 1998.
Figure 2. Trends in age-adjusted mortality rates per 100,000 population attributable to total cardiovascular disease and to leading subtypes of cardiovascular disease as underlying causes of death in the United States with average annual percentage change before and after the inflection point* between 1999 to 2011 and 2011 to 2018.

Declines in age-adjusted mortality rates per 100,000 population attributable to total cardiovascular disease and to leading subtypes of cardiovascular disease as underlying causes of death in the United States with average annual percentage change before and after the inflection point* between 1999 to 2011 and 2011 to 2018.
CVD = Cardiovascular Disease
CHD = Coronary Heart Disease
HD = Heart Disease
Racial/ethnic disparities have been persistent in total CVD mortality and deaths due to leading subtypes of CVD, including CHD and stroke (Figure 3).6 For example, Black and White individuals had similar HD rates in 1968 (1071.6 per 100,000 in Black individuals; 1032.2 per 100,000 in White individuals) but the rates began to diverge around 1975 when the rate of decline became faster for Whites.6 As a result, from 1968 to 2015, the Black-White disparity in HD death rates increased 16.3%. Black individuals continue to have the highest mortality for total CVD, CHD, and stroke compared with all other racial/ethnic groups.7 In particular, the largest Black-White disparities persist in cardiovascular deaths related to hypertension and heart failure (Supplemental Figure 1). In contrast, White individuals have a higher rate of cardiovascular deaths related to atrial fibrillation than Black individuals.8 Disparities are also prominent by urbanization status in the US with rural counties having greater age-adjusted mortality rates for CVD compared with more urban counties (Figure 4).9
Figure 3. Trends in race-sex stratified age-adjusted mortality rates per 100,000 population attributable to total cardiovascular disease and by leading subtypes, coronary heart disease and cerebrovascular disease as underlying causes of death in the United States, 1999–2018.
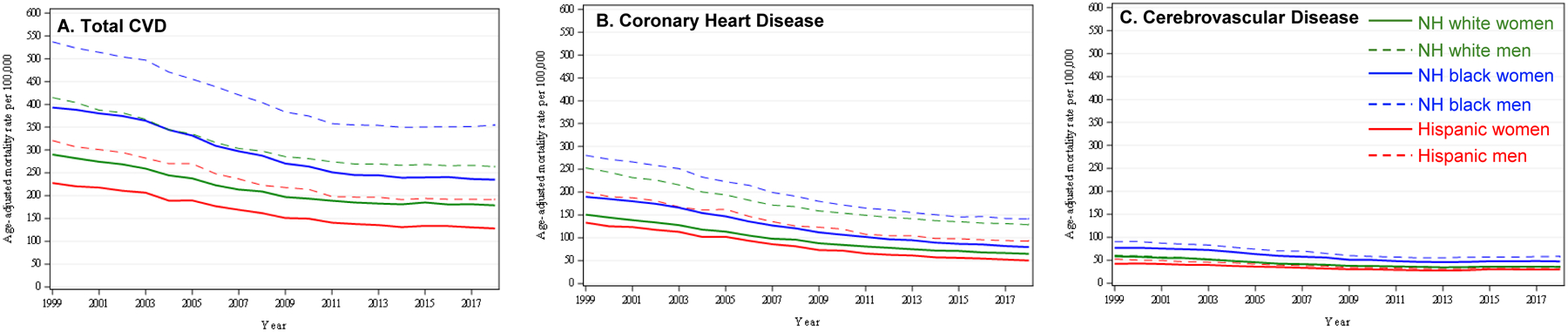
Declines in total cardiovascular disease, coronary heart disease, and cerebrovascular disease mortality rates between 1999–2018
CVD = Cardiovascular Disease
NH = non-Hispanic
Figure 4. Trends in age-adjusted mortality rates per 100,000 population from total cardiovascular disease for both sexes stratified by urbanization status in the United States, 1999–2018.

Declines in cardiovascular mortality rate per 100,000 stratified by county-level urbanization between 1999–2018
In contrast with the continued declining rate of HD (including CHD and all other HD) mortality, the absolute number of deaths from HD has increased steadily from 2011 to 2016 by 6.2%, after declining 16.1% (from 710,760 to 596,577) from 2000 to 2011.10 The increase in absolute numbers is multifactorial and can be attributed, at least in part, to population growth and aging as well as increasing prevalence of obesity and diabetes. Specifically, the population of older adults (65 years and older), which accounts for approximately 80% of all HD deaths, grew by 19% from 2011 to 2016, a 5-fold greater increase than the overall population.10 By 2030, the population of older adults is projected to increase an additional 49% from 49.2 million to 73.1 million. Therefore, the number of HD deaths will continue to grow unless the rate of decline of the HD mortality rate accelerates to overcome the approximately 3% annual increase in the population of older adults. In particular, among HD deaths, the rate of growth of HF deaths is likely to be higher based on the relatively larger increases in HF deaths in older adults since 2011. The projected growth of HD deaths may be further amplified through direct and indirect sequelae of the novel coronavirus 2019 (COVID-19) pandemic based on preliminary reports identifying excess cardiovascular deaths in 2020 to-date.11
Community Surveillance for Non-Fatal CVD Events
The Atherosclerosis Risk in Communities (ARIC) Study12 initiated in 1985 as a response to opportunities identified in the 1978 Bethesda Conference, addressed a fundamental goal of the conference to further our understanding of secular trends in CHD mortality.13, 14 Community surveillance methods used in ARIC were developed in the Community Cardiovascular Surveillance Program (CCSP), which was conducted from 1981 to 1984 with funding from the National Institutes of Health (NIH) with aim of designing, planning, and conducting a feasibility study of a surveillance system for CVD. ARIC community surveillance conducted continuous, population-based retrospective surveillance of hospital discharges and out-of-hospital deaths from 1987 through 2014 in persons age 35–74 (extended to 84 in 2005) in four geographically defined communities (Forsyth County, NC; Jackson, MS; suburban Minneapolis, MN; and Washington County, MD). Deaths due to CHD as well as hospitalized myocardial infarction (MI) events were identified and validated through review of medical records for hospitalization events and interviews with witnesses and other informants for out-of-hospital deaths. A strength of the ARIC community surveillance study was its focus on evaluating and measuring the two main constituents of trends in CHD mortality: trends in the incidence of MI and trends in survival after MI. In an analysis of event trends, simulation-based inference was used to estimate trends in rates of events per 10,000 persons per year in the four communities across age, race, and sex groups. The simulation-based approach allowed testing whether the absolute decrease in event rates per year from 2011 to 2014 was slower compared with 1987–2010. Although rates of validated CHD mortality have declined dramatically in the past 30 years, the models found a high probability that the decline in CHD mortality rates indeed slowed from 2011 to 2014, particularly among individuals older than 50 years of age. The evidence supported the conclusion that the flattening of trends for CHD mortality rates in these communities was a function of flattening of trends for both MI incidence and post-MI survival. These data from community-based surveillance are consistent with the national data described above, provide insight into some of the potential influences underlying those trends, and underscore the importance of revitalizing prevention approaches to reduce CHD burden in the community. They also underscore the ongoing need for active surveillance programs for chronic diseases of aging in the US, since national death data rely on death certification (with its inherent limitations) and there are no current national surveillance systems for incident non-fatal CVD events.
An International Perspective on CVD Morbidity and Mortality Trends
In 2017, CVD was the leading cause of death worldwide, accounting for nearly 32% of all deaths (Of the 55.9 million total deaths, 17.8 million were CVD deaths).15 Nearly 54% of CVD deaths were caused by CHD and 31% by stroke. CVD contributed to 15% of the global burden of disability-adjusted life years (DALY),16 and the onset of CVD tends to occur at significantly younger ages in low- and middle-income countries (LMICs) compared with high-income countries.17 LMICs have the highest prevalence of CVD, accounting for more than 75% of CVD events worldwide. While the absolute burden of CVD has increased globally and has shifted heavily toward LMICs (largely because of population growth and aging), age-adjusted CVD mortality rates have declined in some LMICs, albeit gradually compared with the steep declines observed in many high-income countries (Figure 5). Further, the largest causes of mortality in many of these countries continue to undergo an epidemiological transition from predominantly infectious diseases to non-communicable diseases in a compressed time frame, leading to a dual burden of disease, with substantial variation between countries. Addressing this significant burden of CVD in such a wide variety of rapidly developing and under-developed countries requires an understanding of the complex dynamics of CVD risk factors and their interactions. Particularly relevant are the inequities in access to health care — specifically, availability and distribution of evidence-based and high-quality care in LMICs. However, the capacity to deliver such care is limited, with many LMICs having less than optimal numbers of health care providers (who are also maldistributed between urban and rural locations), which worsens the inequities in cardiovascular care. Furthermore, little research has been conducted in LMICs, with more than 80% of research and research funding available from the high-income countries that bear only 20% of the world’s disease burden.18 As a consequence, the applicability of research findings to LMIC settings is uncertain.
Figure 5. Trends in age-adjusted mortality rates per 100,000 population attributable to cardiovascular disease stratified by Global Burden of Disease Super Regions, 1999–2017.

Age-adjusted mortality rates per 100,000 population across Global Burden of Disease Super Regions, 1999–2017
Likely Contributors to the Decline and Stagnation of US and Global CVD Mortality Rates
Application of IMPACT and CHD Policy Models in the US and Globally
The decline in CVD mortality is one of the most significant epidemiological phenomena of the 20th century, with massive reductions in CHD mortality of more than 60% in multiple western countries over four decades.19 In the Multinational Monitoring of Determinants and Trends in Cardiovascular Disease (MONICA) study populations, CHD mortality rates fell 4% per year. About two-thirds of the observed decline could be attributed to the fall in incident event rates, while one third was attributable to reductions in case fatality.20 Modelling studies are also consistent in identifying the drivers of the CHD epidemic. Both the IMPACT and US CHD policy models suggested that in the US population between 1980 to 2000, risk factor changes and improvements in treatments accounted for roughly equal proportions of the mortality decline. Specifically, the IMPACT model aims to explain the changes in CHD mortality rates observed in the US population and other countries by quantifying the contributions from national trends in CHD risk factors and treatments for CHD. This model found that approximately 44% was attributable to changes in risk factors, including reductions in total cholesterol (24%), systolic blood pressure (20%), smoking prevalence (12%), and physical inactivity (5%) that were offset by increases in the prevalence of obesity and diabetes (−17%).21 These increases in obesity and diabetes continued to be a major concern in children and adults between 1999 to 2015, with an attributable burden for mortality and disability that was substantial.22 Currently, obesity and diabetes contribute to 14% of deaths and 11% of DALYs in the US, an increase of 45% for DALYs from 1990 to 2010.23 Apart from trends in hypertension prevalence, other CHD risk factors in the US show a favorable direction, with declines in tobacco smoking and cholesterol levels and increases in physical activity. However, these risk factors still account for a substantial amount of years of life lost and DALYs in the US. Many of these risk factors are, in large part, determined by unhealthy diets, and 26% of deaths and 14% of DALYs are attributable to the independent contribution of dietary composition.23
Across numerous countries, the IMPACT model has consistently found that 40%−72% of the decline in CHD deaths could be attributed to risk factor changes and 23%−55% to treatments. Particularly powerful drivers included population-wide declines in smoking, blood pressure and cholesterol levels, and improvements in acute care and secondary prevention interventions, including heart failure treatments. In regions where CHD rates were increasing, such as Beijing or Tunisia, adverse population-level trends in smoking, cholesterol and blood pressure drove mortality upwards.24,25 An interesting observation from IMPACT modelling is the halving of the CHD mortality rates in Poland following almost immediately after the massive socioeconomic changes during the 1990s. Risk factor changes at the population level explained about 54% of the decline and 37% was explained by the increased use of evidence-based treatments.26 This is an example of how trends can change swiftly, driven by risk factor changes and social policy at the population-level.
Contribution of Obesity and Diabetes to CVD Trends
Obesity has reached epidemic levels in the present era—affecting 42.4% of the US population over age 20 years in 2017–201827 compared with 15% at the time of the 1978 Bethesda Conference.28, 29 Future projections based on state-level distributions of body mass index from 1993 to 2016 predict a 50% prevalence of obesity by 2030 with some states approaching 60% and no state below 35%. Obesity is associated with multiple cardiovascular and other adverse health outcomes,30 including earlier onset of CVD and all-cause morbidity translating into a shorter health-span and overall life-span. Type 2 diabetes is often cited alongside obesity given their interrelated pathophysiology31 and parallel increases over time.32 Diabetes affects 12.3% of US adults older than 20, and nearly one in four (24.7%) older adults over 65 has diabetes.33 With few exceptions, racial/ethnic minorities and adults and children with fewer socioeconomic resources experience the greatest burden of obesity and diabetes.34 With rising rates of obesity among children and adolescents, and changing demographics of our society (e.g., aging, immigration, and diversification by race and ethnicity),32 populations at risk for obesity and diabetes will continue growing.
The modern obesity epidemic emerged as a result of multiple societal factors that influenced energy balance. With the growth of the workforce in the 1980s and 1990s and longer work hours, the consumption of energy-dense and nutritionally poor “convenience” or “fast” foods and beverages (e.g., processed foods, packaged foods) increased. At the same time, automation of occupational tasks by computers coupled with longer commute times, as families moved to suburban communities and away from cities, reduced physical activity at work and constrained leisure time available for physical activity. What had been societal changes in the 1980s have normalized in the contemporary era, and new threats, including mobile technologies, have emerged that promote sedentary behaviors and interrupt sleep. Despite the clear threat for increases in future obesity prevalence posed by these trends in behavioral factors, few interventions short of bariatric surgery have demonstrated success in sustained weight management.35
A biologically plausible consequence of the obesity epidemic is a leveling—with an eventual increase— of the prevalence of CVD following decades of decline. A rigorous body of research across basic, clinical and population domains describes the action of adipose tissue secreting hormones that damage the vasculature and cause systemic low-grade inflammation.36 Population studies that estimate the contribution of obesity to CVD based on attributable fractions (i.e., the theoretical proportion of disease that could be prevented if the exposure is completely eliminated in the population), report that between 7%−44% of CVD events are due to obesity.37, 38 Obesity and diabetes are two of the four metabolic risk factors (in addition to high blood pressure and dyslipidemia) that are estimated to account for 60% of CVD mortality worldwide.39 These estimated fractions vary by demographic characteristics (e.g., sex, race/ethnicity, age), adjustments for covariates and outcome (e.g., fatal, incident, and type of CVD). Further, the disparate burden of obesity and diabetes experienced by racial/ethnic minorities may underlie the marked disparities in some CVD subtypes such as heart failure.40, 41
Role of Social Determinants Towards CVD Morbidity and Mortality
Recent reports from the Institute of Medicine (IOM)42 and the AHA43 have highlighted the critical role that social determinants of health (SDOH) may play in CVD health outcomes. The World Health Organization (WHO) defines SDOH as the “structural determinants and conditions in which people are born, grow, live, work, and age” that affect health, functioning, and quality of life.44 There are five key domains of SDOH: economic stability, neighborhood and built environment, education, social and community context, and health and health care.45 These constructs cover a number of critical factors including socioeconomic status, adverse life course experiences, social support, family structure, access to care, neighborhood environment, and stress, including stress associated with perceived or actual discrimination.43, 46
Economic stability and education have been linked with individual metrics of CVH and CVD outcomes in cross-sectional and longitudinal studies.43, 47 Those with low education tend to have poorer levels of CVH, including higher rates of smoking, obesity, high blood pressure, and high total cholesterol, compared with well-educated groups.48 Additionally, racial/ethnic differences in modifiable CVH behaviors, such as smoking, physical activity, and diet, may be primarily explained by socioeconomic factors.49 Low socioeconomic status throughout life, and especially starting in childhood, is also associated with poor levels of the CVH component metrics.50 A recent meta-analysis51 found that almost all studies showed a relationship between childhood adversity and adult CVD risk, underscoring the critical role of these factors for primordial prevention of CVD. Several systematic reviews and meta-analyses have also shown that neighborhood disadvantage and features of neighborhood built environment (e.g., healthy food access, walkability/physical activity access) are associated with CVD risk factors, events, and mortality.52–54 Neighborhood and other social factors may explain the substantial geographic variation in CVD mortality even within cities across census-tract levels.8 For example, Figure 6 shows census tract-level heterogeneity in both age-adjusted prevalence of CHD and the Centers for Disease Control and Prevention’s social vulnerability index (SVI) for three major metropolitan cities in the US55 with the largest life expectancy gap (Chicago; Washington, DC; and New Orleans). The marked variation in the age-adjusted prevalence of CHD clearly mirrors that of the SVI at the neighborhood level. Clinical and public health attempts to improve CVH and reduce the burden of CVD are unlikely to be successful unless the background disparities in multi-level social determinants of health over the lifespan are also addressed.
Figure 6. Small-area geographic scale distribution of age-adjusted prevalence of coronary heart disease (per 100,000) and the Centers for Disease Control and Prevention’s Social Vulnerability Index at the census tract level in 3 major metropolitan areas in the United States, both sexes, 2017.

Neighborhood-level differences in age-adjusted prevalence of coronary heart disease and the social vulnerability index in Chicago (A, D), Washington D.C. (B, E), and New Orleans (C, F).
Bending the Curve: Reigniting Progress and Achieving CVH Equity
The following sections highlight several strategies that might be employed more effectively to address the challenge of CVD prevention and CVH promotion. These approaches leverage efforts to understand better and intervene to reverse current adverse trends and promote favorable trends in health care delivery, health behaviors, and social conditions.
Million Hearts
Million Hearts is a national initiative in the US, co-led by the Centers for Disease Control and Prevention and the Centers for Medicare and Medicaid Services and executed by over 100 public and private partners.56 The goal is to prevent one million heart attacks, strokes, and related events over a five-year period through ambitious but achievable targets on a small set of high-impact indicators of CVH. In its first five-year phase (2011–2016), Million Hearts reported no change in population-level measures of aspirin use for secondary prevention, blood pressure control, statin use, or daily sodium consumption. Physical activity and tobacco prevalence improved modestly.57
In total, 213 million opportunities to prevent CVD events were missed during 2011–2016: 9 million people with an indication for aspirin did not take it; 40 million had uncontrolled hypertension (based on a goal <140/<90 mmHg); 39 million were not on a statin despite an indication based on most current evidence; 54 million smoked; and 71 million did not get at least 150 minutes of physical activity a week.57 Notably, middle-aged individuals (35–64 years old) are less likely than those older or younger to take a recommended daily aspirin or statin and they consume more sodium57. A third of middle-aged individuals are physically active for less than ten minutes a week, an only about half of those with hypertension have it under control. Between 2010 to 2015, more than 50% of U.S. counties showed an increase in mortality from cardiac causes in middle-aged individuals.58
Bright spots across the country shed light and hope on this situation. Over 100 “Million Hearts Hypertension Control Champions,” who represent individual practitioners, health systems and community health centers, have been recognized for achieving control rates of over 80%56, and many clinicians are adopting out-of-office monitoring of blood pressure, in accordance with national guideline recommendations.59 However, such success stories are not yet widespread. Even more challenging is how to implement complex policies aimed at obesity prevention and management, such as sugar-sweetened beverage taxation and changes within the built environment to promote physical activity and prevent food insecurity.52–54, 60, 61
Without the systematic spread of preventive actions, over 16 million CVD events will occur between 2017 to 2022, including 2.2 million emergency department visits, 11.8 million hospitalizations, and 2.2 million deaths.62 Prevention, early detection, and control of a few common risk factors would reduce the prevalence of these events and the loss of family members, friends, and co-workers to a largely preventable disease. A sustained, national focus on implementing effective prevention strategies in communities, health care settings, and homes would help bend the curves of CVD rates down again, realizing the vision of the original Bethesda conferees.
Dissemination and Implementation Research
The limited successes to date observed in Million Hearts underscore the importance of research on effective implementation of evidence-based programs and policies in practices and communities. Active dissemination and implementation research in health (DIRH) provides a unique opportunity to reverse the stalled CHD mortality decline and drive progress in CVH. At its core, DIRH addresses the gap between the promise of proven, effective health interventions and their successful, sustained implementation in the real world.63 DIRH provides a framework for accelerated adoption, adaptation, scale-up, and dissemination of effective strategies for the prevention, treatment, and control of diseases and risk factors. Embracing DIRH in CVD prevention and control in acute and chronic management of CVD could help galvanize reversal of the adverse CHD mortality trends. For example, although the hypertension control rate at the national level has stagnated over the past decade and currently is at 48.3%,64 there is compelling evidence that control rates of 80%−90% are achievable in clinical practice, even in large, ethnically diverse populations.65 If a successful national program of active dissemination, implementation, and scale-up of lessons learned from these DIRH studies could be put into practice, an enormous number of lives could be saved or prolonged without morbidity. A similar approach could address dyslipidemia, heart failure, and several other CVD conditions and risk factors for which knowledge exists on effective strategies, barriers, and facilitators of implementation success in clinical and public health practice. DIRH can also be invaluable in addressing CVH promotion and CVD prevention at the community level to help stimulate further CVD mortality decline. A report66 showing declining ideal CVH in White individuals and persistently poor CVH in African Americans and Mexican Americans is an important call to action for all communities to raise awareness about CVD and risk factors, increase the number of people taking action to promote ideal CVH, and reduce related racial and ethnic health disparities.67 DIRH provides successful models for active community engagement for promoting CVH, preventing CVD and risk factors, and addressing the social and environmental determinants of health across the lifespan.68 Examples of all active DIRH grants funded by NHLBI and other NIH institutes can be reviewed using the NIH Research Portfolio Online Reporting Tools from the NIH RePORTER website (n=169 as of 5/6/20).69
Precision Medicine and Public Health
Precision medicine uses information about genes, environment, and lifestyle to tailor and optimize prevention and medical treatment for each individual.70 Researchers have conducted genome-wide association studies and whole-genome sequencing studies to search for genetic variants that can predict risk of disease or response to treatment, and significant variants have been identified on every chromosome.71 For example, a risk score that incorporates information about 27 gene variants may have utility to identify individuals who will benefit more (that is, have lower risk of CHD) from taking a statin drug to lower low density lipoprotein (LDL) cholesterol.72 Precision public health extends the ideas of precision medicine to populations. Whereas precision medicine aims to identify the best treatment for an individual patient, precision public health seeks to identify the best means to promote health in particular communities or strata of the population. For example, a precision public health program might identify (through genetic testing or other means) a segment of the population prone to have high cholesterol or that metabolizes nicotine more quickly and is more susceptible to addiction; once identified, targeted prevention strategies could be delivered to the high-risk group. Those seeking to develop precision medicine and public health interventions face a number of challenges.73 Such measures must prove to be superior (in terms of efficacy and cost-effectiveness) to existing methods. For example, a test that incorporates genetic information must predict risk or treatment response above and beyond well-documented environmental exposures or easily measured physiologic factors linked to CVD. Until precision medicine and public health interventions have been sufficiently vetted, medical and public health professionals continue to rely on traditional, evidence-based measures to reduce CVD risk in the clinic and community.74, 75
Big Data, Mobile Devices, and the Internet of Things
Despite the progress in CVD diagnosis and treatment, the burden of CVD persists or is rising in many communities in the US and around the world, due, in large part, to the low percentage of adults and children who meet ideal levels for all metrics of the AHA characterization of CVH.22, 76, 77 This reality has called attention to the fact that “health” takes place in the real world—outside the healthcare system—and involves daily decisions (e.g., physical activity, dietary intake) that currently go largely unmeasured. Recent advances in technology, particularly the broad consumer availability of mobile, wearable, and connected devices (i.e., Internet of Things), have made real-world, continuous monitoring and promotion of health possible. The accompanying explosion of health-related “big data”—from devices to images to electronic health records (EHR)—has coincided with advances in artificial intelligence (e.g., machine learning) to enable enhanced research, diagnostic, and predictive capabilities at the individual and population level.
A prime example of the opportunity for mobile/wearable devices to advance CVH broadly is helping people achieve recommended levels of physical activity, one of the pillars of AHA’s Life’s Simple 7.76, 78 Mobile devices, including the mobile phones used by the majority of the world’s population, can readily capture quantitative real-world measures of physical activity in individuals and across geographies.79 Recent national guidelines, systematic reviews, and scientific statements document the evidence that mobile phones and wearable devices can increase physical activity80, 81, particularly when partnered with evidence-based behavioral interventions, such as coaching, leveraging social networks, or using behavioral economic nudges.80, 82 These efforts would benefit from better integration into healthcare settings.83 Beyond the utility for integration into healthcare delivery, mobile devices and apps can provide community-level assessment of mobility and the built environment, as well as links to community resources that promote active transportation (e.g., bike share programs) and leisure activity (e.g., parks and hiking trials).84, 85
A vexing issue with the health applications of these novel technologies is the “digital divide”—that they are often more available initially to an already advantaged subset of the population, or based on non-diverse datasets, contributing to health inequity.83 Promoting greater access and equity of health technologies has to be a priority to achieve greater CVH for all. Indeed, the rapid proliferation of mobile and connected devices globally has already had a dramatic impact on technology access for a large majority of the world’s population. Promisingly, we are now in a world where a fitness assessment can be implemented through a smartphone, mobile/wearable devices can detect atrial fibrillation and heart attacks, real and virtual health coaches can “chat” with patients to help them self-manage hypertension and diabetes, and retinal images can be analyzed automatically to provide expert-level diagnosis anywhere and reveal changes predictive of cardiovascular risk.79, 86, 87
A multitude of large-scale research studies are now underway that incorporate mobile/connected device data with clinical and -omics data, to gain a greater understanding of how these real-world data can translate into further evidence to improve disease detection, health promotion, and ultimately health outcomes. The NIH All of Us Research Program incorporates mobile health (mHealth) technology and EHR in its intended recruitment and follow up of at least 1 million diverse participants across the US.88 The new NIH RURAL study89 will also include real-world data from mobile phones and wearables with the intent of understanding why many communities in the southeast U.S. have not seen as much improvements in CVD as other regions. In fact, the AHA has just funded a strategically focused research network (SFRN) aimed at health technology solutions for CVH and brain health.90 Finally, the current COVID-19 pandemic has also forced many health systems to develop and optimize telehealth capacities to deliver care remotely, which may improve both access and quality of cardiovascular care.
Consumers, researchers, and healthcare providers appropriately want transparency about the accuracy, data privacy, and health benefits of these new technologies, with many organizations working on “digital health” guidelines, standards, and regulation.91 Further work is also needed in ensuring equitable access, stronger links to scientific guidelines, and integration into clinical care. There is a shared mission among CVH societies, government funding agencies, technology companies, public health groups, and regulatory bodies to accelerate the translation of these promising technologies to advance equitable CVH for all to achieve a world free of CVD.
New Surveillance Approaches for Monitoring Impact
Disease prevention and health promotion programs are often deployed at the county or city level. Novel approaches to community surveillance open new avenues to monitor the impact of such community interventions, programs, and policies. Limited access to standardized information on local disease occurrence and outcomes hinders the ability of programs like these to evaluate the impact of policy and programmatic changes. This gap defines an opportunity to integrate surveillance into local disease prevention and health promotion efforts to assist stakeholders in establishing priorities and guiding program planning, implementation, and evaluation.92
Digital technology, including mHealth and EHR, offers opportunities to enhance surveillance feasibility and impact. Opportunities and limitations relevant to the application of mHealth to surveillance have been delineated,93 including the challenge of the digital divide, as reflected by disparities in access to technology related to age, race/ethnicity, and social determinants of health.94 While mHealth offers the potential to enable integration of personal sensor and environmental data to other surveillance data sources, privacy concerns could limit the utility of mHealth to surveillance, and its role remains to be defined.
EHRs provide access to dense longitudinal clinical datasets that combined with data science techniques such as machine learning can greatly expand the reach of surveillance while generating new challenges. Phenotypes must be standardized to optimize reliability, and data generated from heterogeneous sources must be integrated. Examples of the utilization of EHRs for surveillance have been published.95, 96 Within the EHR environment, methodological rigor, attention to new sources of bias (e.g., digital divide, differential internet access by geography, populations, socioeconomic status), and medical records shortcomings (e.g., missing data, variations in care-seeking behaviors, data quality) are critically important. To this end, EHR are collected in clinical environments and are influenced by the patient’s health status and care seeking behavior and the clinician’s care and documentation practices. Hence, patients and clinicians, not researchers, determine the time of observation, which affects inferences that can be drawn from the results. Large-scale collection of data as enabled by digital technology and EHR share the limitations and biases inherent to all data generated in the course of care, regardless of sample size. Far from reducing uncertainty, reliance on EHRs can, in fact, amplify it as large numbers will yield tighter confidence intervals without lessening bias or minimizing confounding by indication and other factors. Many large-scale networks, such as the CDC-funded multi-state EHR-based network for disease surveillance (MENDS),97 and the Patient-Centered Outcomes Research Institute (PCORI)-funded PCORNet, developed methods to consolidate EHR data from various healthcare systems and patient populations into a standardized format. Investigators from these EHR “networks of networks” are developing methodology to use these extensive EHR data sources for public health surveillance. Widespread adoption of Fast Healthcare Interoperability Resources (FHIR) and Substitutable Medical Applications, Reusable Technologies (SMART) on FIHR standards may increase the utility of EHR data for research and surveillance purposes.
Future efforts could build on a foundation of new surveillance approaches that integrate data from EHRs, mobile devices, and internet of things, to improve healthcare quality through programs like Million Hearts and to identify opportunities to leverage precision medicine and precision public health (e.g., environmental change). These efforts should leverage knowledge gained from current dissemination and implementation research and identify topics for new research projects. All of these strategies will likely be needed to improve population CVH in ways that will dramatically reduce CVD mortality rates.
A Bold New Goal: 50by50by50
When the AHA defined “ideal CVH” (defined by 7 metrics: non-smoking; ideal healthy diet, physical activity levels, and body weight; and ideal blood pressure, cholesterol, and glucose levels) in its 2020 Strategic Impact Goal, it reframed thinking about CVD.76 From this new perspective, popularized as “Life’s Simple 7”, strategies of prevention became anchored positively in health and not only disease. Prior evidence that ideal CVH at age 50 predicts decades of CVD-free survival and other significant health benefits pointed to a goal of promoting and preserving ideal CVH, through the strategy of primordial prevention. First articulated by Strasser in 197898, his vision of “preserving entire risk-factor-free societies from the penetration of risk factor epidemics” awaits full realization until this strategy is implemented continuously throughout the life course beginning from the earliest years of life.
The rapidly growing body of research on CVH has documented its low prevalence at age 50, due to a decline in prevalence beginning in childhood. Data are presently very limited at pre-adolescent ages but show a loss of ideal CVH from about 50% at age 10 to 10% at age 50. This is highly inconsistent with AHA’s goal “to improve the cardiovascular health of all Americans by 20% by 2020” and even more so with preservation of ideal CVH to age 50.76
A major impetus to mobilizing primordial prevention to promote and preserve ideal CVH was AHA’s One Brave Idea initiative launched in January 2016 that called for proposals for a “moon shot” project to end CHD and its consequences.99 AHA’s call, though lacking a specific target or timeline, raised new possibilities, coming in the context of AHA’s and others’ commitments to improve population CVH, a growing momentum behind community-focused efforts to address the CVD problem, and national and international health organizations setting quantitative time-specific targets to reduce CVD or noncommunicable disease mortality.100
These developments converged to result in a proposed CVD endgame, anchored in ideal CVH for all. If the prevalence of ideal CVH in early life were increased and its loss from childhood were averted, then reaching age 50 in ideal CVH might, in time, become the norm. In the corresponding absence of risk factors, as ample data show, their consequences would no longer occur. This, then, is a potential strategy of the CVD endgame: Promote and preserve ideal CVH for all throughout life, beginning in early life, to sustain a 50% or greater prevalence of ideal CVH at ages 50 years and younger by 2050 or sooner, or “50by50by50”. This could be an interim target of the endgame to be played out beyond 2050, thereby achieving, as nearly as possible, the true end of CVD.
Participant Convergence on Opportunities
After formal presentations by the invited speakers covering the topics above, the audience participated in breakout groups to provide further input. They listed additional challenges and barriers against achieving ideal CVH, such as overall low public awareness of the concept of ideal CVH including the AHA Life’s Simple 7, the typically long lag time in translating scientific findings into actionable policies, insufficient time and compensation for preventive efforts in clinical encounters, and unique challenges faced by communities such as grocery gaps, crime, and unhealthful environmental exposures. The audience recognized and supported concerted efforts to tackle these challenges and overcome barriers in promoting CVH across the lifespan, particularly at the community level, such as the NHLBI’s Disparities Elimination through Coordinated Interventions to Prevent and Control Heart and Lung Disease Risk (DECIPHeR) initiative.101
Participants converged on the idea that the starting age for both additional observational research and interventions should begin in early childhood, ideally in utero or even before conception. For school-aged children, school-based interventions could be explored, including developing simple messages that can readily be remembered for a life-time–an example outside of the cardiovascular arena was the successful “Stop, Drop, and Roll” campaign that taught children simple safety techniques for when clothing catches fire. But beyond schools, other opportunities exist at the community-level that could potentially reach people of all ages.
Conclusions
As reviewed above, the large societal, public health, behavioral, and environmental forces that contributed to the CVD epidemic of the 20th century, as well as the subsequent and dramatic decades-long reductions in CVD mortality, have been well described as a result of initiatives stemming from the initial 1978 Bethesda Conference. The major causal risk factors for CVD that account for the vast majority of CVD cases and mortality, as well as their upstream determinants and downstream consequences, have been delineated, and proven public health and healthcare interventions exist. Nonetheless, we now find ourselves in a period of flattening or stagnated CVD mortality rates, with real concerns that ongoing epidemics of obesity and diabetes (among other factors) are driving an increase in CVD burden (particularly in heart failure and hypertension) among a growing and aging population. Given that so much information was gleaned as a result of the 1978 Bethesda Conference, it is hoped that the Bethesda + 40 Conference agenda and findings, summarized above, can create a roadmap for similar innovations in research, policy, and health care to re-energize CVH promotion and CVD prevention.
Supplementary Material
Acknowledgements:
The authors acknowledge with gratitude the technical assistance of Ms. Ebyan Addou and Mr. Steve Mitchell in the preparation of this manuscript. The authors thank the staff and participants of the ARIC study for their important contributions
Funding Sources:
Dr. Labarthe is supported by # 14SFRN20780002 Trajectories in Ideal Cardiovascular Health from Childhood through Adulthood (07/01/14 - 06/30/21) and # 17SFRN33660752 Early Life Origins of Cardiovascular Health: Healthier Earlier 07/01/17 - 06/30/21 from the American Heart Association
Dr. Loop was supported by The Atherosclerosis Risk in Communities (ARIC) Study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I).
Nonstandard Abbreviations and Acronyms
- AHA
American Heart Association
- ARIC
The Atherosclerosis Risk in Communities Study
- CCSP
Community Cardiovascular Surveillance Program
- CHD
Coronary Heart Disease
- CVH
Cardiovascular Health
- CVD
Cardiovascular Disease
- DALY
Disability-Adjusted Life Year
- DECIPHeR
Disparities Elimination through Coordinated Interventions to Prevent and Control Heart and Lung Disease Risk
- DIRH
Dissemination and Implementation Research in Health
- EHR
Electronic Health Records
- FHIR
Fast Healthcare Interoperability Resources
- HF
Heart Failure
- HD
Heart Diseases
- IOM
Institute of Medicine
- LDL
Low-density lipoprotein
- LMIC
Low and Middle Income Countries
- MENDS
Multi-state EHR-based Network for Disease Surveillance
- MI
Myocardial Infarction
- MONICA
Multinational Monitoring of Determinants and Trends in Cardiovascular Disease
- NHLBI
National Heart, Lung, and Blood Institute
- NIH
National Institutes of Health
- PCORI
Patient-Centered Outcomes Research Institute
- SDOH
Social Determinants of Health
- SFRN
Strategically Focused Research Networks
- SMART
Substitutable Medical Applications, Reusable Technologies
- SVI
Societal Vulnerability Index
- WHO
World Health Organization
Footnotes
Disclosure:
Dr. McConnell reports that he is an employee and stockholder of Google/Alphabet Inc. The other authors have no disclosures.
References
- 1.Thom T, Feinleib M, Havlik RJ, National Heart L, Blood I and National Institutes of H. Proceedings of the Conference on the Decline in Coronary Heart Disease Mortality. [Bethesda, Md.]: Dept. of Health, Education, and Welfare, Public Health Service, National Instiutes of Health; 1979http://hdl.handle.net/2027/pur1.32754081179636 [Google Scholar]
- 2.Shah NS, Lloyd-Jones DM, O’Flaherty M, Capewell S, Kershaw KN, Carnethon M and Khan SS. Trends in Cardiometabolic Mortality in the United States, 1999–2017. JAMA. 2019;322:780–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sidney S, Quesenberry CP Jr., Jaffe MG, Sorel M, Nguyen-Huynh MN, Kushi LH, Go AS and Rana JS. Recent Trends in Cardiovascular Mortality in the United States and Public Health Goals. JAMA Cardiol. 2016;1:594–599 [DOI] [PubMed] [Google Scholar]
- 4.Sidney S, Go AS, Jaffe MG, Solomon MD, Ambrosy AP and Rana JS. Association Between Aging of the US Population and Heart Disease Mortality From 2011 to 2017. JAMA Cardiology. 2019;4:1280–1286. doi: 10.1001/jamacardio.2019.4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glynn P, Lloyd-Jones DM, Feinstein MJ, Carnethon M and Khan SS. Disparities in Cardiovascular Mortality Related to Heart Failure in the United States. J Am Coll Cardiol. 2019;73:2354–2355 [DOI] [PubMed] [Google Scholar]
- 6.Van Dyke M, Greer S, Odom E, Schieb L, Vaughan A, Kramer M and Casper M. Heart Disease Death Rates Among Blacks and Whites Aged >/=35 Years - United States, 1968–2015. MMWR Surveill Summ. 2018;67:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sidney S, Quesenberry CP Jr., Jaffe MG, Sorel M, Go AS and Rana JS. Heterogeneity in national U.S. mortality trends within heart disease subgroups, 2000–2015. BMC Cardiovasc Disord. 2017;17:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth GA, Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, Morozoff C, Naghavi M, Mokdad AH and Murray CJL. Trends and Patterns of Geographic Variation in Cardiovascular Mortality Among US Counties, 1980–2014. JAMA. 2017;317:1976–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrington RA, Califf RM, Balamurugan A, Brown N, Benjamin RM, Braund WE, Hipp J, Konig M, Sanchez E and Joynt Maddox KE. Call to Action: Rural Health: A Presidential Advisory From the American Heart Association and American Stroke Association. Circulation. 2020;141:e615–e644 [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. National Center for Health Statistics Underlying Cause of Death 1999–2017 2018;2019:Data are from the Multiple Cause of Death Files, 1999–2017, as compiled from data provided by the 1957 vital statistics jurisdictions through the Vital Statistics Cooperative Program. http://wonder.cdc.gov/ucd-icd10.html ‘Accessed’ August 2, 2019
- 11.Weinberger DM, Chen J, Cohen T, Crawford FW, Mostashari F, Olson D, Pitzer VE, Reich NG, Russi M, Simonsen L, et al. Estimation of Excess Deaths Associated With the COVID-19 Pandemic in the United States, March to May 2020. JAMA Intern Med. 2020;180:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702 [PubMed] [Google Scholar]
- 13.Rosamond WD, Chambless LE, Heiss G, Mosley TH, Coresh J, Whitsel E, Wagenknecht L, Ni H and Folsom AR. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987–2008. Circulation. 2012;125:1848–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD and Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233 [DOI] [PubMed] [Google Scholar]
- 15.Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prabhakaran D, Singh K, Roth GA, Banerjee A, Pagidipati NJ and Huffman MD. Cardiovascular Diseases in India Compared With the United States. J Am Coll Cardiol. 2018;72:79–95. http://www.onlinejacc.org/content/accj/72/1/79.full.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huffman MD, Baldridge A, Bloomfield GS, Colantonio LD, Prabhakaran P, Ajay VS, Suh S, Lewison G and Prabhakaran D. Global cardiovascular research output, citations, and collaborations: a time-trend, bibliometric analysis (1999–2008). PLoS One. 2013;8:e83440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Murray CJ and Naghavi M. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129:1483–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tunstall-Pedoe H, Kuulasmaa K, Mahonen M, Tolonen H, Ruokokoski E and Amouyel P. Contribution of trends in survival and coronary-event rates to changes in coronary heart disease mortality: 10-year results from 37 WHO MONICA project populations. Monitoring trends and determinants in cardiovascular disease. Lancet. 1999;353:1547–1557 [DOI] [PubMed] [Google Scholar]
- 21.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH and Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398 [DOI] [PubMed] [Google Scholar]
- 22.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596 [DOI] [PubMed] [Google Scholar]
- 23.Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, Dellavalle R, Danaei G, Ezzati M, Fahimi A, et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Critchley J, Liu J, Zhao D, Wei W and Capewell S. Explaining the increase in coronary heart disease mortality in Beijing between 1984 and 1999. Circulation. 2004;110:1236–1244 [DOI] [PubMed] [Google Scholar]
- 25.Critchley J, Capewell S, O’Flaherty M, Abu-Rmeileh N, Rastam S, Saidi O, Sozmen K, Shoaibi A, Husseini A, Fouad F, et al. Contrasting cardiovascular mortality trends in Eastern Mediterranean populations: Contributions from risk factor changes and treatments. Int J Cardiol. 2016;208:150–161 [DOI] [PubMed] [Google Scholar]
- 26.Bandosz P, O’Flaherty M, Drygas W, Rutkowski M, Koziarek J, Wyrzykowski B, Bennett K, Zdrojewski T and Capewell S. Decline in mortality from coronary heart disease in Poland after socioeconomic transformation: modelling study. BMJ. 2012;344:d8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hales CM, Carroll MD, Fryar CD and Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief. 2020:1–8. https://www.cdc.gov/nchs/products/databriefs/db360.htm [PubMed] [Google Scholar]
- 28.Ogden CL and Carroll MD. Prevalence of Overweight, Obestiy and Extreme Obesity among Adults: United States, Trends 1960–1962 through 2007–2008. 2010https://www.cdc.gov/nchs/data/hestat/obesity_adult_07_08/obesity_adult_07_08.pdf
- 29.Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, Long MW and Gortmaker SL. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N Engl J Med. 2019;381:2440–2450 [DOI] [PubMed] [Google Scholar]
- 30.Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, Sweis RN and Lloyd-Jones DM. Association of Body Mass Index With Lifetime Risk of Cardiovascular Disease and Compression of Morbidity. JAMA Cardiol. 2018;3:280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005;307:373–375.https://www.ncbi.nlm.nih.gov/pubmed/15662001 [DOI] [PubMed] [Google Scholar]
- 32.Menke A, Rust KF, Fradkin J, Cheng YJ and Cowie CC. Associations between trends in race/ethnicity, aging, and body mass index with diabetes prevalence in the United States: a series of cross-sectional studies. Ann Intern Med. 2014;161:328–335 [DOI] [PubMed] [Google Scholar]
- 33.Menke A, Casagrande S, Geiss L and Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA. 2015;314:1021–1029 [DOI] [PubMed] [Google Scholar]
- 34.Beckman AL, Herrin J, Nasir K, Desai NR and Spatz ES. Trends in Cardiovascular Health of US Adults by Income, 2005–2014. JAMA Cardiol. 2017;2:814–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Force UPST. Behavioral Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: US Preventive Services Task Force Recommendation StatementUSPSTF Recommendation: Behavioral Interventions to Prevent Adult Obesity-Related OutcomesUSPSTF Recommendation: Behavioral Interventions to Prevent Adult Obesity-Related Outcomes. JAMA. 2018;320:1163–1171. 10.1001/jama.2018.13022 [DOI] [PubMed] [Google Scholar]
- 36.Heymsfield SB and Wadden TA. Mechanisms, Pathophysiology, and Management of Obesity. New England Journal of Medicine. 2017;376:254–266.https://www.nejm.org/doi/full/10.1056/NEJMra1514009 [DOI] [PubMed] [Google Scholar]
- 37.Flegal KM, Panagiotou OA and Graubard BI. Estimating population attributable fractions to quantify the health burden of obesity. Ann Epidemiol. 2015;25:201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng S, Claggett B, Correia AW, Shah AM, Gupta DK, Skali H, Ni H, Rosamond WD, Heiss G, Folsom AR, et al. Temporal trends in the population attributable risk for cardiovascular disease: the Atherosclerosis Risk in Communities Study. Circulation. 2014;130:820–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Global Burden of Metabolic Risk Factors for Chronic Diseases C. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. Lancet Diabetes Endocrinol. 2014;2:634–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bibbins-Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD and Hulley SB. Racial Differences in Incident Heart Failure among Young Adults. New England Journal of Medicine. 2009;360:1179–1190. Doi: 10.1056/NEJMoa0807265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hozawa A, Folsom AR, Sharrett AR and Chambless LE. Absolute and Attributable Risks of Cardiovascular Disease Incidence in Relation to Optimal and Borderline Risk Factors: Comparison of African American With White Subjects—Atherosclerosis Risk in Communities Study. JAMA Internal Medicine. 2007;167:573–579. Doi: 10.1001/archinte.167.6.573 [DOI] [PubMed] [Google Scholar]
- 42.Institute of Medicine and National Research Council. U.S. Health in International Perspective: Shorter Lives, Poorer Health. Washington, DC: The National Academies Press; 2013https://www.nap.edu/catalog/13497/us-health-in-international-perspective-shorter-lives-poorer-health [PubMed] [Google Scholar]
- 43.Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, Davey-Smith G, Dennison-Himmelfarb CR, Lauer MS, Lockwood DW, et al. Social Determinants of Risk and Outcomes for Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2015;132:873–898 [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. Closing the gap in a generation : health equity through action on the social determinants of health : Commission on Social Determinants of Health final report. 2008. https://apps.who.int/iris/bitstream/handle/10665/43943/9789241563703_eng.pdf;jsessionid=DBC93513B47414329D89E1821901500A?sequence=1. Access date: December 30, 2019. [DOI] [PubMed]
- 45.Healthy People 2020. Social Determinants of Health 2020. https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. ‘Accessed’ January 3 2020
- 46.Goosby BJ, Cheadle JE and Mitchell C. Stress-Related Biosocial Mechanisms of Discrimination and African American Health Inequities. Annu Rev Sociol. 2018;44:319–340. Doi: 10.1146/annurev-soc-060116-053403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harper S, Lynch J and Smith GD. Social determinants and the decline of cardiovascular diseases: understanding the links. Annu Rev Public Health. 2011;32:39–69 [DOI] [PubMed] [Google Scholar]
- 48.Winkleby MA, Jatulis DE, Frank E and Fortmann SP. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Am J Public Health. 1992;82:816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitaker KM, Jacobs DR Jr., Kershaw KN, Demmer RT, Booth JN 3rd, Carson AP, Lewis CE, Goff DC Jr., Lloyd-Jones DM, Gordon-Larsen P, et al. Racial Disparities in Cardiovascular Health Behaviors: The Coronary Artery Risk Development in Young Adults Study. Am J Prev Med. 2018;55:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galobardes B, Smith GD and Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol. 2006;16:91–104 [DOI] [PubMed] [Google Scholar]
- 51.Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, Jones L and Dunne MP. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health. 2017;2:e356–e366 [DOI] [PubMed] [Google Scholar]
- 52.Malambo P, Kengne AP, De Villiers A, Lambert EV and Puoane T. Built Environment, Selected Risk Factors and Major Cardiovascular Disease Outcomes: A Systematic Review. PLoS One. 2016;11:e0166846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diez Roux AV, Mujahid MS, Hirsch JA, Moore K and Moore LV. The Impact of Neighborhoods on CV Risk. Glob Heart. 2016;11:353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leal C and Chaix B. The influence of geographic life environments on cardiometabolic risk factors: a systematic review, a methodological assessment and a research agenda. Obes Rev. 2011;12:217–230 [DOI] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention. 500 Cities: Local Data for Better Health, 500 Cities Project Interactive Map. 2020. https://www.cdc.gov/500cities/. ‘Accessed’ April 26, 2020
- 56.U.S. Department of Health and Human Services. Million Hearts. 2020. https://millionhearts.hhs.gov/. ‘Accessed’ January 6, 2020
- 57.Wall HK, Ritchey MD, Gillespie C, Omura JD, Jamal A and George MG. Vital Signs: Prevalence of Key Cardiovascular Disease Risk Factors for Million Hearts 2022 - United States, 2011–2016. MMWR Morb Mortal Wkly Rep. 2018;67:983–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vaughan AS, Ritchey MD, Hannan J, Kramer MR and Casper M. Widespread recent increases in county-level heart disease mortality across age groups. Ann Epidemiol. 2017;27:796–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary. J Am Coll Cardiol. 2018;71:2199. [DOI] [PubMed] [Google Scholar]
- 60.Kontis V, Cobb LK, Mathers CD, Frieden TR, Ezzati M and Danaei G. Three Public Health Interventions Could Save 94 Million Lives in 25 Years. Circulation. 2019;140:715–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roberto CA, Lawman HG, LeVasseur MT, Mitra N, Peterhans A, Herring B and Bleich SN. Association of a Beverage Tax on Sugar-Sweetened and Artificially Sweetened Beverages With Changes in Beverage Prices and Sales at Chain Retailers in a Large Urban Setting. JAMA. 2019;321:1799–1810 [DOI] [PubMed] [Google Scholar]
- 62.Ritchey MD, Wall HK, Owens PL and Wright JS. Vital Signs: State-Level Variation in Nonfatal and Fatal Cardiovascular Events Targeted for Prevention by Million Hearts 2022. MMWR Morb Mortal Wkly Rep. 2018;67:974–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brownson RC, Proctor EK and Colditz GA. Dissemination and Implementation Research in Health: Translating Science to Practice. 2nd ed. New York: Oxford University Press; 2017 [Google Scholar]
- 64.Fryar CD, Ostchega Y, Hales CM, Zhang G and Kruszon-Moran D. Hypertension Prevalence and Control Among Adults: United States, 2015–2016. NCHS data brief. 2017:1–8 [PubMed] [Google Scholar]
- 65.Sim JJ, Handler J, Jacobsen SJ and Kanter MH. Systemic implementation strategies to improve hypertension: the Kaiser Permanente Southern California experience. Can J Cardiol. 2014;30:544–552. http://www.onlinecjc.ca/article/S0828-282X(14)00007-5/abstracthttp://ac.els-cdn.com/S0828282X14000075/1-s2.0-S0828282X14000075-main.pdf?_tid=7e638240-28b8-11e7-9f39-00000aacb35f&acdnat=1493016031_e45d3630e5a8fe77db6cc393b13f9650 [DOI] [PubMed] [Google Scholar]
- 66.Brown A,F, Liang LJ, Vassar SD, Escarce JJ, Merkin SS, Cheng E, Richards A, Seeman T and Longstreth WT Jr. Trends in Racial/Ethnic/Nativity Disparities in Cardiovascular Health among Adults without Prevalent Cardiovascular Disease in the United States, 1988–2014. Ann Intern Med. 2018;168:541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mensah GA, Cooper RS, Siega-Riz AM, Cooper LA, Smith JD, Brown CH, Westfall JM, Ofili EO, Price LN, Arteaga S, et al. Reducing Cardiovascular Disparities Through Community-Engaged Implementation Research: A National Heart, Lung, and Blood Institute Workshop Report. Circ Res. 2018;122:213–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cooper LA, Purnell TS, Ibe CA, Halbert JP, Bone LR, Carson KA, Hickman D, Simmons M, Vachon A, Robb I, et al. Reaching for Health Equity and Social Justice in Baltimore: The Evolution of an Academic-Community Partnership and Conceptual Framework to Address Hypertension Disparities. Ethn Dis. 2016;26:369–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.National Institutes of Health. NIH Research Portfolio Online Reporting Tools Query Form. 2020. https://projectreporter.nih.gov/reporter.cfm ‘Accessed’ April 26, 2020
- 70.Gameiro GR, Sinkunas V, Liguori GR and Auler-Junior JOC. Precision Medicine: Changing the way we think about healthcare. Clinics (Sao Paulo). 2018;73:e723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, McMahon A, Morales J, Mountjoy E, Sollis E, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47:D1005–d1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mega JL, Stitziel NO, Smith JG, Chasman DI, Caulfield M, Devlin JJ, Nordio F, Hyde C, Cannon CP, Sacks F, et al. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet. 2015;385:2264–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bilkey GA, Burns BL, Coles EP, Mahede T, Baynam G and Nowak KJ. Optimizing Precision Medicine for Public Health. Front Public Health. 2019;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khoury MJ, Bowen MS, Clyne M, Dotson WD, Gwinn ML, Green RF, Kolor K, Rodriguez JL, Wulf A and Yu W. From public health genomics to precision public health: a 20-year journey. Genet Med. 2018;20:574–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu X, Luo X, Jiang C and Zhao H. Difficulties and challenges in the development of precision medicine. Clin Genet. 2019;95:569–574 [DOI] [PubMed] [Google Scholar]
- 76.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613 [DOI] [PubMed] [Google Scholar]
- 77.Angell SY, McConnell MV, Anderson CAM, Bibbins-Domingo K, Boyle DS, Capewell S, Ezzati M, de Ferranti S, Gaskin DJ, Goetzel RZ, et al. The American Heart Association 2030 Impact Goal: A Presidential Advisory From the American Heart Association. Circulation. 2020;141:e120–e138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burke LE, Ma J, Azar KM, Bennett GG, Peterson ED, Zheng Y, Riley W, Stephens J, Shah SH, Suffoletto B, et al. Current Science on Consumer Use of Mobile Health for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation. 2015;132:1157–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McConnell MV, Turakhia MP, Harrington RA, King AC and Ashley EA. Mobile Health Advances in Physical Activity, Fitness, and Atrial Fibrillation: Moving Hearts. J Am Coll Cardiol. 2018;71:2691–2701 [DOI] [PubMed] [Google Scholar]
- 80.Afshin A, Babalola D, McLean M, Yu Z, Ma W, Chen CY, Arabi M and Mozaffarian D. Information Technology and Lifestyle: A Systematic Evaluation of Internet and Mobile Interventions for Improving Diet, Physical Activity, Obesity, Tobacco, and Alcohol Use. J Am Heart Assoc. 2016;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.2018 Physical Activity Guidelines Advisory Committee. 2018. Physical Activity Guidelines Advisory Committee Scientific Report. https://health.gov/our-work/physical-activity/current-guidelines/scientific-report ‘Accessed’ October 15, 2020
- 82.Winter SJ, Sheats JL and King AC. The Use of Behavior Change Techniques and Theory in Technologies for Cardiovascular Disease Prevention and Treatment in Adults: A Comprehensive Review. Prog Cardiovasc Dis. 2016;58:605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sim I Mobile Devices and Health. N Engl J Med. 2019;381:956–968 [DOI] [PubMed] [Google Scholar]
- 84.Deville P, Linard C, Martin S, Gilbert M, Stevens FR, Gaughan AE, Blondel VD and Tatem AJ. Dynamic population mapping using mobile phone data. Proc Natl Acad Sci U S A. 2014;111:15888–15893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Katapally TR, Bhawra J, Leatherdale ST, Ferguson L, Longo J, Rainham D, Larouche R and Osgood N. The SMART Study, a Mobile Health and Citizen Science Methodological Platform for Active Living Surveillance, Integrated Knowledge Translation, and Policy Interventions: Longitudinal Study. JMIR Public Health Surveill. 2018;4:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Rajmane A, Cheung L, et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N Engl J Med. 2019;381:1909–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muhlestein JB, Anderson JL, Bethea CF, Severance HW, Mentz RJ, Barsness GW, Barbagelata A, Albert D, Le VT, Bunch TJ, et al. Feasibility of combining serial smartphone single-lead electrocardiograms for the diagnosis of ST-elevation myocardial infarction. Am Heart J. 2020;221:125–135 [DOI] [PubMed] [Google Scholar]
- 88.Denny JC, Rutter JL, Goldstein DB, Philippakis A, Smoller JW, Jenkins G and Dishman E. The “All of Us” Research Program. N Engl J Med. 2019;381:668–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.National Heart Lung and Blood Institute. The RURAL Cohort Study. 2019. http://www.theruralstudy.org/ ‘Accessed’ November 12, 2019
- 90.More than $14 million in research grants awarded for health technology solutions focused on heart and brain health, including special projects related to COVID-19 and CVD. 2020;2020 https://newsroom.heart.org/news/more-than-14-million-in-research-grants-awarded-for-health-technology-solutions-focused-on-heart-and-brain-health-including-special-projects-related-to-covid-19-and-cvd ‘Accessed’ June 1, 2020
- 91.U.S. Department of Health and Human Services. Digital Health Innovation Action Plan. https://www.fda.gov/media/106331/download ‘Accessed’ November 12, 2019
- 92.Goff DC Jr., Brass L, Braun LT, Croft JB, Flesch JD, Fowkes FG, Hong Y, Howard V, Huston S, Jencks SF, et al. Essential features of a surveillance system to support the prevention and management of heart disease and stroke: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Stroke, and Cardiovascular Nursing and the Interdisciplinary Working Groups on Quality of Care and Outcomes Research and Atherosclerotic Peripheral Vascular Disease. Circulation. 2007;115:127–155. [DOI] [PubMed] [Google Scholar]
- 93.Eapen ZJ, Turakhia MP, McConnell MV, Graham G, Dunn P, Tiner C, Rich C, Harrington RA, Peterson ED and Wayte P. Defining a Mobile Health Roadmap for Cardiovascular Health and Disease. J Am Heart Assoc. 2016;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Latulippe K, Hamel C and Giroux D. Social Health Inequalities and eHealth: A Literature Review With Qualitative Synthesis of Theoretical and Empirical Studies. J Med Internet Res. 2017;19:e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reynolds K, Go AS, Leong TK, Boudreau DM, Cassidy-Bushrow AE, Fortmann SP, Goldberg RJ, Gurwitz JH, Magid DJ, Margolis KL, et al. Trends in Incidence of Hospitalized Acute Myocardial Infarction in the Cardiovascular Research Network (CVRN). Am J Med. 2017;130:317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matlock DD, Groeneveld PW, Sidney S, Shetterly S, Goodrich G, Glenn K, Xu S, Yang L, Farmer SA, Reynolds K, et al. Geographic variation in cardiovascular procedure use among Medicare fee-for-service vs Medicare Advantage beneficiaries. JAMA. 2013;310:155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.National Association of Chronic Disease Directors. Multi-State EHR-Based Network for Disease Surveillance (MENDS). 2020https://www.chronicdisease.org/page/MENDSinfo ‘Accessed’ June 2, 2020
- 98.Strasser T Reflections on Cardiovascular Diseases. Interdisciplinary Science Reviews. 1978;3:225–230.https://www.tandfonline.com/doi/abs/10.1179/030801878791925921 [Google Scholar]
- 99.One Brave Idea.https://www.onebraveidea.org/ ‘Accessed’ January 6, 2020
- 100.Labarthe D and Lloyd-Jones Donald M. 50×50×50. Circulation. 2018;138:968–970. 10.1161/CIRCULATIONAHA.118.035985 [DOI] [PubMed] [Google Scholar]
- 101.National Institutes of Health. Disparities Elimination through Coordinated Interventions to Prevent and Control Heart and Lung Disease Risk (DECIPHeR) - Research Coordinating Center (RCC) (U24 Clinical Trial Not Allowed). 2019;2019.https://grants.nih.gov/grants/guide/rfa-files/RFA-HL-20-004.html ‘Accessed’ December 4, 2019
- 102.CDC WONDER. Multiple Cause of Death Files 1999–2018 http://wonder.cdc.gov/mcd-icd10.html ‘Accessed’ April 17, 2020
- 103.National Heart Lung and Blood Institute. Morbidity & Mortality: 2012 Chart Book on Cardiovascular, Lung, and Blood Diseases https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf ‘Accessed’ May 1, 2020
- 104.Institute for Health Metrics and Evaluation (IHME). GBD Compare.http://vizhub.healthdata.org/gbd-compare ‘Accessed’ April 16, 2020
- 105.Centers for Disease Control and Prevention NCfCDPaHP, Division of Population Health,. 500 Citites Project Data.https://www.cdc.gov/500cities ‘Accessed’ October 23, 2018
- 106.Centers for Disease Control and Prevention and Agency for Toxic Substances and Disease Registry. CDC Social Vulnerability Index https://svi.cdc.gov ‘Accessed’ May 2, 2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


