Abstract
Background:
Marijuana and alcohol are both substances that, when used during pregnancy, may have profound effects on the developing fetus. There is evidence to suggest that both drugs have the capacity to affect working memory, one function of the hippocampal formation; however, there is a paucity of data on how perinatal exposure to alcohol or cannabis impacts the process of adult neurogenesis.
Methods:
This systematic review examines immunohistochemical data from adult rat and mouse models that assess perinatal alcohol or perinatal marijuana exposure. A comprehensive list of search terms was designed and used to search 3 separate databases. All results were imported to Mendeley and screened by 2 authors. Consensus was reached on a set of final papers that met the inclusion criteria, and their results were summarized.
Results:
Twelve papers were identified as relevant, 10 of which pertained to the effects of perinatal alcohol on the adult hippocampus, and 2 pertained to the effects of perinatal marijuana on the adult hippocampus. Cellular proliferation in the dentate gyrus was not affected in adult rats and mice exposed to alcohol perinatally. In general, perinatal alcohol exposure did not have a significant and reliable effect on the maturation and survival of adult born granule neurons in the dentate gyrus. In contrast, interneuron numbers appear to be reduced in the dentate gyrus of adult rats and mice exposed perinatally to alcohol. Perinatal marijuana exposure was also found to reduce inhibitory interneuron numbers in the dentate gyrus.
Conclusions:
Perinatal alcohol exposure and perinatal marijuana exposure both act on inhibitory interneurons in the hippocampal formation of adult rats. These findings suggest simultaneous perinatal alcohol and marijuana exposure (SAM) may have a dramatic impact on inhibitory processes in the dentate gyrus.
Keywords: Hippocampus, Dentate Gyrus, Neurogenesis, Alcohol, Marijuana, FASD
With the relaxation of cannabis restrictions across North America, a growing proportion of young adults (19 to 30 years of age) are reporting the simultaneous use of alcohol and marijuana (SAM), and indications are that this trend will continue to rise (Terry-McElrath and Patrick, 2018). This age demographic also coincides with the peak fertility period for males and females (Dunson, Colombo and Baird, 2002), and SAM significantly increases the risk of unplanned pregnancies (Finer and Zolna, 2014). Moreover, the use of illicit drugs in this age group is more common, with cannabis being the most commonly used drug by pregnant women (Chasnoff, Landress and Barrett, 1990). Approximately half of all marijuana users also report alcohol use (Goldschmidt et al., 2004; Jackson, Sher and Schulenberg, 2008; Subbaraman and Kerr, 2015), with recent statistics indicating that over 30% of pregnant females regularly consume alcohol and marijuana (Goldschmidt et al., 2004; Government of Canada, 2017). Although the prevalence rates for SAM are likely to rise (Jackson, Sher and Schulenberg, 2008), the effects of combined perinatal ethanol (EtOH) exposure and THC (Δ9-tetrahydrocannabinol) exposure on the developing brain are not well understood.
Previous studies have shown that working memory is impaired by perinatal alcohol exposure (Goodfellow and Lindquist, 2014; Livy et al., 2003) and similar deficits have been observed in adults exposed to marijuana (Kafaee Razavi et al., 2010; Wright et al., 2017). EtOH is a teratogen and so alcohol consumption during pregnancy can disrupt development leading to facial dysmorphology, pre- and postnatal growth deficiencies, and central nervous system (CNS) dysfunction (May et al., 2013; Riley et al., 2011). Heavy drinking in the second trimester, particularly the tenth to twentieth weeks of human pregnancy, when the brain is growing dramatically, is associated with an increase in the severity of many clinical features (Brocardo et al., 2017; Renwick and Asker, 1983). In rodent models, a portion of the developmental stages that are congruous with the human third trimester occurs up to postnatal day 9 (Livy et al., 2003). For the purpose of this review, postnatal day 10 and older will be considered to be postnatal while rodents at postnatal day 9 and younger will be taken as perinatal. Perinatal alcohol literature contains experiments using multiple exposure paradigms. E1 to E20 and P4–9 are both common models (Kleiber et al., 2013; Livy et al., 2003). A benefit of the E1 to E20 model is that alcohol can be integrated into the mother’s diet without the need for gavage or injection. P4–9 exposure isolated effects to the brain growth spurt and with recent advancements pups can be exposed using vaporized EtOH, which can also decrease the stress related to injection or gavage. For our review, we have chosen to discuss pre-and postnatal exposure paradigms that range from the first gestational day to the ninth postnatal day in rodent models, as these dates coincide with the first to third trimester equivalent in humans (Maier et al., 1999).
It has long been known that the hippocampus is a brain area that is particularly sensitive to the effects of perinatal alcohol exposure (PAE). PAE induces significant cell loss in the hippocampus (Ikonomidou et al., 2000; Redila et al., 2006; Hamilton et al., 2011), and even brief periods of binge exposure can produce significant changes in hippocampal structure and function (Bonthius and West, 1990; Guerri et al., 2009; Patten, Fontaine and Christie, 2014). Both GABAA and NMDA receptors have been implicated in the mechanism of alcohol-related neurodegeneration; GABAA receptors have been shown to become hyperexcitable while NMDARs are blocked (Olney et al., 2002). Since GABA signaling is thought to be integral to spatial and temporal integration of new neurons, it is logical that aberrations of this system lead to severe developmental consequences (Akerman and Cline, 2007). Long-term potentiation deficits have also been reported, and the histamine H3 receptor has been implicated (Varaschin et al., 2018).
Marijuana is one of the most commonly used recreational drugs during pregnancy, yet little is known about how it effects the development of the brain (Vargish et al., 2017). THC is the major psychoactive ingredient in marijuana and is known to readily cross the placental barrier impacting fetal development (Grotenhermen, 2003). Evidence is emerging that perinatal THC, like perinatal alcohol, can impair cognitive functioning of offspring—possibly throughout the lifespan (Huizink and Mulder, 2006). Cannabinoid receptors and their endogenous ligands have been detected at the earliest stages of embryonic development; this indicates that maternal marijuana use can impact the developing brain (Fernández-Ruiz et al., 2000; Harkany et al., 2007). The 2 primary cannabinoid receptors, known as CB1 and CB2, can both act to reduce adenylyl cyclase activity in cells (Galiègue et al., 1995). CB2 receptors are expressed sparsely in microglia, macrophages, and some neurons in the central nervous system, but are more ubiquitous in the peripheral nervous system (Roche and Finn, 2010). There is evidence that alcohol acts to reduce endogenous cannabinoid levels through a CB2 receptor–mediated pathway and that this mechanism is important in alcohol use disorders (Basavarajappa et al., 2019; Martín-Sánchez et al., 2019). CB1 receptors are expressed in both inhibitory and excitatory neurons, at perinatal timepoints in the rodent cortex, basal forebrain, and telencephalon (Scheyer et al., 2019). Due to the fact that CB1 receptors are expressed perinatally and in the hippocampus (part of the telencephalon), it is likely that CB1 receptors will be the major players when it comes to developmental THC exposure (Berrendero et al., 1999). CB1 receptors can impact interneuron development, neuronal proliferation, migration, morphogenesis, synaptogenesis, and the balance of excitation and inhibition in the hippocampus (Berghuis et al., 2005, 2007; Mulder et al., 2008). A recent paper found that parental THC exposure can cause altered hippocampal oscillations, brain hyperexcitability, and spatial memory impairment (de Salas-Quiroga et al., 2020). In this review, we will systematically explore what is known of the effects of perinatal alcohol and marijuana exposure in the dentate gyrus. The entire hippocampus was included in the search parameters; however, the papers returned mainly concerned the dentate gyrus. The dentate gyrus is a good target of this research as it is 1 of 2 sites in the rodent brain that has adult neurogenesis (Praag et al., 2002). Adult neurogenesis in the dentate gyrus is thought to be a mechanism responsible for spatial memory (Clelland et al., 2009). It is worth noting that, while wellestablished in rodents, the existence of adult neurogenesis is still debated in humans due to the type and parameters of assays available for use in humans (Snyder, 2019). Spatial memory is affected by both THC and alcohol consumption in humans and so its corresponding brain structure a logical place to assess deficits caused by these drugs (Green et al., 2009; Mouro et al., 2019).
It is important to consider the actions of both substances alone, as well as in combination, as some work has suggested that the detrimental effects of perinatal alcohol and perinatal marijuana may be synergistic (Boa-Amponsem et al., 2019; Breit, Zamudio and Thomas, 2019; Janisse et al., 2014). Our initial systematic search to investigate documented changes in adult neurogenesis following SAM exposure returned a single result in the hippocampal formation, indicating there is a paucity of data for understanding the cellular consequences of perinatal SAM exposure. This singular paper found that perinatal cannabinoid exposure causes birth defects similar to perinatal alcohol exposure and implicated CB1–Hedgehog interactions as the cause (Fish et al., 2019). While the Fish paper is worth mentioning, it did not satisfy all of the inclusion/exclusion criteria in this review and will not be part of the final result tables. This paper also succinctly discusses the differences between THC and CBD, the 2 main cannabinoids present in marijuana, versus synthetic cannabinoids, which can be hundreds of times more potent and much longer lasting than THC and CBD (Fish et al., 2019). And while it is also worth mentioning that there are many other cannabinoids and terpenes in cannabis, this review will focus on THC and synthetic cannabinoids that bind with the CB1 receptor (Berrendero et al., 1999).
This review will compare cellular data in adult offspring of rats or mice perinatally exposed to alcohol or marijuana. Our goal is to identify how perinatal SAM exposure impacts the structure and function of the adult hippocampus in hopes of directing future research.
MATERIALS AND METHODS
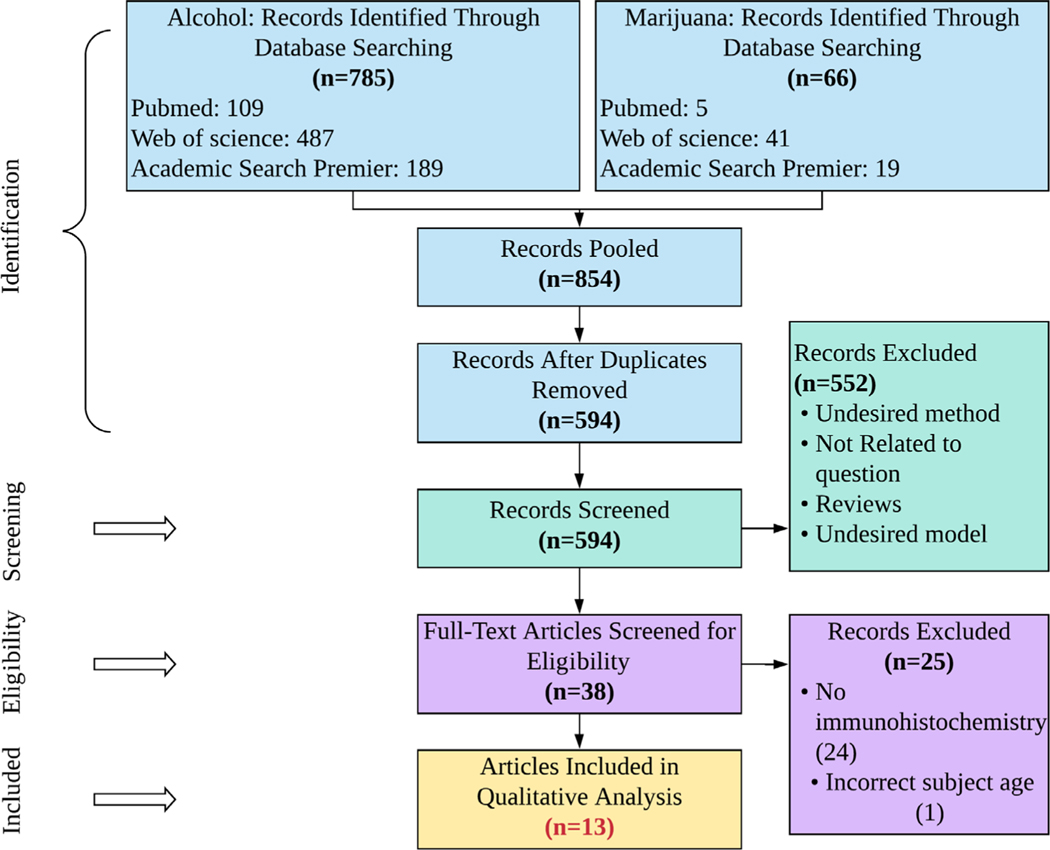
This review was carried out using the stylistic criteria for Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), with minor amendments to the traditional process (e.g., exclusion of bias scoring) due to the nature of cellular studies (Moher et al., 2009). The literature on adult neurogenesis changes in the hippocampal formation after perinatal marijuana exposure is limited; for this reason, marijuana papers selected were compared to selected perinatal alcohol exposure instead of being separately analyzed. This method was used to provide an objective starting point for SAM research aimed at targeting the cellular basis for any developmental changes. In particular, this study was designed to target all papers that investigate adult neurogenic changes in the hippocampal formation under perinatal alcohol conditions or perinatal cannabis conditions. PRISMA search terms were designed to broadly include any cellular study in any age of exposed offspring, and then, papers were selected based on the inclusion and exclusion criteria (S1). Five blocks of search terms were used. Each block included similar terms that contained “OR” as an operator. Between search blocks an “AND” operator was used. The blocks of search terms used here required papers to (i) include perinatal drug exposure, (ii) mention the hippocampal formation, (iii) provide immunohistochemical and other cellular results, (iv) specify alcohol use, and (v) specify marijuana use (see Table S1). Three databases were selected based on their ability to return cellular-level research. The first and second authors individually performed 2 searches, one for perinatal alcohol (search blocks 1, 2, 3, 4) and the other for perinatal marijuana (search blocks 1, 2, 3, 5) using the same terms, then exported all the citations to Mendeley. Duplicates were removed, and all papers were screened using the inclusion/exclusion criteria (S2). Papers selected in the screening process were read in full and assessed for eligibility as defined by the search criteria, and final papers selected were compared between authors. Any discrepancies in paper selection were resolved by discussion. Although outside the scope of this review, the main methods and findings of short-listed papers have been included as a supplementary table (Table S2). The final article numbers for each step of this review are included in Fig. 1. The search period included was January 1, 2000–March 13, 2020.
Fig. 1.
PRISMA flowchart showing the databases used, papers found per database, and number of papers excluded at each review stage.
RESULTS
A total of 12 studies were identified using a predefined criteria (S1), with 10 studies focused on perinatal alcohol exposure and 2 on perinatal marijuana exposure. Eleven of the 12 studies identified included an evaluation of the dentate gyrus subfield of the hippocampus. Datasets for papers that did not satisfy the inclusion and exclusion criteria are not reported in this table.
Perinatal Alcohol Exposure
Effects on Cellular Proliferation.
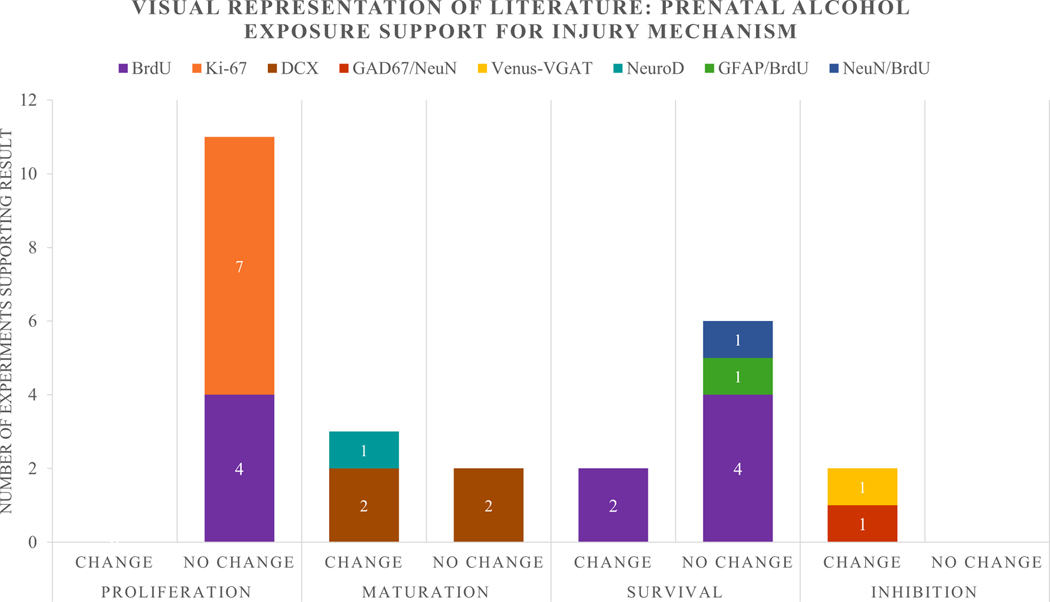
The protein Ki-67, an endogenous marker for cell proliferation in the brain, can be used in conjunction with the administration of BrdU, and exogenous marker that is taken up by the DNA of dividing cells during mitosis, to quantify cell proliferation in the brain (Cameron and Mckay, 2001; Christie and Cameron, 2006). As is depicted in Fig. 2, there were 11 experiments across 10 papers that used Ki-67 or BrdU alone, or in combination, to label proliferating cells in the adult dentate gyrus (Table 1). Seven experiments used the intrinsic marker for cell proliferation, Ki-67, to study how perinatal EtOH exposure affected cell proliferation in the adult hippocampus, but none of the studies showed any change in the number of Ki-67-immunopositive cells. Similarly, the 4 studies that used bromodeoxyuridine (BrdU; 50–200 mg/kg), a thymidine analogue that is injected and incorporated into the DNA of actively dividing cells, failed to document any changes following perinatal EtOH exposure. Thus, whether endogenous or exogenous markers for cell proliferation were quantified, the results are in agreement that BrdU does not induce significant changes in this process in young adult animals.
Fig. 2.
Visual representation of the number of experiments in the above studies that showed an increase and decrease, or showed no change in proliferation, maturation, survival, and inhibition studies.
Table 1.
Summary of Perinatal Alcohol Exposure Experiments Concerning Proliferation. In the Exposure Age and Test Age Column, “P” Indicates Postnatal Day and “E” Indicates Embryonic Day
| Paper | Exposure Age | Model | Sex | Test Age | Marker/ Stain Used | Main Finding |
|---|---|---|---|---|---|---|
| Gil-Mohapel and colleagues (2011) | E1-P9 | Rat | M | P78–82 | BrdU | No Change |
| Ki-67 | No Change | |||||
| Gil-Mohapel and colleagues (2014) | E1–21 | Rat | MF | P386 | Ki-67 | No Change |
| Hamilton and colleagues (2011) | E1-P10 | Rat | MF | P60 | BrdU 2 hours | No Change |
| Ki-67 | No Change | |||||
| P90 | Ki-67 | No Change | ||||
| Klintsova et al. (2007) | P4–9 | Rat | M | P50 | BrdU | No Change |
| Ki-67 | No Change | |||||
| Olateju et al. (2018) | E7-E17 | Mouse | MF | P56 | Ki-67 | No Change |
| Sliwowska et al. (2010) | E1–21 | Rat | M | P60–65 | BrdU | No Change |
| Uban et al. (2010) | E1–21 | Rat | F | P60–65 | BrdU | No Change |
Cell Maturation.
Doublecortin (DCX) is a microtubule-associated protein expressed by neuronal precursor cells and immature neurons. Thus, DCX-positive cells represent a set cells across a broad developmental spectrum, ranging from immature neural progenitor (INP) cells (also known as type 2B cells) to immature granule neurons (IGN) (Kronenberg et al., 2003). Four papers assessed DCX immunoreactivity in the dentate gyrus (Table 2) following perinatal EtOH exposure (Elibol-Can et al., 2014; Gil-Mohapel et al., 2011, 2014; Olateju et al., 2018). Two papers showed no change in DCX immunoreactivity (Elibol-Can et al., 2014; Gil-Mohapel et al., 2014), one showed a significant decrease in DCX-positive cells that was restricted to females (Gil-Mohapel et al., 2011), and one paper found a decrease in DCX cells in both males and females (Olateju et al., 2018) (see Fig. 2). To better elucidate changes in cellular maturation following perinatal EtOH exposure, we also examined papers that examined the basic helix loop helix transcription factor NeuroD (neurogenic differentiation factor 2). NeuroD is a marker expressed continuously by type 2b immature granule neurons once they begin to mature. We found that only one paper used NeuroD as a marker of maturation, and this work found that there was a decrease in the number of NeuroD-positive cells in both male and female rats perinatally exposed to alcohol (Hamilton et al., 2011). This work provides some convergent evidence to support the conclusion that perinatal EtOH exposure does negatively impact neuronal maturation.
Table 2.
Summary of Perinatal Alcohol Exposure Experiments Concerning Maturation. In the Exposure age and Test Age Column, “P” Indicates Postnatal Day and “E” Indicates Embryonic Day
| Paper | Exposure Age | Model | Sex | Test Age | Marker/ Stain Used | Main Finding |
|---|---|---|---|---|---|---|
| Elibol-Can et al. (2014) | E7-E20 | Rat | M | P60 | DCX | No Change |
| Gil-Mohapel et al. (2011) | E1-P9 | Rat | M | P78-82 | DCX | No Change |
| P115 | DCX | No Change | ||||
| Gil-Mohapel et al. (2014) | E1-21 | Rat | MF | P386 | DCX | Decrease in F |
| Hamilton et al. (2011) | P4-P9 | Rat | MF | P60 | NeuroD | Decrease |
| P90 | NeuroD | Decrease | ||||
| Olateju et al. (2018) | E7-E17 | Mouse | MF | P56 | DCX | Decrease |
Cell Survival.
BrdU can be used to examine cell survival if it is injected 3 to 6 weeks prior to tissue being collected for histology (van Praag et al., 1999). This allows sufficient time for new cells to develop and become functional (Praag et al., 2002). BrdU is only available to be incorporated into dividing cells within 2 to 3 hours of being injected (Cameron and Mckay, 2001), so it does not stain cells that are born after this timepoint, allowing researchers to compare the number of cells stained initially (in the immediate perfusion group) to the number of cells present after a given amount of time (a second experimental group). There were 7 papers where BrdU assays were conducted on brain samples collected to study cell survival (see Table 3). In one study, where rats were injected with BrdU (200 mg/kg) at postnatal day 80 (P80) and their brains were collected at P115, a decrease was found in the number of BrdU-positive cells in animals exposed to alcohol perinatally (Hamilton et al., 2011). In a second paper, 2 BrdU experiments were reported. In this work, BrdU (50 mg/kg) was injected every second day from P30 to P50, and then, brains are collected at either P50 or P80 (Klintsova et al., 2007). The number of BrdU-positive cells was found to be equivalent in animals assessed at P50, but a decrease in numbers was observed at P80. One study was performed where BrdU was injected at P60 and brains collected at P90, and no change in BrdU immunoreactivity was found (Gil-Mohapel et al., 2011). Two studies injected BrdU between P60 and P65 and analyzed the brains between P81 and P86 (3 weeks later) and found no change in the number of BrdU-labeled cells relative to controls (Sliwowska et al., 2010; Uban et al., 2010). One study utilized double labeling to assess the survival of new glial cells (GFAP/BrdU) as well as the survival of new granule neurons (NeuN/BrdU) and found no change in the proportion of each, relative to control, in either condition (Uban et al., 2010). Thus, the majority of studies indicate that perinatal alcohol exposure does not have a significant impact on cell survival.
Table 3.
Summary of Perinatal Alcohol Exposure Experiments Concerning Survival. In the Exposure Age and Test Age Column, “P” Indicates Postnatal Day and “E” Indicates Embryonic Day; in the Marker/Stain Used Column, “P” Indicates the Postnatal Day When Subjects Were Injected With BrdU
| Paper | Exposure Age | Model | Sex | Test Age | What was Investigated? | Marker/ Stain Used | Main Finding |
|---|---|---|---|---|---|---|---|
| Gil-Mohapel and colleagues (2011) | E1-P9 | Rat | MF | P90 | Dividing Cells | BrdU, P60 | No Change |
| Hamilton and colleagues (2011) | P4-9 | Rat | MF | P115 | Dividing Cells | BrdU P80 | Decrease |
| Klintsova and colleagues (2007) | P4-9 | Rat | M | P80 | Dividing Cells | BrdU P50 | Decrease |
| Sliwowska and colleagues (2010) | E1-21 | Rat | M | P81-86 | BrdU P60-65 | No Change | |
| Uban and colleagues (2010) | E1-21 | Rat | F | P81-86 | Dividing Cells | BrdU P60-65 | No Change |
| 0- to 3-week-old Glia | GFAP/BrdU P60-65 | No Change | |||||
| 0- to 3-week-old Mature Granule Neurons | NeuN/BrdU P60-65 | No Change |
Changes in Inhibitory Neuron Numbers—
EtOH is known to directly impact inhibitory cells in the brain; however, only a few studies have examined the impact of perinatal EtOH exposure on these cells in the dentate gyrus (see Table 4). In one study, a transgenic mouse model (Venus-VGAT) was used that allowed them to directly visualize inhibitory (GABAergic) interneurons (Bird et al., 2018). This paper found a decrease in the number of interneurons in the granule cell layer (GCL) of the dentate gyrus. The other paper took a more traditional histological approach and labeled cells with NeuN, a mature neuron marker, a marker for the excitatory neurotransmitter glutamate, or with a GAD67, a marker of inhibitory interneurons. This study only assessed males, but in these they found an increase in neurons double labeled with NeuN and glutamate, and an decrease in cells double labeled with NeuN and GAD67 (Lu et al., 2018). Thus, both studies assessing inhibition found perinatal alcohol exposure to result in decreased numbers of interneurons in the granule cells layers.
Table 4.
Summary of Perinatal Alcohol Exposure Experiments Concerning Interneurons. In the Exposure Age and Test Age Column, “P” Indicates Postnatal Day and “E” Indicates Embryonic Day
| Paper | Exposure Age | Model | Sex | Test Age | What was Investigated? | Marker/Stain Used | Main Finding |
|---|---|---|---|---|---|---|---|
| Lu et al. (2018) | E9-20 | Rat | M | P84 | GABAergic Mature Neurons | NeuN and GAD67 | Increase |
| Glutamatergic Mature Neuron | NeuN and Glutamate | Decrease | |||||
| Elibol-Can et al. (2014) | E7-E21 | Rat | MF | P60 | Venus-VGAT | CA1 | Decreased in M |
| CA3 | Decreased in M | ||||||
| GCL | Decreased in MF | ||||||
| Hilus | Decreased in M |
Perinatal Marijuana
Two studies assessing the effects of perinatal marijuana exposure in adult rats and mice were found. One perinatal marijuana study assayed 2 types of interneuron counts in transgenic mouse lines. They found that CCK-positive caudal ganglionic eminence–derived interneurons decreased in adult mice perinatally exposed to THC but Medial Ganglionic Eminence Derived Interneurons showed no change (Vargish et al., 2017). The other perinatal marijuana study assayed CB1 receptor levels and found an increase in the CA1 area of the Hippocampus (Tortoriello et al., 2014).
DISCUSSION
Perinatal Alcohol in the Dentate Gyrus
This review found that in adult rats and mice perinatally exposed to alcohol, most components of adult neurogenesis do not appear to be significantly affected, but that there is evidence for changes in interneurons in the hippocampus (Table 5). The intention of this review was to investigate changes in the hippocampus caused by perinatal alcohol and marijuana exposure; however, a paucity of papers devoted to this topic required a focus on the review of perinatal alcohol exposure effects alone, although 2 papers on perinatal marijuana exposure did meet our criteria. To date, only one paper has been published that assess the interaction of perinatal administration of these substances at the cellular level, but no papers have assessed this in the developing hippocampus (Fish et al., 2019). As this area of research is in its infancy, this review hopes to shed light on possible directions for future SAM and perinatal cannabis research, based upon likely points of interaction.
Table 5.
Results of PRISMA Showing the Impact of Perinatal Marijuana and Perinatal Alcohol in the Hippocampal Formation of Adult Rats and Mice. A/M Indicates if the Perinatal Exposure is Alcohol(A) or Marijuana(M). M and F in the “Main Finding” Column Indicate When There are Sex Differences in the Results. Exposure and Test Ages are Reported as Embryonic (E) Days of Age or Postnatal (P) Days of Age.
| A/M | Exposure Age | Model | Sex | Test Age | What was Investigated? | Marker/ Stain Used | Hippocampal Subregion | Main Finding | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | A | P2-P9 | Mouse | MF | P90 | GABA Interneurons | Venus-VGAT | CA1 | Decreased in M |
| CA3 | Decreased in M | ||||||||
| GCL | Decreased in MF | ||||||||
| Hilus | Decreased in M | ||||||||
| 2 | A | E7-E21 | Rat | M | P60 | Type 2b INP to Immature Granule Neuron | DCX | CA1 CA2 + 3 |
No Change No Change |
| DG | No Change | ||||||||
| 3 | M | E10.5–E17.5 | Mouse | MF | P20-P60 | Cannabinoid receptor type 1 | CB1R | CA1 | Decrease in M |
| Cholecystokinin interneurons | CCK | CA1 | Decrease in M | ||||||
| 4 | A | E1–21 | Rat | M | P78–82 | Dividing Cells | BrdU | DG | No Change |
| Type 2b INP to IGN | DCX | DG | No Change | ||||||
| Dividing Cells | Ki-67 | DG | No Change | ||||||
| P115 | Dividing Cells | BrdU, P80 | DG | Decrease | |||||
| Type 2b INP to IGN | DCX | DG | No Change | ||||||
| 5 | A | E1-P9 | Rat | MF | P386 | Type 2b INP to IGN | DCX | DG | Decrease in F |
| Dividing Cells | Ki-67 | DG | No Change | ||||||
| 6 | A | P4–9 | Rat | MF | P60 | Dividing Cells | BrdU 2 hours | DG | No Change |
| Dividing Cells | Ki-67 | DG | No Change | ||||||
| Type 2b INP to Mature Granule Neuron | NeuroD | DG | Decrease | ||||||
| P90 | Dividing Cells | BrdU P60 | DG | No Change | |||||
| Dividing Cells | Ki-67 | DG | No Change | ||||||
| Type 2b INP to Mature Granule Neuron | NeuroD | DG | Decrease | ||||||
| 7 | A | P4–9 | Rat | M | P80 | Dividing Cells | BrdU | DG | Decrease |
| P50 | Dividing Cells | BrdU | DG | No Change | |||||
| P50 | Dividing Cells | Ki-67 | DG | No Change | |||||
| 8 | A | E9–20 | Rat | M | P84 | GABAergic Mature Neurons | NeuN and GAD67 | CA3 | Increase |
| DG | Increase | ||||||||
| Glutamatergic Mature Neuron | NeuN and Glutamate | CA3 DG |
Decrease Decrease |
||||||
| 9 | A | E7-E17 | Mouse | MF | P56 | Type 2b INP to Immature Granule Neuron | DCX | DG | Decrease |
| Dividing Cells | Ki-67 | DG | No Change | ||||||
| 10 | A | E1–21 | Rat | M | P60–65 | Dividing Cells | BrdU | GCL | No Change |
| Hilus | No Change | ||||||||
| P81–86 | BrdU P60–65 | GCL | No Change | ||||||
| Hilus | No Change | ||||||||
| 11 | M | E5.5–E17.5 | Rat | M | P120 | CB1-positive Boutons | CB1R | CA1 | Increase |
| 12 | A | E1–21 | Rat | F | P60–65 | Dividing Cells | BrdU | GCL | No Change |
| P81–86 | Dividing Cells | BrdU P60–65 | GCL | No Change | |||||
| 0- to 3-week-old Glia | GFAP/BrdU P60–65 | GCL | No Change | ||||||
| 0- to 3-week-old Mature Granule Neurons | NeuN/BrdU P60–65 | GCL | No Change | ||||||
| 13 | M | E10.5–E18.5 | Mouse | MF | P30 | Caudal Ganglionic Eminence–derived Interneurons |
GFP (5HT3AR-GFP transgenic line) | DG/Hilar Border | Decrease |
| Medial Ganglionic Eminence–derived Interneurons |
GFP (NKX2.1-cre: RCE-GFP transgenic line) |
DG/Hilar Border | No Change |
CA1, cornu ammonis area 1; CA2, cornu ammonis area 2; CA3, cornu ammonis area 3; DG, dentate gyrus; GABA, gamma-aminobutyric acid; GCL, granule cell layer; IGN, Immature granule neurons; INP, Immature Neural Progenitor.
Marker purposes are as follows: BrdU, bromodeoxyuridine, a thymidine analogue that labels DNA synthesis; CB1R, cannabinoid receptor type 1; DCX, doublecortin, a neuronal migration protein; GAD67, glutamate decarboxylase 67 kilodalton isoform; GFP, green fluorescent protein; Ki-67, antigen Ki-67, a marker of proliferation; NeuN, neuronal nuclei, a mature neuron marker; NeuroD, neurogenic differentiation factor 2, a marker of type 2b immature up to mature granule neurons; Venus-VGAT, a transgenic construct that labels GABAergic neurons.
Bird et al. (2018); 2. Elibol-Can et al. (2014); 3. de Salas-Quiroga et al. (2020); 4. Gil-Mohapel et al. (2011); 5. Gil-Mohapel et al. (2014); 6. Hamilton et al. (2011); 7. Klintsova et al. (2007); 8. Lu et al. (2018); 9. Olateju et al. (2018); 10. Sliwowska et al. (2010); 11. Tortoriello et al. (2014); 12. Uban et al. (2010); 13. Vargish et al. (2017).
DCX is first expressed in type 2b immature neural progenitor cells but is produced continuously until the cell is an immature granule neuron (Kempermann et al., 2004). BrdU and Ki-67 are both markers of proliferation, and the results of one are often used to validate the other (Kee et al., 2002). To this end, it can be seen that in rats and mice perinatally exposed to alcohol, there is no large long-lasting effect in the numbers of actively dividing cells in the dentate gyrus. NeuroD is also a marker of maturation, and in the prior study that utilized it as a marker, a decrease was found.
BrdU is injected before the animal is euthanized. Cells that incorporate BrdU are actively dividing at the time of injection (Kee et al., 2002). Therefore, when collecting tissue at advanced timepoints, BrdU can assay temporally discrete populations of cells undergoing DNA synthesis (Kee et al., 2002). The studies that used BrdU to assess proliferation (injection immediately before euthanasia) found no change in proliferation; however, half of the studies which followed an adult population of cells over a month-long window found a decreased number of BrdU-stained cells. This indicates that the population of cells dividing at the time of BrdU injection is not surviving in the same proportions of survival rates observed in control animals.
In perinatal alcohol exposure, research suggests that most damage happens due to cell loss early in development, and although some recovery occurs in terms of medically observable phenotype, this is caused by a slow recovery of the affected cell population throughout the individual’s life (Bonthius and West, 1991). A second mechanism proposed suggests that cell death is caused by a loss of inhibitory interneurons and subsequent excitotoxicity or aberrant dendritic pruning (Khaspekov et al., 2005). Two papers were found on this subject: one showed a statistically significant change in the balance of inhibitory and excitatory neurons in the dentate gyrus, and the other suggested that the one specific type of interneuron is decreasing (Lu et al., 2018; Vargish et al., 2017).
Perinatal Marijuana in the Dentate Gyrus
Two papers that assessed the adult effects of perinatal marijuana exposure were identified. One found a decrease in cholecystokinin (CCK)-positive interneurons that arise from the caudal ganglionic eminence, and no change in interneurons arising from the medial ganglionic eminence in the dentate gyrus of mice (Vargish et al., 2017). This suggests that moving forward, more research is required to understand the effects on interneuron subtypes and their implications in disease. The second paper discussed the cannabinoid receptor type 1 (Tortoriello et al., 2014). This receptor is expressed in inhibitory neurons, so an increase in CB1 expression could indicate either that the number of interneurons is increasing, or that CB1 is being upregulated (Han et al., 2012). A new paper found a significant decrease in CB1R expression in males but not females, which is opposite to the previous finding of an increase in CB1-positive boutons (de Salas-Quiroga et al., 2020; Tortoriello et al., 2014). De Salas-Quiroga et al. also found that there was a marked decrease in the number of CCK-positive interneurons in the CA1 region of the hippocampus, which agrees with the paper published by Vargish et al.; however, the effect was only significant in males (de Salas-Quiroga et al., 2020; Vargish et al., 2017).
The data found in this review suggest that the balance between inhibition and excitation may be where the largest effect will be seen in emerging SAM models. It is tempting to hypothesize that the actions of simultaneous perinatal alcohol and marijuana exposure will be synergistic because it appears these substances target 2 different sites. Specifically, alcohol appears to primarily target postsynaptic GABA receptors, whereas cannabinoids seem to target presynaptic CB1 receptors (Kawamura et al., 2006; Sheng and Kim, 2011). Studies done on these receptors in the absence of SAM conditions also support this finding (Chevaleyre and Castillo, 2004; Huang, Lo and Hsu, 2001; Losonczy, Biro and Nusser, 2004; Selvam, Yeh and Levine, 2019).
CONCLUSIONS
This systematic literature review, conducted using PRISMA-style search criteria, suggests that an interaction of alcohol and marijuana in a SAM model of exposure could influence inhibitory interneurons of the dentate gyrus. This study found that in adult rats and mice perinatally exposed to alcohol, within the dentate gyrus, proliferation is not affected but migration, maturation, survival, and interneurons are all affected. The papers pertaining to marijuana exposure suggested differences in interneurons, and thus, interneurons are the likely point of convergence of these 2 drugs. Specifically, CB1 receptors are expressed largely in the second trimester in the hippocampus, are presynaptic, and lead to decreased GABA release. GABA is integral to spatiotemporal integration of developing neurons. Perinatal THC exposure and perinatal alcohol exposure overlap in their ability to affect maturation and integration of pyramidal neurons in the dentate gyrus. Therefore, future studies may show that circuit integration and cell survival in pyramidal neurons in the dentate gyrus of SAM exposed animals. As research begins to acknowledge the patents exposed to both alcohol and marijuana perinatally, an understanding of the underlying mechanism will allow clinicians to better diagnose, and hopefully treat, this understudied population.
Supplementary Material
Table S1. Inclusion and exclusion criteria.
Table S2. Short-listed papers that were excluded from the final PRISMA, with reasons.
Table 6.
Search Terms
| Search Term Blocks | Search Terms |
|---|---|
| Specifies Prenatal | “Antenatal” OR “antepartum” OR “fetal” OR “prenatal” |
| Specifies brain region | “Hippocamp*” OR “dentate” OR “dentate gyrus” OR “CA1” OR “CA2” OR “CA3” OR “LPP” OR “MPP” OR “perforant pathway” OR “fimbria-fornix” OR “Schaffer collaterals” OR “Commisural pathway” |
| Specifies cytoarchitecture | “Genetic” OR “gene” OR “mRNA” OR “methylation” OR “hypermethylation” OR “acetylation”OR “hypomethylation” OR “methyl mark” OR “epigenetic” OR “NDMA” OR “nicotinic” OR “muscarinic” OR “CB2” OR “CB1” OR “GPCR” OR “G-protein” OR “G protein” OR “AMPA” OR “Calcium channel” OR “sodium channel” OR “chloride channel” OR “cAMP” OR “cyclic AMP” OR “PKA” OR “Signal transduction” OR “neurogenesis” OR “granule cell” OR “proliferation” OR “apoptosis” OR “neural stem cell” OR “neural stem cells” OR “cellular migration” OR “Immunohistochemistry” OR “IHC” OR “Ki-67” OR “PCNA” OR “Sox2” OR “BrdU” OR “DCX” OR “GFAP” |
| Specifies Alcohol | “PNEE” OR “Prenatal ethanol” OR “Prenatal alcohol” OR “PAE” OR “prenatal alcohol exposure” OR “FASD” OR “FAS” OR “fetal alcohol spectrum disorder” OR “fetal alcohol syndrome” OR “fetal alcohol exposure” OR “prenatal ethanol” OR “fetal ethanol” OR “ethanol” OR “alcohol” |
| Specifies Cannabis | “cannabis” OR “marijuana” or “THC” OR “Tetrahydrocannabinol” OR “Δ9-tetrahydrocannabinol” OR “cannabinoid” OR “WIN 55,212–2” OR “PME” |
ACKNOWLEDGMENT
We thank C. Fontaine for critical reading of an earlier version of this manuscript. This research was supported by AA025425.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- Akerman CJ, Cline HT (2007) Refining the roles of GABAergic signaling during neural circuit formation. Trends Neurosci 30:382–389. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Joshi V, Shivakumar M, Subbanna S (2019) Distinct functions of endogenous cannabinoid system in alcohol abuse disorders. Br J Pharmacol 176:3085–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis P, Dobszay MB, Wang X, Spano S, Ledda F, Sousa KM, Schulte G, Ernfors P, Mackie K, Paratcha G, Hurd YL, Harkany T (2005) Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc Natl Acad Sci USA 102:19115–19120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T (2007) Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science (80- ) 316:1212–1216. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Sepe N, Ramos JA, Di Marzo V, Fernandez-Ruiz JJ (1999) Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. SYNAPSE 33:181–191. [DOI] [PubMed] [Google Scholar]
- Bird CW, Taylor DH, Pinkowski NJ, Chavez GJ, Valenzuela CF (2018) Long-term reductions in the population of GABAergic interneurons in the mouse hippocampus following developmental ethanol exposure. Neuroscience 383:60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boa-Amponsem O, Zhang C, Mukhopadhyay S, Ardrey I, Cole GJ (2019) Ethanol and cannabinoids interact to alter behavior in a zebrafish fetal alcohol spectrum disorder model. Birth Defects Res 111:775–788. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR (1990) Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin Exp Res 14:107–118. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR (1991) Permanent neuronal deficits in rats exposed to alcohol during the brain growth spurt. Teratology 44:147–163. [DOI] [PubMed] [Google Scholar]
- Breit KR, Zamudio B, Thomas JD (2019) The effects of alcohol and cannabinoid exposure during the brain growth spurt on behavioral development in rats. Birth Defects Res 111:760–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocardo PS, Gil-Mohapel J, Wortman R, Noonan A, McGinnis E, Patten AR, Christie BR (2017) The effects of ethanol exposure during distinct periods of brain development on oxidative stress in the adult rat brain. Alcohol Clin Exp Res 41:26–37. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Mckay RDG (2001) Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol 435:406–417. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ, Landress HJ, Barrett ME (1990) The prevalence of illicit-drug or alcohol use during pregnancy and discrepancies in mandatory reporting in Pinellas County, Florida. N Engl J Med 322:1202–1206. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE (2004) Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron 43:871–881. [DOI] [PubMed] [Google Scholar]
- Christie BR, Cameron HA (2006) Neurogenesis in the adult hippocampus. Hippocampus 16:199–207. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ (2009) A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science (80- ) 325:210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Salas-Quiroga A, García-Rincón D, Gómez-Domínguez D, Valero M, Simón-Sánchez S, Paraíso-Luna J, Aguareles J, Pujadas M, Muguruza C, Callado LF, Lutz B, Guzman M, de la Prida LM, Galve-Roperh I (2020) Long-term hippocampal interneuronopathy drives sex-dimorphic spatial memory impairment induced by prenatal THC exposure. Neuropsychopharmacology 45:877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunson DB, Colombo B, Baird DD (2002) Changes with age in the level and duration of fertility in the menstrual cycle. Hum Reprod 17:1399–1403. [DOI] [PubMed] [Google Scholar]
- Elibol-Can B, Dursun I, Telkes I, Kilic E, Canan S, Jakubowska-Dogru E (2014) Examination of age-dependent effects of fetal ethanol exposure on behavior, hippocampal cell counts, and doublecortin immunoreactivity in rats. Dev Neurobiol 74:498–513. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz J, Berrendero F, Hernandez ML, Ramos JA (2000) The endogenous cannabinoid system and brain development. Trends Neurosci 23:14–20. [DOI] [PubMed] [Google Scholar]
- Finer LB, Zolna MR (2014) Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health 104(S1):S43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Murdaugh LB, Zhang C, Boschen KE, Boa-Amponsem O, Mendoza-Romero HN, Tarpley M, Chdid L, Mukhopadhyay S, Cole GJ, Williams KP, Parnell SE (2019) Cannabinoids exacerbate alcohol teratogenesis by a CB1-Hedgehog interaction. Sci Rep 9:16057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiègue S, Mary S, Marchand J, Dussossoy D, Carrière D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P (1995) Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem 232:54–61. [DOI] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Patten A, Cox A, Kainer L, Giles E, Brocardo PS, Christie BR (2011) Altered adult hippocampal neuronal maturation in a rat model of fetal alcohol syndrome. Brain Res 1384:29–41. [DOI] [PubMed] [Google Scholar]
- Gil-Mohapel J, Titterness AK, Patten AR, Taylor S, Ratzlaff A, Ratzlaff T, Helfer J, Christie BR (2014) Prenatal ethanol exposure differentially affects hippocampal neurogenesis in the adolescent and aged brain. Neuroscience 273:174–188. [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Richardson GA, Cornelius MD, Day NL (2004) Prenatal marijuana and alcohol exposure and academic achievement at age 10. Neurotoxicol Teratol 26:521–532. [DOI] [PubMed] [Google Scholar]
- Goodfellow MJ, Lindquist DH (2014) Significant long-term, but not short-term, hippocampal-dependent memory impairment in adult rats exposed to alcohol in early postnatal life. Dev Psychobiol 56:1316–1326. [DOI] [PubMed] [Google Scholar]
- Government of Canada (2017) Canadian Tobacco, Alcohol and Drugs Survey (CTADS): summary of results for 2017. Available at: https://www.canada.ca/en/health-canada/services/canadian-tobacco-alcohol-drugs-survey/2017-summary.html.
- Green CR, Mihic AM, Nikkel SM, Stade BC, Rasmussen C, Munoz DP, Reynolds JN (2009) Executive function deficits in children with fetal alcohol spectrum disorders (FASD) measured using the Cambridge Neuropsychological Tests Automated Battery (CANTAB). J Child Psychol Psychiatry 50:688–697. [DOI] [PubMed] [Google Scholar]
- Grotenhermen F (2003) Pharmacokinetics and Pharmacodynamics of Cannabinoids. Clin Pharmacokinet 42:327–360. [DOI] [PubMed] [Google Scholar]
- Guerri C, Bazinet A, Riley EP. (2009) Foetal Alcohol Spectrum Disorders and Alterations in Brain and Behaviour. Alcohol Alcohol 44:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GF, Murawski NJ, St.Cyr SA, Jablonski SA, Schiffino Fl, Stanton ME, Klintsova AY (2011) Neonatal alcohol exposure disrupts hippocampal neurogenesis and contextual fear conditioning in adult rats. Brain Res 1412:88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, Koehl M, Abrous DN, Mendizabal-Zubiaga J, Grandes P, Liu Q, Bai G, Wang W, Xiong L, Ren W, Marsicano G, Zhang X (2012) Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 148:1039–1050. [DOI] [PubMed] [Google Scholar]
- Harkany T, Guzmán M, Galve-Roperh I, Berghuis P, Devi LA, Mackie K (2007) The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci 28:83–92. [DOI] [PubMed] [Google Scholar]
- Huang CC, Lo SW, Hsu KS (2001) Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. The Journal of physiology. England 532(Pt 3):731–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC, Mulder EJH (2006) Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev 30:24–41. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW(2000) Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science 287:1056–1060. [DOI] [PubMed] [Google Scholar]
- Jackson KM, Sher KJ, Schulenberg JE (2008) Conjoint developmental trajectories of young adult substance use. Alcohol Clin Exp Res 32:723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janisse JJ, Bailey BA, Ager J, Sokol RJ (2014) Alcohol, tobacco, cocaine, and marijuana use: relative contributions to preterm delivery and fetal growth restriction. Subst Abus 35:60–67. [DOI] [PubMed] [Google Scholar]
- Kafaee Razavi M, Ebrahimpour S, Tehranipour M, Behnam Rasouli M (2010) The investigation of the long-term effects of aquatic extraction of Cannabis sativa on spatial memory consolidation in Rats. Arak Med Univ J 13:125–133. [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M (2006) The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci 26:2991–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boonstra R, Wojtowicz JM (2002) The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods 115:97–105. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G (2004) Milestones of neuronal development in the adult hippocampus. Trends Neurosci 27:447–452. [DOI] [PubMed] [Google Scholar]
- Khaspekov L, Brenz Verca M, Frumkina L, Marsicano D, Hermann H, Heinzmann U, Jennen L, Lutz B, Verca MB, Frumkina L, Marsicano D, Hermann H, Heinzmann U, Jennen L, Lutz B (2005) P.5.028 CB1 cannabinoid receptor-mediatedprotection against excitotoxic damage of hippocampal neurons in vitro: Histological and ultrastructural analysis. Eur Neuropsychopharmacol 15:S216. [Google Scholar]
- Kleiber ML, Mantha K, Stringer RL, Singh SM (2013) Neurodevelopmental alcohol exposure elicits long-term changes to gene expression that alter distinct molecular pathways dependent on timing of exposure. J Neurodev Disord 5(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintsova AY, Helfer JL, Calizo LH, Dong WK, Goodlett CR, Greenough WT (2007) Persistent impairment of hippocampal neurogenesis in young adult rats following early postnatal alcohol exposure. Alcohol Clin Exp Res 31:2073–2082. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G (2003) Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol 467:455–463. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR (2003) Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol 25:447–458. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Biro AA, Nusser Z (2004) Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc Natl Acad Sci U S A 101:1362–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Jiao Z, Yu Y, Zhang C, He X, Li Q, Xu D, Wang H (2018) Programming for increased expression of hippocampal GAD67 mediated the hypersensitivity of the hypothalamic-pituitary-adrenal axis in male offspring rats with prenatal ethanol exposure. Cell Death Dis 9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SE, Miller JA, Blackwell JM, West JR (1999) Fetal alcohol exposure and temporal vulnerability: Regional differences in cell loss as a function of the timing of binge-like alcohol exposure during brain development. Alcohol Clin Exp Res 23:726–734. [DOI] [PubMed] [Google Scholar]
- Martín-Sánchez A, Warnault V, Montagud-Romero S, Pastor A, Mondragón N, La Torre RD, Valverde O (2019) Alcohol-induced conditioned place preference is modulated by CB2 cannabinoid receptors and modifies levels of endocannabinoids in the mesocorticolimbic system. Pharmacol Biochem Behav 183:22–31. [DOI] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais A-S, Gossage JP, Kalberg WO, Joubert B, Cloete M, Barnard R, De Vries M, Hasken J, Robinson LK, Adnams CM, Buckley D, Manning M, Parry CDH, Hoyme HE, Tabachnick B, Seedat S (2013) Maternal alcohol consumption producing fetal alcohol spectrum disorders (FASD): Quantity, frequency, and timing of drinking. Drug Alcohol Depend 133:502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Medicine 6:1–6. [PMC free article] [PubMed] [Google Scholar]
- Mouro FM, Kofalvi A, Andre LA, Baqi Y, Muller CE, Ribeiro JA, Sebastiao AM (2019) Memory deficits induced by chronic cannabinoid exposure are prevented by adenosine A2AR receptor antagonism. Neuropharmacology 155:10–21. [DOI] [PubMed] [Google Scholar]
- Mulder J, Aguado T, Keimpema E, Barabas K, Ballester Rosado CJ, Nguyen L, Monory K, Marsicano G, Di Marzo V, Hurd YL, Guillemot F, Mackie K, Lutz B, Guzman M, Lu, Galve-Roperh I, Harkany T (2008) Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc Natl Acad Sci USA 105:8760–8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olateju OI, Spocter MA, Patzke N, Ihunwo AO, Manger PR (2018) Hippocampal neurogenesis in the C57BL/6J mice at early adulthood following prenatal alcohol exposure. Metab Brain Dis 33:397–410. [DOI] [PubMed] [Google Scholar]
- Olney JW, Wozniak DF, Jevtovic-Todorovic V, Farber NB, Bittigau P, Ikonomidou C (2002) Glutamate and GABA receptor dysfunction in the fetal alcohol syndrome. Neurotox Res 4:315. [DOI] [PubMed] [Google Scholar]
- Patten AR, Fontaine CJ, Christie BR (2014) A comparison of the different animal models of fetal alcohol spectrum disorders and their use in studying complex behaviors. Front Pediatr 2:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila VA, Olson AK, Swann SE, Mohades G, Webber AJ, Weinberg J, Christie BR (2006) Hippocampal cell proliferation is reduced following prenatal ethanol exposure but can be rescued with voluntary exercise. Hippocampus 16:305–311. [DOI] [PubMed] [Google Scholar]
- Renwick JH, Asker RL (1983) Ethanol-sensitive times for the human conceptus. Early Hum Dev 8:99–111. [DOI] [PubMed] [Google Scholar]
- Riley EP, Infante MA, Warren KR, Court A, Diego S, Warren KR (2011) Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev 21:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche M, Finn DP (2010) Brain CB₂ receptors: implications for neuropsychiatric disorders. Pharmaceuticals (Basel) 3:2517–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheyer AF, Melis M, Trezza V, Manzoni OJJ (2019) Consequences of perinatal Cannabis exposure. Trends Neurosci 42:871–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvam R, Yeh ML, Levine ES (2019) Endogenous cannabinoids mediate the effect of BDNF at CA1 inhibitory synapses in the hippocampus. Synapse 73:e22075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Kim E (2011) The postsynaptic organization of synapses. Cold Spring Harbor Perspect Biol 3:a005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwowska JH, Barker JM, Barha CK, Lan N, Weinberg J, Galea LAM (2010) Stress-induced suppression of hippocampal neurogenesis in adult male rats is altered by prenatal ethanol exposure. Stress Int J Biol Stress 13:302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS (2019) Recalibrating the Relevance of Adult Neurogenesis, pp 164–178. Trends in Neurosciences. Elsevier Ltd, London: [DOI] [PubMed] [Google Scholar]
- Subbaraman MS, Kerr WC (2015) Simultaneous versus concurrent use of alcohol and cannabis in the National Alcohol Survey. Alcohol Clin Exp Res 39:872–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry-McElrath YM, Patrick ME (2018) Simultaneous alcohol and Marijuana use among young adult drinkers: age-specific changes in prevalence from 1977 to 2016. Alcohol Exp Res 42:2224–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortoriello G, Morris CV, Alpar A, Fuzik J, Shirran SL, Calvigioni D, Keimpema E, Botting CH, Reinecke K, Herdegen T, Courtney M, Hurd YL, Harkany T (2014) Miswiring the brain: Delta(9)-tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. EMBO J 33:668–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uban KA, Sliwowska JH, Lieblich S, Ellis LA, Yu WK, Weinberg J, Galea LAM (2010) Prenatal alcohol exposure reduces the proportion of newly produced neurons and glia in the dentate gyrus of the hippocampus in female rats. Horm Behav 58:835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH (1999) Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA 96:13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH (2002) Functional neurogenesis in the adult hippocampus. Nature 415:1030–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varaschin RK, Allen NA, Rosenberg MJ, Valenzuela CF, Savage DD (2018) Prenatal alcohol exposure increases histamine H-3 receptor-mediated inhibition of glutamatergic neurotransmission in rat dentate gyrus. Alcohol Exp Res 42:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargish GA, Pelkey KA, Yuan X, Chittajallu R, Collins D, Fang C, McBain CJ (2017) Persistent inhibitory circuit defects and disrupted social behaviour following in utero exogenous cannabinoid exposure. Mol Psychiatry 22:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NE, Maple KE, Lisdahl KM, Preedy VR (2017) Effects of cannabis use on neurocognition in adolescents and emerging adults In Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment, pp 151–159. Elsevier Academic Press, London, UK. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Inclusion and exclusion criteria.
Table S2. Short-listed papers that were excluded from the final PRISMA, with reasons.