Abstract
Background
Higher plasma magnesium concentrations are associated with reduced cardiovascular disease risk in chronic kidney disease (CKD) patients. The importance of plasma magnesium concentration for vascular calcification in earlier stages of CKD remains underexplored. This study investigated whether plasma magnesium is a determinant for the presence and severity of vascular calcification in moderate CKD.
Methods
Retrospective analysis was performed using abdominal aortic calcification (AAC) scores in 280 patients with stage 3 and 4 CKD enrolled in the MASTERPLAN trial. Lateral abdominal X-ray was used to evaluate AAC. Plasma magnesium concentration were measured over time. A zero-inflated Poisson model determined the association between plasma magnesium concentration and AAC.
Results
79 out of 280 patients did not have AAC, and in patients with AAC the median calcification score was 3.5 (interquartile range: 0.0–8.6). The mean plasma magnesium concentration was 0.76 ± 0.10 mmol/L at baseline. A 0.1 mmol/L higher plasma magnesium concentration was associated with lower AAC of 0.07 point (95% CI -0.28 – 0.14). A 0.1 mmol/L higher plasma magnesium lowered the odds of detecting any AAC by 30% (OR = 0.63; 95% CI 0.29–1.37). After 1 year and 4 years (at time of X-ray) of follow-up this association was attenuated (OR = 0.93; 95% CI 0.61–1.43 and 0.93; 95% CI 0.60–1.45, respectively). None of these associations reached statistical significance.
Conclusions
Plasma magnesium concentration at baseline is not associated with the risk for future AAC. Interventions increasing magnesium to avoid vascular calcification may have greatest potential in early CKD stages prior to onset of vascular calcification.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-021-02267-4.
Keywords: Abdominal aortic calcification score, Chronic kidney disease, Magnesium, Vascular calcification
Background
In patients with chronic kidney disease (CKD) cardiovascular complications are the main cause of mortality [1, 2]. These cardiovascular complications are often a consequence of vascular calcification, which occurs in 80% of CKD patients with end-stage disease [3]. Vascular calcification is provoked by disturbances in mineral-bone metabolism in CKD, mainly characterized by hyperphosphatemia [4]. Currently, there is no effective treatment for vascular calcification. Presently used methods aimed at lowering blood phosphate (Pi) concentrations are insufficient to limit vascular calcification or cardiovascular disease risk [5]. Over the past decade, magnesium (Mg2+) has gained attention as a potential modifiable risk factor of vascular calcification in CKD [6]. Indeed, recent data demonstrate that magnesium prevents the formation of secondary calciprotein particles, which contribute to the development of medial calcification. Phosphate is the major determinant of secondary calciprotein particle formation and explains why CKD patients are prone to the development of these particles. Magnesium is a protective factor in the calcification milieu, which may act as a phosphate-buffering system to prevent secondary calciprotein particle development [7].
Increased plasma Mg2+ is associated with reduced risk for all-cause and cardiovascular mortality in the general population and in CKD patients [8–15]. More specifically, Mg2+ effectively prevents vascular calcification in human vascular smooth muscle cells as well as in a variety of rodents [16–18]. A recent clinical trial in CKD patients reported immediate effects of increasing both oral and dialysate Mg2+ on calcification propensity of human serum, as measured by in-vitro analysis [19–21]. Combined, these studies demonstrate that increasing plasma Mg2+ concentrations reduces vascular calcification risk and progression in end-stage CKD patients. Until now, most observational cohort studies on which clinical trials are based have focused on the association between Mg2+ and vascular calcification in hemodialysis patients. However, the potential importance of plasma Mg2+ concentration for vascular calcification in earlier stages of CKD remains underexplored.
The aim of this study was to investigate whether plasma Mg2+ is a determinant for the presence and severity of vascular calcification in moderate CKD. We performed a retrospective study using abdominal aortic calcification (AAC) scores in patients with stage 3 and 4 CKD that were enrolled in the MASTERPLAN (Multifactorial Approach and Superior Treatment Efficacy in Renal Patients with the Aid of Nurse practitioners) trial [22].
Methods
Design and patient inclusion
A comprehensive description of patient selection and the assessment of AAC has been previously reported by Peeters et al. [23] For the reader’s convenience we will briefly summarize the approach. The MASTERPLAN (Multifactorial Approach and Superior Treatment Efficacy in Renal Patients with the Aid of Nurse practitioners) study was a randomized controlled trial that started inclusion in 2004 (ISRCTN73187232). Rationale, design and outcomes are reported elsewhere [22, 24, 25]. In summary, the MASTERPLAN trial was designed as a multifactorial intervention comparing additional renal nurse support to standard care to reduce cardiovascular and renal risk in patients with moderate CKD. Adult patients with moderate to severe CKD (estimated creatinine clearance between 20 and 70 ml/min/1.73m2) were included [22]. Patients with a renal transplant < 1 year before screening, acute kidney injury or rapidly progressing glomerular nephritis, any malignancy < 5 years before screening (other than basocellular or squamous cell carcinoma of the skin) or participating in other clinical trials that required the use of study medication were excluded from the study [22]. The study was performed in accordance with the declaration of Helsinki. All patients provided written informed consent, and medical ethical approval was obtained prior to initiation of the MASTERPLAN trial. In the period of 2008–2009 nephrologists considered to role of evaluating AAC in selected patients, based on the data and discussions that resulted in the recommendation in the 2009 KDIGO CKD-MBD guideline [23, 26]. As the X-ray was not in the initial trial protocol, the decision to take X-rays was left to the treating nephrologist. In total 280 patients had an X-ray. The lateral abdominal X-rays were reviewed by two independent reviewers.
Assessment of abdominal aortic calcification
The presence of AAC was determined and scored according to the method described by Kauppila et al. [27] This calcification score takes into account the anterior and the posterior arterial all separately, and ranges from 0 to 24. A detailed description of the calcification grading in the MASTERPLAN study has been documented previously [23]. The interrater agreement was excellent with a linear weighted kappa of 0.87. In only 8 of the 2240 rated aorta segments the score deviated > 1 point.
Plasma magnesium measurements
Plasma Mg2+ concentrations were determined for all patients in mmol/L using a colorimetric assay according to the manufacturer’s protocol (Roche, Basel, Switzerland) and measured at 600 nm on a Bio-Rad Benchmark plus microplate reader (Bio-Rad Laboratories, Hercules, California, USA). Plasma samples had been frozen and stored at − 80° prior to analysis. All measurements were performed in triplicate. Plasma Mg2+ concentrations were measured at baseline, after one year and at the time of the X-ray (after four years).
Statistical analyses
Baseline data were described by frequencies and proportion for categorical variables, mean and standard deviation (SD) for normally distributed continuous variables, and median and interquartile range for continuous variables with a skewed distribution. We investigated the dose response relation between AAC score and serum Mg2+ by creating a scatterplot and fitting a LOESS smoothed regression line. As the association between AAC and plasma Mg2+ was approximately linear, no transformations were considered. Next, we reviewed missing data patterns (Supplementary Table S1) and used multiple imputation with chained equations to impute missing values using R-package ‘mice’ [28]. Predictive mean matching was used to impute missing values for continuously distributed variables and logistic regression was used to impute missing values for dichotomous variables. For all imputation models, predictors with a bivariate correlation of > 0.15 were considered. Diagnostic plots indicated that the imputations were stable over five iterations. Strip plots showed that imputed values all fell within the range of the observed values and were distributed across the entire range of observed values (Supplementary Figure S1).
In order to obtain a valid estimate for the association between plasma Mg2+ and AAC a multivariate model that adjusts for important confounders is required. To identify the variables that should be adjusted for, a causal model with the hypothesized relation between plasma Mg2+ and AAC was created. A directed acyclic graph was created with dagitty.net software and associated R-package to encode model assumptions [29]. Implied conditional independencies stemming from the model were tested and the model was refined until no gross violations were detected (see Supplementary Table S2 for the results of the conditional independency tests and Supplementary Fig. S2 for the final causal model). We arrived at two possible adjustment sets (Table 2). Both were used to obtain an adjusted estimate for the association between plasma Mg2+ concentration and AAC.
As 79 of the 280 patients had no calcifications we used a zero-inflated Poisson model to determine the association between plasma Mg2+ concentration and AAC. The model assumes that the zeros are generated by another process than the count data, and therefore that these processes can be modeled separately. The model consists of two parts. First, a Poisson model for the continuous data with values > 0, and second a logistic model that estimates the log-odds of a zero observation. Additionally, we included an off-set for the time between Mg2+ measurement and the X-ray in the Poisson sub-model.
All analyses were performed with RStudio (version 1.1.463 –© 2009–2018 RStudio, Inc.), R (version 3.5.3 for Windows) and the following packages: dagitty_0.2–3, boot_1.3–20, mice_3.5.0, V8_2.2, car_3.0–2, survival_2.44–1.1, tableone_0.10.0, ggplot2_3.1.0, dplyr_0.8.1, foreign_0.8–71 (Item S1) [30].
Results
Patient population
Table 1 shows the baseline characteristics of the 280 patients included in the analysis [23]. This cohort consisted of relatively young patients with a mean age of 61 years. With an average eGFR of 41 ml/min/1.73m2 patients had moderately to severely decreased renal function. In total, 79 out of 280 patients did not have AAC (AAC = 0) and the median calcification score was 3.5 (interquartile range: 0.0–8.6) [21]. The majority of the included patients was diagnosed with a hypertensive or renovascular cause of CKD. Only 10% of the patients had diabetic nephropathy as cause of the CKD, while 23% of the patients had diabetes. Around 30% of the patients had cardiovascular disease at time of inclusion. In addition, Pi and fibroblast growth factor 23 (FGF-23) concentrations fell within the normal range and did not differ between patients with or without AAC.
Table 1.
Baseline characteristics
| Total | No AAC | AAC | P | |
|---|---|---|---|---|
| n | 280 | 79 | 201 | |
| Randomized to intervention group | 164 (58.6) | 46 (58.2) | 118 (58.7) | 1.00 |
| Female gender | 88 (31.4) | 28 (35.6) | 60 (29.9) | 0.45 |
| Age (years) | 61.0 [51.7, 68.0] | 49.0 [39.5, 60.0] | 64.0 [57.0, 70.0] | < 0.001 |
| Race | ||||
| Caucasian | 251 (89.6) | 68 (86.1) | 183 (91.0) | |
| Non-Caucasian | 29 (10.4) | 11 (13.9) | 18 (9.0) | |
| Diagnosisa | 0.04 | |||
| Diabetic Nephropathy | 30 (10.7) | 5 (6.3) | 25 (12.4) | |
| Renovascular | 87 (31.1) | 17 (21.5) | 70 (34.8) | |
| Glomerulonephritis | 53 (18.9) | 20 (25.3) | 33 (16.4) | |
| Interstitial Nephritis | 30 (10.7) | 13 (16.5) | 17 (8.5) | |
| Congenital | 25 (8.9) | 9 (11.4) | 16 (8.0) | |
| Unknown | 55 (19.6) | 15 (19.0) | 40 (19.9) | |
| Diabetesb | 64 (22.9) | 9 (11.4) | 55 (27.4) | 0.01 |
| CVDc | 81 (28.9) | 10 (12.7) | 71 (35.3) | < 0.001 |
| Current smoker | 52 (18.6) | 13 (16.5) | 39 (19.5) | 0.68 |
| BMI (kg/m2) | 26.0 [23.6, 28.0] | 24.6 [23.1, 26.5] | 26.5 [24.2, 28.4] | < 0.001 |
| Waist hip ratio | 0.95 (0.08) | 0.94 (0.08) | 0.96 (0.08) | 0.04 |
| SBP (mmHg) | 133 (20) | 128 (17) | 135 (21) | 0.01 |
| DBP (mmHg) | 77 (11) | 78 (10) | 77 (12) | 0.21 |
| eGFRd (mL/min per 1.73m2) | 41.8 (19.0) | 43.1 (20.2) | 41.3 (18.5) | 0.48 |
| Serum creatinine (μmol/L) | 161.3 [129.9, 198.8] | 163.8 [133.4, 195.3] | 160.8 [127.9, 201.8] | 0.72 |
| Serum albumin (g/dL) | 40.5 (3.7) | 41.0 (4.0) | 40.3 (3.5) | 0.15 |
| Total serum cholesterol (mmol/L) | 4.89 (1.08) | 4.99 (1.15) | 4.85 (1.05) | 0.33 |
| LDL cholesterol (mmol/L) | 2.83 (0.98) | 2.87 (1.09) | 2.81 (0.94) | 0.64 |
| Hemoglobin (mmol/L) | 8.3 (0.9) | 8.2 (1.0) | 8.4 (0.9) | 0.09 |
| Ca2+ (mmol/L) | 2.38 (0.14) | 2.39 (0.16) | 2.37 (0.13) | 0.23 |
| Mg2+ (mmol/L) | 0.76 (0.10) | 0.73 (0.10) | 0.77 (0.09) | 0.01 |
| Pi (mmol/L) | 1.07 [0.94, 1.21] | 1.07 [0.92, 1.18] | 1.08 [0.94, 1.24] | 0.20 |
| FGF-23 (RU/L) | 100.3 [58.8, 166.7] | 91.1 [51.4, 161.2] | 108.0 [64.0, 168.0] | 0.18 |
| PTH (pmol/L) | 8.1 [5.2, 12.6] | 8.3 [5.4, 14.1] | 8.0 [5.2, 12.1] | 0.52 |
| hsCRP (mg/dL) | 2.0 [0.8, 51.3] | 1.7 [0.6, 4.3] | 2.1 [1.0, 5.6] | 0.09 |
| Proteinuria (g/24 h) | 0.2 [0.1, 0.6] | 0.2 [0.1, 0.6] | 0.2 [0.1, 0.6] | 0.28 |
| ACEi/ARB use | 239 (85.4) | 62 (78.5) | 177 (88.1) | 0.06 |
| Diuretic use | 137 (48.9) | 31 (39.2) | 106 (52.7) | 0.06 |
| Other antihypertensives | 157 (56.1) | 34 (43.0) | 123 (61.2) | 0.01 |
| Lipid lowering drugs | 204 (72.9) | 46 (58.2) | 158 (78.6) | 0.001 |
| Vitamin D use | 49 (17.5) | 16 (20.3) | 33 (16.4) | 0.56 |
| Phosphate binder use | 25 (8.9) | 8 (10.1) | 17 (8.5) | 0.84 |
| AAC score | 3.50 [0.00, 8.62] | 0.00 [0.00, 0.00] | 6.50 [3.00, 10.50] | |
Studied by logistic regression. Data are given as number (%), mean (SD) or median [interquartile range]
AAC Abdominal aortic calcification, ACEi Angiotensin converting enzyme inhibitor, ARB Angiotensin-II receptor blockers, BMI Body mass index, Ca2+ Calcium (conversion factor /0.2495 for mg/dL), CVD Cardiovascular disease, DPB Diastolic blood pressure, eGFR Estimated glomerular filtration rate, FGF-23 Fibroblast growth factor 23, hsCRP High-sensitivity C-reactive protein, LDL Low-density lipoprotein (conversion factor cholesterol /0.02586 for mg/dL), Mg2+ Magnesium, Pi Phosphate (conversion factor /0.3229 for mg/dL), PTH Parathyroid hormone, SBP Systolic blood pressure. Creatinine conversion factor /88.4 for mg/dL
aDiagnosis of the underlying renal disease was determined by the treating physician using available patient history, clinical course and if available histopathology
bDiabetes was defined as using blood glucose lowering medication or fasting glucose > 7.0 mmol/L
cCardiovascular disease was defined as myocardial infarction, stroke or vascular intervention
dUsing the MDRD (modification of diet in renal disease) eq. (186 x (Creatinine/88.4)-1.154 x (Age)-0.203 x (0.742 if female) x (1.210 if black), re-expressed for standardized serum creatinine
Distribution of plasma magnesium concentrations
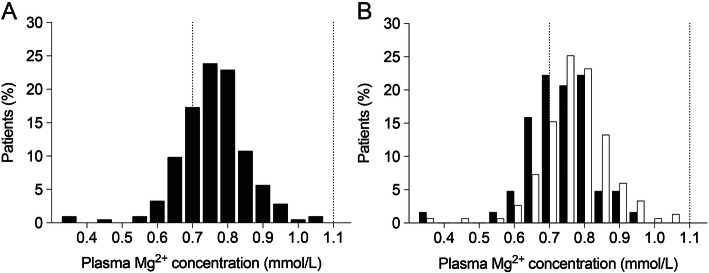
For all 280 patients, plasma Mg2+ concentration was measured at baseline, after one year and after four years. After a median period of 3.7 (interquartile range: 3.1–4.0) years after baseline, the X-rays were performed [21]. The mean plasma Mg2+ concentration was 0.76 ± 0.10 mmol/L at baseline (Fig. 1A). Patients without AAC appeared to have somewhat higher plasma Mg2+ concentrations at baseline (Fig. 1B). No marked differences in plasma Mg2+ concentrations were observed between baseline, after one year (0.76 ± 0.10 mmol/L) and at time of X-ray (0.74 ± 0.10 mmol/L). The lowest and the highest Mg2+ concentration were measured at 0.35 and 1.05 mmol/L, respectively. Approximately 16% of the patients had hypomagnesaemia with a plasma Mg2+ concentration below 0.7 mmol/L.
Fig. 1.
Plasma Mg2+ concentrations in non-dialysis CKD patients. Distribution of Mg2+ concentrations (A). Plasma Mg2+ concentrations for CKD patients with (indicated in white) and without (indicated in black) abdominal aortic calcifications (B). Dotted vertical lines indicate the reference values for Mg2+ concentration (0.7–1.1 mmol/L)
Dose-response relation between magnesium and AAC
The dose-response relationship between plasma Mg2+ concentration and the AAC score determined from X-rays taken three to four years later is shown in Fig. 2. The crude Poisson model demonstrated that a 0.1 mmol/L higher plasma Mg2+ concentration was associated with a 0.07 point lower value of AAC (95% Confidence Interval (CI) -0.28 – 0.014). The crude logistic model showed that a 0.1 mmol/L higher baseline plasma Mg2+ concentration resulted in 30% lower odds for detecting any AAC three to four years later (OR = 0.63; 95% CI 0.29 to 1.37). The association between AAC at year three or four and Mg measurement at year one n was attenuated compared to baseline to 7% per 0.1 mmol/L increase in plasma Mg2+ (OR = 0.93; 95% CI 0.61–1.43). Likewise, when Mg2+ measurements were taken at the same time as the X-ray the odds of absence of a calcification were 7% per 0.1 mmol/L Mg2+ increase (OR = 0.93; 95% CI 0.60–1.45). Adjustment did not substantially change this association (Table 2). None on the association described above reached statistical significance, as can be determined from the 95% confidence intervals overlapping 1.0.
Fig. 2.
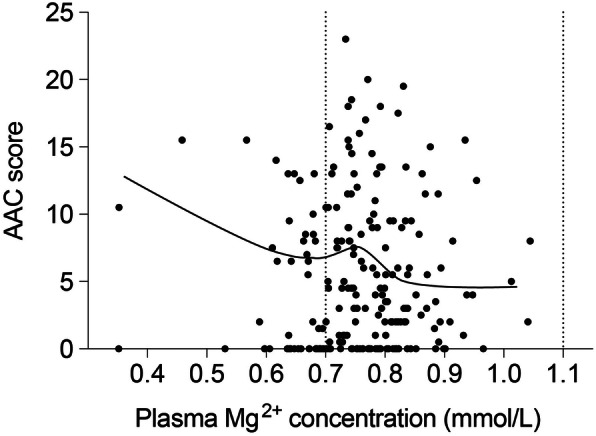
Dose-response relation between Mg2+ and abdominal aortic calcification score. The abdominal aortic calcification (AAC)-score for each patient are presented individually and plotted against their plasma Mg2+ concentration at baseline. Dose-response relation between AAC and plasma Mg2+ concentration was investigated by creating a scatter plot and fitting a LOESS smoothed regression line. No transformations were considered due to linearity of the association. Dotted vertical lines indicate the reference values for Mg2+ concentration (0.7–1.1 mmol/L)
Table 2.
Associations between AAC and plasma Mg2+ in multivariate analysis
| AAC incidence | Per 0.1 mmol/L Mg2+ | 95% CI |
|---|---|---|
| Univariate analysis | ||
| Baseline | ||
| Count (Poisson model) | −0.07 | −0.28 – 0.14 |
| Odds Ratio (zero model) | 0.63 | 0.29–1.37 |
| 1 year | ||
| Count (Poisson model) | −0.08 | − 0.51 – 0.35 |
| Odds Ratio (zero model) | 0.93 | 0.61–1.43 |
| X ray | ||
| Count (Poisson model) | −0.08 | −0.51 – 0.35 |
| Odds Ratio (zero model) | 0.93 | 0.60–1.45 |
| Adjusted for age, calcium, phosphate, cardiovascular disease, and diabetes | ||
| Baseline | ||
| Count (Poisson model) | −0.07 | −0.58 – 0.44 |
| Odds Ratio (zero model) | 0.63 | 0.29–1.37 |
| 1 year | ||
| Count (Poisson model) | −0.08 | − 0.51 – 0.35 |
| Odds Ratio (zero model) | 0.93 | 0.61–1.43 |
| X ray | ||
| Count (Poisson model) | −0.08 | − 0.51 – 0.35 |
| Odds Ratio (zero model) | 0.93 | 0.60–1.45 |
| Adjusted for age, calcium, phosphate, cardiovascular disease, eGFR, and PTH | ||
| Baseline | ||
| Count (Poisson model) | −0.07 | −0.53 – 0.39 |
| Odds Ratio (zero model) | 0.64 | 0.32–1.30 |
| 1 year | ||
| Count (Poisson model) | −0.07 | − 0.45 – 0.32 |
| Odds Ratio (zero model) | 0.94 | 0.63–1.38 |
| X ray | ||
| Count (Poisson model) | -0.07 | − 0.44 – 0.31 |
| Odds Ratio (zero model) | 0.93 | 0.63–1.38 |
| Adjusted for age, eGFR, phosphate, and diuretics | ||
| Baseline | ||
| Count (Poisson model) | −0.08 | −0.53 – 0.38 |
| Odds Ratio (zero model) | 0.65 | 0.34–1.23 |
| 1 year | ||
| Count (Poisson model) | -0.07 | − 0.48 – 0.35 |
| Odds Ratio (zero model) | 0.93 | 0.61–1.41 |
| X ray | ||
| Count (Poisson model) | −0.07 | −0.48 – 0.33 |
| Odds Ratio (zero model) | 0.94 | 0.63–1.40 |
A zero-inflated Poisson model was used to determine the association between plasma Mg2+ concentration and AAC. The model assumes that the zeros are generated by another process than the count data, and therefore that these processes can be modeled separately. The model consists of two parts. First, a Poisson model for the continuous data with values > 0 and second, a logistic model that estimates the Log-odds of a zero observation
Results are presented per 0.1 mmol/L increase in plasma Mg2+
Ca2+ Calcium, CI Confidence interval, Mg2+ Magnesium
Discussion
In this study, we aimed to investigate whether plasma Mg2+ concentration is a determinant for the presence and severity of vascular calcification in moderate CKD. We have identified a modest, not statistically significant association between AAC score and plasma Mg2+ concentration. For every higher value of 0.1 mmol/L plasma Mg2+ the associated AAC score is lower by approximately 0.1 point. In addition, a more pronounced association was present between higher plasma Mg2+ concentration at baseline and the absence of AAC four years later. Specifically, the odds of finding any AAC on the X-ray after four years are 30% lower per 0.1 mmol/L higher value in plasma Mg2+ concentration at baseline. The observed association weakens markedly after one year (shorter period before the X-ray) and is almost absent when plasma Mg2+ concentration is measured at time of the X-ray.
Other studies have reported stronger associations between plasma or serum Mg2+ concentration and calcification score [9, 10, 31–35]. Molnar et al. showed that a 0.1 mmol/L higher serum Mg2+ concentration was associated with a 1.1-point lower AAC score in end-stage renal disease patients [34]. In pre-dialysis CKD patients, every mg/dL (0.4 mmol/L) higher serum Mg2+ concentration was associated with a 0.36 point lower CAC-density score (scale 1–3.5) [33]. Interestingly, Sakaguchi et al. described that the association between Mg2+ and AAC in pre-dialysis patients is dependent on serum Pi concentration. This association was only identified in a sub-group where serum Pi concentration was above 1.1 mmol/L, but not in patients with a Pi concentration below 1.1 mmol/L. [33] In the MASTERPLAN cohort, median plasma Pi concentration was 1.07 mmol/L. In other studies describing an association between Mg2+ concentration and AAC, the mean serum Pi concentration exceeded 1.49 mmol/L (Table 3). The relatively low Pi concentration potentially related to CKD stage may explain the weak correlation between Mg2+ concentration and AAC score found in our study. Of note, with a plasma Mg2+ concentration of 0.76 ± 0.10 mmol/L, plasma Mg2+ concentrations were low in comparison to other studies (Table 3). These relatively low Mg2+ concentrations, in addition to the low variation, could be a reason for the absence of a stronger relationship between plasma Mg2+ concentration and AAC score. Moreover, the average Mg2+ concentrations at baseline was higher in the AAC group. However, in the inferential analysis we accounted for other possible determinants of AAC. These include diabetes, higher age, prior cardiovascular and serum calcium and phosphate. Diabetes prevalence, age, and CVD prevalence were substantially higher in the AAC group, while serum calcium and phosphate concentrations were similar. The crude association between Mg2+ and AAC in our study, may thus be explained by these confounding factors However, absence of evidence does not mean evidence of absence. We hypothesize that the absence of a relation in our cohort may have two reasons: First the serum Mg2+ concentrations were fairly low, possibly insufficient to achieve and effect on AAC. Second, Mg2+ may not have a (clinically meaningful) effect on established AAC. By design we could ascertain if patients had AAC at baseline already. Of note, the plasma Mg2+ concentrations for each patient were stable between the measurements. In advanced CKD, serum Mg2+ concentrations tend to increase, reaching values of around 0.97 mmol/L. [13] In addition, the median AAC score in this study was 3.5, while in stage 5 CKD mean AAC was 8.9 (scale 0–24) [34]. Our study population included patients with moderate CKD, with most in stage 3b. It is possible that a more pronounced association between Mg2+ concentration and AAC is present in more advanced stages of CKD.
Table 3.
Overview of studies assessing the relationship between blood Mg2+ concentration and vascular calcification
| Reference# | Study type | CKD stage | Sample size (% women) | Mg2+ concentration | Pi concentration | Type | Follow-up (years) | Association (P < 0.05) | Associations serum Mg2+ concentration (mmol/L) |
|---|---|---|---|---|---|---|---|---|---|
| Meema et al. 1987 [9] | Prospective | 5 | 44 (0) | 1.16 | 1.88 | Peripheral AC | 2 | Yes | 1.10 ± 0.21 in AC compared to 1.24 ± 0.21 in non-AC |
| Tzanakis et al. 1997 [36] | Cross-sectional | 5 | 56 (39) | 1.23 | 1.63 | MAC | – | Yes | 1.14 ± 0.12 in MAC versus 1.27 ± 0.10 in non-MAC |
| Ishimura et al. 2007 [37] | Cross-sectional | 5 | 390 (42) | 1.14 | 1.87 | AC (hand) | – | Yes | 1.10 ± 0.12 in VC versus 1.14 ± 0.14 in non-VC |
| Matias et al. 2014 [32] | Prospective | 5 | 206 (45) | 1.36 | 1.49 | SVCS | 4 | Yes | β-coefficient 0.17 95% CI 0.08–0.30 (cut-off concentration 1.15)1 |
| Sakaguchi et al. 2016 [33] | Cross-sectional | 3–4 | 109 (33) | 0.85 | ≥ 1.10 | CAC | – | Yes | β-coefficient − 0.36 (CI not reported)2 |
| Molnar et al. 2017 [34] | Cross-sectional | 5 | 80 (30) | 0.84 | 1.70 | AAC | – | Yes | Adjusted R2 0.18, β-coefficient − 12.27, 95% CI − 19.54 – − 5.003 |
| Okamoto et al. 2018 [35] | Retrospective | 5 | 128 (36) | 0.90 | 1.74 | AAC | 1 | Yes | OR 3.11, 95% CI 1.43–5.89 (baseline serum Mg2+ < 0.9) |
| Tamura et al. 2019 [38] | Prospective | 5 | 392 (34.7) | 1.15 | 1.65 | AoAC | 4.2 | No | – |
*(A/C)AC, (abdominal/coronary) arterial calcification; AoAc, aortic arch calcification; MAC, mitral annular calcifications; OR, odds ratio; SVCS, simple vascular calcification score (hands/pelvis); VC, vascular calcification
#Articles were obtained after PubMed search in October 2019 using the following search terms: ((“Renal insufficiency, Chronic”[Mesh] OR “Chronic kidney disease”[TiAb]) AND “Magnesium”[Mesh/TiAb]) AND (“calcinosis”[mesh] OR “calcification”[TiAb])
1Scale of calcification score reported was not quantitative [39]. Score is based on presence in pre-determined locations and scores are made up out of the sum of positive locations, ranging from 0 to 8 [36]
2Scale of calcification density was reported between 0.86–3.33 (Agatston score divided by the total calcified area for each patient)
3Scale of calcification score (AAC) was reported 0–24
Our results suggest that a 0.1 mmol/L higher value of plasma Mg2+ concentration is associated with 30% reduced risk of having any AAC, although not statistically significantly. Because vascular calcification is irreversible once established, determining the optimal window of effective treatment, potentially using Mg2+, is essential [40]. To date, most epidemiological studies have investigated whether plasma Mg2+ concentration is associated with vascular calcification in dialysis patients where calcification has already progressed (Table 3) [9, 31, 32, 34, 37]. Vascular calcification often manifests already in earlier stages of CKD. Our results indicate that, at least in this cohort consisting of 280 non-dialysis CKD patients, the effects of Mg2+ may be lagged. Therefore, supplementation of Mg2+ may be less effective once AAC has already formed. This notion is in line with the study of Bressendorff et al, showing that an increase in blood Mg2+ concentration of 0.34 mmol/L results in reduced calcification propensity, which reflects a lower Ca2+-Pi precipitation risk [19]. The calcification propensity test determines the formation of calcium phosphate particles in human serum in an in vitro setup. Thus, the study of Bressendorff determines the ex vivo formation of calcium precipitates, rather than measuring already formed calcification. As such, the study supports our data and demonstrates that magnesium may prevent the formation of calcification, but will not affect calcification already in place.
A vast body of observational studies has identified associations between the blood Mg2+ concentration and cardiovascular and all-cause mortality in end-stage renal disease patients [6, 11, 13]. While subsequent in vitro and animal study evidence has been compelling, clinical studies assessing the effectiveness of Mg2+ in preventing vascular calcification have been scarce [16–18, 41–43]. Recently, in a randomized clinical study oral Mg2+ supplementation resulted in diminished progression of coronary artery calcification (CAC)-score in pre-dialysis CKD patients [44]. While more clinical studies are currently underway, it is of importance to evaluate the most effective window of intervention, which is likely in early CKD before onset of vascular calcification.
Strengths of our study include the use of a well characterized study cohort that has been followed-up according to standardized procedures and with extensive biobanking. In addition, we used the state-of-the-art methodology for causal inference to create an explicit and testable causal model. Furthermore, plasma Mg2+ concentrations were obtained at several time points which allowed for the determination of the latency of the protective effect of plasma Mg2+ concentration on vascular calcification. The calcifications were scored with high interrater reliability, reducing the possibility of misclassification.
A major limitation of our study was the relatively small sample size and the fact that a lumbar X-ray was only available in a subgroup of patients. A selection bias may have been created by selecting patients that received the X-ray that were relatively healthy. Although the differences were small, patients that received the X-ray had a lower renal risk and a higher cardiovascular risk profile. Moreover, patients in the MASTERPLAN trial were fairly well controlled both at baseline and follow-up. Therefore, this population may not be completely representative of the average CKD population. Furthermore, some variables had missing values, mostly during follow-up. Missingness was handled by using multiple imputation. However, while this approach reduces the likelihood of selection bias, it does introduce noise in the covariate values. As a consequence, residual confounding may remain despite statistical adjustment. Furthermore, as already discussed for the MASTERPLAN cohort study in a previous publication, the sensitivity of AAC measurements by lumbar X-ray is less compared to computed tomography measurements, leading to a potential underestimation of AAC severity [22, 23]. This could lead to misclassification of patients without AAC. Another limitation is the lack of X-ray data at baseline of the study, thus possible effects of Mg2+ on the rate of calcification in patients with established calcifications could not be investigated. Finally, we did not have information about the types of phosphate binding medication that patients used. Some of these may include magnesium salts..
Conclusions
In conclusion, a statistically nonsignificant association between Mg2+ and AAC in this study suggests a limited if any potential preventive effect Mg2+ on the development of AAC in non-dialysis CKD patients.
Supplementary Information
Additional file 1: Table S1. Missingness patterns at baseline. Table S2. Conditional independency tests for the causal assumption model. Figure S1. Diagnostic strip plots. Figure S2. Causal model for identification of adjustment sets. Item S1 Supplementary R-code.
Acknowledgements
We thank participating nephrologists Marjolijn van Buren, Karin A.H. Kaasjager, Yvo W.J. Sijpkens, Peter J.G. van de Ven, Gerald Vervoort, Louis-Jean Vleming and nurse practitioners Hanny Bergsma, Noeleen Berkhout, Paul Gundlach, Lidian Lensen, Simone Mooren, Kathy Schoenmakers, Ans Wieleman, Judith Wierdsma. We also thank Marc G. Vervloet for his valuable consulting. Furthermore, we thank Yelka Koster for reviewing and scoring the X-rays.
Abbreviations
- AAC
Abdominal aortic calcification
- CAC
Coronary Artery Calcification
- CKD
Chronic Kidney Disease
- KDIGO CKD-MBD
Kidney Disease Improving Global Outcomes Chronic Kidney Disease – Mineral Bone Disease
- MASTERPLAN
Multifactorial Approach and Superior Treatment Efficacy in Renal Patients with the Aid of Nurse practitioners trial
- Mg2+
Magnesium
- LOESS
Locally estimated sum of squares
- OR
Odds ratio
- SD
Standard Deviation
Authors’ contributions
ADtB, JHFdB, JGJH and JAGJvdB conceived and designed the study. ADtB and LPG performed the Mg2+ measurements and JAGvdB performed statistical analysis of the data. ADtB, JHFdB and JAGJvdB interpreted the data. MJP, ADvZ, JFMW, PJBAll, JAGJvdB were involved in the original MASTERPLAN cohort study. ADtB and JAGJvdB wrote the manuscript. All authors have critically reviewed the manuscript. The author(s) read and approved the final manuscript.
Funding
This work was financially supported by grants from the Netherlands Organization for Scientific Research (NWO Veni 016.186.012 and VICI 016.130.668) and the Dutch Kidney Foundation (Kolff 14OKG17, 15OP02 and 16TKI02).
Availability of data and materials
Original study data and associated analysis scripts have been stored in a virtual environment on the anDREa platform (https://www.andrea-consortium.org/). Access can be requested via the corresponding author.
Ethics approval and consent to participate
All procedures performed in this study were in accordance with the ethical standards of the institutional research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by Medical Ethics Committees of the participating centers, notably Canisius Wilhelmina Hospital, Nijmegen,
Deventer Hospital, Deventer, Haga Hospital, The Hague, Leiden University Medical Center, Leiden, Medical Center Rijnmond Zuid, Rotterdam, Rijnstate Hospital, Arnhem, Radboud University Nijmegen Medical Center, Nijmegen, University Medical Center Utrecht, Utrecht.
Written informed consent was obtained from all individuals who participated in the MASTERPLAN study.
Consent for publication
No applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Parfrey PS. Cardiovascular disease and mortality in ESRD. J Nephrol. 1998;11:239–245. [PubMed] [Google Scholar]
- 3.Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 4.Mathew S, Tustison KS, Sugatani T, Chaudhary LR, Rifas L, Hruska KA. The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol. 2008;19:1092–1105. doi: 10.1681/ASN.2007070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruospo M, Palmer SC, Natale P, et al. Phosphate binders for preventing and treating chronic kidney disease-mineral and bone disorder (CKD-MBD) Cochrane Database Syst Rev. 2018;8(8):CD006023. doi: 10.1002/14651858.CD006023.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ter Braake AD, Shanahan CM, de Baaij JHF. Magnesium counteracts vascular calcification: passive interference or active modulation? Arterioscler Thromb Vasc Biol. 2017;37:1431–1445. doi: 10.1161/ATVBAHA.117.309182. [DOI] [PubMed] [Google Scholar]
- 7.Ter Braake AD, Vervloet MG, de Baaij JHF, Hoenderop JGJ. Magnesium to prevent kidney disease-associated vascular calcification: crystal clear? Nephrol Dial Transplant. 2020. [DOI] [PMC free article] [PubMed]
- 8.Gartside PS, Glueck CJ. The important role of modifiable dietary and behavioral characteristics in the causation and prevention of coronary heart disease hospitalization and mortality: the prospective NHANES I follow-up study. J Am Coll Nutr. 1995;14:71–79. doi: 10.1080/07315724.1995.10718476. [DOI] [PubMed] [Google Scholar]
- 9.Meema HE, Oreopoulos DG, Rapoport A. Serum magnesium level and arterial calcification in end—stage renal disease. Kidney Int. 1987;32:388–394. doi: 10.1038/ki.1987.222. [DOI] [PubMed] [Google Scholar]
- 10.Ishimura E, Okuno S, Yamakawa T, Inaba M, Nishizawa Y. Serum magnesium concentration is a significant predictor of mortality in maintenance hemodialysis patients. Magnes Res. 2007;20:237–244. [PubMed] [Google Scholar]
- 11.Kanbay M, Yilmaz MI, Apetrii M, et al. Relationship between serum magnesium levels and cardiovascular events in chronic kidney disease patients. Am J Nephrol. 2012;36:228–237. doi: 10.1159/000341868. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi Y, Fujii N, Shoji T, Hayashi T, Rakugi H, Isaka Y. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 2014;85:174–181. doi: 10.1038/ki.2013.327. [DOI] [PubMed] [Google Scholar]
- 13.de Roij van Zuijdewijn CLM, Grooteman MPC, Bots ML, et al. Serum magnesium and sudden death in European hemodialysis patients. PLoS One. 2015;10:e0143104. doi: 10.1371/journal.pone.0143104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu L, Li H, Serum Magnesium WS-X. Mortality in maintenance hemodialysis patients. Blood Purif. 2016;43:31–36. doi: 10.1159/000451052. [DOI] [PubMed] [Google Scholar]
- 15.Cai K, Luo Q, Dai Z, et al. Hypomagnesemia is associated with increased mortality among peritoneal dialysis patients. PLoS One. 2016;11:e0152488. doi: 10.1371/journal.pone.0152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louvet L, Büchel J, Steppan S, Passlick-Deetjen J, Massy ZA. Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol Dial Transplant. 2013;28:869–878. doi: 10.1093/ndt/gfs520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ter Braake AD, Tinnemans PT, Shanahan CM, Hoenderop JGJ, de Baaij JHF. Magnesium prevents vascular calcification in vitro by inhibition of hydroxyapatite crystal formation. Sci Rep. 2018;2069:1–11. doi: 10.1038/s41598-018-20241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz-Tocados JM, Peralta-Ramirez A, Rodríguez-Ortiz ME, et al. Dietary magnesium supplementation prevents and reverses vascular and soft tissue calcifications in uremic rats. Kidney Int. 2017;92:1084–1099. doi: 10.1016/j.kint.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Bressendorff I, Hansen D, Schou M, Pasch A, Brandi L. The effect of increasing dialysate magnesium on serum calcification propensity in subjects with end stage kidney disease. Clin J Am Soc Nephrol. 2018;13:1373–1380. doi: 10.2215/CJN.13921217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bressendorff I, Hansen D, Schou M, et al. Oral magnesium supplementation in chronic kidney disease stages 3 and 4: efficacy, safety, and effect on serum calcification propensity—a prospective randomized double-blinded placebo-controlled clinical trial. Kidney Int Reports. 2017;2:380–389. doi: 10.1016/j.ekir.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasch A, Farese S, Gräber S, et al. Nanoparticle-based test measures overall propensity for calcification in serum. J Am Soc Nephrol. 2012;23(10):1744–1752. doi: 10.1681/ASN.2012030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peeters MJ, van Zuilen AD, van den Brand JAJG, et al. Nurse practitioner care improves renal outcome in patients with CKD. J Am Soc Nephrol. 2014;25:390–398. doi: 10.1681/ASN.2012121222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peeters MJ, van den Brand JA, van Zuilen AD, et al. Abdominal aortic calcification in patients with CKD. J Nephrol. 2017;30:109–118. doi: 10.1007/s40620-015-0260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Zuilen AD, Bots ML, Dulger A, et al. Multifactorial intervention with nurse practitioners does not change cardiovascular outcomes in patients with chronic kidney disease. Kidney Int. 2012;82:710–717. doi: 10.1038/ki.2012.137. [DOI] [PubMed] [Google Scholar]
- 25.Van Zuilen AD, van der Tweel I, Blankestijn PJ, et al. Multifactorial approach and superior treatment efficacy in renal patients with the aid of nurse practitioners. Design of the MASTERPLAN study [ISRCTN73187232] Trials. 2006;7:1–9. doi: 10.1186/1745-6215-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckardt KU, Kasiske BL, Abboud O, et al. KDIGO (2009) KDIGO clinical practice guideline for the diag- nosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int. 2009;76:S1–S2. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 27.Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132:245–250. doi: 10.1016/S0021-9150(97)00106-8. [DOI] [PubMed] [Google Scholar]
- 28.van Buuren S, Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 29.Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package “dagitty”. Int J Epidemiol. 2016;45:1887–1894. doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 30.R Core Team. R: a language and environment for statistical computing: R Foundation for Statistical Computing; 2019.
- 31.Tzanakis I, Virvidakis K, Tsomi A, et al. Intra‐ and extracellular magnesium levels and atheromatosis in haemodialysis patients. Magnes Res. 2004;17:102–108. [PubMed] [Google Scholar]
- 32.Matias PJ, Azevedo A, Laranjinha I, et al. Lower serum magnesium is associated with cardiovascular risk factors and mortality in haemodialysis patients. Blood Purif. 2014;38:244–252. doi: 10.1159/000366124. [DOI] [PubMed] [Google Scholar]
- 33.Sakaguchi Y, Hamano T, Nakano C, et al. Association between density of coronary artery calcification and serum magnesium levels among patients with chronic kidney disease. PLoS One. 2016;11:e0163673. doi: 10.1371/journal.pone.0163673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molnar AO, Biyani M, Hammond I, et al. Lower serum magnesium is associated with vascular calcification in peritoneal dialysis patients: a cross sectional study. BMC Nephrol. 2017;129:1–7. doi: 10.1186/s12882-017-0549-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okamoto T, Hatakeyama S, Hosogoe S, et al. Proton pump inhibitor as an independent factor of progression of abdominal aortic calcification in patients on maintenance hemodialysis. PLoS One. 2018;13:1–14. doi: 10.1371/journal.pone.0199160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tzanakis I, Pras A, Kounali D, et al. Mitral annular calcifications in haemodialysis patients: a possible protective role of magnesium. Nephrol Dial Transplant. 1997;12:2036–2037. doi: 10.1093/ndt/12.9.2036. [DOI] [PubMed] [Google Scholar]
- 37.Ishimura E, Okuno S, Kitatani K, et al. Significant association between the presence of peripheral vascular calcification and lower serum magnesium in hemodialysis patients. Clin Nephrol. 2007;68:222–227. doi: 10.5414/CNP68222. [DOI] [PubMed] [Google Scholar]
- 38.Tamura T, Unagami K, Okazaki M, Komatsu M, Nitta K. Serum magnesium levels and mortality in Japanese maintenance hemodialysis patients. Blood Purif. 2019;47:88–94. doi: 10.1159/000496659. [DOI] [PubMed] [Google Scholar]
- 39.Adragao T, Pires A, Lucas C, et al. A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1480–1488. doi: 10.1093/ndt/gfh217. [DOI] [PubMed] [Google Scholar]
- 40.Wyatt CM, Drueke TB. Vascular calcification in chronic kidney disease: here to stay? Kidney Int. 2017;92:276–278. doi: 10.1016/j.kint.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 41.Kircelli F, Peter ME, Sevinc Ok E, et al. Magnesium reduces calcification in bovine vascular smooth muscle cells in a dose-dependent manner. Nephrol Dial Transplant. 2012;27:514–521. doi: 10.1093/ndt/gfr321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montezano AC, Zimmerman D, Yusuf H, et al. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension. 2010;56:453–462. doi: 10.1161/HYPERTENSIONAHA.110.152058. [DOI] [PubMed] [Google Scholar]
- 43.De Oca AM, Guerrero F, Martinez-Moreno JM, et al. Magnesium inhibits wnt/b-catenin activity and reverses the osteogenic transformation of vascular smooth muscle cells. PLoS One. 2014;9:1–10. doi: 10.1371/journal.pone.0089525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakaguchi Y, Hamano T, Obi Y, et al. A randomized trial of magnesium oxide and oral carbon adsorbent for coronary artery calcification in predialysis CKD. J Am Soc Nephrol. 2019;30:1073–1085. doi: 10.1681/ASN.2018111150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Missingness patterns at baseline. Table S2. Conditional independency tests for the causal assumption model. Figure S1. Diagnostic strip plots. Figure S2. Causal model for identification of adjustment sets. Item S1 Supplementary R-code.
Data Availability Statement
Original study data and associated analysis scripts have been stored in a virtual environment on the anDREa platform (https://www.andrea-consortium.org/). Access can be requested via the corresponding author.