Abstract
The finger-cuff system CNAP (CNSystems Medizintechnik, Graz, Austria) allows non-invasive automated measurement of pulse pressure variation (PPVCNAP). We sought to validate the PPVCNAP-algorithm and investigate the agreement between PPVCNAP and arterial catheter-derived manually calculated pulse pressure variation (PPVINV). This was a prospective method comparison study in patients having neurosurgery. PPVINV was the reference method. We applied the PPVCNAP-algorithm to arterial catheter-derived blood pressure waveforms (PPVINV−CNAP) and to CNAP finger-cuff-derived blood pressure waveforms (PPVCNAP). To validate the PPVCNAP-algorithm, we compared PPVINV−CNAP to PPVINV. To investigate the clinical performance of PPVCNAP, we compared PPVCNAP to PPVINV. We used Bland–Altman analysis (absolute agreement), Deming regression, concordance, and Cohen's kappa (predictive agreement for three pulse pressure variation categories). We analyzed 360 measurements from 36 patients. The mean of the differences between PPVINV−CNAP and PPVINV was −0.1% (95% limits of agreement (95%-LoA) −2.5 to 2.3%). Deming regression showed a slope of 0.99 (95% confidence interval (95%-CI) 0.91 to 1.06) and intercept of −0.02 (95%-CI −0.52 to 0.47). The predictive agreement between PPVINV−CNAP and PPVINV was 92% and Cohen’s kappa was 0.79. The mean of the differences between PPVCNAP and PPVINV was −1.0% (95%-LoA−6.3 to 4.3%). Deming regression showed a slope of 0.85 (95%-CI 0.78 to 0.91) and intercept of 0.10 (95%-CI −0.34 to 0.55). The predictive agreement between PPVCNAP and PPVINV was 82% and Cohen’s kappa was 0.48. The PPVCNAP-algorithm reliably calculates pulse pressure variation compared to manual offline pulse pressure variation calculation when applied on the same arterial blood pressure waveform. The absolute and predictive agreement between PPVCNAP and PPVINV are moderate.
Keywords: Hemodynamic monitoring, Fluid responsiveness, Cardiac preload, Dynamic preload variable, Volume clamp method, Vascular unloading technology
Introduction
Pulse pressure variation (PPV) caused by mechanical ventilation can predict fluid responsiveness [1, 2]. PPV is determined by heart-lung interactions; mechanical ventilation with positive airway pressure causes cyclic changes in venous return and cardiac preload resulting in variable changes in the arterial blood pressure waveform that can be quantified by PPV [3, 4]. Automated measurement of PPV requires continuous recording and analysis of the arterial blood pressure waveform, usually invasively using an arterial catheter.
In recent years, innovative finger-cuff technologies became available that allow continuous recording of the arterial blood pressure waveform and PPV calculation in a non-invasive manner [5–10]. The CNAP system (CNAP Monitor 500; CNSystems Medizintechnik, Graz, Austria) is one commercially available finger-cuff system and has been validated for arterial blood pressure and cardiac output measurements [11]. Using a proprietary algorithm, the CNAP system also automatically calculates PPV (PPVCNAP).
We here sought to (a) validate the PPVCNAP-algorithm and (b) investigate the absolute and predictive agreement between PPVCNAP and arterial catheter-derived manually offline calculated PPV (PPVINV).
Material and methods
Study design
This was a prospective method comparison study comparing non-invasive finger-cuff-derived with invasive arterial catheter-derived arterial blood pressure as well as PPV in patients having neurosurgery. Here, we report PPV results. The arterial blood pressure results will be reported separately. The study was approved by the ethics committee (Ethikkomission der Ärztekammer Hamburg, Hamburg, Germany; registration number PV6048) and conducted in operating rooms of the University Medical Center Hamburg-Eppendorf between April and October 2019. All patients provided written informed consent.
Inclusion and exclusion criteria
We included adult patients (≥ 18 years) who were scheduled for neurosurgery and required invasive arterial blood pressure monitoring using an arterial catheter as part of routine care. We excluded patients with vascular implants at the upper extremities, finger oedema, impairment in peripheral perfusion (e.g., Raynaud syndrome, peripheral artery disease, or arterial-venous shunts), cardiac arrhythmia, valvular heart disease grade 2 or above, excessive movement and/or seizures, or cardiac assist devices. For this analysis of PPV only patients with appropriate ventilator settings (tidal volume ≥ 8 mL kg−1 predicted body weight, respiratory rate ≥ 10 min−1, and positive end-expiratory pressure ≤ 5 cm H2O) were included.
Automated PPVCNAP-algorithm
The PPVCNAP-algorithm is a computer algorithm to detect and analyze ventilation-induced swings in the arterial blood pressure waveform and automatically calculate PPV as illustrated in Fig. 1. In short, the PPVCNAP-algorithm applies an adapted beat detection algorithm [12] to the arterial blood pressure waveform to obtain systolic and diastolic arterial blood pressure, pulse pressure (PP), and pulse interval (PI). PP and PI are compared to the average PP and PI of previous heart beats. If the difference between a new PP or PI value and their average values of previous heart beats exceeds a certain threshold, the beat could be a premature beat and is therefore excluded from further calculation. The tolerance level is adaptively adjusted by the variance of PP and PI. Next, a PP minimum-maximum detector is applied to the time series of PP values. The detector is made somewhat “fuzzy” to ignore small variations in the PP series. The time appearance of minimum PP (PPmin) and maximum PP (PPmax) undergo a plausibility check by using the average of previous verified PPmin and PPmax. After verification, PPV is calculated as: PPV = 200 × (PPmax – PPmin)/(PPmax + PPmin) (%).
Fig. 1.
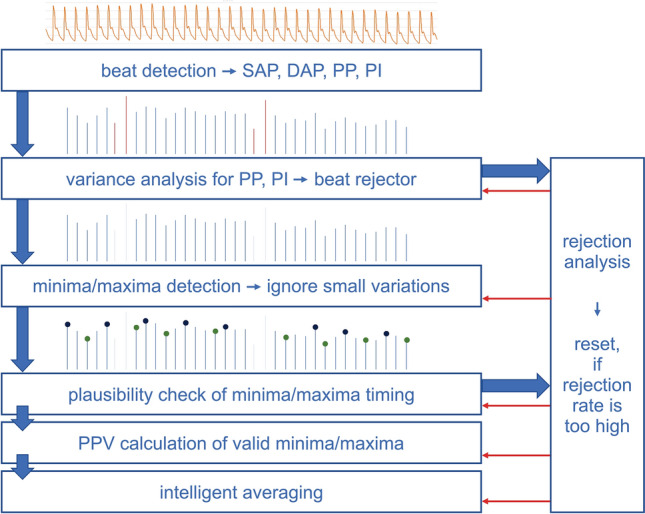
Schematic illustration of the PPVCNAP-algorithm. SAP systolic arterial blood pressure, DAP diastolic arterial blood pressure, PP pulse pressure, PI pulse interval, PPV pulse pressure variation
Note that one PPmax/PPmin pair corresponds to a half of a respiratory cycle. PPVCNAP is calculated by averaging six PPmax/PPmin pairs corresponding to the last three respiratory cycles. Additionally, outlier detection is used; PPV values higher than 40% are completely rejected. Further, if the difference between a new PPV value and the previous one is higher than a certain threshold, this new PPV value is used for calculation only with a 50% weight. If the PPV value is confirmed by the next measurement, the PPV value is then considered with a full 100% weight.
If plausibility checks of new beats or new PPmin or PPmax fail too often, all average and variance variables are reset. The PPVCNAP-algorithm is newly initialized and re-starts the calculation of average and variance variables from scratch.
PPV measurements
After induction of general anesthesia, all patients were ventilated with a tidal volume of 8 mL kg−1 predicted body weight, a respiratory rate of ≥ 10 min−1 adjusted to end-expiratory carbon dioxide, and a positive end-expiratory pressure of ≤ 5 cm H2O. After insertion of the radial arterial catheter, the CNAP system’s upper-arm-cuff was attached on the ipsilateral arm. The CNAP finger-cuff was placed on the index and middle finger of the contralateral arm. CNAP finger-cuff arterial blood pressure measurements were calibrated to oscillometric arterial blood pressure measurements every 30 min in the first 27 patients. We changed this to the maximal calibration interval of 60 min during the study and calibrated every 60 min in the last 9 patients. Arterial blood pressure recording was started after positioning of the patient in the operating room and continued until the end of surgery. The continuous arterial blood pressure waveforms measured non-invasively with the CNAP system and invasively with the arterial catheter were simultaneously displayed and recorded on the patient monitor (Infinity Delta Monitor; Dräger, Lübeck, Germany). Both waveforms were extracted to a personal computer (eData Data Grabber; Dräger) and beat-to-beat measurements were used for further offline analysis.
We randomly selected 10 60-s episodes of each patient. Within these episodes, we identified a period with at least three visible swings in PP in the non-invasive and invasive arterial waveform, which were used for further analysis.
We calculated PPVINV−CNAP by applying the PPVCNAP-algorithm to the arterial blood pressure waveform recorded invasively using the arterial catheter. PPVCNAP was automatically calculated using the PPVCNAP-algorithm based on the arterial blood pressure waveform recorded non-invasively with the CNAP system. PPVINV was calculated manually from the arterial blood pressure waveform recorded invasively using the arterial catheter (reference method).
Statistical analysis
Descriptive data are reported as mean ± standard deviation (SD) for continuous data and as absolute frequency and percentage for categorical data.
Using Bland–Altman analysis accounting for repeated measurements within individuals [13, 14], we compared (a) PPVINV−CNAP and PPVINV to validate the PPVCNAP-algorithm per se and (b) PPVCNAP and PPVINV to investigate the absolute agreement between PPVCNAP and PPVINV. For each comparison, we calculated the mean of the differences between the two methods (test method minus reference method), the SD of the mean of the differences, and the 95% limits of agreement (95%-LoA; i.e., mean of the differences ± 1.96 SD of the mean of the differences) and the 95% confidence intervals (95%-CI) around the 95%-LoA to quantify the trueness and precision of agreement [15, 16]. We additionally describe the correlation between PPVINV−CNAP and PPVINV and between PPVCNAP and PPVINV by Deming regression for scattered plots with 95%-CI [17, 18]. We assessed the predictive agreement for fluid responsiveness across three predefined categories (PPV < 9%, PPV 9 to 13%, PPV > 13%) between PPVCNAP and PPVINV. These PPV categories reflect PPV thresholds used for clinical decision making regarding fluid therapy in clinical practice [19, 20]. The predictive agreement across these three categories was calculated as the number of concordant paired measurements divided by the total number of paired measurements. In addition, we calculated Cohen’s kappa [21]. A Cohen’s kappa of < 0 indicates no agreement, 0–0.20 slight, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 substantial, and 0.81–1.00 almost perfect agreement. Statistical analyses were performed using Microsoft Excel (Microsoft, Redmond, WA, USA), SPSS 25 (IBM, Armonk, NY, USA), and Matlab (The MathWorks, Natick, MA, USA).
Results
Study cohort
A total of 44 patients were available for this analysis, but eight were excluded. We excluded four patients due to cardiac arrhythmia, two patients because of technical failure of the CNAP system, and two patients because of study protocol violations (Fig. 2). We thus included 36 patients with a total of 360 measurements in the final analysis. Patient characteristics are presented in Table 1.
Fig. 2.

Flow chart illustrating patient enrollment and reasons for exclusion.
Table 1.
Patient characteristics
| Sex female | 18 (50) |
| Age, years | 53.4 ± 13.6 |
| Height, cm | 173.3 ± 8.4 |
| Weight, kg | 80.9 ± 19.1 |
| ASA class I/II/III/IV, n | 3/20/12/1 |
| Duration of measurement, min | 167.5 ± 59.3 |
| Type of surgery | |
| Intracranial tumor resection | 24 (66.7) |
| Aneurysm repair surgery | 8 (22.2) |
| Intracranial biopsy | 1 (2.8) |
| Cranial fracture | 1 (2.8) |
| Hippocampectomy | 1 (2.8) |
| Cervical spine surgery | 1 (2.8) |
Data are shown as mean ± standard deviation or absolute numbers (percentages)
ASA class American Society of Anesthesiologists Physical Status class
Validation of the PPVCNAP-algorithm
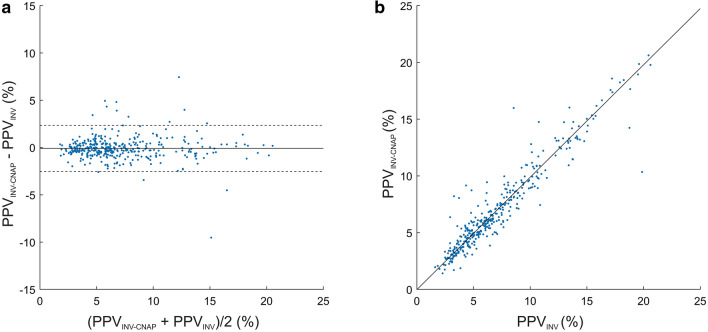
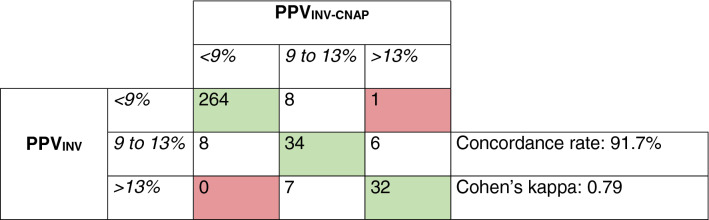
The mean of the differences ± SD between PPVINV−CNAP and PPVINV was −0.1 ± 1.2% (95%-LoA −2.5 to 2.3%) (Fig. 3, Table 2). For the comparison between PPVINV−CNAP and PPVINV, the Deming regression showed a slope of 0.99 (95%-CI 0.91 to 1.06) and an intercept of −0.02 (95%-CI −0.52 to 0.47) (Fig. 3). The predictive agreement for fluid responsiveness between PPVINV−CNAP and PPVINV was 92% with a Cohen’s kappa of 0.79 (Table 3).
Fig. 3.
Bland–Altman and scatter plot comparing PPVINV−CNAP and PPVINV. a Bland–Altman plot illustrating the mean of the differences (bold horizontal line) and 95% limits of agreement (lower and upper dashed horizontal lines) between PPVINV−CNAP and PPVINV. b Scatter plot with Deming regression (bold line) illustrating the relation of PPVINV−CNAP and PPVINV. PPVINV−CNAP arterial catheter-derived automatically calculated pulse pressure variation using the PPVCNAP-algorithm, PPVINV arterial catheter-derived manually calculated pulse pressure variation
Table 2.
Absolute and predictive agreement between PPVINV−CNAP vs. PPVINV and PPVCNAP vs. PPVINV
| Mean of the differences (%) | SD of the mean of the differences (%) | Lower 95%-LoA (95%-CI) (%) | Upper 95%-LoA (95%-CI) (%) | Deming regression | Predictive agreement (%) | Cohen's kappa | |
|---|---|---|---|---|---|---|---|
| PPVINV−CNAP vs. PPVINV | −0.1 | 1.2 |
−2.5 (−2.7 to −2.3) |
2.3 (2.2 to 2.5) |
−0.02 + 0.99x | 92 | 0.79 |
| PPVCNAP vs. PPVINV | −1.0 | 2.7 |
−6.3 (−6.7 to -5.9) |
4.3 (3.9 to 4.7) |
0.10 + 0.85x | 82 | 0.48 |
PPVINV arterial catheter-derived manually calculated pulse pressure variation, PPVINV−CNAP arterial catheter-derived automatically calculated pulse pressure variation using the PPVCNAP-algorithm, PPVCNAP CNAP finger-cuff-derived automatically calculated pulse pressure variation using the PPVCNAP-algorithm, SD standard deviation, LoA limits of agreement, CI confidence interval
Table 3.
Predictive agreement of pulse pressure variation measurements across the three predefined categories
PPVINV arterial catheter-derived manually calculated pulse pressure variation, PPVINV−CNAP arterial catheter-derived automatically calculated pulse pressure variation using the PPVCNAP-algorithm
Agreement between PPVCNAP and PPVINV
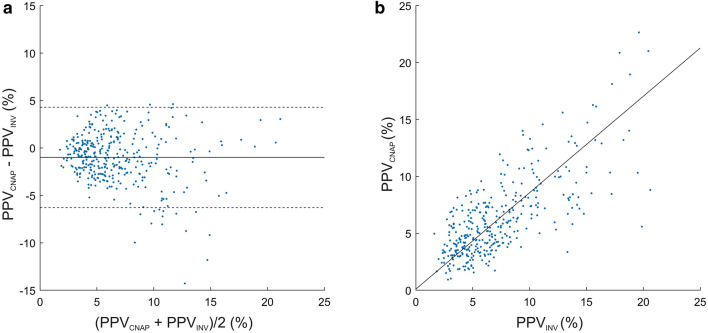
The mean of the differences ± SD between PPVCNAP and PPVINV was −1.0 ± 2.7% (95%-LoA −6.3 to 4.3%) (Fig. 4, Table 2). The Deming regression for the correlation between PPVCNAP and PPVINV showed a slope of 0.85 (95%-CI 0.78 to 0.91) and an intercept of 0.10 (95%-CI −0.34 to 0.55) (Fig. 4). The predictive agreement for fluid responsiveness between PPVCNAP and PPVINV was 82% with a Cohen’s kappa of 0.48 (Table 4).
Fig. 4.
Bland–Altman and scatter plot comparing PPVCNAP and PPVINV. a Bland–Altman plot illustrating the mean of the differences (bold horizontal line) and 95% limits of agreement (lower and upper dashed horizontal lines) between PPVCNAP and PPVINV. b Scatter plot with Deming regression (bold line) illustrating the relation of PPVCNAP and PPVINV. PPVCNAP CNAP finger-cuf-derived automatically calculated pulse pressure variation using the PPVCNAP-algorithm, PPVINV arterial catheter-derived manually calculated pulse pressure variation
Table 4.
Predictive agreement of pulse pressure variation measurements across the three predefined categories
PPVINV arterial catheter-derived manually calculated pulse pressure variation, PPVCNAP CNAP finger-cuff-derived automatically calculated pulse pressure variation using the PPVCNAP-algorithm
Discussion
In this prospective method comparison study, we aimed to validate the PPVCNAP-algorithm and investigate the absolute and predictive agreement between PPVCNAP and PPVINV in patients having neurosurgery.
To validate the PPVCNAP-algorithm per se (independent from waveform recording), we applied the PPVCNAP-algorithm to the arterial blood pressure waveform recorded invasively using an arterial catheter. The absolute agreement—i.e., the trueness and precision of agreement [15, 16]—between PPVINV−CNAP and the manually calculated PPVINV was high. The Deming regression analysis showed a significant correlation between PPVINV−CNAP and PPVINV and the predictive agreement was substantial according to Cohen’s kappa. Our results suggest that the PPVCNAP-algorithm reliably calculates PPV and that its measurements are interchangeable with the reference method—the manual offline calculation of PPV—when applied on the same arterial blood pressure waveform.
As a next step, we compared PPVCNAP to the reference PPVINV. The absolute agreement between PPVCNAP and PPVINV was lower than between PPVINV−CNAP and PPVINV and the Deming regression indicated a minor proportional difference between the methods. Nonetheless, the predictive agreement between PPVCNAP and PPVINV was moderate according to Cohen’s kappa.
In this study, we used arterial catheter-derived manually calculated PPV (PPVINV) as the reference method. There are no consensus guidelines on how to perform PPV validation studies and interpret their results. Specifically, it remains undefined what constitutes clinically acceptable PPV measurement performance. The absolute agreement between PPVCNAP and PPVINV was similar compared with previous studies evaluating the measurement performance of PPVCNAP in critically ill patients [22, 23] and patients having major open abdominal [24] or vascular surgery [25]. A pilot study in only 10 critically ill patients revealed a mean of the differences between PPVCNAP and arterial catheter-derived manually calculated PPV of −2.1% with 95%-LoA of −8.3 to 4.1% [22]. However, the study also included patients who were ventilated with tidal volumes less than 8 mL kg-1, which were excluded in our study. In a cohort of 47 critically ill patients with acute circulatory failure, the mean of the differences between PPVCNAP and PPV calculated manually from a femoral arterial blood pressure waveform was −0.6% with 95%-LoA of −6.3 to 5.2% [23]. The authors excluded 17% of patients because the CNAP system was unable to properly record the non-invasive arterial blood pressure waveform [23]. In contrast, we were able to record an arterial blood pressure waveform with the CNAP system in all patients. Our results are in line with a previous study in 35 patients having vascular surgery which showed similar moderate absolute agreement between PPVCNAP and arterial catheter-derived manually calculated PPV before and after volume expansion [25]. Even though our results are in line with previous findings, it is challenging to interpret the absolute agreement of PPVCNAP with PPVINV as no clearly defined thresholds for clinically acceptable PPV differences exist.
When investigating non-invasively measured dynamic cardiac preload variables, their predictive capabilities regarding the prediction of fluid responsiveness may even be more important than absolute agreement with invasive reference measurements. In the before-mentioned study in vascular surgery patients, volume expansion was performed to investigate the ability of PPVCNAP to predict fluid responsiveness. PPVCNAP predicted fluid responsiveness very well according to receiver operating characteristics curve analysis [25]. This was also shown in other studies directly testing the capabilities of PPVCNAP to predict fluid responsiveness, i.e., an increase in cardiac output after a fluid challenge. PPVCNAP and PPV calculated from an invasive arterial blood pressure waveform seem to have similar predictive value [23, 24]. We did not perform a fluid challenge or passive leg-raising test to directly test how well PPVCNAP predicts fluid responsiveness. Instead, we categorized PPV measurements considering clinical decision making based on predefined PPV thresholds for the prediction of fluid responsiveness [19]. PPVCNAP measurements falling in the same category as the respective PPVINV values would subsequently lead to the same decision regarding fluid therapy. The predictive agreement between PPVCNAP and PPVINV across the three categories was over 90% and Cohen’s kappa indicated a substantial predictive agreement. In line with the results of Bland–Altman analysis, the predictive agreement between PPVCNAP and PPVINV was slightly lower, but still over 80% and Cohen’s kappa indicated moderate agreement.
We did not perform preload-changing interventions such as a fluid challenge or passive leg-raising test to assess fluid responsiveness. Nevertheless, we analyzed the agreement between the test and the reference method stratified by different PPV categories according to clinically established thresholds [19]. Data pairs were selected randomly, but data selection bias cannot be ruled out definitely. We did not perform an a priori sample size calculation. Narrow 95%-CI around the 95%-LoA of the means of the differences between PPVINV−CNAP and PPVINV as well as PPVCNAP and PPVINV suggest that the sample size was sufficient though. Additionally, the change of the calibration interval for the CNAP system during the study may have affected the results. We only included patients having neurosurgery and the results can thus not be generalized to other—especially critically ill—patients.
In conclusion, the PPVCNAP-algorithm reliably calculates PPV compared to manual offline PPV calculation when applied on the same arterial blood pressure waveform. The absolute and predictive agreement between PPVCNAP and PPVINV are moderate.
Funding
The study was supported by an institutional restricted research grant from CNSystems Medizintechnik (Graz, Austria). Open Access funding enabled and organized by Projekt DEAL.
Compliance with ethical standards
Conflict of interest
MF has received honoraria for consulting from CNSystems Medizintechnik (Graz, Austria). PH, JMM, LB, KK, and GG have no conflict of interest to declare. JF is co-founder and CEO of CNSystems Medizintechnik (Graz, Austria). BS has received institutional restricted research grants, honoraria for giving lectures, and refunds of travel expenses from CNSystems Medizintechnik (Graz, Austria). BS has received honoraria for consulting, honoraria for giving lectures, and refunds of travel expenses from Edwards Lifesciences (Irvine, CA, USA). BS has received honoraria for consulting, institutional restricted research grants, honoraria for giving lectures, and refunds of travel expenses from Pulsion Medical Systems (Feldkirchen, Germany). BS has received institutional restricted research grants from Retia Medical (Valhalla, NY, USA). BS has received honoraria for giving lectures from Philips Medizin Systeme Böblingen (Böblingen, Germany). BS has received honoraria for consulting, institutional restricted research grants, and refunds of travel expenses from Tensys Medical (San Diego, CA, USA).
Ethical approval
The study was approved by the ethics committee (Ethikkomission der Ärztekammer Hamburg, Hamburg, Germany; registration number PV6048).
Informed consent
All patients provided written informed consent.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Monnet X, Marik PE, Teboul JL. Prediction of fluid responsiveness: an update. Ann Intensive Care. 2016;6(1):111. doi: 10.1186/s13613-016-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teboul JL, Monnet X, Chemla D, Michard F. Arterial pulse pressure variation with mechanical ventilation. Am J Respir Crit Care Med. 2019;199(1):22–31. doi: 10.1164/rccm.201801-0088CI. [DOI] [PubMed] [Google Scholar]
- 3.Michard F, Chemla D, Richard C, Wysocki M, Pinsky MR, Lecarpentier Y, Teboul JL. Clinical use of respiratory changes in arterial pulse pressure to monitor the hemodynamic effects of PEEP. Am J Respir Crit Care Med. 1999;159(3):935–9. doi: 10.1164/ajrccm.159.3.9805077. [DOI] [PubMed] [Google Scholar]
- 4.Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, Richard C, Pinsky MR, Teboul JL. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162(1):134–8. doi: 10.1164/ajrccm.162.1.9903035. [DOI] [PubMed] [Google Scholar]
- 5.Saugel B, Dueck R, Wagner JY. Measurement of blood pressure. Best Pract Res Clin Anaesthesiol. 2014;28(4):309–22. doi: 10.1016/j.bpa.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Meidert AS, Saugel B. Techniques for non-invasive monitoring of arterial blood pressure. Front Med. 2018;4:231. doi: 10.3389/fmed.2017.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michard F, Sessler DI, Saugel B. Non-invasive arterial pressure monitoring revisited. Intensive Care Med. 2018;44(12):2213–5. doi: 10.1007/s00134-018-5108-x. [DOI] [PubMed] [Google Scholar]
- 8.Teboul JL, Saugel B, Cecconi M, De Backer D, Hofer CK, Monnet X, Perel A, Pinsky MR, Reuter DA, Rhodes A, Squara P, Vincent JL, Scheeren TW. Less invasive hemodynamic monitoring in critically ill patients. Intensive Care Med. 2016;42(9):1350–9. doi: 10.1007/s00134-016-4375-7. [DOI] [PubMed] [Google Scholar]
- 9.Flick M, Schumann R, Hoppe P, Bonney I, Wesselink W, Saugel B. Non-invasive measurement of pulse pressure variation using a finger-cuff method in obese patients having laparoscopic bariatric surgery. J Clin Monit Comput. 2020 doi: 10.1007/s10877-020-00614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Wilde RB, de Wit F, Geerts BF, van Vliet AL, Aarts LP, Vuyk J, Jansen JR. Non-invasive continuous arterial pressure and pulse pressure variation measured with Nexfin® in patients following major upper abdominal surgery: a comparative study. Anaesthesia. 2016;71(7):788–97. doi: 10.1111/anae.13503. [DOI] [PubMed] [Google Scholar]
- 11.Saugel B, Hoppe P, Nicklas JY, Kouz K, Korner A, Hempel JC, Vos JJ, Schon G, Scheeren TWL. Continuous noninvasive pulse wave analysis using finger cuff technologies for arterial blood pressure and cardiac output monitoring in perioperative and intensive care medicine: a systematic review and meta-analysis. Br J Anaesth. 2020;125(1):25–37. doi: 10.1016/j.bja.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Zong W, Heldt T, Moody GB, Mark RG. An open-source algorithm to detect onset of arterial blood pressure pulses. Computers in Cardiology, 2003. 30:259–262. 10.1109/CIC.2003.1291140.
- 13.Altman DG, Bland JM. Statistics notes: variables and parameters. BMJ. 1999;318(7199):1667. doi: 10.1136/bmj.318.7199.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17(4):571–82. doi: 10.1080/10543400701329422. [DOI] [PubMed] [Google Scholar]
- 15.Squara P, Scheeren TWL, Aya HD, Bakker J, Cecconi M, Einav S, Malbrain M, Monnet X, Reuter DA, van der Horst ICC, Saugel B. Metrology part 1: definition of quality criteria. J Clin Monit Comput. 2020. [Epub ahead of print]. 10.1007/s10877-020-00494-y. [DOI] [PMC free article] [PubMed]
- 16.Squara P, Scheeren TWL, Aya HD, Bakker J, Cecconi M, Einav S, Malbrain M, Monnet X, Reuter DA, van der Horst ICC, Saugel B. Metrology part 2: procedures for the validation of major measurement quality criteria and measuring instrument properties. J Clin Monit Comput. 2020. [Epub ahead of print] 10.1007/s10877-020-00495-x. [DOI] [PMC free article] [PubMed]
- 17.Linnet K. Evaluation of regression procedures for methods comparison studies. Clin Chem. 1993;39(3):424–32. doi: 10.1093/CLINCHEM/39.3.424. [DOI] [PubMed] [Google Scholar]
- 18.Martin RF. General deming regression for estimating systematic bias and its confidence interval in method-comparison studies. Clin Chem. 2000;46(1):100–104. doi: 10.1093/clinchem/46.1.100. [DOI] [PubMed] [Google Scholar]
- 19.Cannesson M, Le Manach Y, Hofer CK, Goarin JP, Lehot JJ, Vallet B, Tavernier B. Assessing the diagnostic accuracy of pulse pressure variations for the prediction of fluid responsiveness: a "gray zone" approach. Anesthesiology. 2011;115(2):231–41. doi: 10.1097/ALN.0b013e318225b80a. [DOI] [PubMed] [Google Scholar]
- 20.Joosten A, Jacobs A, Desebbe O, Vincent JL, Sarah S, Rinehart J, Van Obbergh L, Hapfelmeier A, Saugel B. Monitoring of pulse pressure variation using a new smartphone application (Capstesia) versus stroke volume variation using an uncalibrated pulse wave analysis monitor: a clinical decision making study during major abdominal surgery. J Clin Monit Comput. 2019;33(5):787–93. doi: 10.1007/s10877-018-00241-4. [DOI] [PubMed] [Google Scholar]
- 21.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 22.Schmid M, Prettenthaler H, Weger C, Smolle KH. Evaluation of a novel automated non-invasive pulse pressure variation algorithm. Comput Biol Med. 2013;43(10):1583–9. doi: 10.1016/j.compbiomed.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 23.Monnet X, Dres M, Ferre A, Le Teuff G, Jozwiak M, Bleibtreu A, Le Deley MC, Chemla D, Richard C, Teboul JL. Prediction of fluid responsiveness by a continuous non-invasive assessment of arterial pressure in critically ill patients: comparison with four other dynamic indices. Br J Anaesth. 2012;109(3):330–8. doi: 10.1093/bja/aes182. [DOI] [PubMed] [Google Scholar]
- 24.Renner J, Gruenewald M, Hill M, Mangelsdorff L, Aselmann H, Ilies C, Steinfath M, Broch O. Non-invasive assessment of fluid responsiveness using CNAP technology is interchangeable with invasive arterial measurements during major open abdominal surgery. Br J Anaesth. 2017;118(1):58–67. doi: 10.1093/bja/aew399. [DOI] [PubMed] [Google Scholar]
- 25.Biais M, Stecken L, Martin A, Roullet S, Quinart A, Sztark F. Automated, continuous and non-invasive assessment of pulse pressure variations using CNAP((R)) system. J Clin Monit Comput. 2017;31(4):685–92. doi: 10.1007/s10877-016-9899-4. [DOI] [PubMed] [Google Scholar]