Abstract
Aims:
The primary objective of this systematic review was to estimate the prevalence and temporal changes in chronic comorbid conditions reported in heart failure (HF) clinical trials.
Methods and results:
We searched MEDLINE for HF trials enrolling more than 400 patients published between 2001 and 2016. Trials were divided into HF with reduced ejection fraction (HFrEF), HF with preserved ejection fraction (HFpEF), or trials enrolling regardless of ejection fraction. The prevalence of baseline chronic comorbid conditions was categorized according to the algorithm proposed by the Chronic Conditions Data Warehouse, which is used to analyse Medicare data. To test for a trend in the prevalence of comorbid conditions, linear regression models were used to evaluate temporal trends in prevalence of comorbidities. Overall, 118 clinical trials enrolling a cumulative total of 215 508 patients were included. Across all comorbidities examined, data were reported in a mean of 35% of trials, without significant improvement during the study period. Reporting of comorbidities was more common in HFrEF trials (51%) compared with HFpEF trials (27%). Among trials reporting data, hypertension (63%), ischaemic heart disease (44%), hyperlipidaemia (48%), diabetes (33%), chronic kidney disease (25%) and atrial fibrillation (25%) were the major comorbidities. The prevalence of comorbidities including hypertension, atrial fibrillation and chronic kidney disease increased over time while the prevalence of smoking decreased in HFrEF trials.
Conclusion:
Many HF trials do not report baseline comorbidities. A more rigorous, systematic, and standardized framework needs to be adopted for future clinical trials to ensure adequate comorbidity reporting and improve recruitment of multi-morbid HF patients.
Keywords: Clinical trials, Comorbidities, Heart failure, Trends
Introduction
Patients with heart failure (HF) are remarkably complex with a large burden of cardiac and non-cardiac comorbidities. More than half of patients with HF have coexisting comorbid conditions such as obesity, chronic kidney disease, diabetes mellitus, hypertension, and atrial fibrillation.1,2 Among Medicare beneficiaries with HF, about 50% have >5 non-cardiac comorbidities, a percentage that has increased dramatically over the last two decades.3 These comorbidities are associated with higher overall symptom burden and worse clinical outcomes.4–6 Moreover, by virtue of comorbidity-based exclusion criteria in many HF clinical trials, there are concerns that data from such trials may not fully generalize to multi-morbidity patients seen in routine clinical practice.3,7,8 Indeed, the presence and number of comorbidities could conceivably alter the biologic response to a trial therapy and/or the balance of risks and benefits, as well as the ability to adhere to and tolerate a given therapy or care strategy. As such, both US and European HF guidelines have reinforced to recognize multiple comorbidities in HF patients and tailor patient care accordingly.1,9–11 Nonetheless, despite the implications for generalizing clinical trial data to real-world practice, little is known regarding the patterns and reporting of comorbidities in HF trials. Knowing these trends can have important implications for future clinical trial design and setup of standardized framework to ensure adequate comorbidity reporting. Modification in comorbidity reporting can also be important in improving clinical outcomes in select subgroups. Thus, the primary objective of this systematic review was to estimate the prevalence and temporal changes in chronic comorbid conditions reported in HF clinical trials.
Methods
Identification of clinical trials
We performed a systematic search utilizing two strategies to identify major HF clinical trials published between 2001 and 2016: (i) PubMed/MEDLINE query with the following limits: publication year, ‘heart failure’, ‘trial*’, and ‘randomized’, and (ii)www.ClinicalTrials.gov query with the following limits: adult (18 years and older), interventional, phase II–IV, ‘heart failure’. Potential eligible publications were reviewed individually for inclusion based on titles and abstracts. The exclusion criteria included: (i) phase I or pilot trials; (ii) trials enrolling paediatric populations; (iii) trials where hospitals were the units of randomization; (iv) publications reporting interim, secondary or post-hoc analyses; and (v) trials enrolling less than 400 patients as smaller trials are more likely to be single-centre, early-phase studies and unlikely to inform clinical practice. These selected larger trials represented approximately the top fifth of trials identified by the systemic query and enrolled about 80% of the overall population included in all HF trials. Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines were followed for all procedures and reporting. Two independent reviewers screened selected studies for inclusion; a third reviewer resolved discordant assessments.
Data abstraction
The following data were abstracted: (i) journal; (ii) year of publication; (iii) HF type (based on ejection fraction and hospitalization status); (iv) trial intervention; (v) enrolment duration (estimated from starting and ending dates); (vi) total sample size; (vii) mean or median age; (viii) proportion of women; (ix) race and ethnicity (if reported); (x) body mass index (BMI); (xi) number of participating centres; (xii) number of countries; and (xiii) funding sources. For incomplete data, additional data were obtained from secondary publications identified by ClinicalTrials.gov, if available.
Trials were divided into chronic HF with reduced ejection fraction (HFrEF), chronic HF with preserved ejection fraction (HFpEF), chronic HF regardless of ejection fraction (in cases where ejection fraction eligibility criteria were not specified), and trials enrolling acute HF. Trials were divided into three categories based on primary intervention: (i) medications; (ii) invasive therapies (intracoronary gene therapy, ultrafiltration, implantable haemodynamic monitors, pacemakers, left ventricular assist systems, cardiac resynchronization therapy, implantable cardioverter-defibrillators, or intra-aortic balloon pumps, surgical procedures such as coronary artery bypass graft surgery or ventricular reconstruction); or (iii) others (exercise training, continuous positive airway pressure, patient education, multidisciplinary management programme, and behavioural or lifestyle interventions, testing/imaging). Based on the ClinicalTrials.gov description, funding source was categorized as (i) industry, (ii) government, or (iii) university or other non-profit or non-federal organizations. Government funding was further classified into those funded by the National Institute of Health(NIH)/National Heart, Lung, and Blood Institute (NHLBI) and non-US agencies. Regions were divided into (i) exclusively North America including United States, Canada, and Mexico; (ii) exclusively Western Europe including Austria, Belgium, Bermuda, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, and United Kingdom; (iii) exclusively outside of North America and Western Europe – rest of the world; and (iv) mixed/multiregional. Enrolment rates, expressed as patients per site per month, were estimated based on the reported study duration (completion dates minus start dates).
Comorbid condition data
The prevalence of relevant baseline chronic comorbid conditions was categorized according to the algorithm proposed by the Chronic Conditions Data Warehouse, which is used to analyse Medicare data.12 Data for the following variables were extracted for each trial: (i) current and former smoking history; (ii) alcohol intake; (iii) coronary artery disease; (iv) depression; (v) dementia; (vi) anaemia; (vii) diabetes mellitus; (viii) hypertension; (ix) hyperlipidaemia; (x) chronic kidney disease or estimated glomerular filtration rate; (xi) atrial fibrillation; (xii) chronic liver disease; (xiii) stroke; (xiv) myocardial infarction (xv) peripheral arterial disease; (xvi) cancer; (xvii) chronic obstructive pulmonary disease or asthma; (xviii) obstructive sleep apnoea. Data regarding exclusion criteria for 10 comorbid conditions were extracted for each trial: (i) dementia; (ii) anaemia; (iii) diabetes mellitus; (iv) severe or uncontrolled hypertension; (v) chronic kidney disease; (vi) atrial fibrillation; (vii) chronic liver disease; (viii) stroke; (ix) cancer; (x) chronic obstructive pulmonary disease.
Statistical analysis
Trials were assigned to four 4-year blocks based on publication date (2001–2004, 2005–2008, 2009–2012, and 2013–2016). Continuous variables were described as mean and standard deviation, or as median and interquartile interval (IQI), and categorical variables as number (%). Mean age was calculated as weighted mean, given varying sample sizes across trials. Similarly, proportion of women, various racial/ethnic groups, and BMI were calculated and indexed to sample size of each trial. Spearman rank correlation coefficients were used to define the relationships between trial-level variables and comorbid conditions. Categorical variables were compared using Chi-square testing. We performed analyses of temporal trends in comorbidities by HF setting studied, primary funding mechanism, and primary enrolment location.
To test for a trend in the prevalence of comorbid conditions across our study period, we performed simple linear regression models using year of publication as the independent variable. Moreover, to test for heterogeneity of variance for reporting comorbidities by HF type, we performed Levene’s test. The analyses were adjusted for mean age and sample size of the trial. Analyses were performed with IBM SPSS 25 (IBM Corporation).
Results
General characteristics
In total, 5488 studies were screened and 118 clinical trials that cumulatively enrolled 215 508 patients were included. Online supplementary Figure S1 shows the detailed literature search process while online supplementary Table S1 lists all the included trials. Online supplementary Table S2 shows the detailed characteristics of the trials while Table 1 lists the prevalence of comorbidities of patients enrolled in all HF trials. Overall, across all comorbidities examined, data were reported in a mean of 35% of trials, without significant improvement during the study period. All trials reported at least one comorbidity. However, <10% of the trials reported all of the main comorbidities. Online supplementary Table S3 shows comorbidity reporting per trial.
Table 1.
Trends in comorbidities of heart failure clinical trials between 2001–2016
| 2001–2004 |
2005–2008 |
2009–2012 |
2013–2016 |
2001–2016 |
P-value* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients with reported data | Average (range) of patients with comorbidity (%)a | Patients with reported data | Average (range) of patients with comorbidity (%) | Patients with reported data | Average (range) of patients with comorbidity (%) | Patients with reported data | Average (range) of patients with comorbidity (%) | Patients with reported data | Average (range) of patients with comorbidity (%) | ||
| Cardiac comorbidities | |||||||||||
| Current smoking | 35% | 33.3 (17.5–66.5) | 51% | 13.7 (5.1–30.1) | 49% | 21.8 (8.6–54) | 19% | 10 (6–17.6) | 39% | 20.4 (5.1 –66.5) | 0.13 |
| Former smoking | 35% | 40.8 (28.3–72.1) | 51% | 16.6 (5.1 –43.3) | 49% | 27.4 (8.6–59.4) | 19% | 20.5 (6–36.8) | 39% | 26 (5.1 –72.1) | 0.13 |
| Alcohol intake | 31% | 2.5 (1.7–6.6) | 1% | 9.6 (9.6–9.6) | 22% | 18.8 (2.2–36) | 11% | 18.2 (10.3–22) | 16% | 10.3 (1.7–36) | 0.83 |
| CAD or ischaemic aetiology | 95% | 44.4 (23–67) | 87% | 61.7 (25–104) | 98% | 58.7 (20–87) | 85% | 58.3 (27–75) | 92% | 55.5 (20–104) | 0.10 |
| Diabetes | 93% | 28.3 (14.5–47.2) | 91% | 32.5 (20–100) | 91% | 35.9 (22.6–107.2) | 87% | 35.1 (16–63.6) | 91% | 32.7(14.5–107.2) | 0.97 |
| Hypertension | 70% | 54.3 (31.6–69.9) | 75% | 65.9 (15.3–88.4) | 95% | 64.2 (20.9–87.8) | 83% | 67.6 (17.4–91.7) | 80% | 63.1 (15.3–91.7) | 0.03 |
| Hyperlipidaemia | 32% | 32.1 (29.9–43.2) | 21% | 56.5 (45.8–83.9) | 12% | 67 (37.8–77.8) | 16% | 56.8 (37.8–65.8) | 21% | 47.9 (29.9–83.9) | 0.79 |
| Chronic kidney disease | 29% | 3.8 (1.8–24.8) | 29% | 36.5 (13.5–56.9) | 19% | 27.5 (8.5–53.2) | 27% | 33.3 (13.3–72) | 26% | 24.6 (1.8–72) | 0.61 |
| GFR (mL/min/1.73 m2) | 50% | – | 57% | 64 (51.4–71) | 54% | 61.8 (50.7–70.8) | 62% | 67 (47–79) | 55% | 64.9 (47–79) | 0.02 |
| BMI (kg/m2) | 61% | 27.2 (26–28) | 75% | 28 (26–29.7) | 80% | 28.2 (25.4–33.1) | 76% | 28 (24.4–31) | 72% | 27.9 (24.4–33.1) | 0.01 |
| Atrial fibrillation | 66% | 14.5 (6.3–34.6) | 58% | 31.2 (15.5–100) | 77% | 22.7 (3.7–54.7) | 84% | 31.7 (5.2–54.5) | 71% | 24.9 (3.7–100) | 0.15 |
| Non-cardiac comorbidities | |||||||||||
| Cancer | 26% | 2.2 (2.2–2.2) | 12% | 3.6 (3.6–3.6) | 3% | 11.4 (10.9–11.9) | 5% | 7.6 (7.6–7.6) | 12% | 3.6 (2.2–11.9) | 0.93 |
| Stroke | 33% | 6.9 (6.1 –20.1) | 67% | 11.1 (0–18) | 60% | 10.4 (2.2–24.1) | 62% | 8.2 (4.3–13.5) | 55% | 9.5 (0–24.1) | 0.19 |
| Chronic liver disease | 0% | – | 0% | – | 3% | 2.1 (2.1–2.1) | 3% | 6.8 (6.8–6.8) | 1% | 4.2 (2.1 –6.8) | 0.65 |
| Peripheral arterial disease | 26% | 8.4 (8.4–8.4) | 21% | 12.9 (8.7–21) | 27% | 11.4 (3 –17.5) | 20% | 11 (7.8–16.7) | 23% | 10.8 (3 –21) | 0.22 |
| Anaemia | 0% | – | 16% | 19.5 (1.8–30.9) | 9% | 23.4 (6.2–31.5) | 5% | 29.6 (21.3–35.3) | 8% | 22 (1.8–35.3) | 0.41 |
| COPD | 29% | 10.3 (8.6–31) | 33% | 17.1 (10–31) | 42% | 15.9 (7.4–27.2) | 27% | 17 (3.3–34.7) | 33% | 15.1 (3.3–34.7) | 0.33 |
| Asthma | 1% | 6.4 (6.4–6.4) | 0% | – | 0% | – | 0% | – | 0% | 6.4 (6.4–6.4) | 0.19 |
| OSA | 0% | – | 0% | – | 0% | – | 1% | 8.8 (8.8–8.8) | 0% | 8.8 (8.8–8.8) | 0.43 |
| Depression | 1% | 8 (8–8) | 3% | 11.6 (9.1 –14) | 21% | 10.2 (6–29.4) | 14% | 20.4 (6.3–27) | 9% | 13.6 (6–29.4) | 0.52 |
| Dementia | 0% | – | 1% | 1.7 (1.7–1.7) | 0% | – | 0% | – | 0% | 1.7 (1.7–1.7) | 0.41 |
BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; OSA, obstructive sleep apnoea.
P-values compare trial-level reporting of comorbidities.
Prevalence of comorbidities was determined among trial reporting data.
Body mass index was reported in 72% of trials with improvement from 61% in 2001–2004 to 76% in 2013–2016. In trials with reported data, hypertension (63%), ischaemic heart disease (44%), hyperlipidaemia (48%) and diabetes mellitus (33%) were the major comorbidities. The estimated prevalence of atrial fibrillation and chronic kidney disease was 25% each. BMI increased slightly from 27.2 kg/m2 to 28.0 kg/m2 over the study period. Overall temporal trends in comorbidities are summarized in Figure 1.
Figure 1.

Trends of key comorbidities across all clinical trials of heart failure. The prevalence of smoking decreased over time while the prevalence of cardio-metabolic comorbidities increased. CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease.
Temporal trends in comorbidities in trials of heart failure by inclusion criteria
Reporting of comorbidities was more common in HFrEF trials (51% on average) and HF trials regardless of ejection fraction (48%), as compared with acute HF (27%) or HFpEF trials (27%). BMI was reported in all HFrEF trials vs. 63% of HFpEF trials and 71% of acute HF trials. The estimated prevalence of hypertension and atrial fibrillation was highest in HFrEF trials (89% and 30%, respectively) whereas hyperlipidaemia and chronic obstructive pulmonary disease were more common in HFpEF trials (72% and 23%, respectively). Prevalence of ischaemic heart disease or coronary artery disease was highest in acute HF trials (60%) and lowest in HFrEF trials (39%). Comparison of comorbidities among trials with different HF settings is shown in Table 2 and Figure 2. Among the 94 trials investigating treatments for HFrEF, the prevalence of comorbidities including hypertension, atrial fibrillation, chronic kidney disease increased over time while smoking decreased (online supplementary Table S4 and Figure S2). Similarly, in acute HF trials, comorbid conditions including diabetes, hypertension, ischaemic heart disease, chronic kidney disease, hyperlipidaemia and chronic obstructive pulmonary disease increased over time (online supplementary Table S5 and Figure S3). Due to the low number of HFpEF trials, it was not possible to analyse trends for HFpEF patients. BMI was highest in the HFrEF population (30.0 kg/m2) compared to 27.9 kg/m2 in acute HF and 26.9 kg/m2 in HFpEF. Online supplementary Table S6 shows the results of Levene’s test to check for heterogeneity in comorbidity reporting by HF type.
Table 2.
Comorbidities of heart failure clinical trials between 2001–2016 by heart failure setting
| Acute HF |
HFrEF |
HFpEF |
HF regardless of EF |
P-value* | |||||
|---|---|---|---|---|---|---|---|---|---|
| Patients with reported data | Average (range) of patients with comorbidity (%)a | Patients with reported data | Average (range) of patients with comorbidity (%) | Patients with reported data | Average (range) of patients with comorbidity (%) | Patients with reported data | Average (range) of patients with comorbidity (%) | ||
| Cardiac comorbidities | |||||||||
| Current smoking | 36% | 18.7 (5.1–66.5) | 39% | 10.4 (10.4–1 0.4) | 40% | 13 (6–29.6) | 52% | 32.2 (13.2–64.8) | 0.98 |
| Former smoking | 36% | 26 (5.1–72.1) | 39% | 36.8 (36.8–36.8) | 40% | 13 (6–29.6) | 52% | 32.3 (13.2–64.8) | 0.98 |
| Alcohol intake | 7% | 16.6 (2.2–36) | 39% | 22 (22–22) | 0% | 0 (0–0) | 63% | 5.8 (1.7–14.1) | 0.37 |
| CAD or ischaemic aetiology | 93% | 60.2 (20–104) | 100% | 39.1 (25–59) | 73% | 50.6 (42–70) | 100% | 44.4 (22–68) | 0.03 |
| Diabetes | 91% | 33.1 (14.5–107.2) | 100% | 28.2 (16.6–32.5) | 83% | 31.3 (20–50.1) | 95% | 33.5 (22.8–47.5) | 0.52 |
| Hypertension | 81% | 60.9 (15.3–87.8) | 100% | 88.8 (78.8–91.7) | 52% | 61.5 (42.9–86.6) | 94% | 64.9 (52.4–86.6) | 0.16 |
| Hyperlipidaemia | 14% | 55.8 (37.8–83.9) | 44% | 60.7 (60.2–64.7) | 10% | 72.5 (64–77.8) | 52% | 32.8 (29.9–56.9) | 0.21 |
| Chronic kidney disease | 19% | 31.5 (8.5–72) | 86% | 34 (30.2–38.7) | 14% | 41.6 (15.2–53.2) | 53% | 6.7 (1.8–36) | 0.80 |
| GFR (mL/min/1.73 m2) | 54% | 65.1 (47–74) | 64% | 66.9 (65.4–79) | 60% | 69.1 (55–71) | 53% | 51.8 (50.7–53.5) | 0.25 |
| BMI (kg/m2) | 71% | 27.9 (24.6–33.1) | 100% | 30 (28–31) | 63% | 26.9 (24.4–30.1) | 81% | 27.7 (27.2–29.3) | 0.19 |
| Atrial fibrillation | 64% | 25.0(3.7–100) | 100% | 29.6 (5.2–35.2) | 68% | 21.8 (6.9–37.3) | 92% | 25.2 (6.5–54.7) | 0.13 |
| Non-Cardiac comorbidities | |||||||||
| Cancer | 3% | 9 (7.6–11.9) | 0% | 0 (0–0) | 25% | 3.6 (3.6–3.6) | 42% | 2.2 (2.2–2.2) | 0.89 |
| Stroke | 49% | 10.2 (0–24.1) | 86% | 8.8 (7.7–9.7) | 46% | 6.3 (4.3–20.1) | 81% | 9.6 (6.1–19.7) | 0.64 |
| Chronic liver disease | 0% | 0 (0–0) | 0% | 0 (0–0) | 11% | 4.2 (2.1–6.8) | 0% | 0 (0–0) | 0.01 |
| Peripheral arterial disease | 9% | 13.9 (3–21) | 39% | 9.3 (9.3–9.3) | 35% | 10.7 (8.7–17.5) | 70% | 9.5 (8.4–14.4) | 0.17 |
| Anaemia | 8% | 24 (1.8–31.5) | 47% | 12.5 (12.5–12.5) | 5% | 35.3 (35.3–35.3) | 0% | 0 (0–0) | 0.37 |
| COPD | 15% | 14.4 (7.4–31) | 90% | 10.4 (3.3–11.7) | 42% | 23.4 (17.3–34.7) | 83% | 13.4 (8.6–31) | 0.02 |
| Asthma | 0% | 0 (0–0) | 0% | 0 (0–0) | 0% | 0 (0–0) | 1% | 6.4 (6.4–6.4) | 0.09 |
| OSA | 0% | 8.8 (8.8–8.8) | 0% | 0 (0–0) | 0% | 0 (0–0) | 0% | 0 (0–0) | 0.94 |
| Depression | 4% | 14.1 (6–29.4) | 39% | 27 (27–27) | 7% | 10.7(9.1–11.7) | 22% | 7.9 (7.9–8) | 0.85 |
| Dementia | 0% | 1.7 (1.7–1.7) | 0% | 0 (0–0) | 0% | 0 (0–0) | 0% | 0 (0–0) | 0.94 |
BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; GFR, glomerular filtration rate; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; OSA, obstructive sleep apnoea.
P-values compare trial-level reporting of comorbidities.
Prevalence of comorbidities was determined among trial reporting data.
Figure 2.

Trends of key comorbidities across clinical trials by heart failure (HF) setting. Prevalence of hypertension and atrial fibrillation was highest in HF with reduced ejection fraction (HFrEF) trials whereas hyperlipidaemia and chronic obstructive pulmonary disease (COPD) were more common in HF with preserved ejection fraction (HFpEF) trials. AHF, acute heart failure; CAD, coronary artery disease; EF, ejection fraction.
Temporal trends of exclusion of comorbid conditions
Data regarding exclusion criteria for 10 comorbid conditions (dementia, anaemia, diabetes mellitus, severe or uncontrolled hypertension, chronic kidney disease, atrial fibrillation, chronic liver disease, stroke, cancer, chronic obstructive pulmonary disease) were extracted from the eligibility criteria for each of the trials. Overall, 72% of trials excluded patients with ≥1 of these comorbid conditions, with an increase over time from 67% in 2001–2004 to 88% in 2012–2016. Chronic kidney disease was the most common exclusion criterion (47% of trials) and increased from 38% in 2001–2004 to 63% in 2012–2016. Similarly, median glomerular filtration rate cutoff for exclusion used in trials published between 2001–2008 was 20 (IQI 15–30) mL/min/1.73 m2 compared to 30 (IQI 16.5–30) mL/min/1.73 m2 in trials 2009–2016 (P =0.17). The remaining comorbid conditions were excluded less often (rage 3–19%) in individual HF trials (online supplementary Table S7). Similar to chronic kidney disease, exclusion of patients with other comorbid conditions including anaemia, atrial fibrillation, chronic liver disease, cancer, or chronic obstructive pulmonary disease increased over time (online supplementary Table S6). Overall, across all trials, there was no association between trials with stricter exclusion criteria and reporting of individual comorbid conditions (P =0.65).
Temporal trends in comorbidities in trials of heart failure by region
Reporting of comorbidities was highest in trials conducted in Western Europe and multiregional trials (37% and 34% respectively for all comorbidities included in our study), followed by trials conducted in North America (26%) and the rest of the world (24%). BMI was reported in 79% of trials conducted in Western Europe compared with 77% in multiregional trials and 59% in North America. The estimated prevalence of smoking, diabetes mellitus, chronic kidney disease, and chronic obstructive pulmonary disease was more common in trials conducted exclusively in North America while atrial fibrillation was more common in multiregional trials (Table 3, Figure 3). BMI was highest in North America (28.8 kg/m2) compared to multiregional (28.0) and Western European (27.0 kg/m2), and was lowest in trials conducted exclusively in the rest of the world (24.5 kg/m2).
Table 3.
Comorbidities of heart failure clinical trials between 2001–2016 by region
| Rest of the world |
Multiregional |
North America |
Western Europe |
P-value* | |||||
|---|---|---|---|---|---|---|---|---|---|
| Patients with reported data | Patients with comorbidity (%)a | Patients with reported data | Patients with comorbidity (%) | Patients with reported data | Patients with comorbidity (%) | Patients with reported data | Patients with comorbidity (%) | ||
| Cardiac comorbidities | |||||||||
| Current smoking | 49% | 8.1 (6–17.6) | 42% | 21.3 (5.1–66.5) | 22% | 23.4 (9.4–64.8) | 52% | 18.1 (11.6–34) | 0.53 |
| Former smoking | 49% | 8.1 (6–17.6) | 42% | 25.4 (5.1–66.5) | 22% | 49 (9.4–72.1) | 52% | 18.2 (11.6–34) | 0.53 |
| Alcohol intake | 0% | 0 (0–0) | 1 8% | 9.4 (1.7–24.8) | 20% | 8.3 (6.6–10.3) | 2% | 2.2 (2.2–2.2) | 0.56 |
| CAD or ischaemic aetiology | 39% | 46.1 (27–70) | 97% | 56.3 (20–88) | 68% | 56.9 (23–104) | 94% | 48.6 (22–59) | 0.49 |
| Diabetes | 53% | 29.8 (16–50.1) | 96% | 32.5 (20.6–100) | 73% | 40.1 (30.4–49.9) | 91% | 29.3 (16.6–107.2) | 0.02 |
| Hypertension | 30% | 58.3 (56.3–59.1) | 91% | 64.3 (15.3–91.3) | 78% | 62.8 (17.4–87.8) | 52% | 51.1 (20.9–91.7) | 0.03 |
| Hyperlipidaemia | 9% | 37.8 (37.8–37.8) | 21% | 44.2 (29.9–72.2) | 40% | 57.9 (34–83.9) | 9% | 59.4 (37.8–74.4) | 0.74 |
| Chronic kidney disease | 9% | 14.3 (14.3–14.3) | 30% | 23.1 (1.8–72) | 24% | 33.6 (13.5–53.2) | 19% | 31 (16.5–44.2) | 0.80 |
| GFR (mL/min/1.73 m2) | 16% | 70.2 (70.2–70.2) | 19% | 63.1 (47–74) | 0% | 0 (0–0) | 52% | 69.2 (55–79) | 0.04 |
| BMI (kg/m2) | 0% | 0 (0–0) | 0% | 8.8 (8.8–8.8) | 0% | 0 (0–0) | 0% | 0 (0–0) | 0.81 |
| Atrial fibrillation | 30% | 1 (0.9–1.1) | 52% | 1.2 (1–1.6) | 34% | 1.3 (1.1–1.5) | 23% | 1.2 (1–1.4) | |
| Non-Cardiac comorbidities | |||||||||
| Cancer | 9% | 16.7 (16.7–16.7) | 23% | 10.9 (8.4–21) | 12% | 15.6 (13.2–17.5) | 50% | 8.5 (3–12.2) | 0.34 |
| Stroke | 0% | 0 (0–0) | 0% | 0 (0–0) | 12% | 4.2 (2.1–6.8) | 0% | 0 (0–0) | 0.12 |
| Chronic liver disease | 63% | 10.9 (6.9–27) | 79% | 26.3 (3.7–100) | 46% | 21.5 (11.2–52.8) | 78% | 21.8 (5.2–36.4) | 0.00 |
| Peripheral arterial disease | 49% | 5.9 (4.3–13.1) | 64% | 10 (0–18.3) | 21% | 9.5 (6.6–20.1) | 61% | 8 (2.2–24.1) | 0.04 |
| Anaemia | 0% | 0 (0–0) | 9% | 20.8 (1.8–30.9) | 5% | 35.3 (35.3–35.3) | 11% | 21.2 (6.2–31.5) | 0.41 |
| COPD | 0% | 0 (0–0) | 11% | 2.9 (2.2–7.6) | 0% | 0 (0–0) | 42% | 4.9 (3.6–11.9) | 0.06 |
| Asthma | 0% | 0 (0–0) | 31 % | 12.4 (7.4–23.8) | 32% | 23.8 (17.3–34.7) | 63% | 20.5 (3.3–31) | 0.14 |
| OSA | 0% | 0 (0–0) | 0% | 0 (0–0) | 0% | 0 (0–0) | 2% | 6.4 (6.4–6.4) | 0.18 |
| Depression | 9% | 23.7 (23.7–23.7) | 8% | 13 (6–27) | 14% | 17 (11.7–29.4) | 14% | 9.7 (6.3–15.7) | 0.32 |
| Dementia | 0% | 0 (0–0) | 0% | 0 (0–0) | 2% | 1.7 (1.7–1.7) | 0% | 0 (0–0) | 0.42 |
BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; OSA, obstructive sleep apnoea.
P-values compare trial-level reporting of comorbidities.
Prevalence of comorbidities was determined among trial reporting data.
Figure 3.
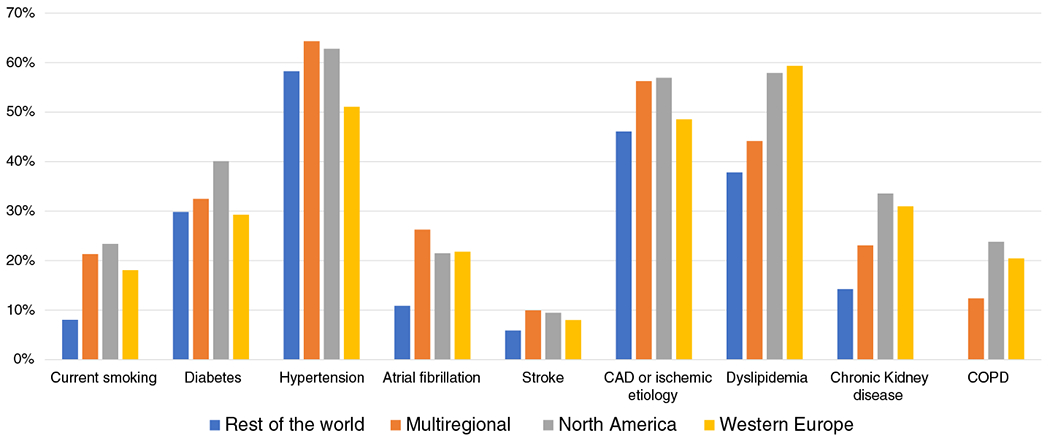
Trends of key comorbidities across clinical trials by region. The estimated prevalence of smoking, diabetes mellitus, chronic kidney disease, and chronic obstructive pulmonary disease (COPD) was more common in trials conducted exclusively in North America. CAD, coronary artery disease.
Association between comorbidities and enrolment rates
Higher enrolment rates correlated positively with the prevalence of stroke (R = 0.45, P =0.003), chronic obstructive pulmonary disease (R = 0.35, P =0.04) and negatively with prevalent ischaemic heart disease (R = −0.24, P =0.05) and previous smoking (R = −0.49, P =0.006). Higher prevalence of diabetes mellitus correlated with higher proportion of non-White race (R = 0.31, P =0.03), which was mainly driven by enrolment of Blacks (R = 0.50, P =0.002) (online supplementary Figure S4A). Higher BMI correlated with higher proportion of enrolled Black patients (R = 0.54, P =0.02) (online supplementary Figure S4B).
Discussion
In this systematic review of 118 HF clinical trials, the rate of comorbidity reporting was low and did not improve significantly over time. Reporting of comorbidities was more common in HFrEF trials as compared with acute HF or HFpEF trials. In trials reporting data, hypertension, ischaemic heart disease, hyperlipidaemia, diabetes, atrial fibrillation and chronic kidney disease were the most common comorbidities. Conditions with the largest increases over time included hypertension, atrial fibrillation, and chronic kidney disease while the prevalence of smoking decreased in HFrEF trials. These findings have important implications for future clinical design, complexity of clinical care in HF patients and patient outcomes in terms of identifying subgroups for whom the treatment will provide the greatest benefit–risk ratio.
Overall low rates of comorbidity reporting in HF clinical trials may be due to comorbidity-specific trial exclusion criteria of certain comorbidities in efforts to attenuate competing risks of non-cardiovascular death and/or to improve tolerability of the study therapy.13 For example, we observed that the reporting of cancer in trials as a comorbidity was extremely low. This is likely due to active or recent cancer being an exclusion criterion for most trials. Nonetheless, comorbidity reporting was not significantly different between trials with and without comorbidity-based exclusion criteria, suggesting comorbidity-specific eligibility criteria were not predominant factors in the rates of reporting.
Even among trials with sufficient reported details regarding comorbidities, rates of multi-morbidity enrolment were generally low. Previous studies have suggested that multi-morbidity is associated with lower rates of clinical trial discussions between patients and healthcare providers leading to decreased trial participation.14,15 Moreover, the presence of multiple disease states and associated polypharmacy may curb enthusiasm to add to therapeutic complexity. Multi-morbid patients may further have associated frailty or limited transportation options that may complicate participation in trials.
Most of the increased burden of comorbidity was in concordant cardiometabolic comorbidities such as hypertension, hyperlipidaemia, atrial fibrillation and chronic kidney disease, which are known to cluster together.6,16 This increase in comorbid burden over time may be partially explained by increased life expectancy, population-level aging and increased awareness.17 Although the proportion of patients with HF who are elderly is increasing over time, the mean age pertrial in our study remained stable suggesting that age is not the key driver of the increasing burden. Consistent with our trial-level experience, previous studies have also shown an increase in the number of HF patients with multiple comorbidities over time.18 Taken together, increasing comorbidity burden in HF may partially explain the recent resurgence in HF mortality in the US.19 These data also have implications for health policies in assessment of penalty burden; ongoing efforts will need to more completely capture and risk adjust HF metrics (such as readmission rates) in the context of this increasing comorbidity trajectory.20
Results from the Quality of Adherence to Guideline Recommendations for Life-Saving Treatment in Heart Failure (QUALIFY) survey showed that 43% patients had at least three comorbidities.21 Similarly, the European Heart Failure Survey reported at least 74% of patients to have one comorbidity with chronic kidney disease (41%), anaemia (29%), diabetes and chronic obstructive pulmonary disease (15%) being the most prevalent.22 Concordant findings have been seen in other studies.23–25 In the European Heart Failure Survey, presence of diabetes, chronic kidney disease and anaemia were independently related to increased mortality and HF hospitalization, with the highest population attributable risk for chronic kidney disease and anaemia. In a contemporary community European population with chronic HF, non-cardiac comorbidities were similarly high in both HFrEF and HFpEF, except obesity and hypertension which were more common in HFpEF.26 Similarly, non-cardiac comorbidities conferred a similar contribution to worse outcomes in HFrEF and HFpEF. Additionally, reporting frailty may be particularly important to characterize HF populations. Sanders et al.27 showed that frailty is extremely common in HFpEF patients with greater frailty associated with higher risk of cardiovascular outcomes and mortality.
Moreover, recent data from 207 984 patients in the Get With The Guidelines-Heart Failure registry showed that the prevalence of 0, 1, 2, and ≥3 non-cardiovascular comorbidities was 18%, 30%, 27%, 25%, respectively. Moreover, from 2005 to 2014, there was a decline in patients with 0 non-cardiovascular comorbidities (22–16%; P <0.0001) and an increase in patients with ≥3 non-cardiovascular comorbidities (18–29%; P <0.0001).18 The greatest absolute increase was for chronic obstructive pulmonary disease (9% increase from 2005 to 2014) and obesity (8% increase from 2005 to 2014). The most common comorbidities noted were hypertension (85.6%), hyperlipidaemia (62.6%), diabetes mellitus (47.1%), chronic obstructive pulmonary disease (30.9%) and atrial fibrillation (28.8%). These data derived from a real-world acute HF registry estimate comorbidity burden to be higherthan observed in recent clinical trials based on our experience.18 This has important implications because these comorbidities contribute to worsening HF and higher readmission rates but may not be uniformly modified by HF therapies.
Although the presence of comorbidities in patients with HF is seen widely, significant gaps remain in the evidence base and guidelines for caring of patients with HF and multiple comorbidities.
Management of medical comorbidities is addressed in both US and European guidelines, although the European guideline discussion of treatment of comorbidities is more extensive on selected comorbidities not mentioned in the US guidelines such as cancer, diabetes mellitus, renal dysfunction, obesity, and pulmonary disease. A better understanding of the prevalence and interaction of concomitant diseases in HF is required to bridge the gap between routine practice and guideline-directed medical therapy.
The employment of guideline-directed medical therapy has been reported to be considerably low in routine medical practice further questioning the generalizability of HF trials.28,29 The Change the Management of Patients with Heart Failure (CHAMP-HF) registry found a significant gap between guideline-directed medical therapy and prescription of medicines in routine practice.29 Although less than 2% patients had an absolute contraindication to any medication, the optimal use of guideline-directed medication was <75%.29 Data from the QUALIFY survey also reported that only 27.1% of patients treated with angiotensin-converting enzyme inhibitors and 14.8% of patients treated with beta-blockers received the recommended target dose mentioned in the guidelines.21 Patient factors such as age and comorbidities contribute to under-dosing in such patients.
We also found that the percentage of reported comorbidities was higher in Western Europe and multiregional trials. Such geographic variation in patient characteristics has been found in previous studies, and may be attributed to multiple possible reasons.30 First, the increased reporting of comorbidities in Western Europe could be a result of less stringent inclusion criteria in trials allowing for a diverse study group including patients with multiple chronic diseases. Furthermore, the prevalence of comorbidities may vary among the population due to differences in genetic risks and environmental exposures of different regions.31 Varied approaches to management of HF in terms of resource availability and standard of care have also been reported in different regions of the world.32 This would also include the differences in insurance and healthcare policies and explain why least number of comorbidities were reported in the rest of the world; disparities in patients’ insurance coverage have an impact on utilization of proper health care and thus enrolment in clinical trials.
Although the prevalence of comorbidities is generally regarded as higher in HFpEF, our results showed comorbidities were reported more often in patients with HFrEF.33,34 This may be due to the intentional exclusion criteria, as patients with HFpEF are perceived to be older and with more underlying chronic conditions.6,35 The increase in atrial fibrillation, hypertension and chronic kidney disease in patients with HFrEF may have been due to improved and early diagnostic screening and awareness amongst the physician and general population. The standard application of natriuretic peptides may also bias against enrolling certain comorbidities, such as obesity, that are known to have lower distributions of natriuretic peptide levels.36 Obesity may occur more commonly among HFpEF patients in real-world settings, but appears under-represented in these clinical trials. Certain HFpEF trials have applied a BMI ceiling for eligibility; for instance, the recently presented Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor With Angiotensin Receptor Blocker Global Outcomes in HFpEF (PARAGON-HF) trial excluded patients who had a BMI >40kg/m2.37
A more rigorous, systematic, and standardized framework needs to be adopted to ensure adequate comorbidity reporting in future clinical trials. It is important to note that there is a difference between collection of data and reporting data. It is likely that extensive comorbidity data are collected for regulatory purposes but just not published in full, implying that a standardized method of reporting comorbidities is warranted. Moreover, there is a need to improve recruitment of multi-morbid HF patients in future trials. Partnering with primary care providers and community clinics can be beneficial to better engage diverse patient populations with higher longitudinal care needs. Pragmatic clinical trials can also be an important solution to the problem. However, the conflict of greater external validity with pragmatic trials vs. greater internal validity with mechanistic trials will remain. Moreover, while there has been substantial regulatory emphasis on ensuring enrolment of women and ethnic/racial minority populations, new efforts are needed to improve representation of key comorbidities observed in clinical practice.
Limitations
Limitations in this study should be considered. Due to the time range of our study, some important HF trials published before 2000, and trials recently published such as PARAGON-HF and Dapagliflozin And Prevention of Adverse-outcomes in Heart Failure (DAPA-HF) were not included in our analyses. Many trials did not report data for specific comorbidities, thus our overall results for comorbidity prevalence are potentially subject to reporting bias. Likewise, analyses were limited to published data available and unpublished comorbidities or patient characteristics may have changed these findings. The baseline criteria used to define different comorbidities may have varied between trials and data regarding these definitions were not readily available. For instance, although BMI was reported in some studies, the percentage of obese patients could not be extrapolated. Given the heterogeneity of reporting of comorbidities among studies, differences in the prevalence of comorbidities between HF types should be interpreted with caution. In addition, the participating study population may not be proportional to the size of the participating countries or an accurate representation of the HF population which was used to estimate the prevalence of diseases. Also, we could not gauge the severity of comorbidities together with the prevalence. Finally, ejection fraction-based definitions of HFrEF and HFpEF varied across the study period. The term ‘HF with mid-range ejection fraction’ was also introduced in the 2016 European Society of Cardiology guidelines for patients with a left ventricular ejection fraction of 40–49%. Moreover, measurement of left ventricular ejection fraction is only accurate within 5–10%. All of these factors could have resulted in some inconsistencies in our subgroup analyses on the subdivisions of HF based on ejection fraction.
Conclusion
In this systematic review of HF trials, we demonstrate that reporting of comorbid conditions remains low and incomplete. When reported, the prevalence of comorbidities in HF patients has increased over time suggesting a possible increasing complexity of trial populations over time. Further efforts are needed to ensure future HF clinical trials adequately describe and account for the burden of cardiovascular and non-cardiovascular comorbidities in their respective study populations. Development of a standardized framework to ensure adequate comorbidity reporting and improve recruitment of multi-morbid HF patients is critical.
Supplementary Material
Table S1. Phase II–IV heart failure clinical trials published between January 2001 and December 2016 with sample sizes over 400 patients identified by systematic search.
Table S2. Baseline characteristics of the included trials.
Table S3. Trends in comorbidities reported per trial.
Table S4. Trends in comorbidities of contemporary clinical trials of chronic heart failure with reduced ejection fraction over the last 16 years.
Table S5. Trends in comorbidities of contemporary clinical trials of acute heart failure over the last 16 years.
Table S6. Results of Levene’s test to check for heterogeneity in comorbidity reporting by heart failure type.
Table S7. Trends in comorbidities as exclusion criteria in contemporary clinical trials of heart failure over the last 16 years.
Figure S1. PRISMA flow diagram detailing trial selection and key inclusion/exclusion criteria.
Figure S2. Trends of key comorbidities across clinical trials of chronic heart failure with reduced ejection fraction.
Figure S3. Trends of key comorbidities across clinical trials of acute heart failure.
Figure S4. (A) Association of diabetes with enrolment of black patients. (B) Association of obesity with enrolment of black patients.
Acknowledgments
Funding
Dr. Ayman Samman Tahhan is supported by the Abraham J. & Phyllis Katz Foundation (Atlanta, GA) and NIH/NIA grant AG051633. Dr. Muthiah Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541). Dr. Stephen J. Greene is supported by the National Heart Lung and Blood Institute T32 postdoctoral training grant (T32HL069749–14) and a Heart Failure Society of America/Emergency Medicine Foundation Acute Heart Failure Young Investigator Award funded by Novartis.
Conflict of interest: M.V serves on advisory boards for Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, and Boehringer Ingelheim, and participates on clinical endpoint committees for studies sponsored by Novartis and the NIH. S.J.G. has received research support from Amgen, Bristol-Myers Squibb, and Novartis, and serves on an advisory board for Amgen. S.D.A. has received research support from Vifor International & Abbott Vascular, and fees for consultancy and/or speaking from AstraZeneca, Bayer, Boehringer Ingelheim, Respicardia, Impulse Dynamics, Janssen, Novartis, Servier and Vifor International. G.C.F. reports consulting for Abbott, Amgen, Bayer, Janssen, Medtronic, and Novartis. J.B. has received research support from the NIH and European Union; and has been a consultant for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, CVRx, Janssen, Luitpold Pharmaceuticals, Medtronic, Merck, Novartis, Relypsa, Vifor Pharma, and ZS Pharma. The other authors have nothing to disclose.
Footnotes
Supplementary Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- 1.Iyngkaran P, Majoni W, Cass A, Sanders P, Ronco C, Brady S, Kangaharan N, Ilton M, Hare DL, Thomas MC. Northern territory perspectives on heart failure with comorbidities – understanding trial validity and exploring collaborative opportunities to broaden the evidence base. Heart Lung Circ 2015;24:536–543. [DOI] [PubMed] [Google Scholar]
- 2.Mentz RJ, Felker GM. Noncardiac comorbidities and acute heart failure patients. Heart Fail Clin 2013;9:359–367 vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong CY, Chaudhry SI, Desai MM, Krumholz HM. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med 2011. ;124:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee CS, Chien CV, Bidwell JT, Gelow JM, Denfeld QE, Masterson Creber R, Buck HG, Mudd JO. Comorbidity profiles and inpatient outcomes during hospitalization for heart failure: an analysis of the U.S. nationwide inpatient sample. BMC Cardiovasc Disord 2014;14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandesara PB, O’Neal WT, Kelli HM, Samman-Tahhan A, Hammadah M, Quyyumi AA, Sperling LS. The prognostic significance of diabetes and microvascular complications in patients with heart failure with preserved ejection fraction. Diabetes Care 2018;41:150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 2012;59:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Triposkiadis FK, Skoularigis J. Prevalence and importance of comorbidities in patients with heart failure. Curr Heart Fail Rep 2012;9:354–362. [DOI] [PubMed] [Google Scholar]
- 8.Van Spall HG, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA 2007;297:1233–1240. [DOI] [PubMed] [Google Scholar]
- 9.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC Jr. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002;40:976–982. [DOI] [PubMed] [Google Scholar]
- 10.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891 –975. [DOI] [PubMed] [Google Scholar]
- 11.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017. ACC/AHA/HFSA Focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 12.Gorina Y, Kramarow EA. Identifying chronic conditions in Medicare claims data: evaluating the Chronic Condition Data Warehouse algorithm. Health Serv Res 2011;46:1610–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherubini A, Oristrell J, Pla X, Ruggiero C, Ferretti R, Diestre G, Clarfield AM, Crome P, Hertogh C, Lesauskaite V, Prada GI, Szczerbinska K, Topinkova E, Sinclair-Cohen J, Edbrooke D, Mills GH. The persistent exclusion of older patients from ongoing clinical trials regarding heart failure. Arch Intern Med 2011;171:550–556. [DOI] [PubMed] [Google Scholar]
- 14.Yee KW, Pater JL, Pho L, Zee B, Siu LL. Enrollment of older patients in cancer treatment trials in Canada: why is age a barrier? J Clin Oncol 2003;21:1618–1623. [DOI] [PubMed] [Google Scholar]
- 15.Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol 2005;23:3112–3124. [DOI] [PubMed] [Google Scholar]
- 16.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC; ADHERE Scientific Advisory Committee and Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) database. J Am Coll Cardiol 2006;47:76–84. [DOI] [PubMed] [Google Scholar]
- 17.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med 2002;347:1397–1402. [DOI] [PubMed] [Google Scholar]
- 18.Sharma A, Zhao X, Hammill BG, Hernandez AF, Fonarow GC, Felker GM, Yancy CW, Heidenreich PA, Ezekowitz JA, DeVore AD. Trends in noncardiovascular comorbidities among patients hospitalized for heart failure: insights from the Get With The Guidelines-Heart Failure registry. Circ Heart Fail 2018;11:e004646. [DOI] [PubMed] [Google Scholar]
- 19.Glynn P, Lloyd-Jones DM, Feinstein MJ, Carnethon M, Khan SS. Disparities in cardiovascular mortality related to heart failure in the United States. J Am Coll Cardiol 2019;73:2354–2355. [DOI] [PubMed] [Google Scholar]
- 20.Tsugawa Y, Figueroa JF, Papanicolas I, Orav EJ, Jha AK. Assessment of strategies for managing expansion of diagnosis coding using risk-adjustment methods for Medicare data. JAMA Intern Med 2019;179:1287–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komajda M, Anker SD, Cowie MR, Filippatos GS, Mengelle B, Ponikowski P, Tavazzi L; QUALIFY Investigators. Physicians’ adherence to guideline-recommended medications in heart failure with reduced ejection fraction: data from the QUALIFY global survey. Eur J Heart Fail 2016;18:514–522. [DOI] [PubMed] [Google Scholar]
- 22.van Deursen VM, Urso R, Laroche C, Damman K, Dahlstrom U, Tavazzi L, Maggioni AP, Voors AA. Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail 2014; 16: 103–111. [DOI] [PubMed] [Google Scholar]
- 23.Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP; ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005;149:209–216. [DOI] [PubMed] [Google Scholar]
- 24.O’Connor CM, Abraham WT, Albert NM, Clare R, Gattis Stough W, Gheorghiade M, Greenberg BH, Yancy CW, Young JB, Fonarow GC. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Hean J 2008;156:662–673. [DOI] [PubMed] [Google Scholar]
- 25.Streng KW, Nauta JF, Hillege HL, Anker SD, Cleland JG, Dickstein K, Filippatos G, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zwinderman AH, Zannad F, Damman K, van der Meer P, Voors AA. Non-cardiac comorbidities in heart failure with reduced, mid-range and preserved ejection fraction. Int J Cardiol 2018;271:132–139. [DOI] [PubMed] [Google Scholar]
- 26.Iorio A, Senni M, Barbati G, Greene SJ, Poli S, Zambon E, Di Nora C, Cioffi G, Tarantini L, Gavazzi A, Sinagra G, Di Lenarda A. Prevalence and prognostic impact of non-cardiac co-morbidities in heart failure outpatients with preserved and reduced ejection fraction: a community-based study. Eur J Heart Fail 2018;20:1257–1266. [DOI] [PubMed] [Google Scholar]
- 27.Sanders NA, Supiano MA, Lewis EF, Liu J, Claggett B, Pfeffer MA, Desai AS, Sweitzer NK, Solomon SD, Fang JC. The frailty syndrome and outcomes in the TOPCAT trial. Eur J Heart Fail 2018;20:1570–1577. [DOI] [PubMed] [Google Scholar]
- 28.Fonarow GC, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, McBride ML, Inge PJ, Mehra MR, O’Connor CM, Reynolds D, Walsh MN, Yancy CW. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation 2010;122:585–596. [DOI] [PubMed] [Google Scholar]
- 29.Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF, Fonarow GC. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol 2018;72:351–366. [DOI] [PubMed] [Google Scholar]
- 30.Kristensen SL, Kober L, Jhund PS, Solomon SD, Kjekshus J, McKelvie RS, Zile MR, Granger CB, Wikstrand J, Komajda M, Carson PE, Pfeffer MA, Swedberg K, Wedel H, Yusuf S, McMurray JJ. International geographic variation in event rates in trials of heart failure with preserved and reduced ejection fraction. Circulation 2015;131:43–53. [DOI] [PubMed] [Google Scholar]
- 31.Freedman BI, Divers J, Palmer ND. Population ancestry and genetic risk for diabetes and kidney, cardiovascular, and bone disease: modifiable environmental factors may produce the cures. Am J Kidney Dis 2013;62:1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poole-Wilson PA. Differences in European and North American approaches to the management of heart failure. Cardiol Clin 2008;26:107–112 viii. [DOI] [PubMed] [Google Scholar]
- 33.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol 2004;43:317–327. [DOI] [PubMed] [Google Scholar]
- 34.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251 –259. [DOI] [PubMed] [Google Scholar]
- 35.Mentz RJ, Kelly JP, von Lueder TG, Voors AA, Lam CS, Cowie MR, Kjeldsen K, Jankowska EA, Atar D, Butler J, Fiuzat M, Zannad F, Pitt B, O’Connor CM. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2014;64:2281 –2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myhre PL, Vaduganathan M, Claggett BL, Anand IS, Sweitzer NK, Fang JC, O’Meara E, Shah SJ, Desai AS, Lewis EF, Rouleau J, Pitt B, Pfeffer MA, Solomon SD. Association of natriuretic peptides with cardiovascular prognosis in heart failure with preserved ejection fraction: secondary analysis of the TOPCAT randomized clinical trial. JAMA Cardiol 2018;3:1000–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solomon SD, Rizkala AR, Lefkowitz MP, Shi VC, Gong J, Anavekar N, Anker SD, Arango JL, Arenas JL, Atar D, Ben-Gal T, Boytsov SA, Chen CH, Chopra VK, Cleland J, Comin-Colet J, Duengen HD, Echeverria Correa LE, Filippatos G, Flammer AJ, Galinier M, Godoy A, Goncalvesova E, Janssens S, Katova T, Kober L, Lelonek M, Linssen G, Lund LH, O’Meara E, Merkely B, Milicic D, Oh BH, Perrone SV, Ranjith N, Saito Y, Saraiva JF, Shah S, Seferovic PM, Senni M, Sibulo AS Jr, Sim D, Sweitzer NK, Taurio J, Vinereanu D, Vrtovec B, Widimsky J Jr, Yilmaz MB, Zhou J, Zweiker R, Anand IS, Ge J, Lam CS, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, Van Veldhuisen DJ, Zannad F, Zile MR, JJ MM. Baseline characteristics of patients with heart failure and preserved ejection fraction in the PARAGON-HF trial. Circ Heart Fail 2018;11: e004962. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Phase II–IV heart failure clinical trials published between January 2001 and December 2016 with sample sizes over 400 patients identified by systematic search.
Table S2. Baseline characteristics of the included trials.
Table S3. Trends in comorbidities reported per trial.
Table S4. Trends in comorbidities of contemporary clinical trials of chronic heart failure with reduced ejection fraction over the last 16 years.
Table S5. Trends in comorbidities of contemporary clinical trials of acute heart failure over the last 16 years.
Table S6. Results of Levene’s test to check for heterogeneity in comorbidity reporting by heart failure type.
Table S7. Trends in comorbidities as exclusion criteria in contemporary clinical trials of heart failure over the last 16 years.
Figure S1. PRISMA flow diagram detailing trial selection and key inclusion/exclusion criteria.
Figure S2. Trends of key comorbidities across clinical trials of chronic heart failure with reduced ejection fraction.
Figure S3. Trends of key comorbidities across clinical trials of acute heart failure.
Figure S4. (A) Association of diabetes with enrolment of black patients. (B) Association of obesity with enrolment of black patients.


