Abstract
Background:
Patients with a compromised immune system are at risk for converting from latent tuberculosis infection (LTBI) to active tuberculosis (TB) infection. Multiple sclerosis (MS) therapies may put individuals with LTBI at higher risk of TB.
Methods:
Patients at the Beth Israel Deaconess Medical Center MS Clinic were screened for TB as part of routine testing with the QuantiFERON-TB Gold In-Tube (QFT-GIT) assay (Cellestis Ltd) from 2013 to 2017. Patients were tested either before or during immunomodulatory therapy.
Results:
Four of 222 patients (1.8%; 95% CI, 0.1%–3.6%) had positive QFT-GIT results; three patients had risk factors for TB, having emigrated from TB-endemic countries or worked in the health care industry. Twenty-eight of 222 patients (12.6%) had an indeterminate assay result, and 75.0% of these occurred in patients taking dimethyl fumarate. Fingolimod, natalizumab, or anti-CD20 treatments showed 0% to 7.7% indeterminate results.
Conclusions:
The prevalence of LTBI was 1.8% in the Beth Israel Deaconess Medical Center MS Clinic. Not all LTBI cases were associated with known risk factors for TB. Screening for LTBI before starting immunosuppressive agents for MS could help prevent activation of TB. Dimethyl fumarate use is associated with indeterminate QFT-GIT results, possibly due to functional effects on lymphocytes and levels of cytokines, such as interferon gamma. In contrast, fingolimod use was rarely associated with indeterminate QFT-GIT results despite a high rate of lymphopenia in virtually all patients.
Keywords: Dimethyl fumarate, Fingolimod, Interferon gamma, Latent tuberculosis infection, Multiple sclerosis (MS), Tuberculosis (TB)
Up to 13 million people have latent tuberculosis infection (LTBI) in the United States, corresponding to a prevalence of 4.7% (95% CI, 3.4%–6.3%) based on the tuberculin skin test and 5.0% (95% CI, 4.2%–5.8%) based on an interferon gamma release assay (IGRA).1 Another study suggests a prevalence of 3.1% estimated by annual tuberculosis (TB) cases by county coupled with estimates of reactivation from LTBI.2 The prevalence of LTBI in multiple sclerosis (MS) clinic patients is not well established, although a German study suggests a prevalence of 3.85% (95% CI, 2%–8%).3 Individuals who have gone to prison, worked in health care, or lived outside of the United States in countries with higher rates of TB are at increased risk for LTBI. Of those who have LTBI, 5% to 15% will go on to develop infectious TB.4 Patients with a compromised immune system (eg, HIV infection, medicines affecting the immune system, or diabetes) are at higher risk for converting from LTBI to active TB infection. Treating LTBI reduces the chance of developing active TB by 90%.4,5
The QuantiFERON-TB Gold In-Tube (QFT-GIT) assay (Cellestis Ltd) is an enzyme-linked immunosorbent assay–based whole-blood test that measures the release of interferon gamma (IFN-γ) by T cells in response to Mycobacterium tuberculosis antigens. It is one of the IGRA tests that evaluate for a cellular immune response to M tuberculosis. The IGRAs are more specific than tuberculin skin tests and are not affected by a history of the Bacillus Calmette-Guérin vaccine. However, IGRAs may give false-negative results in the setting of immunosuppression.6
Multiple treatments for MS are now commercially available, including the interferon class, glatiramer acetate, teriflunomide, dimethyl fumarate (DMF), fingolimod, siponimod, cladribine, natalizumab, rituximab, ocrelizumab, and alemtuzumab. Immunomodulatory therapies for MS may put individuals with LTBI at higher risk of IGRA diagnostic inaccuracy as well as TB. In particular, DMF has anti-inflammatory and neuroprotective effects apparently mediated by activation of the nuclear factor erythroid 2–related factor 2 (Nrf2) transcriptional pathway. Dimethyl fumarate is well-known to cause lymphopenia in patients with MS. In addition, nonspecific stimulation of T cells from DMF-treated patients results in a reduction in cytokine production, including IFN-γ.7,8 Through its action on sphingosine-1-phosphate receptors, fingolimod also can inhibit the production of IFN-γ from activated T cells.9 Although in vivo effects are unknown for teriflunomide, in vitro exposure to teriflunomide reduces IFN-γ production by T cells from patients with LTBI.10 In contrast, natalizumab mildly upregulates IFN-γ expression in activated T cells from healthy donors.11 Also, IFN-γ messenger RNA is upregulated in peripheral blood mononuclear cells from natalizumab-treated patients.12 With other MS immunomodulatory or immunosuppressive treatments, it is unclear whether IFN-γ is upregulated or downregulated in response to treatment in patients with MS.
In this work, we aimed to quantify the prevalence of LTBI in a US-based MS population and to assess how immunomodulatory therapies affect IGRAs. We hypothesized that MS therapies, including DMF and fingolimod, would reduce the reliability of IGRAs by causing lymphopenia and reducing cytokine production.
Methods
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Data collection and analysis was approved by the Beth Israel Deaconess Medical Center (BIDMC) institutional review board. Patients with MS from the BIDMC MS Clinic in Boston, Massachusetts, were screened for TB using the QFT-GIT assay from December 2013 to December 2017 as part of an evolving routine clinical practice at this MS clinic. Many patients included in this study were already initiated on therapy before testing. Screening was repeated in a subset of patients with indeterminate results. For patients receiving natalizumab or anti-CD20 treatments, we considered these treatments current and ongoing if patients were compliant with prescribed dosing schedules. All patients with MS satisfied the revised McDonald criteria for MS. We did not study alemtuzumab-treated patients. There were no additional requirements for laboratory data. All blood collections for testing were performed at one time, except when blood testing was repeated for indeterminate results. We excluded patients with concurrent methylprednisolone treatments for MS relapses within a month of blood testing. Patients receiving non-MS immunomodulatory or immunosuppressive treatments were excluded from analysis. Most outpatients with MS were tested in the BIDMC MS Clinic during this study.
Injectables include glatiramer acetate and interferon therapies, and anti-CD20 therapy includes rituximab and ocrelizumab therapies. Lymphopenia grades were defined using the National Cancer Institute Common Toxicity Criteria, where patients with grade 1 lymphopenia have 800 to 999 cells/mm3; patients with grade 2 lymphopenia, 500 to 799 cells/mm3; patients with grade 3 lymphopenia, 200 to 499 cells/mm3; and patients with grade 4 lymphopenia, less than 200 cells/mm3.13 If not otherwise stated, lymphopenia refers to at least grade 1 lymphopenia.
Statistical tests were performed using JMP Pro 13.0.0 (SAS Institute Inc). Herein we define diagnostic results as both positive and negative results to distinguish them from indeterminate results. The Wilcoxon rank sum test was used to compare nonparametric continuous data. The Pearson χ2 test was used to compare categorical data; the Fisher exact test was used when any cell contained fewer than ten observations. A P value less than .05 was considered statistically significant.
Results
We collected QFT-GIT assay results for 222 unique patients: 216 (97.3%) with MS, three (1.4%) with clinically isolated syndrome, and three (1.4%) with neuromyelitis optica. The median age of all the patients was 47.0 (interquartile range, 38.0–54.3) years; 144 were female (64.9%). Ethnicities were 79.3% White, 12.6% African American, 3.6% Middle Eastern, 2.7% Latin American, and 1.8% Asian.
One hundred thirty-eight (62.2%) were currently receiving immunomodulatory therapy, and 163 (73.4%) had a history of receiving immunomodulatory therapy.
Four of 222 patients (1.8%; 95% CI, 0.1%-3.6%) had positive QFT-GIT test results, and 190 patients (85.6%) had negative results. One of the positive results occurred in a patient who had emigrated from Russia. Another patient with LTBI had emigrated from China and had been treated for TB in the past. A third patient with LTBI worked in the health care industry.
Twenty-eight of 222 patients (12.6%) had an indeterminate assay result. Twenty-one of these patients were taking DMF, four were untreated, and one each were taking fingolimod, methotrexate, and interferon. Characteristics of those with and without indeterminate results are presented in Table 1. Fifteen of the 28 patients (53.6%) with indeterminate results were women. The median age of participants with indeterminate results was 49.0 (range, 44.3–55.8) years. Patients with indeterminant results were more likely to be receiving immunomodulatory therapy (83.3%; odds ratio [OR] = 3.6, P = .008) or have a history of immunomodulatory therapy (90.0%; OR = 3.7, P = .027). This effect was largely due to DMF use because patients with indeterminate results were more likely to be currently taking DMF (OR = 9.3, P < .001) or to have previously taken DMF (OR = 9.9, P < .001). In contrast, only one of the 28 indeterminate results (3.6%) occurred in a patient taking fingolimod, and there was no association between fingolimod use and indeterminate results.
Table 1.
Characteristics of the patients stratified by QuantiFERON-TB Gold In-Tube assay results
| Characteristic | Indeterminate result (n = 28) | Diagnostic result (n = 194) | P value |
|---|---|---|---|
| Female sex | 15 (53.6) | 129 (66.5) | NSa |
| Disease duration, y | 15 [1–20.8] | 9 [3–17] | NSb |
| Age, y | 49 [44.3–55.8] | 46.5 [36.0–54.0] | NSb |
| African American race | 5 (17.9) | 23 (11.9) | NSa |
| Current therapy | 24 (85.7) | 114 (58.8) | <.007a |
| History of MS therapy | 24 (85.7) | 139 (71.6) | <.004a |
| Current DMF use | 21 (75.0) | 40 (20.6) | <.0001a |
| History of DMF use | 23 (82.1) | 57 (29.4) | <.0001a |
| Current fingolimod | 1 (3.6) | 12 (6.2) | NSa |
| Current treatment duration, y | 2.1 [1.1–3.0] | 2.4 [1.2–3.7] | <.001b |
| White blood cell count, K/μL | 5.3 [4.6–10.5] | 6.4 [5.0–8.1]c | NSb |
| Absolute lymphocyte count,/μL | 729 [586–1107] | 1676 [1203–2242]c | <.0001b |
| Lymphopenia | 20 (71.4) | 40 (21.1)c | <.0001a |
Note: Values are given as number (percentage) or median [interquartile range] unless otherwise indicated; interquartile range represented by 25th–75th percentiles.
Abbreviations: DMF, dimethyl fumarate; MS, multiple sclerosis; NS, not significant.
By χ2 test.
By Wilcoxon rank sum test.
Based on 190 patients.
Patients with indeterminate results had decreased absolute lymphocyte, CD3, CD4, and CD8 counts (P < .001), as well as a higher CD4/CD8 ratio (P = .002). Patients with indeterminate results were also much more likely to have lymphopenia, with 20 of 28 (71.4%) having lymphopenia (OR = 13.1, P < .001).
In the patients actively taking DMF, an elevated CD4/CD8 ratio was associated with indeterminate results (Table 2). For patients taking DMF, lymphopenia was also strongly associated with an indeterminate test result, with 17 of the 21 patients (81.0%) taking DMF having lymphopenia (OR = 10.8, P < .001).
Table 2.
Characteristics of patients taking dimethyl fumarate stratified by QuantiFERON-TB Gold In-Tube assay results
| Characteristic | Indeterminate result (n = 21) | Diagnostic result (n = 40) | P valuea |
|---|---|---|---|
| Female sex | 12 (57.1) | 30 (75.0) | NSb |
| Age, y | 52.0 [46.5–56.0] | 48.0 [39.0–56.8] | NSa |
| Current treatment duration, y | 2.4 [1.4–3.1] | 2.3 [1.5–3.2] | NSa |
| White blood cell count, K/μL | 5.1 [4.4–6.8] | 5.3 [4.5–6.6] | NSa |
| Lymphocytes, % | 13.3 [10.1–18.5] | 23.3 [17.6–28.3] | <.0001a |
| Absolute lymphocyte count,/μL | 714 [587–925] | 1225 [974–1668] | <.0001a |
| Lymphopenia | 17 (81.0) | 12 (30.0) | <.0003b |
Note: Values are given as number (percentage) or median [interquartile range] unless otherwise indicated; interquartile range represented by 25th–75th percentiles.
Abbreviation: NS, not significant.
By Wilcoxon rank sum test.
By χ2 test.
Table 3 gives a breakdown of the indeterminate results by immunomodulatory therapy. There were four patients who had indeterminate testing while off therapy. Three of these four patients with indeterminate results had repeated testing with negative results; we were unable to repeat testing for the fourth patient. Although 12 of 13 patients (92.3%) taking fingolimod in the study had lymphopenia, only one of these patients (7.7%) had an indeterminate result (Figure 1).
Table 3.
Breakdown of QuantiFERON-TB Gold In-Tube assay indeterminate results and lymphopenia by immunomodulatory therapy
| Therapy | Patients, n/N (%) | ||
|---|---|---|---|
| Indeterminate results | Lymphopenia | Indeterminate results with lymphopenia | |
| Dimethyl fumarate | 21/61 (34.4) | 29/61 (47.5) | 17/21 (81.0) |
| Other treatments | 1/6 (16.7)a | 3/6 (50.0) | 1/1 (100)a |
| Fingolimod | 1/13 (7.7)a | 12/13 (92.3) | 1/1 (100)a |
| Injectables | 1/37 (2.7)a | 3/35 (8.6) | 1/1 (100)a |
| No therapy | 4/84 (4.8) | 2/84 (2.4) | None |
| Natalizumab | 0/10 | 0/10 | None |
| Anti-CD20 therapy | 0/10 | 0/10 | None |
Patients in these groups with indeterminate results with lymphopenia had been previously treated with dimethyl fumarate.
Figure 1.
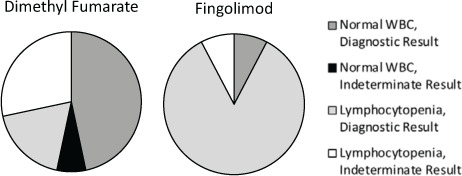
Comparison of lymphocytopenia and QuantiFERON-TB Gold In-Tube assay results for patients taking dimethyl fumarate and fingolimod
WBC, white blood cell.
Of the 37 patients receiving injectable therapies, one (2.7%) had an indeterminate result; this patient was taking peginterferon beta-1a, having been being switched from DMF for lymphopenia 9 months before testing, and had persistent lymphopenia, which was considered primarily a carryover from DMF exposure. Results of repeated QFT-GIT testing after this patient had subsequently been switched to rituximab therapy were negative. In addition, retesting of all patients with indeterminate results to date yielded negative results in nine of 13 patients (69.2%).
We had one patient taking methotrexate, a treatment used in MS not approved by the US Food and Drug Administration, who had an indeterminate result. Although we had fewer patients receiving teriflunomide, natalizumab, or anti-CD20 treatments, there were no indeterminate results recorded for these patients.
Discussion
Routine screening in our MS population revealed four positive results (1.8%; 95% CI, 0.1%–3.6%) for TB using QFT-GIT testing. Of the four patients, two were at higher risk because they had emigrated from countries with higher rates of TB, and another worked in the health care industry. The present finding of 1.8% prevalence in the BIDMC MS Clinic is lower than estimates of general prevalence in the United States (3%–5%).1,2Another study showed a 3.95% prevalence of LTBI in German patients with MS.3 It is thus important to check LTBI status in the MS patient population because MS treatments could place patients at higher risk for reactivation of their LTBI.
There is a risk of reactivation from LTBI in MS, but this risk is poorly quantified. Mulero et al14 reported six patients with MS in Spain with positive tuberculin skin test results receiving natalizumab therapy; these patients did not have evidence of reactivation at the time of publication. Cases of TB have been reported in patients with MS after treatment with teriflunomide,15 fingolimod,16 alemtuzumab,17,18 natalizumab,19 and daclizumab.20 Because LTBI is still a risk in the United States at 3% to 5% prevalence,1,2 screening for LTBI before starting patients on immunomodulatory therapy could potentially reduce the risk of reactivation of TB. The Colombian Association of Neurology Committee of Multiple Sclerosis has recommended screening for TB before starting oral and infusion therapies.21 In fact, the full prescribing information for cladribine recommends screening for TB before initiating therapy. Interferon beta and glatiramer acetate are thought to be safer agents in patients with LTBI or active TB, and screening may not be necessary before initiation of these therapies.21,22
In the present study, we screened for LTBI with most of the patient population receiving immunomodulatory therapy. Study limitations include the real-world retrospective nature of the study, the fact that this is a single-center study, and the fact that several patients were receiving MS treatment at the time of blood testing. We found that DMF use was associated with indeterminate IGRA results, with 34.4% of QFT-GIT testing on patients taking DMF yielding indeterminate results. This represented 75.0% of the total indeterminate results from the present study. These effects on the reliability of IGRA may be long-lasting as well because a few patients in this study had persistent indeterminate IGRA results. Two patients continued to have indeterminate results on retesting even after DMF was discontinued, one at 4 months after treatment and one at 9 months after treatment.
Of patients taking DMF, those with lymphopenia were significantly more likely to have indeterminate results. In contrast, although lymphopenia was seen in almost all the patients receiving fingolimod, there was only one indeterminate result in this group. A possible explanation for this difference is the different mechanisms of action of the two therapies. Although fingolimod sequesters lymphocytes peripherally, this may not affect the cellular immune response activated by the M tuberculosis antigens via IGRA testing. Conversely, DMF is known to promote lymphopenia as well as alterations in cytokines, including IFN-γ, which is paramount to the functioning of IGRAs.8,23
Screening for LTBI in patients with MS before initiating immunomodulatory therapy could reduce the risk of reactivation. The yield of IGRAs is most likely highest in treatment-naive patients, particularly patients who have not had exposure to DMF.
PRACTICE POINTS
The prevalence of latent tuberculosis is low at 1.8% (95% CI, 0.1%–3.6%) in the Beth Israel Deaconess Medical Center MS Clinic.
Finding indeterminate QuantiFERON-TB Gold In-Tube (QFT-GIT) (Cellestis Ltd) results may depend on the type of disease-modifying therapy used.
Dimethyl fumarate use is associated with reduced reliability of the QFT-GIT assay result, presumably due to a dysfunction of lymphocytes rather than lymphopenia.
Despite fingolimod-associated lymphopenia, fingolimod use did not compromise the QFT-GIT assay results in virtually all patients tested.
Footnotes
Financial Disclosures: Dr Sloane has served as a consultant for Biogen, Serono/Merck, Celgene, Genzyme, Genentech, and Teva. The other authors declare no conflicts of interest.
Funding/Support: Dr Sloane has received grant funding from Biogen, Genzyme, and the National MS Society.
Prior Presentation: Aspects of this study have been presented in abstract form at the Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC); May 30–June 2, 2018; Nashville, TN.
References
- 1.Mancuso JD, Diffenderfer JM, Ghassemieh BJ, Horne DJ, Kao TC. The prevalence of latent tuberculosis infection in the United States. Am J Respir Crit Care Med. 2016;194:501–509. doi: 10.1164/rccm.201508-1683OC. [DOI] [PubMed] [Google Scholar]
- 2.Haddad MB, Raz KM, Lash TL et al. Simple estimates for local prevalence of latent tuberculosis infection, United States, 2011–2015. Emerg Infect Dis. 2018;24:1930–1933. doi: 10.3201/eid2410.180716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graf J, Leussink VI, Dehmel T et al. Infectious risk stratification in multiple sclerosis patients receiving immunotherapy. Ann Clin Transl Neurol. 2017;4:909–914. doi: 10.1002/acn3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiazyk S, Ball TB. Latent tuberculosis infection: an overview. Can Commun Dis Rep. 2017;43:62–66. doi: 10.14745/ccdr.v43i34a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis. 1999;3:847–850. [PubMed] [Google Scholar]
- 6.Pai M, Behr M. Latent Mycobacterium tuberculosis infection and interferon-gamma release assays. Microbiol Spectr. 2016:4. doi: 10.1128/microbiolspec.TBTB2-0023-2016. [DOI] [PubMed] [Google Scholar]
- 7.Mills EA, Ogrodnik MA, Plave A, Mao-Draayer Y. Emerging understanding of the mechanism of action for dimethyl fumarate in the treatment of multiple sclerosis. Front Neurol. 2018;9:5. doi: 10.3389/fneur.2018.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross CC, Schulte-Mecklenbeck A, Klinsing S, Posevitz-Fejfar A, Wiendl H, Klotz L. Dimethyl fumarate treatment alters circulating T helper cell subsets in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016;3:e183. doi: 10.1212/NXI.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazzola MA, Raheja R, Murugaiyan G et al. Identification of a novel mechanism of action of fingolimod (FTY720) on human effector T cell function through TCF-1 upregulation. J Neuroinflammation. 2015;12:245. doi: 10.1186/s12974-015-0460-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bua A, Ruggeri M, Zanetti S, Molicotti P. Effect of teriflunomide on QuantiFERON-TB Gold results. Med Microbiol Immunol. 2017;206:73–75. doi: 10.1007/s00430-016-0482-x. [DOI] [PubMed] [Google Scholar]
- 11.Benkert TF, Dietz L, Hartmann EM et al. Natalizumab exerts direct signaling capacity and supports a pro-inflammatory phenotype in some patients with multiple sclerosis. PLoS One. 2012;7:e52208. doi: 10.1371/journal.pone.0052208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khademi M, Bornsen L, Rafatnia F et al. The effects of natalizumab on inflammatory mediators in multiple sclerosis: prospects for treatment-sensitive biomarkers. Eur J Neurol. 2009;16:528–536. doi: 10.1111/j.1468-1331.2009.02532.x. [DOI] [PubMed] [Google Scholar]
- 13.National Cancer Institute Common Terminology Criteria for Adverse Events v50. Bethesda, MD: National Institutes of Health; 2017. [Google Scholar]
- 14.Mulero P, Caminero AB, Neri Crespo MJ, Fernandez-Herranz R, Tellez Lara N. Latent tuberculosis seems not to reactivate in multiple sclerosis patients on natalizumab. J Neuroimmunol. 2012;243:103–105. doi: 10.1016/j.jneuroim.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Confavreux C, O’Connor P, Comi G et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:247–256. doi: 10.1016/S1474-4422(13)70308-9. [DOI] [PubMed] [Google Scholar]
- 16.Calabresi PA, Radue EW, Goodin D et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:545–556. doi: 10.1016/S1474-4422(14)70049-3. [DOI] [PubMed] [Google Scholar]
- 17.Cohen JA, Coles AJ, Arnold DL et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380:1819–1828. doi: 10.1016/S0140-6736(12)61769-3. [DOI] [PubMed] [Google Scholar]
- 18.Coles AJ, Twyman CL, Arnold DL et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380:1829–1839. doi: 10.1016/S0140-6736(12)61768-1. [DOI] [PubMed] [Google Scholar]
- 19.Dahdaleh D, Altmann DM, Malik O, Nicholas RS. Breathlessness, night sweats, and weight loss on natalizumab. Lancet. 2012;380:726–727. doi: 10.1016/S0140-6736(12)61401-9. [DOI] [PubMed] [Google Scholar]
- 20.Gold R, Radue EW, Giovannoni G et al. Safety and efficacy of daclizumab in relapsing-remitting multiple sclerosis: 3-year results from the SELECTED open-label extension study. BMC Neurol. 2016;16:117. doi: 10.1186/s12883-016-0635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navas C, Torres-Duque CA, Munoz-Ceron J, et al. ; on behalf of the Colombian Association of Neurology, Committee of Multiple Sclerosis Diagnosis and treatment of latent tuberculosis in patients with multiple sclerosis, expert consensus. Mult Scler J Exp Transl Clin. 2018;4 doi: 10.1177/2055217317752202. 2055217317752202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fragoso YD, Adoni T, Anacleto A et al. How do we manage and treat a patient with multiple sclerosis at risk of tuberculosis? Expert Rev Neurother. 2014;14:1251–1260. doi: 10.1586/14737175.2014.962517. [DOI] [PubMed] [Google Scholar]
- 23.Montes Diaz G, Fraussen J, Van Wijmeersch B, Hupperts R, Somers V. Dimethyl fumarate induces a persistent change in the composition of the innate and adaptive immune system in multiple sclerosis patients. Sci Rep. 2018;8:8194. doi: 10.1038/s41598-018-26519-w. [DOI] [PMC free article] [PubMed] [Google Scholar]


