Abstract
Background
There is evidence for an inherited contribution to primary brain cancer. Linkage analysis of high-risk brain cancer pedigrees has identified candidate regions of interest in which brain cancer predisposition genes are likely to reside.
Methods
Genome-wide linkage analysis was performed in a unique set of 11 informative, extended, high-risk primary brain cancer pedigrees identified in a population genealogy database, which include from 2 to 6 sampled, related primary brain cancer cases. Access to formalin-fixed paraffin embedded tissue samples archived in a biorepository allowed analysis of extended pedigrees.
Results
Individual high-risk pedigrees were singly informative for linkage at multiple regions. Suggestive evidence for linkage was observed on chromosomes 2, 3, 14, and 16. The chromosome 16 region in particular contains a promising candidate gene, pyridoxal-dependent decarboxylase domain-containing 1 (PDXDC1), with prior evidence for involvement with glioblastoma from other previously reported experimental settings, and contains the lead single nucleotide polymorphism (rs3198697) from the linkage analysis of the chromosome 16 region.
Conclusions
Pedigrees with a statistical excess of primary brain cancers have been identified in a unique genealogy resource representing the homogeneous Utah population. Genome-wide linkage analysis of these pedigrees has identified a potential candidate predisposition gene, as well as multiple candidate regions that could harbor predisposition loci, and for which further analysis is suggested.
Keywords: linkage analysis, PDXDC1, primary brain cancer, UPDB
Key Points.
A resource of pedigrees exhibiting a significant excess of individuals with primary brain cancer was analyzed.
High-risk pedigrees were identified in a genealogic resource with large numbers of biosamples available.
Analysis of high-risk brain cancer pedigrees identified a candidate primary brain tumor predisposition gene.
Importance of the Study.
This innovative study used a unique genealogic resource linked to statewide cancer data to identify stored biospecimens representing primary brain cancer cases in pedigrees with a significant excess of brain cancer. Genetic analysis of the pedigrees identified evidence supporting PDXDC1 as a candidate primary brain cancer predisposition gene.
Heredity in brain tumors has been a challenging topic for study. Primary tumors are relatively rare, and pedigrees with multiple affected members are scarce, outside of known syndromes.1 The Gliogene Consortium has utilized linkage analysis methods to study genotypic predisposition in “high-risk” families (defined as proband glioma cases with first- or second-degree relatives with glioma).2 Further studies in the last decade have identified heritable chromosomal alterations which are associated with significantly increased incidence for brain tumors, as high as 2- to 6-fold relative risk for certain variants.3
We previously demonstrated evidence for an inherited contribution to primary brain cancer and identified multiple high-risk Utah primary brain tumor pedigrees.4 Linkage analysis of high-risk pedigrees is recognized to be a powerful method to localize predisposition genes.5–7 This high-risk pedigree approach has proven successful for a variety of tumor types in Utah, where a genealogic database linked to statewide cancer data has allowed identification of multiple predisposition genes, including breast cancer (BRCA)1, BRCA2, cyclin-dependent kinase inhibitor 2A (CDKN2A),8–10 and more recently, GOLM1 (Golgi membrane protein 1).11 Few resources of extended pedigrees exhibiting high risk for primary brain cancer have been reported; the lethality and relative rarity of brain tumors make ascertainment and recruitment of brain cancer cases in such pedigrees difficult.12,13 Here we have taken advantage of a genealogy resource representing the entire state of Utah, linked to a statewide cancer registry and to the health care records of the largest health care provider in Utah and its associated decades-old biorepository, to identify and obtain germline DNA samples for multiple pedigrees exhibiting a higher-than-population rate of primary brain tumors. Our Utah experience with a number of different cancer predisposition genes has shown us that pathogenic variants are most often associated with not only different subtypes of a specific cancer site, but with different cancer sites (eg, BRCA1 which predisposes to both breast and ovarian cancers, and CDKN2A which predisposes to both melanoma and pancreas cancers). For this reason, our hypothesis and study design included all primary brain tumors, regardless of subtype. We used genome-wide linkage analysis of these high-risk pedigrees to identify potential candidate brain cancer predisposition genes and candidate regions of interest that may harbor brain cancer predisposition genes.
Materials and Methods
Utah Genealogy Resource
The Utah Population Data Base, or UPDB, is a unique resource representing the Utah population.14 The UPDB includes the genealogy of the founders of Utah from the mid-1800s and their modern-day descendants. The genealogy has been linked to various statewide data resources, including the Utah Cancer Registry (UCR), and electronic health data for the largest health care provider in Utah, Intermountain Healthcare (Intermountain), among others. The UCR was established in 1966 and became a National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) registry in 1973. The UCR maintains records of all independent primary cancers diagnosed or treated in Utah and is linked to UPDB genealogy regularly. Intermountain serves 60–80% of the Utah population. The UPDB includes demographic data for over 9 million individuals. Approximately 3 million of these individuals have at least 3 generations of genealogy connecting to Utah founders, including approximately 1.8 million patients within the Intermountain system. Institutional review board approval was in place for this study.
Brain Cancer Cases and Samples
All individuals with diagnoses of primary brain cancer who were linked to at least 3 generations of genealogy data were identified in the UCR with International Classification of Diseases for Oncology site coding for brain (primary site 710–719; histology 8000–9529, 9540–9589). The Intermountain Healthcare Biorepository houses over 5 million archival formalin-fixed paraffin embedded (FFPE) tissue samples extending back to the 1960s. These samples are linked to more than 1.8 million individuals, including over 1900 individuals with diagnoses of primary brain cancer. Tissue scrolls or directed punches were prepared from FFPE blocks containing normal tissue for DNA extraction. When available, normal tissue was obtained from nonbrain tissue (preferred sample type; n = 34) or normal brain tissue farthest from the tumor (n = 13) when another normal sample was not available. The Genetic Epidemiology Laboratory at the University of Utah had an additional 75 stored DNA samples extracted from whole blood from primary brain cancer cases identified and recruited as members of high-risk cancer pedigrees. These samples were obtained from over 30 years of high-risk cancer pedigree studies for multiple different cancer types.
High-Risk Primary Brain Cancer Pedigrees
All relationships among all primary brain cancer cases with a stored sample at Intermountain or Genetic Epidemiology who were linked to at least 3 generations of genealogy data were identified. We attempted to gather available stored samples of primary brain tumor and noncancerous (normal) tissues from the largest high-risk brain cancer pedigrees identified in this fashion from both Genetic Epidemiology and Intermountain biorepositories. We obtained samples for 83 primary brain cancer cases.
These sampled brain cancer cases clustered in 26 sets of descending individuals from a common ancestor pair (pedigrees) including at least 2 sampled primary brain cancer cases. Since such pedigrees could occur by chance clustering, each pedigree was tested for a statistical excess of primary brain cancer cases among the descendants as follows. All ~3 million individuals in the UPDB with at least 3 generations of genealogy were assigned to a sex, 5-year birth year range, and birth state (Utah or not) cohort (n = 136 total cohorts). Cohort-specific rates of primary brain cancer were estimated by counting all primary brain cancer cases in each cohort and dividing by the total number of individuals in each cohort. To determine whether a specific pedigree had a significant excess of primary brain cancer among the descendants, the number of observed primary brain cancer cases in the pedigree was compared with the expected number of primary brain cancer cases in the pedigree, using the internal UPDB primary brain cancer rates estimated as described above. To estimate the expected number of primary brain cancer cases in a pedigree, all descendants were counted by cohort; the number of descendants in each cohort was multiplied by the cohort-specific rate of primary brain cancer and summed over all cohorts. All pedigrees with a significant excess of observed to expected primary brain cancer cases (P < 0.05) were considered pedigrees at high risk for primary brain cancer; 6 pedigrees included 2 sampled primary brain cancer cases, 3 pedigrees included 3 sampled primary brain cancer cases, 1 pedigree included 5 sampled primary brain cancer cases, and 1 pedigree included 6 sampled primary brain cancer cases. We attempted to gather available stored samples of brain tumor and noncancerous (normal) tissues from the largest high-risk brain cancer pedigrees identified in this fashion from both Genetic Epidemiology and Intermountain biorepositories.
Genome-wide Genotype Data
Data for the Illumina OmniExpress 720k single nucleotide polymorphism (SNP) marker set, commonly used for genome-wide association studies (GWAS), were generated for the selected sampled primary brain cancer cases, allowing performance of standard linkage analysis with dense coverage. Many fewer markers are necessary for genome-wide linkage analysis than are needed for GWAS. For linkage analysis, markers that are associated, or in linkage disequilibrium (LD), are not included because of the risk of false positives from allele sharing that is a result of LD, rather than linkage. A much smaller subset of SNP markers can represent the entire genome. The set of 720 000 genotyped SNPs was reduced to a low-LD subset of markers by selecting SNPs with higher heterozygosity and low or no LD using publicly available HapMap data for 30 trios of US residents of northern and western European ancestry (CEU). Specifically, markers with a minimum spacing of 0.1 centimorgans (cM), and minimum heterozygosity (0.3) were selected; using a sliding window of 500 000 base pairs, r2 between markers was minimized (max r2 of 0.16). This strategy resulted in selection of 27 157 markers genome-wide with a median heterozygosity of 0.49 and median spacing of 0.14 cM. Quality control excluded all SNPs with a call rate <98%, and all individuals with a call rate <98%. Illumina OmniExpress 720k SNP data were available for 32 primary brain cancer cases related in 11 extended high-risk pedigrees after quality control.
Linkage Analysis
MCLINK15 software was used for linkage analysis. MCLINK uses Monte Carlo Markov chain techniques including blocked Gibbs sampling to generate haplotype reconstructions that are used to extract inheritance information in pedigrees.15–17 In addition to calculating standard multipoint logarithm of the odds (LOD) scores, the program also calculates robust multipoint theta LOD scores (TLODs18). Uncertainties about the genetic model, locus heterogeneity, and misdiagnosis can complicate conventional multipoint LOD score analysis. However, the TLOD incorporates optimization of the recombination fraction (theta) in the statistic’s parameterization (similar to the two-point linkage statistic), so it retains the robustness of the two-point LOD to model misspecification, while also benefiting from multipoint information. Because these extended Utah high-risk pedigrees are typically large enough to be singly informative for linkage analysis, and because a given case could be included in more than one high-risk pedigree, we considered evidence for each pedigree separately as well as a statistic for all pedigrees combined.
All pedigrees were analyzed using an “affecteds-only” model that assumed a disease allele frequency of 0.005 for a dominant model and 0.05 for a recessive model. The penetrance estimates for carriers and noncarriers were 0.5 and 0.0005, respectively. LOD scores >1.86 (corresponding to a false-positive rate of 1 per genome) were considered as suggestive evidence for linkage, and scores >3.30 as significant, as defined by Lander and Kruglyak,19 whether considering evidence for 1 pedigree or for multiple pedigrees.
Results
Multipoint Linkage Analysis
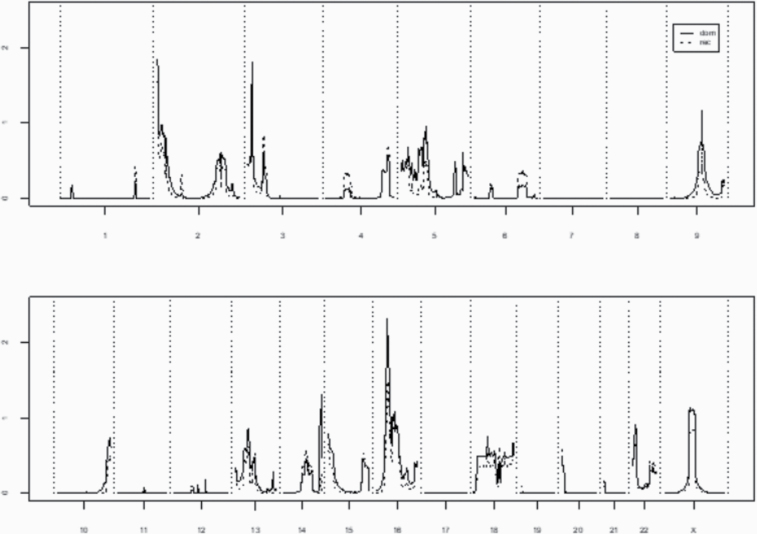
A total of 11 high-risk primary brain cancer pedigrees with at least 2 primary brain cancer cases with good quality genotype data were analyzed. Summary genome-wide het-TLODs for both the dominant and recessive models are shown in Figure 1. Although no regions reached significant evidence for linkage (LOD > 3.30), one region had suggestive evidence for linkage (chromosome 16, marker rs8056212, Hetlod = 2.31).
Fig. 1.
Genome-wide HetLOD scores for 11 high-risk primary brain cancer pedigrees combined.
Pedigree-Specific Multipoint Linkage
Although overall multipoint consideration of Utah high-risk pedigrees did not give significant evidence for linkage, some of the Utah high-risk pedigrees could be sufficiently informative to provide significant evidence for linkage when analyzed alone. Analyzing such pedigrees independently could allow identification of rare (private) segregating variants. Table 1 shows results of linkage analysis for the 2 pedigrees which exhibited suggestive evidence of linkage (LOD > 1.86) for the dominant model; suggestive evidence for linkage was observed twice for each pedigree, on 2 different chromosomes. These 2 pedigrees included 5 and 6 sampled cases, respectively, with no overlap. Table 1 shows chromosome, pedigree identification, TLOD score, and chromosome band. No single pedigrees were observed to exhibit significant evidence for linkage (TLOD > 3.30). No significant or suggestive evidence of linkage was observed at any locus for the recessive model.
Table 1.
Pedigree-specific linkage results for the dominant model for high-risk primary brain cancer pedigrees
| Chromosome | Pedigree ID | TLOD | Chromosome Band |
|---|---|---|---|
| 2 | K5100 | +2.29 | 2p25.3 |
| 3 | K5100 | +2.40 | 3p26.1 |
| 14 | K5101 | +1.93 | 14q32.32 |
| 16 | K5101 | +2.44 | 16p13.11 |
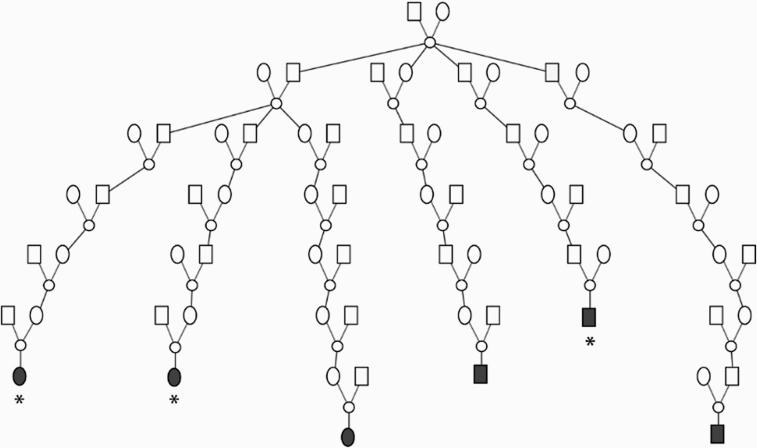
The pedigree in which the linkage evidence for chromosome 16 was observed is shown in Figure 2. The male founder was born in New York in the late 1700s and has over 30 000 descendants represented in the UPDB dating to modern day; descendants from 2 marriages are represented in the UPDB and both include primary brain cancer cases (only 1 marriage shown). Among the descendants of the pedigree founder, 39 brain cancer patients were observed and 26.4 were expected (P = 0.0128). Table 2 shows these primary brain cancers considered by histologic type. Only one type of primary brain cancer was observed in significant excess of what was expected among all descendants: 5 ependymomas were observed (1.2 expected; P = 0.007). Six of the 39 primary brain cancers in this pedigree had available samples; Table 3 shows details for these 6 primary brain cancer cases; details have been generalized to prevent identification.
Fig. 2.
High-risk primary brain cancer pedigree K5101 showing evidence for linkage on chromosome 16; only descendants connected to genotyped primary brain cancer cases (fully shaded) are shown. *Inferred haplotype carriers.
Table 2.
Total primary brain cancers recorded in UPDB in linked pedigree K5101 (Figure 2), by histologic type
| Type | Histology Coding | Obs | Exp | P-value |
|---|---|---|---|---|
| Astrocytoma | 9380–82, 9400–1, 9410–11, 9420–1, 9423–24, 9430 | 14 | 11.7 | 0.283 |
| Glioblastoma | 9440–9442 | 11 | 6.7 | 0.077 |
| Oligodendroglioma | 9450–51 | * | 2.3 | 0.657 |
| Ependymoma | 9391–92 | 5 | 1.2 | 0.0071 |
| Medulloblastoma | 9470–71, 9473 | * | 1.8 | 0.276 |
| Oligoastrocytoma | 9382 | * | 0.9 | 0.602 |
| Unclassified | 4 | NA | NA |
*Counts <5 censored to protect privacy.
Table 3.
Characteristics of the 6 genotyped primary brain cancer cases in K5101, the chromosome 16-linked pedigree shown in Figure 2
| Primary | Age at Diagnosis | Survival | ||
|---|---|---|---|---|
| Site | Stage | Years | Months | Histology |
| 717& | 1 | <10 | 36–48 | 9470-medulloblastoma |
| 715 | 1 | 60–70 | <12 | 9391-ependymoma |
| NA | 1 | 50–60 | <12 | 9440-glioblastoma |
| 713* ^ | 1 | 50–60 | <12 | 9401-astrocytoma |
| 715* & | 1 | 50–60 | 24–36 | 9390-choroid plexus carcinoma |
| 711* | 1 | >80 | <12 | 9440-glioblastoma |
Stage 1 = localized.
* Haplotype sharing cases from linkage analysis.
^ Positive for IDH1.
& Negative for IDH1.
Discussion
While it is recognized that there is an inherited contribution to predisposition to primary brain cancer, it is also clear that it has been difficult to create a powerful resource of sampled high-risk pedigrees due to the relative rarity, lethality and heterogeneity of this cancer. Using a unique Utah resource consisting of a population-based genealogy linked to a statewide cancer registry and decades-old biorepositories, a resource of extended, sampled high-risk primary brain cancer pedigrees has been created, and genome-wide linkage analysis for evidence of a primary brain cancer predisposition gene has been performed.
Paunu et al reported the first genome-wide linkage analysis of familial glioma in a Finnish population, and noted a novel susceptibility locus at 15q23–q26.3.13 The Genetic Epidemiology of Glioma International Consortium (Gliogene) has created a worldwide resource of families with samples from 2 or more confirmed glioma cases that have been interrogated for evidence for predisposition genes.20,21 Shete et al21 presented the Gliogene genome-wide linkage analysis of 46 US families selected for the presence of 2 or more cases of histologically confirmed glioma; significant evidence for linkage was observed at 17q12–21.32 (P = 0.0005); this finding was replicated in 29 additional independent US families (P = 0.008). Three other regions had maximum nonparametric linkage scores >2.0, including 6p22.3, 12p13.33-12.1, and 18q23. The tumor protein 53 variant has been associated with a 2-fold increase in relative risk for hereditary glioma. Association with a variant on chromosome 8 has shown as high as a 6-fold relative risk for isocitrate dehydrogenase (IDH)–mutated glioma.3 The analysis of high-risk Utah pedigrees did not identify any LOD scores >1.0 (nominal evidence) at any of these previously reported chromosomal locations.
There are several significant differences between previous linkage studies and the Utah analysis presented here that might be responsible for differences in findings. All Gliogene and Finnish cases were confirmed gliomas, while Utah pedigrees included independent primary brain cancers of any histology, identified by primary site. Gliogene and Finnish pedigrees were selected for the presence of 2 or more related sampled cases, while Utah pedigrees were confirmed as a set of descendants from a founder pair among which a significant excess of primary brain cancers (exceeding Utah population rates) was observed, and were thus identified as high-risk primary brain cancer pedigrees, not just clusters of related primary brain cancer cases. Utah pedigrees primarily included individuals who were deceased and unavailable for sampling (but who had stored samples available at a large hospital biorepository) and were therefore not subject to survival bias; Gliogene and Finnish cases were available for sampling, which could have led to bias toward longer surviving cases.
This analysis of Utah pedigrees identified 4 suggestive regions based on evidence from single pedigree analyses; one of these regions was also suggestive based on analysis of all pedigrees combined. The chromosome band 16p13.11 region was supported by both analyses, and is perhaps most interesting, with the evidence from the single pedigree most likely almost entirely responsible for the evidence from all pedigrees combined. This region has been previously reported to be of interest for gliomas. Zheng et al22 reported chromosome 16p13.1–13.3 as a region of common loss in ependymomas. The maximum linkage evidence in the pedigree with suggestive evidence for linkage in this study occurred at an SNP (rs3198697) located in the pyridoxal-dependent decarboxylase domain-containing protein 1 gene (PDXDC1). Melin et al23 reported significant association of glioblastoma at 16p13.3 rs2562152, P = 1.9e-8, odds ratio = 1.21) in a large meta-analysis of GWAS data for glioma. Online Mendelian Inheritance in Man reports that PDXDC1 expression is detected in all human tissues and cell lines examined (https://www.omim.org/entry/614244). The Cancer Genome Atlas reports antibody staining of the protein in 17 different cancers, including glioma (https://www.proteinatlas.org/ENSG00000179889-PDXDC1/pathology). Feldcamp et al24 reported that PDXDC1 mRNA and protein are both strongly expressed in the hippocampus. The Human Protein Atlas shows antibody staining of the protein in 20 different cancers, including gliomas (https://www.proteinatlas.org/ENSG00000179889-PDXDC1/pathology). Huang et al25 performed a gene-centric integrative GWAS of glioma risk, combining transcriptomics and genetics, and identified PDXDC1 as one of 4 novel glioma susceptibility genes with internal and external validation. The linkage evidence from the current study mapping to this same locus strengthens the evidence for a possible role for PDXDC1 in glioma risk.
Potential limitations of this analysis include data censoring of any primary brain cancer cases diagnosed outside the state of Utah or before 1973, and censoring of any Utah primary brain cancer cases which did not link to the Utah genealogy data. This would have resulted in censoring some brain cancer cases in the pedigrees studied, and would likely not affect the high-risk classification of the pedigrees or the analysis. An additional limitation is that findings may only be applicable to the relatively homogeneous Utah population whose founders were largely from northern Europe. Strengths of the study include the histopathologic confirmation of all primary brain cancer cases recorded in the UCR, the validation of each pedigree as exhibiting a statistically significant excess of primary brain cancer over population rates, and the distant relationships between related cases in high-risk pedigrees, which adds power to genetic analysis. While many studies of brain cancer families limit focus to the specific glioblastoma subtype, here we have considered all primary brain cancers, regardless of subtype, as potentially associated with the same inherited predisposition.
This analysis of DNA from high-risk Utah primary brain cancer pedigrees shows the value of the combination of 2 unique resources, a computerized genealogy of the Utah population and tissues stored for decades at a biorepository representing the largest health care provider in the state and the principal investigator’s smaller biorepository. These resources allowed analysis of powerful and informative high-risk primary brain cancer pedigrees that are a rare resource for such a fatal disorder. This resource has the potential to allow similar analyses for other disorders with limited opportunity to obtain biosamples.
This analysis has identified 4 regions of interest for primary brain cancer predisposition genes which can all be pursued. Sequence analysis of PDXDC1 in the available brain cancer samples from the linked high-risk pedigree could identify a variant responsible for the excess of primary brain cancers observed in this pedigree, and validation of association with risk for primary brain cancer could be established with an independent set of brain cancer cases and controls.
Acknowledgments
Special thanks to the Intermountain Biorepository staff who identified and prepared samples.
Conflict of interest statement. There are no conflicts of interest for any authors.
Authorship statement. Experimental design: LAC-A, JMF, CCT. Implementation: MHC, KR. Analysis/interpretation of data: LAC-A, JMF, JS, CCT, CAP. Writing draft and revision of manuscript: LAC-A, JMF, JS, CAP, CCT, MHC, KR, DTB.
Funding
This research was supported by the Utah Cancer Registry, which is funded by the National Cancer Institute’s SEER Program, Contract No. HHSN261201800016I, the US Centers for Disease Control and Prevention’s National Program of Cancer Registries, Cooperative Agreement No. NU58DP0063200-01, with additional support from the University of Utah and Huntsman Cancer Foundation. Partial support for all datasets within the Utah Population Database is provided by the University of Utah, Huntsman Cancer Institute and the Huntsman Cancer Institute Cancer Center Support grant, P30 CA42014 from the National Cancer Institute. LAC-A acknowledges support from the Huntsman Cancer Institute Cancer Center Support grant, P30 CA42014 from the National Cancer Institute. Partial support for sample and data acquisition and analysis was from a research grant from the Intermountain Research and Medical Foundation.
References
- 1. Johansson G, Andersson U, Melin B. Recent developments in brain tumor predisposing syndromes. Acta Oncol. 2016;55(4):401–411. [DOI] [PubMed] [Google Scholar]
- 2. Sun X, Vengoechea J, Elston R, et al. ; Gliogene Consortium . A variable age of onset segregation model for linkage analysis, with correction for ascertainment, applied to glioma. Cancer Epidemiol Biomarkers Prev. 2012;21(12):2242–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rice T, Lachance DH, Molinaro AM, et al. Understanding inherited genetic risk of adult glioma—a review. Neurooncol Pract. 2016;3(1):10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blumenthal DT, Cannon-Albright LA. Familiality in brain tumors. Neurology. 2008;71(13):1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461(7265):747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wijsman EM. The role of large pedigrees in an era of high-throughput sequencing. Hum Genet. 2012;131(10):1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ott J, Wang J, Leal SM. Genetic linkage analysis in the age of whole-genome sequencing. Nat Rev Genet. 2015;16(5):275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266(5182):66–71. [DOI] [PubMed] [Google Scholar]
- 9. Tavtigian SV, Simard J, Rommens J, et al. The complete BRCA2 gene and mutations in chromosome 13q-linked kindreds. Nat Genet. 1996;12(3):333–337. [DOI] [PubMed] [Google Scholar]
- 10. Kamb A, Shattuck-Eidens D, Eeles R, et al. Analysis of the p16 gene (CDKN2) as a candidate for the chromosome 9p melanoma susceptibility locus. Nat Genet. 1994;8(1):23–26. [DOI] [PubMed] [Google Scholar]
- 11. Teerlink CC, Huff C, Stevens J, et al. A non-synonymous variant in GOLM1 in cutaneous malignant melanoma. J Natl Cancer Inst. 2018;110(12):1380–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malmer B, Haraldsson S, Einarsdottir E, Lindgren P, Holmberg D. Homozygosity mapping of familial glioma in Northern Sweden. Acta Oncol. 2005;44(2):114–119. [DOI] [PubMed] [Google Scholar]
- 13. Paunu N, Lahermo P, Onkamo P, et al. A novel low-penetrance locus for familial glioma at 15q23-q26.3. Cancer Res. 2002;62(13):3798–3802. [PubMed] [Google Scholar]
- 14. Cannon Albright LA. Utah family-based analysis: past, present and future. Hum Hered. 2008;65(4):209–220. [DOI] [PubMed] [Google Scholar]
- 15. Thomas A, Gutin A, Abkevich V, Bansal A. Multilocus linkage analysis by blocked Gibbs sampling. Stat Comput. 2000;10:259–269. [Google Scholar]
- 16. Camp NJ, Gutin A, Abkevich V, Farnham JM, Cannon-Albright L, Thomas A. A new nonparametric linkage statistic for mapping both qualitative and quantitative trait loci. Genet Epidemiol. 2001;21(Suppl 1):S461–S466. [DOI] [PubMed] [Google Scholar]
- 17. Abkevich V, Camp NJ, Gutin A, Farnham JM, Cannon-Albright L, Thomas A. A robust multipoint linkage statistic (tlod) for mapping complex trait loci. Genet Epidemiol. 2001;21(Suppl 1):S492–S497. [DOI] [PubMed] [Google Scholar]
- 18. Göring HH, Terwilliger JD. Linkage analysis in the presence of errors I: complex-valued recombination fractions and complex phenotypes. Am J Hum Genet. 2000;66(3):1095–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet. 1995;11(3):241–247. [DOI] [PubMed] [Google Scholar]
- 20. Malmer B, Adatto P, Armstrong G, et al. GLIOGENE—an international consortium to understand familial glioma. Cancer Epi Bio Prev. 2007;16(9):1730–1734. [DOI] [PubMed] [Google Scholar]
- 21. Shete S, Lau CC, Houlston RS, et al. ; Gliogene Consortium . Genome-wide high-density SNP linkage search for glioma susceptibility loci: results from the Gliogene Consortium. Cancer Res. 2011;71(24):7568–7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zheng PP, Pang JC, Hui AB, Ng HK. Comparative genomic hybridization detects losses of chromosomes 22 and 16 as the most common recurrent genetic alterations in primary ependymomas. Cancer Genet Cytogenet. 2000;122(1):18–25. [DOI] [PubMed] [Google Scholar]
- 23. Melin BS, Barnholtz-Sloan JS, Wrensch MR, et al. ; GliomaScan Consortium . Genome-wide association study of glioma subtypes identifies specific differences in genetic susceptibility to glioblastoma and non-glioblastoma tumors. Nat Genet. 2017;49(5):789–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feldcamp LA, Boutros PC, Raymond R, Fletcher PJ, Nobrega JN, Wong AHC. PDXDC1 modulates prepulse inhibition of acoustic startle in the mouse. Transl Psychiatry. 2017;7(5):e1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang YT, Zhang Y, Wu Z, Michaud DS. Genotype-based gene signature of glioma risk. Neuro Oncol. 2017;19(7):940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]