Abstract
Background
We investigated differences in radiation-induced grade 3+ lymphopenia (G3+L), defined as an absolute lymphocyte count (ALC) nadir of <500 cells/µL, after proton therapy (PT) or X-ray (photon) therapy (XRT) for patients with glioblastoma (GBM).
Methods
Patients enrolled in a randomized phase II trial received PT (n = 28) or XRT (n = 56) concomitantly with temozolomide. ALC was measured before, weekly during, and within 1 month after radiotherapy. Whole-brain mean dose (WBMD) and brain dose-volume indices were extracted from planned dose distributions. Univariate and multivariate logistic regression analyses were used to identify independent predictive variables. The resulting model was evaluated using receiver operating characteristic (ROC) curve analysis.
Results
Rates of G3+L were lower in men (7/47 [15%]) versus women (19/37 [51%]) (P < 0.001), and for PT (4/28 [14%]) versus XRT (22/56 [39%]) (P = 0.024). G3+L was significantly associated with baseline ALC, WBMD, and brain volumes receiving 5‒40 Gy(relative biological effectiveness [RBE]) or higher (ie, V5 through V40). Stepwise multivariate logistic regression analysis identified being female (odds ratio [OR] 6.2, 95% confidence interval [CI]: 1.95‒22.4, P = 0.003), baseline ALC (OR 0.18, 95% CI: 0.05‒0.51, P = 0.003), and whole-brain V20 (OR 1.07, 95% CI: 1.03‒1.13, P = 0.002) as the strongest predictors. ROC analysis yielded an area under the curve of 0.86 (95% CI: 0.79–0.94) for the final G3+L prediction model.
Conclusions
Sex, baseline ALC, and whole-brain V20 were the strongest predictors of G3+L for patients with GBM treated with radiation and temozolomide. PT reduced brain volumes receiving low and intermediate doses and, consequently, reduced G3+L.
Keywords: GBM, glioblastoma, lymphopenia, proton therapy
Key Points.
Protons, versus photons, reduce irradiated brain volumes and thus severe lymphopenia.
Patients with low baseline lymphocyte counts and women are at high risk.
GBM patients at high risk of severe lymphopenia could benefit from proton therapy.
Importance of the Study.
Severe radiation-induced lymphopenia (RIL) is associated with reduced survival in GBM and other forms of cancer. Lymphocytes are highly radiosensitive. Proton therapy, because of the unique dosimetric characteristics of protons, can considerably reduce the volumes of tissues receiving low and intermediate radiation doses, thereby substantially sparing lymphocytes. Here we compared RIL among GBM patients given protons or photons and investigated associations between severe RIL and dosimetric and patient-specific factors. We developed a predictive model of severe RIL for patients with GBM treated with radiotherapy plus temozolomide and found that female patients and those with low baseline lymphocyte counts are at high risk of severe RIL. This model may be useful for identifying patients at high risk of RIL who could benefit from proton therapy, and such models could also be incorporated into the optimization of treatment plans to mitigate RIL.
Glioblastoma (GBM) is the most common and aggressive primary brain tumor among adults. The median survival time after standard X-ray (photon) radiotherapy (XRT) and concurrent and adjuvant temozolomide (TMZ) is approximately 15 months. Radiotherapy, with or without chemotherapy, has been associated with radiation-induced lymphopenia (RIL) in patients with numerous tumor types, including GBM.1–9 High-grade RIL has been associated with reduced overall survival,3,6,8,10–14 increased risk of recurrence,15,16 reduced response rates,17 and possibly opportunistic infections. Such consequences extend across cancer types, including gliomas, breast, pancreas, lung, hepatocellular, head and neck, esophageal, cervical, and bladder cancers.7,9,14,18–23
The incidence and severity of RIL are associated with several baseline and dosimetric factors, such as treatment duration (number of fractions), target volume size, age, baseline absolute lymphocyte count (ALC), body mass index (BMI), and, more recently, treatment modality (ie, protons vs photons). In a previous study of patients with esophageal cancer receiving concurrent chemotherapy and either intensity-modulated (photon) radiotherapy (IMRT) or passively scattered proton therapy (PSPT), 35% of patients had Common Terminology Criteria for Adverse Events v4 (CTCAE) grade 4 RIL, which was associated with both disease-specific and overall survival.23 Patients treated with protons had 70% lower rates of grade 4 RIL compared with those treated with IMRT. This and other reports comparing lymphopenia among patients receiving protons versus photons9,23–27 suggest that protons, because of the compact nature of their dose distributions, could spare the lymphocyte-bearing tissues to a greater degree and, therefore, reduce the risk of severe lymphopenia and potentially improve disease outcomes. (See the Supplementary Figure 1 for an explanation.)
Grossman et al7 studied immunosuppression in patients treated for high-grade glioma with XRT and TMZ and found reductions in CD4 counts to be common, treatment related, long-lasting, and associated with early death from tumor progression. In a separate study, Huang et al28 reported that being female, being older, having lower baseline ALC, and having higher brain volume receiving ≥25 Gy were significant predictors of acute severe lymphopenia (ASL) during radiotherapy plus TMZ. In this study, we use the term “G3+L” instead of ASL, defining it as an ALC nadir of <500 cells/µL, and investigated its association with baseline patient-specific and dosimetric factors and treatment modality (ie, protons or photons) for GBM patients treated on a randomized phase II trial.
Materials and Methods
Patients and Treatment
Patients selected for this retrospective analysis had been enrolled in a randomized phase II GBM trial of proton versus photon therapy (NCT01854554, Glioblastoma Multiforme Proton vs Intensity-Modulated Radiotherapy) and treated from March 2014 through March 2016. The trial was approved by The MD Anderson Cancer Center institutional review board, and all patients had provided written informed consent before enrollment. Inclusion criteria included histologically confirmed GBM and age >18 years. All patients received the prescribed regimen of concurrent and adjuvant TMZ, with standard dose modifications determined at the discretion of the treating medical oncologists. The primary objective of the trial was to investigate differences in time to cognitive failure between protons and photons. The manuscript describing the analysis of the primary outcomes is in preparation. The inclusion criteria for the present retrospective analysis included having at least 4 documented weekly ALC measurements, specifically at baseline (before treatment), at least twice during treatment, and at approximately one month after treatment. For radiation treatment planning, the gross tumor volume (GTV) was defined as tumor cavity and any T1 tumor enhancement. The clinical target volume (CTV) included the GTV + a 2 cm margin customized to include fluid attenuated inversion recovery enhancement (if considered by the radiation oncologist to be tumor) and excluded bone, fascia, and other anatomic barriers. Planning target volume (PTV) included a PTV-50 composed of CTV + a 3‒5 mm expansion treated to 50 Gy(RBE) and a PTV-60 composed of GTV + a 3‒5 mm expansion treated to 60 Gy(RBE) in 30 fractions. Because of the greater sensitivity of proton dose distributions to positioning and proton range uncertainties, the volumes used for planning proton treatments are different from those for photons. Nevertheless, for consistency, the PTVs used for the analyses and intercomparisons of proton and photon data were defined identically. The “simultaneous integrated boost” technique was used to deliver different prescribed doses to both the PTVs. In this paper we use units of Gy(relative biological effectiveness [RBE]); for protons, the RBE is assumed to be 1.1, whereas for photons, the RBE by definition is 1. A total of 89 patients met the inclusion criteria; however, 5 were excluded for having received mixed proton and photon treatments.
Lymphopenia was graded according to the CTCAE v4.0 as grade 0 (greater than or equal to the lower limit of normal [LLN]), grade 1 (lower than the LLN to ≥0.8 × 109 cells/L), grade 2 (<0.8 to ≥0.5 × 109 cells/L), grade 3 (<0.5 to ≥0.2 × 109 cells/L), and grade 4 (<0.2 × 109 cells/L), all nadir values.29 The time frame for assessing G3+L was defined as from the start of to 1 month after the completion of radiation therapy.
Photon treatment plans were produced with a Pinnacle system (Philips Radiation Oncology Systems), whereas proton treatment plans were produced with an Eclipse system (Varian Medical Systems). Planning target volumes (PTV-50 and PTV-60), the WBMD, and the whole-brain volumes receiving 5, 10, 15, . . . 50 Gy(RBE) or higher (denoted as V5 through V50) were extracted from the treatment plans.
Correlative Studies and Statistical Analyses
The primary endpoint for this retrospective analysis was G3+L. Patient-specific baseline characteristics including age, sex, body mass index (as surrogate for total blood volume), baseline ALC, baseline white blood cell count, receipt of steroids before radiation, tumor location, GTV, CTV, PTV-50 and PTV-60; and treatment-related factors and dosimetric factors including radiation modality, WBMD, and V5, V10, V15, . . . , V50 were evaluated using standard descriptive statistics with mean and standard deviation for continuous variables and with frequency and proportions for categorical variables. The Wilcoxon rank-sum test was used to examine differences in continuous variables between patient-characteristic groups as well as between radiation modalities. Associations between categorical variables were assessed using chi-squared or Fisher’s exact tests, as appropriate. Correlation analysis was performed with the Spearman method. Univariate logistic regression analysis was used to provide odds ratios (ORs) with corresponding 95% confidence intervals (CIs) for investigating the association of a variable with lymphopenia. Stepwise logistic regression multivariate analysis (MVA) was then performed to determine the most significant predictors from the candidate set of univariate analysis variables. Ten-fold cross-validation was carried out to evaluate the robustness of the MVA prediction model. The receiver operating characteristic (ROC) curve analysis, which summarized G3+L classification accuracy with the area under the curve (AUC), was used to assess the performance of the final prediction model. P-values less than 0.05 were considered to indicate statistical significance. All statistical analyses were done with R v3.4.0.
Results
Of the 84 patients enrolled in the study, 47 were men and 37 women; 28 received protons (20 intensity-modulated proton therapy [IMPT], 5 PSPT, and 3 a combination of IMPT and PSPT), and 56 received photons (IMRT or volumetric modulated arc therapy) (Table 1). The imbalance in patient numbers between the proton and photon arms was due primarily to the denial of insurance coverage for proton therapy. The randomized trial (the source of patient data for this study) was powered to study differences in cognition. Because this was a phase II trial, enrollment continued until sufficient numbers of patients were in the proton arm. Descriptive statistics indicated that sex, baseline ALC, treatment modality, receipt of steroids before radiation, WBMD, and whole-brain V5 through V40 were significantly associated with G3+L. (See also Supplementary Figure 2.) A total of 26 patients developed G3+L, 4 of whom (14%) were treated with protons and 22 (39%) with photons. Nineteen of 37 women (51%) and 7 of 47 men (15%) developed G3+L (P < 0.001). Baseline ALC values (mean ± (SD)) were 1.3 ± 0.6 × 103/µL among patients who developed G3+L versus 1.7 ± 0.5 × 103/µL (P < 0.001) among those who did not. The incidence of G3+L among patients treated with photons was significantly higher than that for patients treated with protons. Mean WBMD was 28.1 ± 6.3 Gy(RBE) for patients with G3+L and 23.2 ± 6.5 Gy(RBE) for those without (P = 0.001). Brain volumes V5 through V40 were all associated with G3+L. Of the 41 patients who received pretreatment steroids, 18 (44%) developed G3+L, as opposed to 8 (19%) of 43 who did not (P = 0.009). However, pretreatment steroids were found to influence the baseline ALC (mean 1.47 × 103/µL with steroids vs 1.69 × 103/µL without steroids, P = 0.042; Supplementary Figure 3). Other factors listed in Table 1 were not significantly different between patients with or without G3+L.
Table 1.
Baseline and dosimetric characteristics grouped according to the occurrence of grade 3+ lymphopenia*
| Characteristic | Total (n = 84) | G0–2L (n = 58) | G3+L (n = 26) | P value |
|---|---|---|---|---|
| Sex (%) | <0.001* | |||
| Male | 47 (56.0) | 40 (69.0) | 7 (26.9) | |
| Female | 37 (44.0) | 18 (31.0) | 19 (73.1) | |
| Age , mean (SD) | 52.6 (12.4) | 52.7 (11.7) | 52.3 (13.9) | 0.892 |
| BMI, mean (SD) | 28.3 (7.5) | 29.3 (8.3) | 26.2 (4.8) | 0.077 |
| GTV, mean (SD) | 47.0 (35.3) | 43.9 (35.7) | 53.7 (34.2) | 0.240 |
| CTV, mean (SD) | 231.1 (93.5) | 221.7 (91.7) | 252.1 (95.8) | 0.167 |
| PTV-50, mean (SD) | 331.4 (125.2) | 317.9 (122.7) | 361.4 (128.0) | 0.139 |
| PTV-60, mean (SD) | 91.6 (65.7) | 87.3 (69.9) | 101.0 (55.4) | 0.378 |
| Baseline ALC, mean (SD) | 1.6 (0.6) | 1.7 (0.5) | 1.3 (0.6) | <0.001* |
| Baseline WBC, mean (SD) | 8.5 (3.8) | 8.2 (3.8) | 9.1 (3.8) | 0.332 |
| Modality (%) | 0.024* | |||
| Protons | 28 (33.3) | 24 (41.4) | 4 (15.4) | |
| Photons | 56 (66.7) | 34 (58.6) | 22 (84.6) | |
| Pre-radiation steroids | 0.023* | |||
| No | 43 (51.2) | 35 (60.3) | 8 (30.8) | |
| Yes | 41 (48.8) | 23 (39.7) | 18 (69.2) | |
| Location (%) | 0.916 | |||
| Left | 37 (44.0) | 25 (43.1) | 12 (46.2) | |
| Right | 44 (52.4) | 31 (53.4) | 13 (50.0) | |
| Bilateral | 3 (3.6) | 2 (3.4) | 1 (3.8) | |
| Whole Brain DVH, mean (SD) | ||||
| Mean, Gy(RBE) | 24.7 (6.8) | 23.2 (6.5) | 28.1 (6.3) | 0.001* |
| V5 (%) | 76.8 (21.1) | 72.8 (22.2) | 85.6 (15.5) | 0.008* |
| V10 (%) | 68 (20.3) | 63.4 (20.4) | 78.2 (16.2) | 0.001* |
| V15 (%) | 58 (18.1) | 54.1 (17.6) | 66.8 (16.2) | 0.002* |
| V20 (%) | 48.2 (15.2) | 44.7 (14.1) | 56.1 (14.9) | <0.001* |
| V25 (%) | 40.9 (12.8) | 38.3 (11.8) | 46.7 (13.2) | 0.004* |
| V30 (%) | 35.7 (11.1) | 33.6 (10.3) | 40.3 (11.8) | 0.009* |
| V40 (%) | 29 (9.6) | 27.5 (8.9) | 32.3 (10.3) | 0.028* |
| V50 (%) | 22.1 (8.2) | 21 (7.2) | 24.6 (9.7) | 0.064 |
*Quantities in parentheses are standard deviations for continuous variable and percentage of patients for categorical variables. Asterisks denote statistically significant parameters
Abbreviations: G0–2L, grade 0‒2 lymphopenia; G3+L, grade 3+ lymphopenia; BMI, body mass index; GTV, gross tumor volume; CTV, clinical target volume; PTV-50 and PTV-60, planning target volumes receiving higher than 50 and 60 Gy respectively; ALC: absolute lymphocyte count; WBC, white blood cells count (in units of 100 cells per liter); DVH, dose-volume histogram; V5, V10, . . . , brain volumes receiving greater than 5, 10, . . . Gy(RBE) dose.
Table 2 summarizes baseline and dosimetric characteristics of the study population grouped according to treatment modality (protons or photons). Baseline characteristics were generally well balanced between the 2 groups, whereas the dosimetric characteristics generally tended to favor protons. Although the mean baseline ALC values between the proton and photon groups were not significantly different (1.54 vs 1.6 × 103/µL, P = 0.675), the mean ALC nadir was significantly higher for the proton group than for the photon group (0.86 vs 0.69 × 103/µL, P = 0.018; Supplementary Figure 4).
Table 2.
Baseline and dosimetric characteristics grouped according to treatment modality*
| Characteristic | Total (n = 84) | Protons (n = 28) | Photons (n = 56) | P-value |
|---|---|---|---|---|
| Sex, n (%) | 1 | |||
| Male | 47 (56.0) | 16 (57.1) | 31 (55.4) | |
| Female | 37 (44.0) | 12 (42.9) | 25 (44.6) | |
| Age, mean (SD) | 52.6 (12.4) | 55.1 (10.7) | 51.3 (13) | 0.177 |
| BMI, mean (SD) | 28.3 (7.5) | 30 (8.6) | 27.5 (6.8) | 0.139 |
| GTV, mean (SD) | 47.0 (35.3) | 41.7 (28.1) | 49.6 (38.4) | 0.339 |
| CTV, mean (SD) | 231.1 (93.5) | 215.1 (83.0) | 239.2 (98.0) | 0.264 |
| PTV-50, mean (SD) | 331.4 (125.2) | 295.4 (96.1) | 349.4 (134.7) | 0.058 |
| PTV-60, mean (SD) | 91.6 (65.7) | 74.0 (41.0) | 100.4 (73.9) | 0.079 |
| Baseline ALC, mean (SD) | 1.6 (0.6) | 1.5 (0.6) | 1.6 (0.6) | 0.675 |
| Baseline WBC, mean (SD) | 8.5 (3.8) | 8.2 (4.2) | 8.6 (3.6) | 0.663 |
| G3+L, n (%) | 0.024* | |||
| No | 58 (69.0) | 24 (85.7) | 34 (60.7) | |
| Yes | 26 (31.0) | 4 (14.3) | 22 (39.3) | |
| Location, n (%) | 0.916 | |||
| Left | 37 (44.0) | 13 (46.4) | 24 (42.9) | |
| Right | 44 (52.4) | 14 (50.0) | 30 (53.6) | |
| Bilateral | 3 (3.6) | 1 (3.6) | 2 (3.6) | |
| Preradiation steroids | 1 | |||
| No | 43 (51.2) | 14 (50.0) | 29 (51.8) | |
| Yes | 41 (48.8) | 14 (50.0) | 27 (48.2) | |
| Whole brain DVH, mean (SD) | ||||
| Mean, Gy(RBE) | 24.7 (6.8) | 20.1 (5.7) | 27.0 (6.1) | <0.001* |
| V5 (%) | 76.8 (21.1) | 51.9 (13.3) | 89.2 (10.8) | <0.001* |
| V10 (%) | 68 (20.3) | 46.2 (13) | 78.9 (13.3) | <0.001* |
| V15 (%) | 58 (18.1) | 42 (12.7) | 66.1 (14.8) | <0.001* |
| V20 (%) | 48.2 (15.2) | 37.4 (11) | 53.6 (14.2) | <0.001* |
| V25 (%) | 40.9 (12.8) | 35.3 (10.7) | 43.8 (13) | 0.003* |
| V30 (%) | 35.7 (11.1) | 32.5 (9.9) | 37.3 (11.5) | 0.059 |
| V40 (%) | 29 (9.6) | 27.6 (8.9) | 29.7 (9.9) | 0.363 |
| V50 (%) | 22.1 (8.2) | 21.1 (7.2) | 22.6 (8.6) | 0.410 |
Abbreviations: BMI, body mass index; GTV, gross tumor volume; CTV, clinical target volume; PTV-50 and PTV-60, planning target volumes receiving higher than 50 and 60 Gy respectively; ALC: absolute lymphocyte count; WBC, white blood cells count (in units of 100 cells per liter); G3+L, grade 3+ lymphopenia; DVH, dose-volume histogram; V5, V10, . . . , brain volumes receiving greater than 5, 10, . . . Gy(RBE) dose.
The WBMD and brain V5‒V40 were significantly lower for the proton group than for the photon group, but the volumes at higher doses were not significantly different. Treatment modality and irradiated volumes were found to correlate with each other (Fig. 1). In general, irradiated volumes are expected to be smaller for proton therapy (as explained in Supplementary Figure 1); however, depending on the PTV size, anatomy, and beam configurations, a subset of patients treated with photons may also have small irradiated volumes, and the reverse may be true for protons. The larger low- and intermediate-dose bath from photons may be responsible for the greater depletion of highly radiosensitive lymphocytes in the photon versus proton groups.
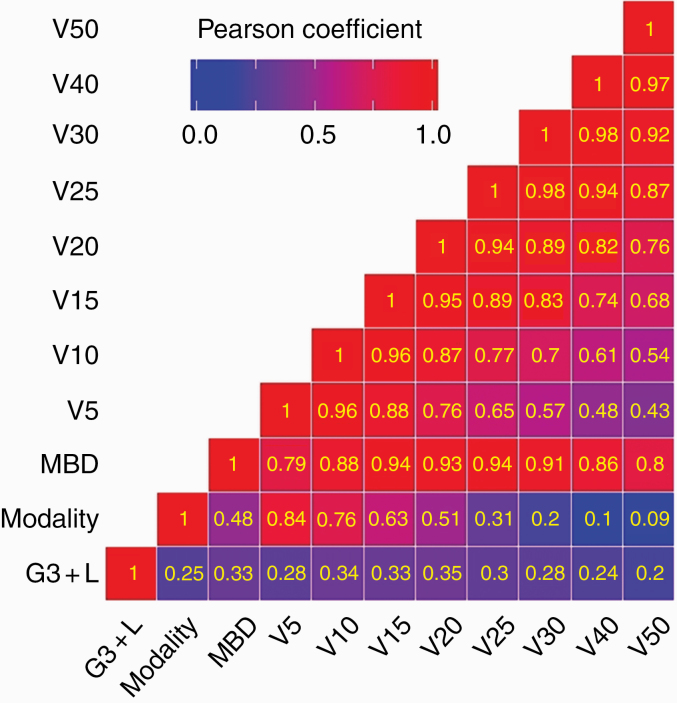
Fig. 1.
Pearson correlation matrix showing associations of G3+RIL with modality, mean brain dose (MBD), and volumes irradiated. Numbers in the boxes are correlation coefficients. G3+RIL was most strongly associated with V20 (volume receiving 20 Gy[RBE] or higher), and, to a somewhat lesser extent, with other volumes. Similarly, treatment modality was strongly associated with lower dose volumes but the association weakens as the dose increases. V20 is associated to varying degrees with all other volumes. In other words, if V20 is larger, then other volumes would be as well.
Results of the univariate and multivariate logistic regression analyses are shown in Table 3. Variables found to be significant in univariate analyses were sex, baseline ALC, treatment modality, steroids before treatment, WBMD, and whole-brain V5–V40. In MVA, stepwise elimination during the model development identified the strongest predictors of G3+L to be sex, baseline ALC, and whole-brain V20. Notably, however, the treatment modality and steroids were absent from the final model. The absence of modality may reflect the strong correlation between irradiated volumes and treatment modality (see Fig. 1). An MVA that omitted irradiated volumes resulted in the treatment modality being among the strongest predictors (Supplementary Table 1). Interactions between whole-brain dosimetric variables and the modality were tested in logistic regression analyses. Although the study showed the relative predictive strength to be different among different whole-brain dosimetric variables versus modality, the results of analysis were not statistically significant. For example, the interaction between V20 and modality resulted in OR 0.95, 95% CI: 0.82‒1.06, P = 0.373 (Supplementary Table 2). Therefore, interaction terms were not included in multivariable regression modeling.
Table 3.
Univariate and multivariable logistic regression analyses to determine associations with G3+L
| Univariate Regression Analysis | ||
|---|---|---|
| Variable | OR (95% CI) | P-value |
| Sex (Female) | 6.03 (2.24–17.89) | 0.001* |
| Age | 1.00 (0.96–1.04) | 0.89 |
| BMI | 0.92 (0.84–1.00) | 0.084 |
| GTV | 1.01 (0.99–1.02) | 0.246 |
| CTV | 1.00 (1.00–1.01) | 0.173 |
| PTV50 | 1.00 (1.00–1.01) | 0.146 |
| PTV60 | 1.00 (1.00–1.01) | 0.381 |
| Baseline ALC | 0.23 (0.08–0.57) | 0.003* |
| Baseline WBC | 1.06 (0.94–1.20) | 0.332 |
| Modality (photons) | 3.88 (1.29–14.56) | 0.025* |
| Preradiation steroids (yes) | 3.49 (1.20–11.14) | 0.026 |
| Location | ||
| Right | 0.79 (0.29–2.20) | 0.655 |
| Bilateral | 1.35 (0.06–15.67) | 0.815 |
| Whole brain DVH | ||
| Mean, Gy(RBE) | 1.00 (1.00–1.00) | 0.004* |
| V5 (%) | 1.03 (1.01–1.07) | 0.014* |
| V10 (%) | 1.04 (1.02–1.08) | 0.003* |
| V15 (%) | 1.04 (1.02–1.08) | 0.004* |
| V20 (%) | 1.06 (1.02–1.10) | 0.003* |
| V25 (%) | 1.06 (1.02–1.10) | 0.007* |
| V30 (%) | 1.06 (1.01–1.11) | 0.014* |
| V40 (%) | 1.06 (1.01–1.12) | 0.035* |
| V50 (%) | 1.06 (1.00–1.13) | 0.071 |
| Multivariate Regression Analysis | ||
| Variable | OR (95% CI) | P-value |
| Sex (Female) | 6.193 (1.951–22.37) | 0.0029 |
| Baseline ALC (K/µL) | 0.179 (0.052–0.511) | 0.0027 |
| Whole brain V20 (%) | 1.072 (1.028–1.125) | 0.0021 |
*Variables with statistically significant association.
Abbreviations: BMI, body mass index; GTV, gross tumor volume; CTV, clinical target volume; PTV-50 and PTV-60, planning target volumes receiving higher than 50 and 60 Gy respectively; DVH, dose-volume histogram; ALC, absolute lymphocyte count; WBC, white blood cells count (in units of 109 cells per liter); V5, V10, . . . , brain volumes receiving greater than 5, 10, . . . Gy(RBE) dose.
A Pearson correlation matrix showing correlations between G3+L versus treatment modality and irradiated volumes is displayed in Fig. 1. The treatment modality, WBMD, and the irradiated volumes from V5 to V30 were associated with G3+L, although the association with V20 was slightly stronger. At the same time, the irradiated volumes, especially at the lower end, depended on the treatment modality. Moreover, as might be expected, the irradiated volumes were strongly interdependent; for example, if a treatment design had a larger V20, then generally other volumes, especially neighboring ones, would also be larger.
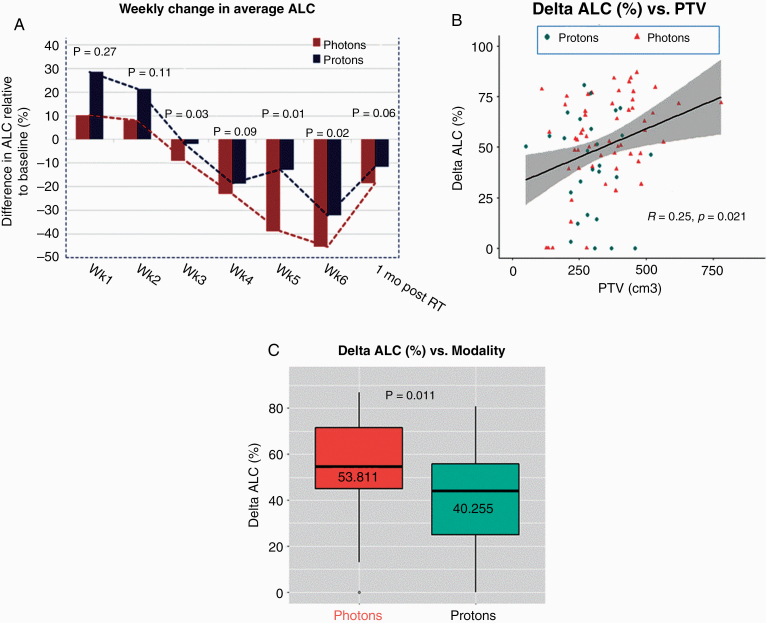
In addition to the association between radiation modality and G3+L, we investigated the effect of protons versus photons on changes in lymphocyte counts over time and the percent change in ALCs from the baseline to nadir (Δ-ALC; Fig. 2). Regarding changes in mean ALCs over time relative to baseline (Fig. 2A), the mean values at baseline were essentially the same between protons and photons (see also Table 2 and Supplementary Figure 4), but the ALCs in patients treated with photons declined by a larger magnitude by the end of the treatment course. ALC values in both treatment groups recovered at approximately the same rate after treatment. Interestingly, the ALC values actually increased (over baseline levels) at week 1, and the change seemed to be higher for protons than for photons (Supplementary Figure 5). Percent change in ALCs relative to baseline remained positive at weeks 1 and 2 for protons and photons and then started becoming negative. The association between the percentage change from baseline ALC to ALC nadir (ie, the Δ-ALC) with PTV is shown in Fig. 2B, and between Δ-ALC and treatment modality in Fig. 2C. Representing the data in terms of Δ-ALC reduced the influence of interpatient variations in baseline ALC and revealed the significance of independent variables.
Fig. 2.
(A) Weekly percent changes, relative to baseline, in absolute lymphocyte counts (ALCs) for patients treated with protons and photons. The P-values reflect the significance of differences between protons and photons. (B) Scatter plot of % differences between baseline and posttreatment ALCs (Δ-ALC) for each treatment modality as a function of PTV. A larger PTV means greater decline in ALCs over the course of radiotherapy. (C) Mean Δ-ALC for photon and proton populations are significantly different even though the baseline ALCs are essentially the same (Table 2).
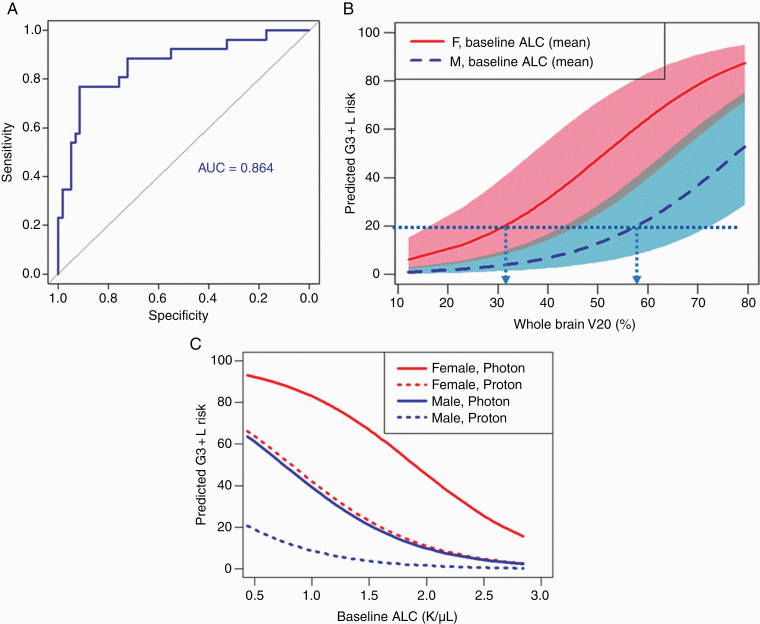
Results from the multivariate logistic regression analysis (Table 3) indicated that being female (OR 6.2, 95% CI: 1.95‒22.4, P = 0.003), baseline ALC (OR 0.18, 95% CI: 0.05‒0.51, P = 0.003), and whole-brain V20 (OR 1.07, 95% CI: 1.03‒1.13, P = 0.002) were the most significant model predictors of G3+L. Validation tests of the final predictive model are shown in Fig. 3. The AUC value of the ROC analysis was found to be 0.86 (Fig. 3A). The AUC for the corresponding ROC analysis excluding irradiated volumes (Supplementary Figure 6), which led to modality being a strong predictor, was similar. The probability of G3+L risk predicted by the model as a function of whole-brain V20 for men and women, with shaded regions corresponding to ranges of baseline ALC values within 1 (SD) from the mean, is shown in Fig. 3B. The G3+L risk as a function of baseline ALC value for protons and photons for men versus women is shown in Fig. 3C.
Fig. 3.
(A) ROC curve analyses showing the predictive power of the final model that includes sex, baseline absolute lymphocyte counts (ALCs), and whole-brain V20. (B) Predicted probability of severe (grade ≥3) lymphopenia as a function of baseline ALC values for patients treated with protons and photons. Dotted lines and arrows indicate constraints on V20 for female and male patients with medium baseline ALC to maintain the probability of G3+L risk to below 20%. The shaded regions represent variation of probability over one standard deviation of the baseline ALC count. (C) Predicted probability of grade ≥3 lymphopenia as a function of baseline ALC values for men and women.
Discussion
We sought here to determine whether proton therapy, with its compact dose distribution patterns, would afford greater sparing of the immune system, and correspondingly lower incidence of high-grade lymphopenia, among patients with GBM relative to those treated with conventional (X-ray) radiation.
This retrospective analysis of data collected prospectively from a randomized phase II trial focused on rates of severe (grade ≥3) lymphopenia (G3+L) among GBM patients treated with either protons or photons and TMZ. In univariate analysis, we found that being female, having a low baseline ALC value, treatment modality, steroids before treatment, WBMD, and whole-brain V5 through V40 were significantly associated with G3+L. Our MVA produced a model in which sex, baseline ALC, and whole-brain V20 were the strongest predictors of G3+L.
Among the dosimetric characteristics, V20 was found to have the strongest correlation with G3+L in our analyses (Fig. 1), but other volumes receiving low and intermediate doses as well as the WBMD were also nearly as well correlated. Although these dosimetric correlations were statistically significant, they were weak individually. Quite possibly, a composite dose-volume index other than the mean dose, such as “effective dose” represented by the expression ,30,31 may be more strongly correlated with G3+L than any of the individual dosimetric factors we investigated. In this expression, vi is the fractional subvolume of the brain receiving dose Di and n is a parameter that can be obtained by the maximum likelihood approach. We plan to investigate the association with Deff in future studies
Although we found that proton therapy, relative to photon therapy, led to significantly smaller whole-brain V5 through V30 as well as WBMD (Tables 2 and 3), treatment modality itself did not emerge among the model variables. This may be due to the possibility that some of the patients receiving photons, such as those with smaller target volumes, may have had smaller irradiated brain volumes. Omitting dosimetric factors from the MVA did lead to modality being a strong predictor and a model variable (see Supplementary Table 1).
Our results, showing association of high-grade lymphopenia with volumes irradiated, are consistent with prior studies of patients with GBM treated with photons. For instance, Huang et al28 and Rudra et al32 reported that mean brain dose and brain V25 were associated with severe lymphopenia and that reducing the brain V25 reduced the risk of severe RIL. It may be argued that the size of the PTV should also influence the volumes irradiated and, therefore, have an association with RIL. However, our univariate analysis did not find any such association. To further explore the role of the PTV size, we subdivided the PTVs into quartiles and repeated the analysis. We found that the highest quartile PTV was indeed significantly associated with G3+L; however, it dropped out from the final model after MVA (see Supplementary Table 2).
Similar to the findings by Huang et al,28 we found that women were at a strikingly higher risk of G3+L than men (Tables 1 and 3), despite the fact that the baseline ALC was the same for both sexes (Supplementary Figure 7). This was somewhat surprising and needs further investigation but could be related to sex-based differences in cerebral perfusion. Amen et al33 pointed out that healthy women generally have higher rates of regional cerebral blood flow34–37 and regional cerebral metabolic rates for glucose than men,38,39 which might mean greater exposure of circulating lymphocytes to radiation. Another explanation may be the higher sensitivity of females to TMZ. In our study, all patients received concurrent TMZ. However, Lin et al40 have reported on lymphopenia in lower-grade gliomas where concurrent TMZ is not always used and found that concurrent chemotherapy (as opposed to adjuvant) is associated with a higher incidence of early lymphopenia. They also identified female sex as a risk factor for lymphopenia; however, it appears that patient numbers were too low to determine if these sex-related differences could potentially be due to increased sensitivity to TMZ. On the other hand, Schmetzer and Florcken41 have noted that there are “clear gender-dependent differences in response rates and the probability of side effects in patients treated with chemotherapy.” Nevertheless, it seems that for women with GBM (and possibly women with other types of brain tumors), protons may be the preferred modality.
Of the variables we identified that were associated with lymphopenia, those factors that can be controlled to reduce its incidence and severity were the dosimetric variables, ie, WBMD and the irradiated volumes. Reduction in these variables can be achieved by the choice of treatment modality and by the optimization of beam intensities and beam configurations. Although many patients with GBM may benefit from proton therapy, patients at particularly high risk of G3+L after photon therapy may benefit the most. The model we developed may be useful for defining criteria for treatment-plan optimization with the goal of maintaining the risk of G3+L to below a specified acceptable threshold. The findings in Fig. 3B, for instance, show that if our requirement is to limit the risk of G3+L to 20%, then the whole-brain V20 should be limited to 32% for women and 58% for men whose baseline ALC is equal to the population mean. Similarly, our model can be used to set constraints for patients with different baseline ALCs. Notably, protons are more likely to achieve such constraints than photons.
An interesting result shown in Fig. 2A and Supplementary Figure 4 is the observed increase in ALC values at week 1 of treatment. The increase resulting from the combined effect of protons and photons was statistically significant (P = 0.028), though the increase from each separately was not. One might hypothesize that this may have resulted from the stimulation of the immune system during the initial few fractions, but that the depletion of lymphocytes with continued radiotherapy overwhelmed any stimulatory effect. This needs further study in larger groups of patients with brain tumors.
We and others have also compared the effects of protons versus photons on lymphopenia and outcomes for cancer of other sites (eg, esophagus). Generally protons have been found to be advantageous even when they are delivered with PSPT. More advanced techniques, such as IMPT, with its greater power to control and tailor dose distributions, should offer greater ability to mitigate lymphopenia. However, studies such as this are essential to understand the complex relationships between lymphopenia, detailed dose distributions, and patient-specific factors and to develop models for predicting lymphopenia. The patient-specific dosimetric constraints defined based on such models can then be incorporated into IMPT optimization criteria to ensure optimal sparing of lymphocytes.
It should also be noted that this study may have important implications for immunotherapy as it is being actively investigated for brain tumors. Immunotherapy for brain metastasis from cancers such as melanoma or non-small-cell lung cancer have shown great success and demonstrate that the CNS is not an immune-privileged organ as previously believed.42,43 However, immunotherapy for GBM has shown little success due to low mutational burden, tumor-mediated immunosuppression, and multiple other factors.44 Regardless, avoiding radiation-induced immune suppression utilizing techniques such as proton therapy may preserve immune function and ultimately enhance the efficacy of immunotherapy for GBM.
The main limitation of this study was its small sample size, which, coupled with the short survival time for patients with GBM, limited our ability to assess potential differences in protons versus photons in survival and other clinically relevant outcomes. Nevertheless, we analyzed the overall survival data and have included sample results in Supplementary Figure 8. Although the current study is the first, to our knowledge, to address the issue of proton- versus photon-induced lymphopenia in GBM and to show the benefit of proton therapy, we did not have ALC data at all time points. This, together with small sample size (which was calculated based on projected cognitive outcomes, the primary endpoint of the trial), may have obscured more subtle clinical variables for specific tissues that may be associated with lymphopenia. Nevertheless, we did observe significant associations between G3+L versus modality and dosimetric factors for the whole brain. Ideally, specific immune organs at risk in the brain should also be considered. As mentioned above, until recently, the CNS was considered an immune-privileged site, in that it lacks a traditional lymphatic system, and lymphopenia resulting from brain irradiation has been assumed to result from the exposure of lymphocytes in the circulating blood. However, some evidence suggests that the CNS undergoes continuous immune surveillance through the lymphatic vessels lining the dural sinuses.45,46 The rich lymphatic network in the dura absorbs and transports craniospinal fluid into the cervical lymph nodes.47,48 Thus, the irradiation of lymphatics in the brain, in addition to blood, may also be responsible for the incidence and severity of lymphopenia.
In conclusion, our results reaffirm previous findings that lymphopenia is common after radiotherapy plus TMZ for GBM. We also found WBMD and whole-brain V5 through V40 to be significantly associated with G3+L. G3+L was also strongly associated with being female and having a low baseline ALC. A model developed based on our data analyses was able to predict with sufficient specificity and sensitivity the probability of G3+L for the population studied. Importantly, we further found that protons can significantly reduce WBMD and the irradiated volume of the whole brain and, therefore, the incidence of G3+L, implying that patients with GBM (and, plausibly, patients with other types of brain tumors) who are at high risk of severe lymphopenia, namely women and those with low baseline ALC, stand to benefit from proton therapy. In the future, models of the type developed here could be applied to individual patients, with their own individual pretreatment factors, to define the patient-specific dose-volume constraints required to maintain the probability of G3+L to within acceptable limits. Such constraints may be more readily achievable with protons than photons, especially with IMPT.
Funding
This work was supported by the National Cancer Institute (NCI U19 CA021239, NCI P30 CA016672).
Supplementary Material
Acknowledgments
The authors thank Ani Yalamanchali of Indiana University for useful discussions and Christine Wogan, MS, ELS, of MD Anderson for reviewing and editing the manuscript.
Conflict of interest statement. PDB, personal fees from UpToDate (contributor), not relevant to this paper. EPS, institutional grants from Novocure and AbbVie; consulting fees from Merck, Novocure, Zai Lab, Blue Earth Diagnostic; honoraria from Physician’s Education Resource, BrainLab; travel support from Merck, Novocure, Zai Lab.
Authorship statement. Study design: RM, AYL, DRG. Implementation and analyses (including statistical): AYL, RM, DRG. Interpretation of data: all. Manuscript writing, feedback, and revisions: all.
References
- 1. Ahmed MM, Hodge JW, Guha C, Bernhard EJ, Vikram B, Coleman CN. Harnessing the potential of radiation-induced immune modulation for cancer therapy. Cancer Immunol Res. 2013;1(5):280–284. [DOI] [PubMed] [Google Scholar]
- 2. Balmanoukian A, Ye X, Herman J, Laheru D, Grossman SA. The association between treatment-related lymphopenia and survival in newly diagnosed patients with resected adenocarcinoma of the pancreas. Cancer Invest. 2012;30(8):571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yovino S, Grossman SA. Severity, etiology and possible consequences of treatment-related lymphopenia in patients with newly diagnosed high-grade gliomas. CNS Oncol. 2012;1(2):149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campian JL, Ye X, Brock M, Grossman SA. Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer. Cancer Invest. 2013;31(3):183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campian JL, Sarai G, Ye X, Marur S, Grossman SA. Association between severe treatment-related lymphopenia and progression-free survival in patients with newly diagnosed squamous cell head and neck cancer. Head Neck. 2014;36(12):1747–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grossman SA, Ellsworth S, Campian J, et al. Survival in patients with severe lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. J Natl Compr Canc Netw. 2015;13(10):1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grossman SA, Ye X, Lesser G, et al. ; NABTT CNS Consortium . Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wild AT, Ye X, Ellsworth SG, et al. The association between chemoradiation-related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma. Am J Clin Oncol. 2015;38(3):259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang C, Liao Z, Gomez D, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys. 2014;89(5):1084–1091. [DOI] [PubMed] [Google Scholar]
- 10. Fumagalli LA, Vinke J, Hoff W, Ypma E, Brivio F, Nespoli A. Lymphocyte counts independently predict overall survival in advanced cancer patients: a biomarker for IL-2 immunotherapy. J Immunother. 2003;26(5):394–402. [DOI] [PubMed] [Google Scholar]
- 11. Ku GY, Yuan J, Page DB, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116(7):1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu J, Zhao QQ, Deng WY, et al. Radiation-related lymphopenia is associated with spleen irradiation dose during radiotherapy in patients with hepatocellular carcinoma. Radiat Oncol. 2017;12(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wild AT, Herman JM, Dholakia AS, et al. Lymphocyte-sparing effect of stereotactic body radiation therapy in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2016;94(3):571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grassberger C, Hong TS, Hato T, et al. Differential association between circulating lymphocyte populations with outcome after radiation therapy in subtypes of liver cancer. Int J Radiat Oncol Biol Phys. 2018;101(5):1222–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pillai R, Balaram P, Nair BS, Hareendran NK, Padmanabhan TK, Nair MK. Lymphocyte subset distribution after radiation therapy for cancer of the uterine cervix. Possible prognostic implications and correlation with disease course. Cancer. 1991;67(8):2071–2078. [DOI] [PubMed] [Google Scholar]
- 16. Rand RJ, Jenkins DM, Bulmer R. T- and B-lymphocyte subpopulations following radiotherapy for invasive squamous cell carcinoma of the uterine cervix. Clin Exp Immunol. 1978;33(1):159–165. [PMC free article] [PubMed] [Google Scholar]
- 17. Kitayama J, Kawai K, Yasuda K, Sunami E, Nagawa H. Relationship of lymphocyte count to effectiveness of preoperative radiotherapy in advanced rectal cancer. J Clin Oncol. 2010;28(15_suppl). doi:10.1200/jco.2010.28.15_suppl.e14101 [Google Scholar]
- 18. Chadha AS, Liu G, Chen HC, et al. Does unintentional splenic radiation predict outcomes after pancreatic cancer radiation therapy? Int J Radiat Oncol Biol Phys. 2017;97(2):323–332. [DOI] [PubMed] [Google Scholar]
- 19. Carr BI, Metes DM. Peripheral blood lymphocyte depletion after hepatic arterial 90Yttrium microsphere therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;82(3):1179–1184. [DOI] [PubMed] [Google Scholar]
- 20. Moon H, Roh JL, Lee SW, et al. Prognostic value of nutritional and hematologic markers in head and neck squamous cell carcinoma treated by chemoradiotherapy. Radiother Oncol. 2016;118(2):330–334. [DOI] [PubMed] [Google Scholar]
- 21. Cho O, Chun M, Chang SJ, Oh YT, Noh OK. Prognostic value of severe lymphopenia during pelvic concurrent chemoradiotherapy in cervical cancer. Anticancer Res. 2016;36(7):3541–3547. [PubMed] [Google Scholar]
- 22. O’Toole C, Unsgaard B. Clinical status and rate of recovery of blood lymphocyte levels after radiotherapy for bladder cancer. Cancer Res. 1979;39(3):840–843. [PubMed] [Google Scholar]
- 23. Davuluri R, Jiang W, Fang P, et al. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2017;99(1):128–135. [DOI] [PubMed] [Google Scholar]
- 24. Fang P, Shiraishi Y, Jiang W, Song J, Hobbs BP, Lin S. Lymphocyte-sparing effect of proton therapy in patients with esophageal cancer. Int J Radiat Oncol. 2017;98(2):E6–E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jensen GL, Blanchard P, Gunn GB, et al. Prognostic impact of leukocyte counts before and during radiotherapy for oropharyngeal cancer. Clin Transl Radiat Oncol. 2017;7:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fang P, Jiang W, Davuluri R, et al. High lymphocyte count during neoadjuvant chemoradiotherapy is associated with improved pathologic complete response in esophageal cancer. Radiother Oncol. 2018;128(3):584–590. [DOI] [PubMed] [Google Scholar]
- 27. Shiraishi Y, Fang P, Xu C, et al. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: a propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother Oncol. 2018;128(1):154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang J, DeWees TA, Badiyan SN, et al. Clinical and dosimetric predictors of acute severe lymphopenia during radiation therapy and concurrent temozolomide for high-grade glioma. Int J Radiat Oncol Biol Phys. 2015;92(5):1000–1007. [DOI] [PubMed] [Google Scholar]
- 29. NCI Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Bethesda, MD: U.S. Department of Health and Human Services;2009. [Google Scholar]
- 30. Mohan R, Mageras GS, Baldwin B, et al. Clinically relevant optimization of 3-D conformal treatments. Med Phys. 1992;19(4):933–944. [DOI] [PubMed] [Google Scholar]
- 31. Tucker SL, Xu T, Paganetti H, et al. Validation of effective dose as a better predictor of radiation pneumonitis risk than mean lung dose: secondary analysis of a randomized trial. Int J Radiat Oncol Biol Phys. 2019;103(2):403–410. [DOI] [PubMed] [Google Scholar]
- 32. Rudra S, Hui C, Rao YJ, et al. Effect of radiation treatment volume reduction on lymphopenia in patients receiving chemoradiotherapy for glioblastoma. Int J Radiat Oncol Biol Phys. 2018;101(1):217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Amen DG, Trujillo M, Keator D, et al. Gender-based cerebral perfusion differences in 46,034 functional neuroimaging scans. J Alzheimers Dis. 2017;60(2):605–614. [DOI] [PubMed] [Google Scholar]
- 34. Slosman DO, Chicherio C, Ludwig C, et al. (133)Xe SPECT cerebral blood flow study in a healthy population: determination of T-scores. J Nucl Med. 2001;42(6):864–870. [PubMed] [Google Scholar]
- 35. Jones K, Johnson KA, Becker JA, Spiers PA, Albert MS, Holman BL. Use of singular value decomposition to characterize age and gender differences in SPECT cerebral perfusion. J Nucl Med. 1998;39(6):965–973. [PubMed] [Google Scholar]
- 36. Gur D, Good WF, Wolfson SK Jr, Yonas H, Shabason L. In vivo mapping of local cerebral blood flow by xenon-enhanced computed tomography. Science. 1982;215(4537):1267–1268. [DOI] [PubMed] [Google Scholar]
- 37. Devous MD Sr, Stokely EM, Chehabi HH, Bonte FJ. Normal distribution of regional cerebral blood flow measured by dynamic single-photon emission tomography. J Cereb Blood Flow Metab. 1986;6(1):95–104. [DOI] [PubMed] [Google Scholar]
- 38. Andreason PJ, Zametkin AJ, Guo AC, Baldwin P, Cohen RM. Gender-related differences in regional cerebral glucose-metabolism in normal volunteers. Psychiat Res. 1994;51(2):175–183. [DOI] [PubMed] [Google Scholar]
- 39. Baxter LR Jr, Mazziotta JC, Phelps ME, Selin CE, Guze BH, Fairbanks L. Cerebral glucose metabolic rates in normal human females versus normal males. Psychiatry Res. 1987;21(3):237–245. [DOI] [PubMed] [Google Scholar]
- 40. Lin AJ, Campian JL, Hui C, et al. Impact of concurrent versus adjuvant chemotherapy on the severity and duration of lymphopenia in glioma patients treated with radiation therapy. J Neurooncol. 2018;136(2):403–411. doi: 10.1007/978-3-642-30726-3_19 [DOI] [PubMed] [Google Scholar]
- 41. Schmetzer O and Florcken A. Sex differences in the drug therapy for oncologic diseases. Handb Exp Pharmacol. 2012(214):411–442. [DOI] [PubMed] [Google Scholar]
- 42. Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goldberg SB, Schalper KA, Gettinger SN, et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2020;21(5):655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Majd N, Dasgupta P, de Groot J. Immunotherapy for neuro-oncology. Adv Exp Med Biol. 2020;1244:183–203. [DOI] [PubMed] [Google Scholar]
- 45. Demaria S, Formenti SC. Sensors of ionizing radiation effects on the immunological microenvironment of cancer. Int J Radiat Biol. 2007;83(11-12):819–825. [DOI] [PubMed] [Google Scholar]
- 46. Absinta M, Ha SK, Nair G, et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Louveau A, Harris TH, Kipnis J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 2015;36(10):569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.