The nomogram represents a simple and applied graphical tool that can be used to estimate individualized survival probabilities, develop individualized treatment options, and decide the interval of follow-up. We recognize that 2 nomogram models have been previously developed for glioblastoma (GBM) and lower-grade glioma (LGG), respectively.1,2 We praise the authors for developing and validating these 2 nomogram models in similar US populations. However, these models may be not generalizable to populations outside of the United States or subsets of populations (such as elderly patients). Subsequently, 2 nomograms had been validated in different races (Asian cohort) and showed some limitations.3,4 For example, although the effectiveness of Gittleman’s nomogram1 for newly diagnosed GBM was tested in a Chinese cohort and obtained similar results, a new nomogram incorporating isocitrate dehydrogenase (IDH) status and treatment strategy revealed the greater validity for Chinese patients.3 In addition, a survival nomogram for individuals with IDH-wild-type GBM was developed by Gittleman et al,5 whereas another study revealed that the validation of this nomogram for IDH-wild-type GBM in an elderly cohort showed some evidence of overestimating 24-month survival probability.6
Due to the rarity of thalamic gliomas, however, the characteristics, treatments, and prognosis of these tumors are not well characterized. Also, we note that none or a minority of thalamic gliomas may be included in these previous studies. Recently, our team comprehensively summarized the characteristics and prognosis of patients with adult thalamic gliomas (ATG) in a relatively large-scale surgical cohort.7 For these rare gliomas, to our knowledge, no clinical predictive model has been reported or exclusively discussed in the previous nomograms. Therefore, the above conditions raise a question on predicting the survival probability of these gliomas located in thalamus.
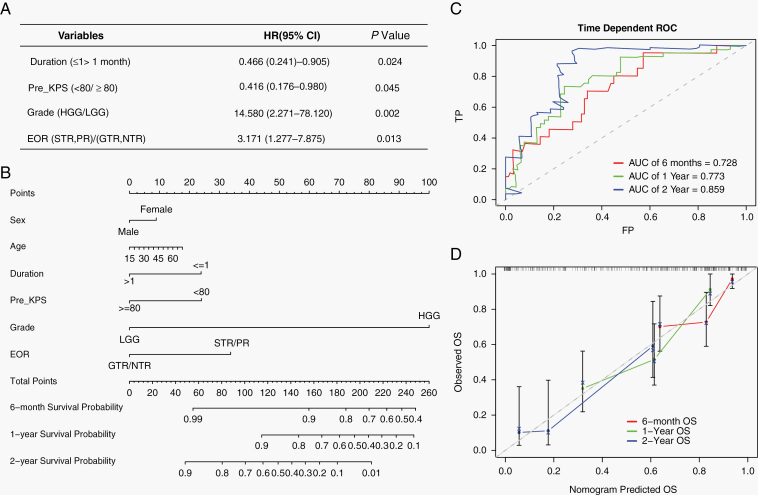
In our previous study,7 a total number of 102 patients with ATG between 2009 and 2017 were included; the median age was 41 years (range: 18–68 y) and 56 (54.9%) patients were males. The informed consents were provided by all patients or family members. The ethics committee review for this study was approved by our Institutional Review Board. Four independent prognostic factors (duration [duration of symptoms], Pre_KPS [preoperative KPS], grade, and EOR [extent of resection]) were identified by multivariate Cox regression analysis (Figure 1A). Besides, our data revealed distinct molecular characteristics (such as IDH1, H3 K27M) of rare ATGs compared with common hemispheric gliomas, although these molecular markers were not significantly associated with the overall survival (OS) of ATG patients. Based on the above results,7 we further construct a prognostic nomogram model for patients with ATG in a relatively large-scale surgical cohort (Figure 1B). Our nomogram is established to estimate 6-month, 1-year, and 2-year survival probabilities and shows that glioma grade is the largest contributor to OS, followed by EOR, Pre_KPS, duration of symptoms, age, and sex. Internal validation of our nomogram shows that the concordance index (C-index) is 0.736, showing relatively reliable predictive performance. Furthermore, time-dependent receiver operating characteristic (ROC) curves reveal that the values of area under the curve (AUC) of 6 months, 1 year, and 2 years are 0.728, 0.773, and 0.859, respectively (Figure 1C). The calibration plots of 6 months, 1 year, and 2 years show that the curves of nomogram predicted OS and observed OS are moderately aligned (Figure 1D). These validation results reveal that this nomogram model has moderate discrimination and relatively reliable predictive performance.
Fig. 1.
(A) Multivariate Cox regression analysis showing 4 independent prognostic factors of overall survival (OS) in adult thalamic glioma (ATG) patients. (B) Nomogram model predicting the 6-mo, 1-y, and 2-y survival probabilities. (C) Time-dependent receiver operating characteristic (ROC) curves of 6-mo, 1-y, and 2-y OS. (D) Calibration curves of 6-mo, 1-y, and 2-y OS.
In conclusion, we firstly develop a prognostic nomogram model to predict the survival probability of rare ATG patients. Importantly, we demonstrate a simple and applied graphic tool that may expand the clinical use of the nomogram in midline gliomas. Due to the rarity of ATGs, although relatively large-scale samples are included in the present nomogram, the multicentric studies with the larger sample size and/or other midline locations are still needed to validate and optimize the nomogram model in future work. In general, this nomogram should be an applied tool that can be used to predict the risk assessments of the survival probability of ATG patients and provide references regarding treatment options, follow-up, and prognosis in clinical practice. In this context, we have further developed a free online prediction tool for this nomogram (https://mydemos.shinyapps.io/Nomo_ATG/).
Funding
None declared.
Conflict of interest statement. The authors have no relevant competing interests to disclose.
References
- 1. Gittleman H, Lim D, Kattan MW, et al. An independently validated nomogram for individualized estimation of survival among patients with newly diagnosed glioblastoma: NRG Oncology RTOG 0525 and 0825. Neuro Oncol. 2017;19(5):669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gittleman H, Sloan AE, Barnholtz-Sloan JS. An independently validated survival nomogram for lower-grade glioma. Neuro Oncol. 2020;22(5):665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng W, Zhang C, Ren X, et al. Treatment strategy and IDH status improve nomogram validity in newly diagnosed GBM patients. Neuro Oncol. 2017;19(5):736–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han MZ, Huang B, Ni SL, Wang J, Li XG, Bjerkvig R. A validated prognostic nomogram for patients with newly diagnosed lower-grade gliomas in a large-scale Asian cohort. Neuro Oncol. 2020;22(5): 729–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gittleman H, Cioffi G, Chunduru P, et al. An independently validated nomogram for isocitrate dehydrogenase-wild-type glioblastoma patient survival. Neurooncol Adv. 2019;1(1):vdz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shen E, Johnson MO, Lee JW, et al. Performance of a nomogram for IDH-wild-type glioblastoma patient survival in an elderly cohort. Neurooncol Adv. 2019;1(1):vdz036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Niu X, Wang T, Zhou X, et al. Surgical treatment and survival outcome of patients with adult thalamic glioma: a single institution experience of 8 years. J Neurooncol. 2020;147(2):377–386. [DOI] [PubMed] [Google Scholar]