Abstract
Background
The Delphi consensus statements on the management of germ cell tumors (GCTs) failed to reach agreements on the statement that the cases with (i) pineal and neurohypophyseal bifocal lesion, (ii) with diabetes insipidus, and (iii) with negative tumor markers can be diagnosed as germinoma without histological verification. To answer this, multicenter retrospective analysis was performed.
Methods
A questionnaire on clinical findings, histological diagnosis, and details of surgical procedures was sent to 86 neurosurgical and 35 pediatrics departments in Japan.
Results
Fifty-one institutes reported 132 cases that fulfilled the 3 criteria. Tissue sampling was performed in 91 cases from pineal (n = 44), neurohypophyseal (n = 32), both (n = 6), and distant (n = 9) lesions. Histological diagnosis was established in 89 cases: pure germinoma or germinoma with syncytiotrophoblastic giant cells in 82 (92.1%) cases, germinoma and mature teratoma in 2 cases, and granulomatous inflammation in 2 cases. Histological diagnosis was not established in 2 cases. Although no tumors other than GCTs were identified, 3 (3.4%) patients had non-germinomatous GCTs (NGGCTs). None of the patients developed permanent complications after endoscopic or stereotactic biopsy. Thirty-nine patients underwent simultaneous procedure for acute hydrocephalus without permanent complications, and hydrocephalus was controlled in 94.9% of them.
Conclusion
All patients who fulfilled the 3 criteria had GCTs or granulomatous inflammation, but not other types of tumors. However, no fewer than 3.4% of the patients had NGGCTs. Considering the safety and the effects of simultaneous procedures for acute hydrocephalus, biopsy was recommended in such patients.
Keywords: diabetes insipidus, germinoma, neurohypophysis, pineal body, tumor marker
Key Points.
Approximately 3.4% of the patients who fulfilled the 3 criteria had NGGCTs.
Biopsy had high diagnostic rate and was considered safe.
Histological diagnosis was recommended for such cases.
Importance of the Study.
Because of the lack of previous studies based on a large number of cases, whether patients with pineal and neurohypophyseal bifocal lesion, diabetes insipidus, and negative tumor markers can be diagnosed with germinoma without histological diagnosis remains unclear. To elucidate this clinical question, this study reported the distribution of histological diagnosis of such cases and the safety and diagnostic rate of tissue sampling in 91 patients. Histological diagnosis was established in 89 of 91 patients (97.8%), and 3.4% had NGGCTs. No permanent complications developed in 50 and 9 patients who underwent endoscopic and stereotactic biopsies, respectively. Moreover, 39 simultaneous procedures for acute hydrocephalus were performed safely, and hydrocephalus was controlled in 94.9% of the patients. These results suggested that histological diagnosis was recommended in patients with bifocal tumors, diabetes insipidus, and negative tumor markers.
Intracranial germ cell tumors (GCTs) are relatively rare brain tumors comprising 1.9% and 0.4% of the primary brain tumors in Japan and the United States, respectively.1,2 Although GCTs develop commonly as an isolated lesion in either the pineal or neurohypophyseal region,1 13–41% of the patients with GCTs had synchronous lesions in both regions. These synchronous lesions have been described as bifocal tumor.3–7
Empirically, the presence of bifocal tumor in the setting of alpha-fetoprotein (AFP) within normal value and undetectable to modestly elevated human chorionic gonadotropin (HCG) is considered to be pathognomonic of germinoma. This is supported by the report demonstrating that all 11 patients had pure germinoma or germinoma with syncytiotrophoblastic giant cells (STGCs).6 Additionally, stereotactic or endoscopic biopsy in the pineal or neurohypophyseal region is an invasive procedure, and biopsy of small tissues could miss the malignant component due to the heterogeneity of the tumor.8 From this background, some physicians and clinical trials, including those conducted in North America (ACNS1123) and Europe (SIOP CNS GCT II), do not require histological verification of cases with bifocal lesions and negative tumor markers.
Recently, multidisciplinary Delphi processes were undertaken to reach a consensus in the clinical management of intracranial GCTs.9 In this process, the statement was rejected that synchronous lesions occurring in both the neurohypophyseal and the pineal region with typical imaging characteristics, presence of diabetes insipidus (DI), and normal values of serum and cerebrospinal fluid (CSF) AFP were consistent with a diagnosis of germinoma, receiving only 49% support.9 Participants were concerned that there might be cases of non-germinomatous GCTs (NGGCTs) or non-GCTs. Certainly, 3 patients with mixed GCTs of germinoma and yolk sac tumor, 2 with primitive neuroectodermal tumor, and 1 with papillary tumor of the pineal region had bifocal lesions and negative tumor markers.10–13 To determine the optimal diagnostic strategy for these patients, identifying the frequency of germinoma histology is necessary in those with clinically estimated bifocal tumors as well as the diagnostic rate and safety of biopsy.
This retrospective multicenter study aimed to clarify the necessity for histological diagnosis in patients with (i) bifocal lesion, (ii) DI, and (iii) negative tumor markers. To this end, we analyzed the proportion of germinoma and exceptional type of tumors in patients who fulfilled the 3 criteria, the diagnostic rate and surgical complication at tissue sampling, and the outcome and complications of simultaneous intervention for acute hydrocephalus.
Materials and Methods
A multicenter retrospective study was conducted after obtaining the necessary ethical clearance from the institutional ethics board for study on humans. A questionnaire containing items related to the clinical findings including age, sex, symptoms, radiological findings, value of tumor markers, histological diagnosis, and details of tissue sampling procedures was sent to 121 institutes in Japan, including 86 neurosurgical and 35 pediatric departments. Patients included had fulfilled the 3 criteria and were treated from January 1990 to December 2015. These institutes belong to the intracranial Germ Cell Tumor Consortium or Japan Children’s Cancer Group or were training hospitals of the Japan Neurosurgical Society.
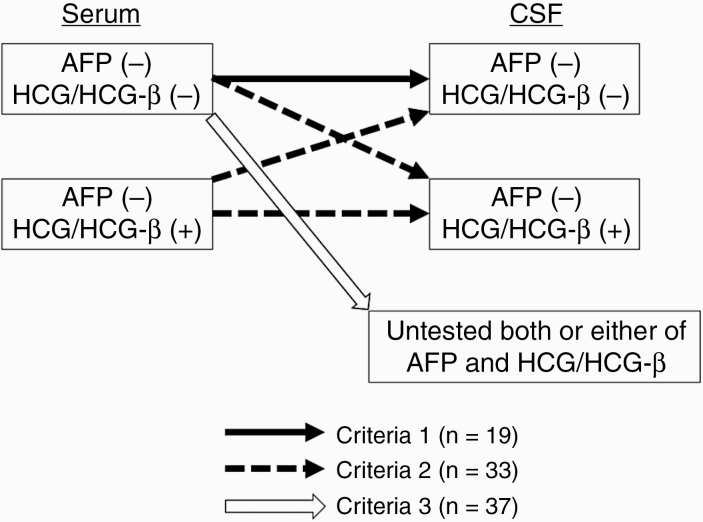
In this study, the newly diagnosed patients with bifocal lesion, DI, and negative tumor markers were registered. Bifocal tumors implied synchronous pineal and neurohypophyseal lesions irrespective of the presence or absence of distant lesion. “Negative tumor markers” have various definitions. When distinguishing germinoma and NGGCTs, the modest elevation of HCG/HCG-β is also regarded as a negative tumor marker because this elevation of HCG/HCG-β is often observed in pure germinoma.6 This study focused on the differential diagnosis of germinoma, NGGCT, and other types of tumors, so that negative tumor markers were defined as follows. The pretreatment values of AFP and HCG/HCG-β in serum and CSF were obtained. All patients with elevated serum or CSF AFP (≥10 ng/mL) were excluded from this study. Patients who met one of the following tumor marker criteria were selected (Fig. 1): criteria 1: AFP within normal value (<10 ng/mL) and undetectable HCG/HCG-β in both serum and CSF (black arrow in Fig. 1); criteria 2: AFP within normal value in serum and CSF and modestly elevated HCG/HCG-β in serum and/or CSF (HCG <50 mIU/mL and HCG-β <5 ng/mL: HCG-β 1 ng/mL = HCG-β 1 mIU/mL (personal communication from Bio Medical Laboratories, Tokyo)6,14,15 (dashed arrows in Fig. 1); and criteria 3: AFP within normal value and undetectable HCG/HCG-β in serum and incomplete estimation of either or both of the tumor markers in CSF as follows (white arrow in Fig. 1): AFP within normal value and undetectable HCG/HCG-β in serum and without evaluation of tumor markers in CSF, and AFP within normal value and undetectable HCG/HCG-β in serum and either AFP within normal value/unexamined HCG/HCG-β in CSF or undetectable HCG/HCG-β in CSF/unexamined AFP in CSF.
Fig. 1.
Three definitions of negative tumor markers and the distribution of the cases. AFP (−) = alpha-fetoprotein <10 ng/mL; HCG/HCG-β (−) = undetected human chorionic gonadotropin/human chorionic gonadotropin-β subunit; HCG/HCG-β (+) = human chorionic gonadotropin detected but <50 mIU/mL or human chorionic gonadotropin-β subunit detected but <5 ng/mL (mIU/mL).
Results
Patient Demographics
Fifty-one institutes (42.1%) answered the inquiry and reported 172 cases. Forty-6 patients were excluded: 29 had positive tumor markers, 6 had absence of bifocal lesions and DI, 4 had absence of bifocal lesions, 3 had positive tumor markers and absence of DI, 2 had absence of DI, and 2 had absence of bifocal lesion and positive tumor markers. A total of 126 patients met the following criteria: (i) pineal and neurohypophyseal bifocal tumor, (ii) presence of DI, and (iii) negative tumor markers evaluated according to the criteria described in Materials and Methods.
Histological diagnosis was performed in 91 patients. The method and site of tissue sampling are summarized in Table 1. Tissue for histological diagnosis was obtained by biopsy (n = 74) or tumor resection (n = 17). Tissue samples from pineal, neurohypophyseal, and distant lesions were obtained in 44 (48.3%), 32 (35.2%), and 9 (9.9%) patients, respectively. Bifocal tumors with or without distant lesion were included, so that some cases had more than 2 lesions. Thirty-three patients had distant lesion, including the ventricular wall, brain parenchyma, subarachnoid space, or the spinal cord, and 9 of these patients underwent biopsy from distant lesion. The biopsy site in 8 cases was a distant lesion in the ventricular wall, while in one case, the biopsy site was a distant lesion in the frontal lobe. Simultaneous biopsy of pineal and neurohypophyseal lesions was performed in 6 (6.6%) patients. Histological diagnosis was established in 89 (97.8%) patients, but not in 2 patients (2.2%) in whom the specimens were obtained from a neurohypophyseal tumor by stereotactic or open biopsy.
Table 1.
Distribution of the method and site of tissue sampling
| Endoscopic Biopsy | Stereotactic Biopsy | Open Biopsy | Tumor Resection | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Cases | (%) | Number of Cases | Number of Cases | (%) | Number of Cases | (%) | Number of Cases | (%) | |||
| Number of cases | 51 | 9 | 14 | 17 | 91 (2) | ||||||
| Site of biopsy | Pineal | 25 | 49.0 | 4 | 44.4 | 7 | 50.0 | 8 | 47.1 | 44 | 48.3 |
| Neurohypophyseal | 16 | 31.4 | 3 (1) | 33.3 | 6 (1) | 42.9 | 7 | 41.1 | 32 (2) | 35.2 | |
| Pineal and neurohypophyseal | 6 | 11.8 | 0 | 0.0 | 0 | 0 | 0 | 0.0 | 6 | 6.6 | |
| Distant | 4 | 7.8 | 2 | 22.2 | 1 | 7.1 | 2 | 11.8 | 9 | 9.9 |
Parentheses = the number of patients whose histological diagnosis could not be established.
Of 89 patients with histological diagnosis, 67 were males and 22 were females. Age at diagnosis was 8–59 years (median, 18 y). A total of 19, 33, and 37 patients met criteria 1, 2, and 3, respectively, for negative tumor marker (Fig. 1). In the group that met criteria 2, modestly elevated HCG/HCG-β levels were observed in serum and CSF of 8 patients, in CSF only in 23 patients, and in serum only in 2 patients (Supplementary Table 1). In the group that met criteria 3, twenty-nine cases had AFP within normal value and undetectable HCG/HCG-β in serum, and evaluation CSF AFP/HCG/HCG-β was not done. Four cases had AFP within normal value and undetectable HCG/HCG-β in serum; CSF AFP was within normal value; and CSF HCG/HCG-β was not examined. Further, 4 more cases had AFP within normal value and undetectable HCG/HCG-β in serum; CSF AFP was not examined; and CSF HCG/HCG-β was undetectable (Supplementary Table 1).
Histological Diagnosis Before Treatment
In total, pure germinoma was histologically diagnosed in 79 (88.8%) patients and germinoma with STGC in 3 (3.4%) patients. The other histological diagnoses were germinoma and mature teratoma in 2 patients (2.2%); immature teratoma (IMT) in 1 (1.1%); mixed GCT consisting of germinoma, IMT, and embryonal carcinoma (EC) in 1 (1.1%); EC in 1 (1.1%); and granulomatous inflammation in 2 (2.2%) (Supplementary Figure 1A). None of the patients had tumors other than GCTs.
The distribution of histological diagnosis was compared by tissue sampling, the definition of negative tumor markers, and the site of tissue sampling. By tissue sampling, NGGCTs were detected in patients diagnosed by tumor resection and biopsy, and no significant difference was observed between these patients (5.9% and 2.8%, P = 0.48: Fisher’s exact test) (Supplementary Figure 1B and Supplementary Table 2). Based on the definition of negative tumor markers, all 19 patients who fulfilled criteria 1, and 32 (97.0%) of 33 patients who fulfilled criteria 2 had pure germinoma, germinoma with STGC, or germinoma and mature teratoma, whereas 3 (8.1%) of 37 patients who fulfilled criteria 3 had NGGCTs (Supplementary Figure 1C and Table 2). Based on the site of tissue sampling, NGGCTs were found in the pineal, neurohypophyseal, and distant lesion, and the frequent site for NGGCTs was not determined (Supplementary Figure 1D and Table 3). By contrast, granulomatous inflammation was found only in neurohypophyseal lesion (Supplementary Figure 1D and Table 3). Six patients underwent endoscopic biopsy from both pineal and neurohypophyseal lesion. Histological diagnosis was pure germinoma in 5 patients and germinoma and mature teratoma in 1, and the diagnosis of all patients coincided with each other.
Table 2.
Histological diagnosis by definition of negative tumor markers
| Criteria 1 (n = 19) | Criteria 2 (n = 33) | Criteria 3 (n = 37) | ||||
|---|---|---|---|---|---|---|
| Number of Cases | (%) | Number of Cases | (%) | Number of Cases | (%) | |
| Pure germinoma | 17 | 89.5 | 29 | 87.9 | 33 | 89.2 |
| Germinoma with STGC | 1 | 5.3 | 2 | 6.1 | 0 | 0 |
| Germinoma and mature teratoma | 1 | 5.3 | 1 | 3.0 | 0 | 0 |
| IMT | 0 | 0 | 0 | 0 | 1 | 2.7 |
| Germinoma and IMT and EC | 0 | 0 | 0 | 0 | 1 | 2.7 |
| EC | 0 | 0 | 0 | 0 | 1 | 2.7 |
| Granulomatous inflammation | 0 | 0 | 1 | 3.0 | 1 | 2.7 |
Abbreviations: STGC = syncytiotrophoblastic giant cells; IMT = immature teratoma; EC = embryonal carcinoma.
Table 3.
Histological diagnosis by the site of tissue sampling
| Pineal Lesion (N = 50*) | Neurohypophyseal Lesion (N = 36*) | Distant Site (N = 9) | ||||
|---|---|---|---|---|---|---|
| Number of Patients | (%) | Number of Patients | (%) | Number of Patients | (%) | |
| Pure germinoma | 46 | 92.0 | 30 | 83.3 | 8 | 88.9 |
| germinoma with STGC | 1 | 2.0 | 2 | 5.6 | 0 | 0.0 |
| Germinoma and mature teratoma | 2 | 4.0 | 1 | 2.8 | 0 | 0.0 |
| IMT | 0 | 0.0 | 1 | 2.8 | 0 | 0.0 |
| Germinoma and IMT and EC | 0 | 0.0 | 0 | 0 | 1 | 11.1 |
| EC | 1 | 2.0 | 0 | 0 | 0 | 0.0 |
| Granulomatous inflammation | 0 | 0.0 | 2 | 5.6 | 0 | 0.0 |
Abbreviations: STGC = syncytiotrophoblastic giant cells; IMT = immature teratoma; EC = embryonal carcinoma; *simultaneous tissue sampling of pineal and neurohypophyseal lesion in 6 cases.
Clinical Course of the Patients with NGGCTs and Granulomatous Inflammation
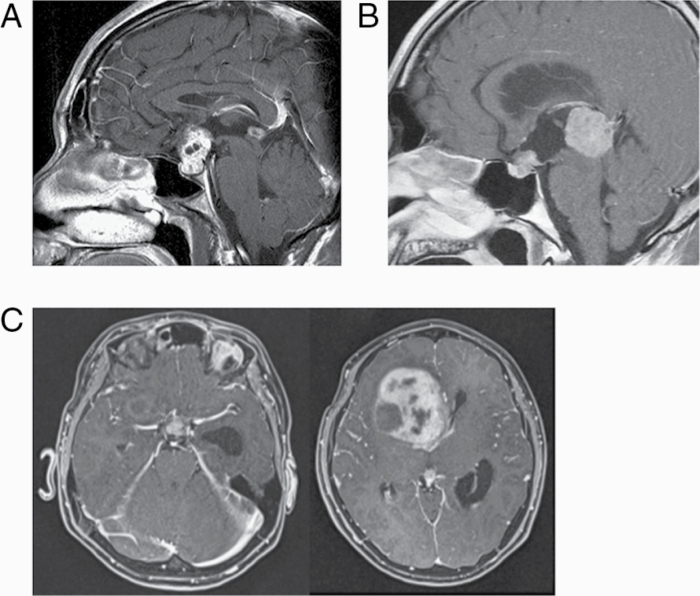
The clinical features of 3 patients with NGGCTs are demonstrated in Fig. 2 and Table 4. All of them fulfilled criteria 3 for negative tumor markers. Histological diagnosis was performed with biopsy in 2 and resection of the large lesion in the anterior horn of the lateral ventricle in 1. Patients 1 and 2 received 3 cycles of ifosfamide, cisplatin, and etoposide chemotherapy and radiation therapy to the craniospinal axis and local tumor site. Salvage surgery to residual mature teratoma were performed in patient 1. Patient 3 had bifocal and large intraventricular tumor. He had a neurological exacerbation prior to surgery and underwent emergency surgery but eventually died due to rapid progressive disease.
Fig. 2.
MR images of 3 cases with NGGCTs. (A) Patient 1: A 32-year-old man with immature teratoma. (B) Patient 2: A 27-year-old man with embryonal carcinoma. (C) Patient 3: An 11-year-old boy with mixed GCTs of germinoma, immature teratoma, and embryonal carcinoma.
Table 4.
Summary of patients with histologically diagnosed NGGCTs
| Patient Number | Age/ Sex | DI at Presentation | Tumor Markers | Surgery (Site of Biopsy) | Histological Diagnosis Before Treatment | Chemotherapy | Radiation (Gy) | Histological Diagnosis at Salvage Surgery To Residual Lesion | Survival (mo) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum | CSF | |||||||||||
| AFP (ng/ mL) | HCG-b (ng/mL) | AFP (ng/ mL) | HCG-b (ng/mL) | |||||||||
| 1 | 32M | yes | 2 | <0.1 | N.E. | <0.1 | Biopsy (neurohypophysis) | IMT | ICE x3 | CSI 24, WVI 6.4, LBI 20.8 | MT | 106 (NED) |
| 2 | 27M | yes | 2.8 | <0.1 | N.E. | N.E. | Biopsy (pineal) | EC | ICE x3 | CSI 30, LBI 30 | No salvage surgery | 21 (NED) |
| 3 | 11M | yes | 5 | <0.1 | N.E. | N.E. | Resection (lateral ventricle) | Germinoma/IMT/EC | N.D. | N.D. | No salvage surgery | 1 (dead) |
Abbreviations: M = male; DI = diabetes insipidus; AFP = alpha-fetoprotein; HCG-b = human chorionic gonadotropin-b subunit; CSF = cerebrospinal fluid; N.E. = not examined; IMT = immature teratoma; EC = embryonic carcinoma; ICE = combination chemotherapy of ifosfamide, cisplatin, and etoposide; N.D. = not done; CSI = craniospinal irradiation; WVI = whole ventricle irradiation; LBI = local brain irradiation; MT = mature teratoma; NED = no evidence of disease.
Two patients diagnosed with granulated inflammation received chemotherapy and radiation therapy because they were clinically diagnosed with germinoma. Neither of them had disease progression.
Morbidity and Mortality of Tissue Sampling
To elucidate the morbidity and mortality of tissue sampling, we analyzed the surgical complication of 86 of 91 patients who underwent tissue sampling (Supplementary Table 3). The data regarding surgical complications were not available in 5 patients from 3 institutions. None of the patients developed permanent or symptomatic complications due to endoscopic biopsy and stereotactic biopsy. By contrast, permanent or long-standing complications occurred in 1 (7.7%) of the 13 patients who underwent open biopsy and 3 (21.4%) of the 14 patients who underwent tumor resection. None of the patients died due to complications of tissue sampling, including craniotomy resection. From these findings, tissue sampling with endoscopic or stereotactic biopsy was considered as a safe procedure for patients with bifocal tumors, DI, and negative tumor markers.
Simultaneous Procedures for Acute Hydrocephalus
We also obtained the data on the procedure for acute hydrocephalus performed with tissue collection (Supplementary Table 3). Thirty-nine (45.3%) patients underwent various procedures for acute hydrocephalus simultaneously. A total of 26 patients underwent endoscopic third ventriculostomy (ETV), 14 underwent continuous ventricular drainage (CVD), 3 underwent implantation of Ommaya reservoir, and 2 ventriculoperitoneal (VP) shunt. ETV was performed safely without permanent complications. CVD were transiently placed until hydrocephalus improved following the treatment of tumors. None of the patients developed infections, seizures, or bleeding during the transient placement of CVD. A total of 37 of 39 (94.9 %) patients did not require additional treatment for hydrocephalus, whereas 2 (7.7 %) required VP shunt after ETV (Supplementary Table 3).
Discussion
In this study, we demonstrated the result of histological diagnosis, the diagnostic rate and complication at biopsy procedures, and the effect of simultaneous intervention for acute hydrocephalus in the cases with bifocal neurohypophyseal and pineal tumors, DI, and negative tumor markers.
Histological Diagnosis Before Initial Treatment
We analyzed the proportion of germinoma in the patients with AFP within normal value and undetectable HCG/HCG-β in serum and CSF (criteria 1), AFP within normal value in serum and CSF and modestly elevated HCG/HCG-β in serum and/or CSF (criteria 2), or AFP within normal value and undetectable HCG/HCG-β in serum with incomplete assessment of tumor markers in CSF (criteria 3). In total, this study revealed that 92.1% of the patients were pure germinoma or germinoma with STGC on histological diagnosis. However, 3 (3.4%) patients had NGGCTs, including IMT; mixed GCT consisting of germinoma, IMT, and EC; and EC. This proportion was comparable to that of intracranial GCT, not limited to bifocal tumors, with AFP within normal value and HCG in serum and CSF (2.6%: 1 of 38 patients with germinoma and immature teratoma).6 The findings of MR images were reviewed to discriminate NGGCT from germinoma. Three patients with NGGCT did not have certain trends in MR images. One case had a homogeneous enhanced lesion, while the other 2 patients had intratumoral cyst formation (Fig. 2). As germinomas have cystic components in 43–46%,16,17 this finding did not help the differential diagnosis. Ventricular enhancement was found in both tumor groups: 1 of 3 patients with NGGCTs and 28 of 84 with germinomatous tumors in this study. These results suggested that diagnostic strategies based on radiological, serological, and clinical findings without histological diagnosis could lead to underdiagnosis, and that histological diagnosis was essential to establishing the optimal treatment strategy. In the future, novel tumor markers should be developed to distinguish germinoma from NGGCTs. In this regard, Aihara et al demonstrated the diagnostic value of placental alkaline phosphatase (PLAP) in CSF for the detection of a germinoma component.18,19 Unfortunately, their reports did not include EC and few cases of IMT, and the presence of a malignant component in germinoma could not be predicted by measuring the PLAP in CSF.
Mixed GCTs consisted of germinoma and mature teratoma in 2.2% of the patients, which was comparable to that of the recent report.6 In that study, mixed germinoma and teratoma verified through central pathological review were reported in 3.6–5.0% of the patients with negative tumor markers. In clinical practice, there were no differences in treatment strategy between patients with a tumor-mature teratoma component and those without. If there were residual lesions after chemotherapy and radiation therapy, residual or growing tumors can be followed with a watch-and-see strategy or can be resected safely to control the tumor.20–22
Granulomatous inflammation lacking a germinoma component was observed in 2 patients. The histological diagnosis of the tumors with significant infiltration of lymphocytes to germinoma are sometimes difficult to establish.23–26 Hence, infundibular neurohypophysitis, sarcoidosis, and other idiopathic inflammatory disease must be differentiated in patients with neurohypophyseal lesion. To the best of our knowledge, no study has demonstrated that these diseases involved both the neurohypophyseal and pineal regions. In addition, a previous report suggested that increased anterior pituitary size with stalk thickening is strongly suggestive of germinoma, not other inflammatory diseases.27 From these reports, the histological diagnosis of “granulomatous inflammation” could be germinoma when bifocal lesion and stalk thickening are present. This does not mean that pathological examination is unnecessary but that a diagnosis was made based on both pathological findings of significant lymphocyte infiltration and radiological findings.
None of the patients in this study had a tumor other than GCTs. In previous studies,12,13 2 patients with primitive neuroectodermal tumor and 1 with papillary tumor of the pineal region with bifocal lesions were reported, but all of them did not present DI. Similarly, a 15-year-old patient with bifocal tumor was excluded from this study due to the absence of DI. He had pineal mixed GCTs containing teratoma with malignant transformation and pituitary prolactinoma. Although this case could be a rare collision tumor, it also suggested the diagnostic value of DI. Considering that most of the patients with neurohypophyseal germinoma have DI,28 presence of DI could provide an important clue to differentiate germinoma from pathologies other than GCTs. In summary, inflammatory disease and tumors other than GCTs can be excluded by radiological findings and the absence of DI, but it was difficult to differentiate NGGCTs from germinoma without histological verification.
Consideration for Procedures for Histological Diagnosis and for Acute Hydrocephalus
The endoscopic and stereotactic biopsy for pineal or neurohypophyseal tumors, and simultaneous procedures, has been considered to have acceptable diagnostic value and safety; however, these issues are not considered controversial in this study. Consistent with previous reports,8,29,30 the outcomes of these procedures were acceptable. We reviewed the data to elucidate whether the distribution of histological diagnosis was different based on the tissue sampling site. We were unable to determine the specific location where pathologies other than germinoma developed. It remains unclear whether simultaneous biopsies from pineal and neurohypophyseal lesion were recommended or not. All 6 patients in whom simultaneous biopsies were performed had identical histological diagnosis, although histological diagnosis was not confirmed in most of the cases in this series.
This study included 17 tumor resections. As shown in Supplementary Table 2, the proportion of mixed tumor of mature teratoma, STGC, or NGGCT component with germinoma background was 4.3% and 18.8% in the biopsy and tumor resection, respectively. This suggested that tumor resection could detect the component of mature teratoma, STGC, and NGGCT in the germinoma component more frequently than biopsy. Because the presence of NGGCT, not mature teratoma or STGC, influences treatment strategies, only 1 of 17 patients (5.9%) benefited from tumor resection over biopsy.
Moreover, data of patients with germinoma, germinoma with STGC, germinoma and teratoma with modest elevation of HCG/HCG-β, or incomplete examination of tumor marker in the CSF from the points of clinical outcomes were reviewed because most patients with histological evidence of germinoma were diagnosed using biopsy. This could have a sampling error problem. To test this, overall survival was analyzed in patients who received standard treatment of reduced-dose radiation therapy and chemotherapy. Reduced-dose radiation therapy and chemotherapy were performed in 22 of 33 patients satisfying criteria 2 and 21 of 37 patients satisfying criteria 3, respectively. Survival data were available in 17 patients of each group. Patients satisfying criteria 2 had a 5- and 10- year overall survival rate of 100% and 89%, respectively, and 100% and 100%, respectively, in patients satisfying criteria 3. One patient satisfying criteria 2 with pure germinoma died of recurrent immature teratoma. This finding suggested that a small proportion of patients with histological diagnosis of germinoma could have an unexpected clinical course, but most of these patients had a consistent course as those with germinoma.
Considering the high morbidity of tumor resection and the rarity of the patients who benefited from tumor resection in this study, a biopsy is recommended rather than tumor resection. However, this result should be considered carefully because the neurosurgeons in Japan have more opportunities to master performing endoscopic biopsy for pineal and neurohypophyseal tumors because of the higher incidence of GCTs. The indication for biopsy might differ according to the availability of experts in the area where the patient might present in the world.
Limitations
Certain limitations should be noted in this study. First, our data were collected retrospectively, so that the measurement of tumor markers including the source of samples and the detection threshold for HCG and its β-subunit, the surgical procedure and site of tissue sampling, and criteria of histological diagnosis were not standardized. Second, this cohort included heterogeneous cases based on 3 different criteria for negative tumor markers. Considering that 3 patients with NGGCT had incomplete CSF examination (criteria 3), incomplete estimation of the CSF tumor markers could possibly lead to the incorrect inclusion of positive tumor markers in this study. To verify this, data of the patients satisfying criteria 3 were reviewed. This group had 33 patients without data of AFP and HCG/HCG-β in the CSF (Supplementary Table 1). As for the value of AFP in CSF, the value in serum was higher than that in the CSF,31 and all 30 patients with AFP within normal value in serum had AFP within normal value in the CSF.6 Based on these facts, AFP in the CSF seemed likely to be negative in patients who fulfilled criteria 3. As for the value of HCG/HCG-β in CSF, the value of HCG/HCG-β in serum was lower than that in the CSF,31 so that Patients 2 and 3 in Table 4 could have had higher HCG/HCG-β value in the CSF than the threshold of modest elevation of HCG/HCG-β. Similarly, 33 of 37 patients satisfying criteria 3 did not have the data of HCG/HCG-β in the CSF, and they may have higher HCG/HCG-β value in the CSF. However, it seemed unlikely because linear correlations were found between the CSF and serum HCG-β value after logarithmic transformation and all 16 patients with low HCG levels (<1 mIU/mL) in serum had HCG levels <50 mIU/mL in the CSF (0.1–32.4 mIU/mL, median: 0.6 mIU/mL).6 In this manner, HCG levels of Patients 2 and 3 and 33 patients without the data of HCG/HCG-β in CSF could be <50 mIU/mL. We presumed that this study included patients with AFP within normal value and undetectable HCG/HCG-β in both serum and the CSF or that those with AFP within normal value in both serum and the CSF had modestly elevated HCG/HCG-β in serum and/or the CSF.
Conclusion
This study reports a novel finding that all cases with the bifocal tumors with DI and negative tumor markers were GCTs or granulomatous inflammation, but not other types of tumors. However, it is noteworthy that no fewer than 3.4% of the patients who fulfilled the 3 criteria had NGGCT. Since biopsy had high diagnostic rate and low morbidity and can also treat acute hydrocephalus safely, we recommend histological diagnosis in patients with bifocal tumors, DI, and negative tumor markers.
Supplementary Material
Acknowledgments
The following individuals made significant contributions to the study but are not included in the author list due to the exclusion of their cases. We here express gratitude for their contribution: Yasuo Aihara (Department of Neurosurgery, Tokyo Women’s Medical University, Tokyo, Japan), Yoshitaka Narita (Departments of Neurosurgery and Neuro-Oncology, National Cancer Center Hospital, Tokyo, Japan), Yuichi Sato (Department of Neurosurgery, Iwate Medical University, Morioka, Japan), Kenichiro Asano (Department of Neurosurgery, Hirosaki University Graduate School of Medicine, Hirosaki, Japan), Sadahiro Nomura (Department of Neurosurgery, Yamaguchi University School of Medicine, Yamaguchi, Japan), Atsushi Kikuta (Department of Pediatric Oncology, Fukushima Medical University, Fukushima, Japan), Tsuyoshi Ito (Department of Pediatrics, Toyohashi Municipal Hospital, Toyohashi, Japan), and Keisuke Miyake (Department of Neurological Surgery, Kagawa University Faculty of Medicine, Kagawa, Japan).
Conflict of interest statement. The authors declare that they have no conflicts of interest pertaining to this work.
Authorship statement. Conceptualization and design: M.K, H.T., K.I., and R.N. Acquisition of data: M.K., S.Y., T.S., K.Y., T.T., A.I., N.I., A.K., T.K., M.M., S.T., M.N., K.M., M.N., K.J., M.Y., N.K., N.S., T.N., Y.N., Y.A., S.H., H.S., A.Y., H.A., J.A., Y.K., T.S., A.N., M.N., Y.A., D.K., T.F., T.T., K.K., T.T., S.I., M.N., D.K., S.Y., R.A., T.U., J.F., N.K., and K.T. Analysis of data: M.K. and H.T. Manuscript writing: M.K, H.T., and R.N. Final editing and approval of the manuscript: M.K, H.T., S.Y., T.S., K.Y., T.T., A.I., N.I., A.K., T.K., M.M., S.T., M.N., K.M., M.N., K.J., M.Y., N.K., N.S., T.N., Y.N., Y.A., S.H., H.S., A.Y., H.A., J.A., Y.K., T.S., A.N., M.N., Y.A., D.K., T.F., T.T., K.K., T.T., S.I., M.N., D.K., S.Y., R.A., T.U., J.F., N.K., K.T., K.I., and R.N.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- 1. The Committee of Brain Tumor Registry of Japan. Report of brain tumor registry of Japan (2005–2008). Neurol Med Chir (Tokyo). 2017;57(Suppl):14–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ostrom QT, Gittleman H, Truitt G, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20(Suppl 4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sugiyama K, Uozumi T, Kiya K, et al. Intracranial germ-cell tumor with synchronous lesions in the pineal and suprasellar regions: report of six cases and review of the literature. Surg Neurol. 1992;38(2):114–120. [DOI] [PubMed] [Google Scholar]
- 4. Cuccia V, Alderete D. Suprasellar/pineal bifocal germ cell tumors. Childs Nerv Syst. 2010;26(8):1043–1049. [DOI] [PubMed] [Google Scholar]
- 5. Lafay-Cousin L, Millar BA, Mabbott D, et al. Limited-field radiation for bifocal germinoma. Int J Radiat Oncol Biol Phys. 2006;65(2):486–492. [DOI] [PubMed] [Google Scholar]
- 6. Takami H, Fukuoka K, Fukushima S, et al. Integrated clinical, histopathological, andmolecular data analysis of 190 central nervous system germ cell tumors from the iGCT Consortium. Neuro Oncol. 2019;21(12):1565–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Phi JH, Kim SK, Lee J, et al. The enigma of bifocal germ cell tumors in the suprasellar and pineal regions: synchronous lesions or metastasis? J Neurosurg Pediatr. 2013;11(2):107–114. [DOI] [PubMed] [Google Scholar]
- 8. Balossier A, Blond S, Reyns N. Endoscopic versus stereotactic procedure for pineal tumor biopsies: focus on overall efficacy rate. World Neurosurg. 2016;92:223–228. [DOI] [PubMed] [Google Scholar]
- 9. Murray MJ, Bartels U, Nishikawa R, Fangusaro J, Matsutani M, Nicholson JC. Consensus on the management of intracranial germ-cell tumours. Lancet Oncol. 2015;16(9):e470–e477. [DOI] [PubMed] [Google Scholar]
- 10. Aizer AA, Sethi RV, Hedley-Whyte ET, et al. Bifocal intracranial tumors of nongerminomatous germ cell etiology: diagnostic and therapeutic implications. Neuro Oncol. 2013;15(7):955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cunliffe CH, Fischer I, Karajannis M, et al. Synchronous mixed germ cell tumor of the pineal gland and suprasellar region with a predominant angiomatous component: a diagnostic challenge. J Neurooncol. 2009;93(2):269–274. [DOI] [PubMed] [Google Scholar]
- 12. Phuakpet K, Larouche V, Hawkins C, et al. Bifocal pineal and suprasellar tumor as a rare presentation of supratentorial primitive neuroectodermal tumor: case report. Neuro Oncol. 2014;16(Suppl 1):i86. [Google Scholar]
- 13. Mobley BC, Kumar M, Sanger WG, et al. Papillary tumor of the pineal region with synchronous suprasellar focus and novel cytogenetic features. Hum Pathol. 2015;46(8):1232–1236. [DOI] [PubMed] [Google Scholar]
- 14. Calaminus G, Kortmann R, Worch J, et al. SIOP CNS GCT 96: final report of outcome of aprospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol. 2013;15(6):788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bamberg M, Kortmann RD, Calaminus G, et al. Radiation therapy for intracranial germinoma: results of the German cooperative prospective trials MAKEI 83/86/89. J Clin Oncol. 1999;17(8):2585–2592. [DOI] [PubMed] [Google Scholar]
- 16. Kanagaki M, Miki Y, Takahashi JA, et al. MRI and CT findings of neurohypophyseal germinoma. Eur J Radiol. 2004;49(3):204–211. [DOI] [PubMed] [Google Scholar]
- 17. Dumrongpisutikul N, Intrapiromkul J, Yousem DM. Distinguishing between germinomas and pineal cell tumors on MR imaging. AJNR Am J Neuroradiol. 2012;33(3):550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Watanabe S, Aihara Y, Kikuno A, et al. A highly sensitive and specific chemiluminescent enzyme immunoassay for placental alkaline phosphatase in the cerebrospinal fluid of patients with intracranial germinomas. Pediatr Neurosurg. 2012;48(3):141–145. [DOI] [PubMed] [Google Scholar]
- 19. Aihara Y, Watanabe S, Amano K, et al. Placental alkaline phosphatase levels in cerebrospinal fluid can have a decisive role in the differential diagnosis of intracranial germ cell tumors. J Neurosurg. 2018;131(3):687–694. [DOI] [PubMed] [Google Scholar]
- 20. Kanamori M, Kumabe T, Watanabe M, et al. Indications for salvage surgery during treatment for intracranial germ cell tumors. J Neurooncol. 2018;138(3):601–607. [DOI] [PubMed] [Google Scholar]
- 21. Kanamori M, Kumabe T, Saito R, et al. Optimal treatment strategy for intracranial germ cell tumors: a single institution analysis. J Neurosurg Pediatr. 2009;4(6):506–514. [DOI] [PubMed] [Google Scholar]
- 22. Kanamori M, Kumabe T, Tominaga T. Is histological diagnosis necessary to start treatment for germ cell tumours in the pineal region? J Clin Neurosci. 2008;15(9):978–987. [DOI] [PubMed] [Google Scholar]
- 23. Moon KS, Jung S, Lee MC, et al. Two cases of pineal germinoma with granulomatous inflammation. J Clin Neurosci. 2005;12(3):310–313. [DOI] [PubMed] [Google Scholar]
- 24. Kasper CS, Schneider NR, Childers JH, Wilson JD. Suprasellar germinoma. Unresolved problems in diagnosis, pathogenesis, and management. Am J Med. 1983;75(4):705–711. [DOI] [PubMed] [Google Scholar]
- 25. Konno S, Oka H, Utsuki S, et al. Germinoma with a granulomatous reaction. Problems of differential diagnosis. Clin Neuropathol. 2002;21(6):248–251. [PubMed] [Google Scholar]
- 26. Kraichoke S, Cosgrove M, Chandrasoma PT. Granulomatous inflammation in pineal germinoma. A cause of diagnostic failure at stereotaxic brain biopsy. Am J Surg Pathol. 1988;12(9):655–660. [PubMed] [Google Scholar]
- 27. Maghnie M. Diabetes insipidus. Horm Res. 2003;59(Suppl 1):42–54. [DOI] [PubMed] [Google Scholar]
- 28. Ramelli GP, von der Weid N, Stanga Z, Mullis PE, Buergi U. Suprasellar germinomas in childhood and adolescence: diagnostic pitfalls. J Pediatr Endocrinol Metab. 1998;11(6):693–697. [DOI] [PubMed] [Google Scholar]
- 29. Kim K, Yeon JY, Seol HJ, Shin HJ. Transventricular endoscopic biopsy of suprasellar tumors: a pediatric case series. Childs Nerv Syst. 2013;29(8):1285–1291. [DOI] [PubMed] [Google Scholar]
- 30. Shono T, Natori Y, Morioka T, et al. Results of a long-term follow-up after neuroendoscopic biopsy procedure and third ventriculostomy in patients with intracranial germinomas. J Neurosurg. 2007;107(3 Suppl):193–198. [DOI] [PubMed] [Google Scholar]
- 31. Hu M, Guan H, Lau CC, et al. An update on the clinical diagnostic value of β-hCG and αFP for intracranial germ cell tumors. Eur J Med Res. 2016;21:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.