Abstract
Background
In Radiation Therapy Oncology Group (RTOG) 0825, a phase III trial of standard therapy with bevacizumab or without (placebo) in newly diagnosed glioblastoma, 44 patients underwent dynamic contrast enhanced (DCE) and/or dynamic susceptibility contrast (DSC) MRI in the American College of Radiology Imaging Network (ACRIN) trial 6686. The association between early changes in relative cerebral blood volume (rCBV) and volume transfer constant (Ktrans) with overall survival (OS) was evaluated.
Methods
MRI was performed at postop baseline (S0), immediately before (S1), 1 day after (S2), and 7 weeks after (S3) bevacizumab or placebo initiation. Mean normalized and standardized rCBV (nRCBV, sRCBV) and Ktrans were measured within contrast-enhancing lesion. Wilcoxon rank sum tests compared parameter changes from S1–S2 and S1–S3. Association with OS and progression-free survival (PFS) were determined using Kaplan–Meier and log-rank tests. Treatment response for groups stratified by pretreatment nRCBV (S0, S1) was explored. The intraclass correlation coefficient and repeatability coefficient for the placebo arm (S1–S2) were used to assess repeatability.
Results
Evaluable were 27–36 datasets per time point. Significant differences between treatment arms were found for changes in nRCBV and sRCBV from S1–S2 and S1–S3, and in Ktrans for S1–S3. Improved PFS (P = 0.05) but not OS (P = 0.46) was observed. High pretreatment rCBV predicted improved OS for bevacizumab-treated patients. Based on the intraclass correlation coefficient, sRCBV (0.92) was more repeatable than nRCBV (0.71) and Ktrans (0.75), consistent with repeatability coefficient values.
Conclusions
Bevacizumab significantly changes rCBV but not Ktrans as early as 1 day posttreatment in newly diagnosed glioblastoma unrelated to outcomes. Improvements in clinical trial design to maximize rCBV benefit are indicated.
Keywords: bevacizumab, brain tumor, clinical trial, Ktrans, rCBV
Key Points.
This is the first multicenter trial of CBV and Ktrans in bevacizumab-treated new glioblastoma.
Measures of rCBV and Ktrans repeatability are provided.
Evidence suggests use of rCBV for future trial stratification.
Importance of the Study.
This is the first multicenter study to address the use of DSC-MRI and DCE-MRI in bevacizumab-treated newly diagnosed glioblastoma. The results show rCBV but not Ktrans to be a sensitive biomarker of early biological changes following initiation of bevacizumab therapy, yet without an association with outcomes. Future clinical trials should consider using advanced MRI biomarkers to stratify treatment groups, rather than the standard approach of randomized assignment where subpopulations of responding patients might be missed.
Each year, nearly 70,000 patients are newly diagnosed with primary central nervous system tumors. Of these, one-third are malignant, of which 80% are gliomas.1 Glioblastomas are the most common and most aggressive gliomas in adults, with a median overall survival (OS) of 15 months.2 Standard-of-care treatment consists of maximal safe resection, followed by chemoradiation therapy (CRT) using temozolomide (TMZ).3 At tumor recurrence, common4 treatment options include re-irradiation, tumor treating fields, and bevacizumab, which inhibits angiogenesis.5 Bevacizumab received accelerated FDA approval for recurrent glioblastoma based on 2 prospective phase II clinical trials where the 6-month progression-free survival (PFS), based on MacDonald6 and Response Assessment in Neuro-Oncology (RANO)7 criteria, was 42.6% and 29%, respectively, compared with the historical control rate of 15%, but with no apparent improvement in OS.8 Similarly, in a large randomized trial of radiation plus chemotherapy with and without bevacizumab for newly diagnosed glioblastoma (Radiation Therapy Oncology Group [RTOG] 0825),9 the PFS was significantly improved (10.7 vs 7.3 mo, P = 0.007) but the OS was not (15.7 and 16.1 mo, P = 0.21). Currently, standard practice considers bevacizumab a treatment option for recurrent but not newly diagnosed glioblastoma.
Understanding the true efficacy of bevacizumab for both newly diagnosed and recurrent glioblastoma is further nuanced by the growing understanding that conventional MRI insufficiently evaluates the response of glioblastoma to anti-angiogenic therapies such as bevacizumab.10,11 Since these agents decrease blood–brain barrier permeability to gadolinium-based contrast agents (GBCAs), decreased contrast enhancement on T1-weighted MRI and fluid-attenuated inversion recovery (FLAIR) hyperintense signal on post-bevacizumab MRI may not reflect cytotoxic effect. This phenomenon, termed “pseudoresponse,” 12 may also partly explain why improved PFS but not OS was observed in early bevacizumab-treated glioblastoma trials.
Recent studies measuring relative cerebral blood volume (rCBV) using dynamic susceptibility contrast (DSC) MRI demonstrated improved OS with bevacizumab treatment for subsets of patients with recurrent and newly diagnosed glioblastoma in both single institution13–17 and multicenter trials.18 These results suggest that meaningful prediction of response to bevacizumab requires an advanced imaging marker that interrogates tumor hemodynamics. However, it remains unclear when rCBV should be measured or whether absolute or relative change in rCBV is most predictive. For example, some studies show utility in measuring rCBV pretreatment13,15,17 or at 2 or 4 weeks posttreatment,18 but others produced mixed results at 7–8 weeks posttreatment,13,18–20 a finding dependent on whether absolute rCBV or rCBV changes were measured. In another study, changes in both DSC-MRI and dynamic contrast enhanced (DCE) MRI perfusion parameters, including rCBV and Ktrans (volume transfer coefficient), were noted as early as 1 day posttreatment with the anti-angiogenic agent cediranib.21 In this context, the planned objective of the American College of Radiology Imaging Network (ACRIN) trial 6686 was to assess the association between OS and changes in rCBV and Ktrans 1 day after treatment initiation with bevacizumab compared with placebo, in patients with newly diagnosed glioblastoma. Parameter changes measured at 7–8 weeks posttreatment with bevacizumab or placebo were also assessed.
Methods
RTOG (now NRG Oncology), in collaboration with ACRIN (now the Eastern Cooperative Oncology Group [ECOG]-ACRIN), both funded by the National Cancer Institute, conducted a prospective, phase III double-blind placebo-controlled multicenter trial comparing conventional concomitant chemoradiation and adjuvant TMZ without (placebo arm) versus with bevacizumab in patients with newly diagnosed glioblastoma (RTOG 0825/ACRIN 6686). Each participating institution obtained institutional review board approval before subject accrual and conducted the trial in compliance with the Health Insurance Portability and Accountability Act of 1996. Informed consent was obtained for all subjects.
Patients
As described in the primary report for this study,22 all patients had newly diagnosed, histologically proven glioblastoma or gliosarcoma (World Health Organization [WHO] grade IV astrocytoma) confirmed on central review. Inclusion and exclusion criteria are available at https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0825. Patients were randomly assigned to receive standard CRT plus bevacizumab or standard therapy alone (referred to as the placebo arm). Bevacizumab (for the bevacizumab arm) was administered (10 mg/kg i.v., days 1 and 15 of a 28-day cycle) at the end of week 3 of CRT, until disease progression, severe treatment-related toxicity, or completion of adjuvant therapy (24 doses over 12 cycles, maximum). Maintenance chemotherapy (TMZ) began 4 weeks after completion of CRT.23
MRI Acquisition
MRI was performed at both 1.5 T (Siemens Espree, Siemens Magnetom Avanto, GE Signa Excite, GE Signa HDx) and 3T (GE Signa HDx, GE Signa Excite). Conventional MRI included precontrast T1-weighted, T2-weighted, FLAIR, and diffusion-weighted imaging.24 Following intravenous injection of GBCA (0.1 mmol/kg), axial 2D spin-echo postcontrast T1-weighted images with or without 3D volumetric T1-weighted images were acquired. The imaging protocol remained fixed at each site and across all time points.
For patients participating in the advanced component of the trial, DCE-MRI and/or DSC-MRI was also performed. For DCE-MRI, pre-injection T1 data were obtained using the multiple flip angle (FA) 3D T1-weighted spoiled gradient echo/magnetization prepared gradient echo (SPGR/MPRAGE ) technique (repetition time [TR] = 4.2–8.3 ms for all FA, minimum echo time [TE], and 2°, 10°, 15°, 20°, and 30° FA). Dynamic 3D T1-weighted SPGR/MPRAGE images (TR = 4–7.4 ms, minimum TE, 20–30° FA) were then obtained every <6.3 sec, for a total imaging time of 4–6 min during the first power-injected bolus of GBCA administered at a rate of 3–5 cc/sec. This was followed by standard postcontrast imaging, using the same precontrast T1-weighted technique described above. Next, DSC-MRI was performed using gradient-echo or spin-echo echo-planar imaging with gradient-echo TE = 30–40 msec and spin-echo TE = 70–105 msec; TR = 1.0–1.5 sec, with 30–60 sec of acquisition before and 1 min of acquisition after a power-injected bolus of 0.1 mmol/kg dose of GBCA, for a total of 120 acquired time points. The first GBCA dose used for DCE-MRI and postcontrast imaging served as a preload for DSC-MRI, thereby diminishing confounding GBCA leakage effects.25
Imaging Timeline
ACRIN 6686 enrolled 44 patients for advanced imaging, and OS information was available for 42 of these patients. Of these, 22 patients received bevacizumab (bevacizumab arm) and 20 did not (placebo arm). DSC-MRI and DCE-MRI were performed 0–5 days pre-CRT (S0), 0–3 days before (S1), 0–1 day (S2), and 7 weeks (S3) after treatment initiation with bevacizumab or placebo. (Note the S1 and S2 time points take place during week 4 of the 6 weeks of CRT.) DSC-MRI data from 6 of the 42 patients was excluded because gradient-echo data were not available at any of the 4 time points. Spin-echo data was excluded due to the well-known differences in susceptibility contrast between gradient-echo and spin-echo methods26 and the small number of spin-echo datasets obtained. For the remaining 36 patients, datasets for the bevacizumab/placebo arms at S0, S1, S2, and S3 were 27 (14/13), 31 (16/15), 31 (17/14), and 29 (15/14). (See Supplementary Figure 1.) For the following reasons, 26 individual datasets were excluded: no DSC data (n = 6), spin-echo data collected (n = 1), poor DSC signal due to lesion location (n = 9), incorrect or no contrast agent administration (n = 2), no contrast agent preload given or precontrast T1 not collected (n = 4), an enhancing lesion was not present (n = 4). Overall, this resulted in a small number of excluded datasets (11 of 144 total) for direct DSC-MRI issues, such as poor signal or difficulties with contrast agent injection. For DCE-MRI, the number of evaluable datasets of acceptable image quality for the bevacizumab/placebo arms at S0, S1, S2, and S3 are 30 (16/14), 35 (18/17), 36 (17/19) and 33 (15/18), respectively. As listed in Supplementary Figure 1, 34–39 DCE-MRI datasets were obtained at each time point. Datasets were excluded if the DCE-MRI data were incomplete (n = 5) due to shortened imaging durations, lack of baseline images, or use an incorrect FA, and when the arterial input function could not be determined (n = 3) or the precontrast T1 map was not obtained (n = 5).
Image Analysis
Central reader analysis.
—As previously described,9,22 all local imaging was transmitted to ACRIN for central review with each distinct contrast-enhancing target lesion (≥1 cm diameter, ≥1 cm from other enhancing lesions), evaluated according to RANO guidelines.7 MRI at postop/pre-CRT baseline (S0) and pre–cycle 4 of adjuvant TMZ (about 22 wk after starting CRT and 19 wk after beginning bevacizumab/placebo treatment) were used to determine response. Consistent with RANO criteria, progression on 2D-T1 occurred when there was a >25% increase with respect to the nadir, or in this case postop baseline, or new measurable enhancing tumor. Radiologic response was defined as a ≥50% decrease with respect to baseline, confirmed on the subsequent time point. Steroid dosage and clinical status were unavailable to the readers for this study.
Advanced MRI analysis.
—For DSC-MRI analysis, rCBV maps normalized (nRCBV) to normal-appearing white matter (NAWM) and standardized (sRCBV) to a universal scale27 were corrected for contrast agent leakage effects25,28 using IB Neuro TM (Imaging Biometrics LLC, Elm Grove, WI). For consistency of analysis across patients, NAWM was selected by one person (M.A.P.) for all datasets within deep frontal white matter free of FLAIR hyperintensity.
DCE-MRI analysis was performed by first computing the pre-injection T1 map from the multi-FA T1-weighted SPGR data, and then using this map to convert signal intensities from the dynamic acquisition into estimates of GBCA concentrations over time (∆R1(t)). Parameter maps of Ktrans were computed using a matrix-based linearization method to fit ∆R1(t) to the extended Tofts model.29
Using IB DeltaSuite TM (Imaging Biometrics LLC, Elm Grove, WI), contrast-enhancing regions of interest (ROIs) were defined from deltaT1 (dT1) maps, which are calibrated, quantitative difference maps computed from standardized post- and standardized precontrast T1-weighted images.30 The dT1 maps facilitate visualization of enhancing lesion, free of intrinsically increased T1 signal from blood products or proteinaceous material. Because dT1 maps are quantitative, a single threshold can be applied to all cases for consistent delineation of contrast-enhancing tumor ROI. The tumor ROIs were applied to both rCBV and Ktrans parameter maps, which were coregistered to the T1-weighted images and the mean and median parameter values extracted.
Statistical Analysis
Descriptive statistics were computed for nRCBV, sRCBV, and Ktrans. The Wilcoxon rank sum tests were used to compare the percent changes in nRCBV, sRCBV, and Ktrans from time points S1–S2 and S1–S3 between bevacizumab and placebo-treated patients. The absolute change was also determined for parameter changes from S1 to S2. The Kaplan–Meier method and log-rank test were used to compare PFS and OS between patients treated with bevacizumab versus placebo, and between patients with increasing versus no change/decreasing nRCBV, sRCBV, and Ktrans. The choice for this comparison was based on a previous multicenter study using rCBV, where positive versus negative/no-change differences demonstrated a significant difference in outcomes.18
An exploratory analysis was performed where the patients were divided into “high” and “low” nRCBV groups, defined as the pretreatment nRCBV being above or below the median nRCBV value. This analysis was performed for nRCBV obtained pre-CRT (S0) and pre-bevacizumab/placebo (S1). Within each “low” and “high” nRCBV group, the Kaplan–Meier method and log-rank test were used to compare OS between patients treated with bevacizumab versus placebo.
Finally, the repeatability of nRCBV, sRCBV, and Ktrans measurements was determined from imaging data collected for the placebo arm, obtained 1 day apart (at S1 and S2), by constructing Bland–Altman plots and computing the repeatability coefficient (RC) and intraclass correlation coefficient (ICC).31 Statistical computations were performed using SAS v9.4 software or R v3.4.4 software (R project; https://www.r-project.org), with P-values <0.05 considered statistically significant.
Results
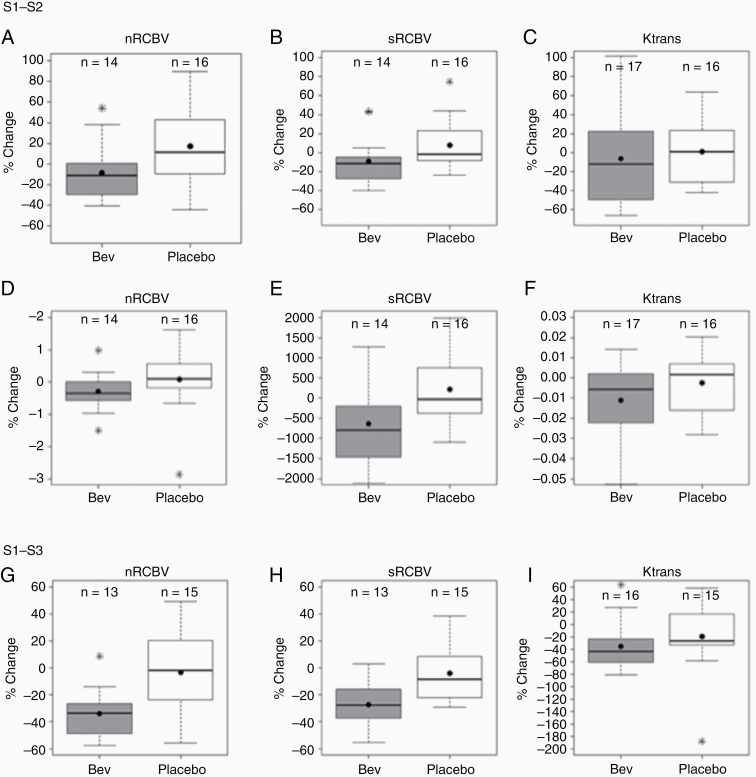
Example images and parameter maps from one patient are shown in Fig. 1. Summary statistics for nRCBV, sRCBV, and Ktrans for each treatment arm are provided as Supplementary Table 1. The percent and absolute changes in parameter values from pretreatment (S1) to 1 day posttreatment (S2) are also given in Supplementary Tables 2–5. Comparing treatment arms from S1–S2, significant differences in percent change were noted for nRCBV (P = 0.03) and sRCBV (P = 0.03) (Fig. 2A-B). Likewise, significant differences in absolute change resulted for nRCBV (P = 0.03) and sRCBV (P = 0.02) (Fig. 2D-E). From S1–S3, significant differences in percent change were noted for nRCBV (P = 0.007) and sRCBV (0.01) (Fig. 2G-H). (The absolute change was not computed for S1–S3.) There were no significant differences between treatment arms for Ktrans for S1–S2 percent change (P = 0.28) (Fig. 2C) or absolute change (P = 0.23), (Fig. 2F) but there was a significant difference in percent change Ktrans for S1–S3 (P = 0.045) (Fig. 2I).
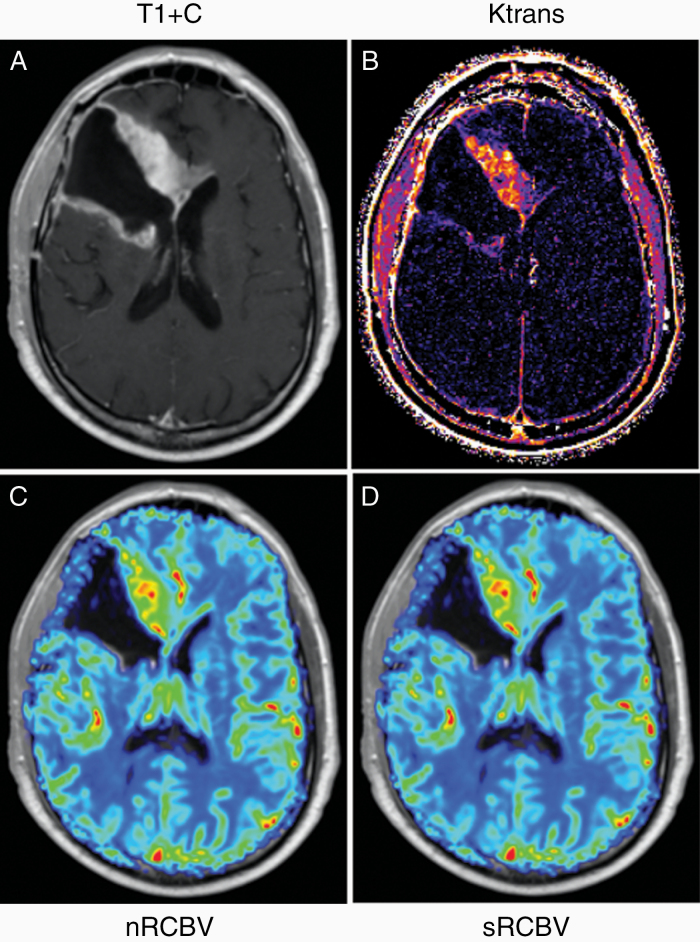
Fig. 1.
Example images and parameter maps for case 193 prior to bevacizumab/placebo treatment. Shown are the (A) postcontrast T1-weighted images along with the corresponding (B) Ktrans map from the DCE-MRI data and (C) normalized rCBV (nRCBV) and (D) standardized rCBV (sRCBV) maps from DSC-MRI data.
Fig. 2.
Percent and absolute change in parameter values. From S1 to S2, significant differences between treatment arms were found for percent change for (A) nRCBV (P = 0.03) and (B) sRCBV (P = 0.03) but not (C) Ktrans (P = 0.28) and absolute change for (D) nRCBV (P = 0.03) and (E) sRCBV (P = 0.02) but not (F) Ktrans (P = 0.23). From S1 to S3, significant differences between treatment arms were found for percent change for nRCBV (P = 0.007), sRCBV (P = 0.01), and Ktrans (P = 0.045). The Wilcoxon rank sum test was used for all comparisons.
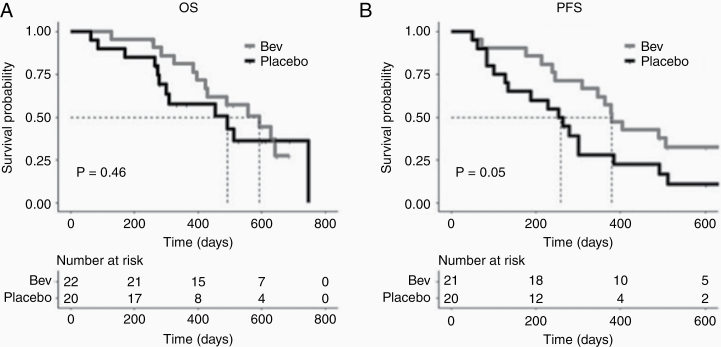
Although the median survival of the bevacizumab-treated group was 593 days compared with 492 days for the placebo arm, there was no significant difference in OS (P = 0.46) (Fig. 3A). Yet marginally significant differences in PFS (P = 0.05) were observed (Fig. 3B), with 380 versus 258.5 days for the bevacizumab and placebo treatment arms, respectively. There was no significant difference in OS between those with increasing compared with decreasing percent change in nRCBV, sRCBV, or Ktrans from S1–S2 or S1–S3, for individual treatment arms or when combining data from both arms. However, due to the small sample size of this study, firm conclusions cannot be made. Larger studies addressing the utility of rCBV to predict treatment-based survival differences are still needed.
Fig. 3.
Kaplan–Meier survival estimates of OS and PFS. (A) The median OS with placebo versus bevacizumab treatment was 492 versus 593 days, respectively, but was not statistically significant (P = 0.46). (B) Borderline statistically significant differences in PFS were observed as a function of treatment arm (P = 0.05) with median PFS of 380 and 258.5 days for bevacizumab and placebo treatment arms, respectively.
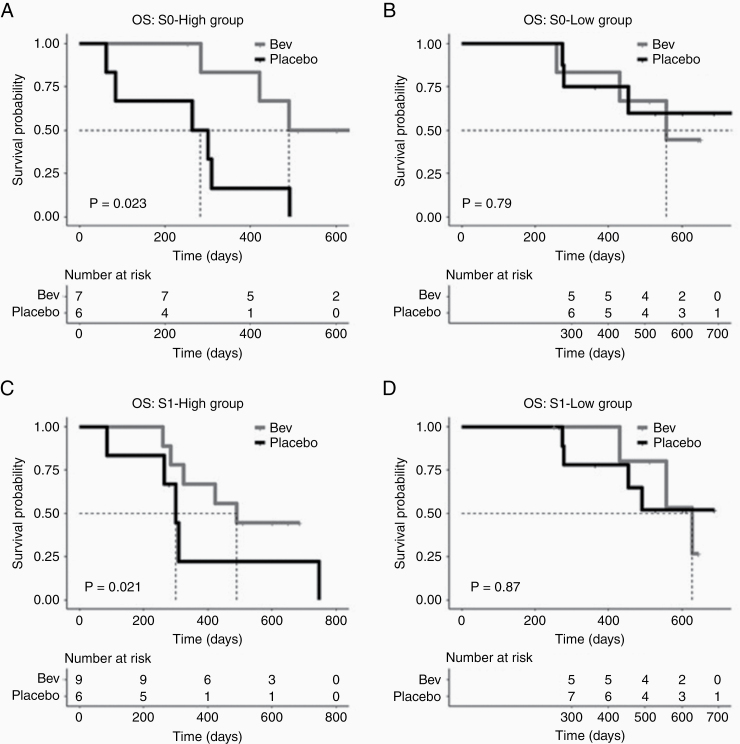
The exploratory analysis (Fig. 4) revealed a significant prolongation for bevacizumab-treated patients with high pre-CRT nRCBV (S0) (Fig. 4A). For those with low pre-CRT nRCBV, bevacizumab did not show an improvement in OS compared with placebo (Fig. 4B). A significant difference in treatment-based outcomes did not result when using nRCBV measured after CRT and before bevacizumab/placebo treatment (S1) (Fig. 4C, D). Though these results show that rCBV can be used to identify patients with newly diagnosed glioblastoma whose survival will be prolonged with bevacizumab, these results are not conclusive given the small sample size for this analysis.
Fig. 4.
Exploratory analysis using pretreatment rCBV for stratification. (A) Treatment groups stratified by nRCBV, measured at S0 (pre-CRT), above and below median nRCBV suggest that those with high nRCBV (A) may respond better to bevacizumab than those with low nRCBV (B). Treatment groups stratified by post-CRT/pre-bevacizumab (or placebo) nRCBV do not show different outcomes in response to treatment (C, D).
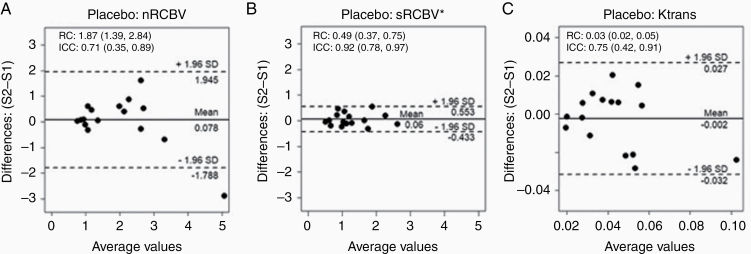
The ICC was highest for the sRCBV parameter (0.92) compared with the nRCBV and Ktrans parameters (0.71 and 0.75, respectively); however, the 95% confidence intervals overlapped. Likewise, the best repeatability coefficient was found for sRCBV (RC = 0.49) compared with nRCBV (1.87) meaning that it will be easier to detect a given change using sRCBV than with nRCBV because of its greater reproducibility.31 The Bland–Altman plots given in Fig. 5 show the reliability of each parameter.
Fig. 5.
Bland–Altman plots for (A) normalized rCBV, (B) standardized rCBV, and (C) Ktrans with RC and ICC listed. The rCBV measurement with the best (lowest) RC (sRCBV = 0.49) and best ICC (0.92) demonstrates the best consistency.
Discussion
This study demonstrates an immediate (1 day) biologic response to bevacizumab treatment compared with placebo, with a significant decrease in absolute and percent change in rCBV measured with DSC-MRI. This significant difference is maintained at 7 weeks posttreatment initiation. Conversely, Ktrans measured with DCE-MRI did not show a difference between treatment groups at the early time point but was significantly different at the later time point. Both PFS and OS were longer for patients receiving bevacizumab treatment compared with placebo, the with marginal significance for PFS. Also, the rCBV differences between treatment arms are not associated with PFS or OS. Yet this deserves further investigation, since an important limitation to this study is that ACRIN 6686 had a very low accrual rate and was ended without sufficient participants to power the study. Consequently, we performed an ad-hoc power analysis to compare the percent change in nRCBV and sRCBV from S1 to S2 between the 2 arms. Two-sided, 2-sample Wilcoxon rank sum tests were used at a significance level of 0.05. Given 30 available participants, the powers were 51.5% and 37.3% for the percent change in nRCBV and sRCBV, respectively. We therefore do not have sufficient power to confirm that nRCBV or sRCBV are not associated with PFS or OS, and additional studies are warranted.
What also might explain the results, at least in part, is the fact that the rCBV differences measured at the 7–8 week time point might be less sensitive to effects of bevacizumab compared with other time points. In a previous multicenter trial of bevacizumab-treated recurrent glioblastoma, changes in rCBV at 2 and 16 weeks after bevacizumab initiation were predictive of both PFS and OS, whereas changes at 7–8 weeks were not.18 Likewise, a single-center study of bevacizumab-treated recurrent glioblastoma found that although changes in rCBV at the 7–8 week time point were not predictive of OS, stratification by baseline absolute rCBV values were predictive of outcome.13 Patients with the best outcomes had rCBV values that started low and remained low, whereas patients with the worst outcomes had high rCBV levels that remained high even after treatment with bevacizumab. These results suggest that, although not all patients respond to bevacizumab, rCBV may help identify those that do, but there might also be a time-dependent sensitivity of rCBV to predict outcome. These findings deserve further investigation.
Overall, the significant changes found for absolute and percent change endorse rCBV as a sensitive and early measure of biologic response to bevacizumab treatment, with Ktrans demonstrating a later sensitivity. These differences did not, however, translate into a significant association with PFS and OS. This result may be at least partially explained by a recent study32 that underscores our incomplete understanding of combination therapies. Specifically, they demonstrated that in patients with recurrent glioblastoma, treated with bevacizumab plus CRT, there were regional differences in tumor TMZ uptake, with decreased uptake in regions with low permeability, as measured by Ktrans, and regions of improved delivery that correlated with increased cerebral blood flow. Note that in the current study, bevacizumab was administered after 4 weeks of CRT. These results demonstrate the need for an improved understanding and application of treatment strategies, for which imaging biomarkers, such as CBV, cerebral blood flow, and Ktrans can play a critical role.
Essentially all previous attempts to establish a role for rCBV in prognosticating response to bevacizumab have been made for recurrent and not newly diagnosed glioblastoma. Thus, this is the first multicenter study to address the use of rCBV in bevacizumab-treated newly diagnosed glioblastoma. Studies in newly diagnosed glioblastoma are likely lacking due to the standard neuro-oncology practice of prescribing bevacizumab for recurrent but not newly diagnosed glioblastoma. This practice is primarily based on 2 studies of 621 and 921 patients with newly diagnosed glioblastoma where significant improvements in PFS but not OS were realized.23,33 However, due to the crossover nature of both studies, 48.3% and 48.2% of patients in the placebo arm were treated with bevacizumab at progression. Therefore, as described by Gilbert et al, “the end point of overall survival was used to determine whether early first-line use of bevacizumab was superior to use as a salvage regimen.” 23 In short, whether up-front bevacizumab improves OS compared with not using bevacizumab treatment up-front was never fully addressed. Moreover, only standard imaging methods were used to assess treatment response, which can be insufficient or even inaccurate measures of response.
In seeming contradiction to these earlier studies and our current results, a previous study34 did show an OS improvement in response to bevacizumab treatment for newly diagnosed glioblastoma. Patients were separated into 2 cohorts based on the angiogenic profile of pretreatment tumor (ie, before CRT), defined in part by DSC-MRI perfusion parameters. Of the patients with an aggressive angiogenic profile, those treated with bevacizumab showed a significant improvement in OS compared with those that did not (placebo arm). This result suggests that whereas simple randomization between treatment arms may fail to demonstrate a survival advantage for newly diagnosed glioblastoma on bevacizumab therapy,23 at least a subpopulation of patients will have a survival advantage in response to bevacizumab, and these patients can be identified by pretreatment measures of rCBV.
The exploratory analysis performed in the present study is consistent with the results of this previous study. Specifically, when patients were separated into 2 groups defined as those with high versus low nRCBV measured pre-CRT, a significant improvement in OS was noted for those with high pretreatment nRCBV treated with bevacizumab. Interestingly, the nRCBV measured after CRT and just prior to treatment with bevacizumab or placebo did not show the same ability to stratify treatment-based outcomes. Though the results of this analysis are not conclusive given the small sample size, they are in agreement with those of Liu et al,34 who also used a pre-CRT measure of rCBV. Clearly, a well-designed study with an up-front measurement of rCBV to stratify patients into treatment arms may help to better determine whether first-line bevacizumab treatment is actually beneficial in a subset of patients.
Other important observations resulting from our study include the repeatability of the parameter measurements. Although normalized rCBV and Ktrans demonstrated good repeatability, standardized rCBV was clearly superior, with an ICC of 0.92. This improved consistency is likely a result of the fact that standardizing rCBV precludes the need for selecting a reference ROI,27 which is a well-known source of substantial variability. It is also a good choice for clinical trials, since far fewer subjects would be required to power a study.35
Study limitations include the fact that molecular features were not obtained. It is now well-known that the isocitrate dehydrogenase 1 (IDH1) mutation and O6-methylguanine-DNA methyltransferase (MGMT) methylation status independently associate with improved PFS and OS in patients with glioblastoma.36 In a more recent study,37 recurrent glioblastoma treated with bevacizumab showed improved PFS for those with MGMT promoter methylation and IDH1 positive mutations. In a similar way, the presence or absence of these molecular features may have influenced the analysis of the present study. However, since this information was not widely available or routinely obtained when this study was formulated, it was not collected.
The protocol for this study did not include a specific recommendation for the FA to be used when collecting the DSC-MRI data. However, a recent study using a DSC-MRI digital reference object38,39 provided computational evidence to show that when using a single dose preload followed by a single DSC-MRI dose (as was used for this study), the rCBV accuracy is negligibly affected by the choice of FA. Therefore, it is safe to surmise that the FA settings used for this study will not hamper the general applicability of the results.
The results from this study should help to improve the design of future clinical trials. First, because the standard-of-care imaging assessment of brain tumors typically takes place at 7–8 weeks posttreatment initiation, this was data obtained at this imaging time point was available to this study. However, based on the results of this and previous studies, this also seems to be a time point where the ability of rCBV to predict response is more variable, and therefore an earlier time point should be considered. For this reason, the currently active ECOG-ACRIN EAF151 trial includes obtaining rCBV at the 2-week time point after treatment initiation. EAF151 is also the first multicenter clinical trial for which the primary aims include advanced imaging, such that statistically justified larger numbers of patients can be enrolled for the evaluation of these imaging biomarkers. Also, given recent studies,32 and variations in reported outcomes, more attention should be given to understanding the impact of timing and combination of therapies and when additional biomarkers such as Ktrans may be most useful. Finally, provocative early evidence is provided that supports the use of rCBV as a method of patient stratification, rather than random stratification, which may alter our understanding of the benefit of using bevacizumab for newly diagnosed glioblastoma.
Conclusions
This study demonstrates that rCBV measured with DSC-MRI is sensitive to early biological changes following initiation of bevacizumab therapy. Use of rCBV for patient and treatment stratification is recommended for future clinical trials as its potential to predict patient response to bevacizumab remains high.
Supplementary Material
Funding
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Peter J. O’Dwyer, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: U10CA180820, U10CA180794. Funding support was also provided by the National Institutes of Health/National Cancer Institute U01 CA176110 (KMS, MAP) and R01CA082500 (KMS, MAP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Conflict of interest statement. Past ownership interest and current financial interest in Imaging Biometrics LLC (KMS) and ownership interest in IQ-AI Ltd (KMS).
Authorship statement. Author contributions to the experimental design (KMS, HM, EK, DPB, JLB), implementation (KMS, MAP, HM, EK, DPB, JLB), analysis (KMS, MAP, HM, EK, DPB, JLB), and interpretation of data (KMS, MAP, HM, EK, DPB, JLB). All authors were involved in the writing of the manuscript at both the draft and revision states and have read and approved this final version.
References
- 1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20(suppl_4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thakkar JP, Dolecek TA, Horbinski C, et al. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014;23(10):1985–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in randomized phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet. 2009;10:459–466. [DOI] [PubMed] [Google Scholar]
- 4. Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–2543. [DOI] [PubMed] [Google Scholar]
- 5. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 6. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 7. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 8. Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilbert MR, Sulman EP, Mehta MP. Bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(21):2048–2049. [DOI] [PubMed] [Google Scholar]
- 10. Henson JW, Ulmer S, Harris GJ. Brain tumor imaging in clinical trials. AJNR Am J Neuroradiol. 2008;29(3):419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wen PY, Norden AD, Drappatz J, Quant E. Response assessment challenges in clinical trials of gliomas. Curr Oncol Rep. 2010;12(1):68–75. [DOI] [PubMed] [Google Scholar]
- 12. Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22(6):633–638. [DOI] [PubMed] [Google Scholar]
- 13. Schmainda KM, Prah M, Connelly J, et al. Dynamic-susceptibility contrast agent MRI measures of relative cerebral blood volume predict response to bevacizumab in recurrent high-grade glioma. Neuro Oncol. 2014;16(6):880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leu K, Enzmann DR, Woodworth DC, et al. Hypervascular tumor volume estimated by comparison to a large-scale cerebral blood volume radiographic atlas predicts survival in recurrent glioblastoma treated with bevacizumab. Cancer Imaging. 2014;14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kickingereder P, Wiestler B, Burth S, et al. Relative cerebral blood volume is a potential predictive imaging biomarker of bevacizumab efficacy in recurrent glioblastoma. Neuro Oncol. 2015;17(8):1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu TT, Achrol AS, Mitchell LA, et al. Magnetic resonance perfusion image features uncover an angiogenic subgroup of glioblastoma patients with poor survival and better response to antiangiogenic treatment. Neuro Oncol. 2017;19(7):997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bennett IE, Field KM, Hovens CM, et al. Early perfusion MRI predicts survival outcome in patients with recurrent glioblastoma treated with bevacizumab and carboplatin. J Neurooncol. 2017;131(2):321–329. [DOI] [PubMed] [Google Scholar]
- 18. Schmainda KM, Zhang Z, Prah M, et al. Dynamic susceptibility contrast MRI measures of relative cerebral blood volume as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 multicenter trial. Neuro Oncol. 2015;17(8):1148–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harris RJ, Cloughesy TF, Hardy AJ, et al. MRI perfusion measurements calculated using advanced deconvolution techniques predict survival in recurrent glioblastoma treated with bevacizumab. J Neurooncol. 2015;122(3):497–505. [DOI] [PubMed] [Google Scholar]
- 20. Leu K, Boxerman JL, Lai A, et al. Bidirectional contrast agent leakage correction of dynamic susceptibility contrast (DSC)-MRI improves cerebral blood volume estimation and survival prediction in recurrent glioblastoma treated with bevacizumab. J Magn Reson Imaging. 2016;44(5):1229–1237. [DOI] [PubMed] [Google Scholar]
- 21. Batchelor TT, Gerstner ER, Emblem KE, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci U S A. 2013;110(47):19059–19064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boxerman JL, Zhang Z, Safriel Y, et al. Prognostic value of contrast enhancement and FLAIR for survival in newly diagnosed glioblastoma treated with and without bevacizumab: results from ACRIN 6686. Neuro Oncol. 2018;20(10):1400–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boxerman JL, Zhang Z, Safriel Y, et al. Early post-bevacizumab progression on contrast-enhanced MRI as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 Central Reader Study. Neuro Oncol. 2013;15(7):945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmainda KM, Rand SD, Joseph AM, et al. Characterization of a first-pass gradient-echo spin-echo method to predict brain tumor grade and angiogenesis. AJNR Am J Neuroradiol. 2004;25(9):1524–1532. [PMC free article] [PubMed] [Google Scholar]
- 26. Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM. MR contrast due to intravascular magnetic susceptibility perturbations. Magn Reson Med. 1995;34(4):555–566. [DOI] [PubMed] [Google Scholar]
- 27. Bedekar D, Jensen T, Schmainda KM. Standardization of relative cerebral blood volume (rCBV) image maps for ease of both inter- and intrapatient comparisons. Magn Reson Med. 2010;64(3):907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol. 2006;27(4):859–867. [PMC free article] [PubMed] [Google Scholar]
- 29. Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10(3):223–232. [DOI] [PubMed] [Google Scholar]
- 30. Schmainda KM, Prah MA, Zhang Z, et al. Quantitative Delta T1 (dT1) as a replacement for adjudicated central reader analysis of contrast-enhancing tumor burden: a subanalysis of the American College of Radiology Imaging Network 6677/Radiation Therapy Oncology Group 0625 Multicenter Brain Tumor Trial. AJNR Am J Neuroradiol. 2019;40(7):1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barnhart HX, Barboriak DP. Applications of the repeatability of quantitative imaging biomarkers: a review of statistical analysis of repeat data sets. Transl Oncol. 2009;2(4):231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gerstner ER, Emblem KE, Chang K, et al. Bevacizumab reduces permeability and concurrent temozolomide delivery in a subset of patients with recurrent glioblastoma. Clin Cancer Res. 2020;26(1): 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chinot OL, de La Motte Rouge T, Moore N, et al. AVAglio: phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv Ther. 2011;28(4):334–340. [DOI] [PubMed] [Google Scholar]
- 34. Liu TT, Achrol AS, Mitchell LA, et al. Magnetic resonance perfusion image features uncover an angiogenic subgroup of glioblastoma patients with poor survival and better response to antiangiogenic treatment. Neuro Oncol. 2017;19(7):997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prah MA, Stufflebeam SM, Paulson ES, et al. Repeatability of standardized and normalized relative CBV in patients with newly diagnosed glioblastoma. AJNR Am J Neuroradiol. 2015;36(9):1654–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang P, Zhang W, Wang Y, et al. IDH mutation and MGMT promoter methylation in glioblastoma: results of a prospective registry. Oncotarget. 2015;6(38):40896–40906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cardona AF, Rojas L, Wills B, et al. A comprehensive analysis of factors related to carmustine/bevacizumab response in recurrent glioblastoma. Clin Transl Oncol. 2019;21(10):1364–1373. [DOI] [PubMed] [Google Scholar]
- 38. Semmineh NB, Bell LC, Stokes AM, Hu LS, Boxerman JL, Quarles CC. Optimization of acquisition and analysis methods for clinical dynamic susceptibility contrast MRI using a population-based digital reference object. AJNR Am J Neuroradiol. 2018;39(11): 1981–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Semmineh NB, Stokes AM, Bell LC, Boxerman JL, Quarles CC. A population-based digital reference object (DRO) for optimizing dynamic susceptibility contrast (DSC)-MRI methods for clinical trials. Tomography. 2017;3(1):41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.