In this study, we identified that more favorable neighborhood opportunities in midchildhood predict better cardiometabolic health from midchildhood to early adolescence.
Abstract
Video Abstract
BACKGROUND AND OBJECTIVES:
The Child Opportunity Index (ChOI) is a publicly available surveillance tool that incorporates traditional and novel attributes of neighborhood conditions that may promote or inhibit healthy child development. The extent to which ChOI relates to individual-level cardiometabolic risk remains unclear.
METHODS:
We geocoded residential addresses obtained from 743 participants in midchildhood (mean age 7.9 years) in Project Viva, a prebirth cohort from eastern Massachusetts, and linked each location with census tract-level ChOI data. We measured adiposity and cardiometabolic outcomes in midchildhood and early adolescence (mean age 13.1 years) and analyzed their associations with neighborhood-level ChOI in midchildhood using mixed-effects models, adjusting for individual and family sociodemographics.
RESULTS:
On the basis of nationwide distributions of ChOI, 11.2% (n = 83) of children resided in areas of very low overall opportunity (ChOI score <20 U) and 55.3% (n = 411) resided in areas of very high (ChOI score ≥80 U) overall opportunity. Children who resided in areas with higher overall opportunity in midchildhood had persistently lower levels of C-reactive protein from midchildhood to early adolescence (per 25-U increase in ChOI score: β = .14 mg/L; 95% confidence interval, .28 to .00). Additionally, certain ChOI indicators, such as greater number of high-quality childhood education centers, greater access to healthy food, and greater proximity to employment in midchildhood, were associated with persistently lower adiposity, C-reactive protein levels, insulin resistance, and metabolic risk z scores from midchildhood to early adolescence.
CONCLUSIONS:
Our findings suggest more favorable neighborhood opportunities in midchildhood predict better cardiometabolic health from midchildhood to early adolescence.
What’s Known on This Subject:
The Child Opportunity Index is a publicly available surveillance tool that incorporates traditional and novel attributes of neighborhood conditions that may affect child development. It is still unclear whether this neighborhood-level index relates to individual-level cardiometabolic outcomes in early adolescence.
What This Study Adds:
In our study, we broaden the scope of research on neighborhood environments by showing that more favorable neighborhood opportunities (reflected by higher Child Opportunity Index scores) in midchildhood predicted better cardiometabolic health from midchildhood to early adolescence.
Family socioeconomic status and the environments in which children reside (ie, their homes, neighborhoods, and schools) are known to be important contributors to health in children and adolescents.1–4 These environments confer both opportunities and challenges for children to follow a healthy developmental trajectory.5 For example, children living in deprived neighborhoods (ie, high poverty and unemployment rates, lack of healthy food choices) are less likely to stay on a healthy developmental trajectory.6,7 However, existing indices commonly used in research on neighborhood environments represent only selected aspects of socioeconomic disadvantage, such as poverty, low income level, and unemployment.1,8 They do not adequately describe other conditions embedded in the same neighborhood that may counteract the risks associated with living in disadvantaged neighborhoods, such as access to healthy food choices.9,10
To circumvent these limitations of existing measures, researchers developed the Child Opportunity Index (ChOI), a multidimensional, population-level surveillance tool that incorporates both traditional (eg, median household income) and novel (eg, access to healthy food choices) attributes of neighborhood conditions that may promote or inhibit healthy child development.11 Thus, the ChOI reflects the combined contributions of these positive and negative components and provides a measure of overall neighborhood opportunity. To date, it has been used to examine disparities in acute health care use among pediatric patients and has shown to be associated with children’s stress physiology.12,13 However, it is still unclear whether the ChOI relates to individual-level cardiometabolic outcomes in early adolescence. Understanding these relationships may help public health professionals to better strategize or implement policies that aim to reduce neighborhood inequalities in children’s cardiometabolic health.
To address these gaps, we used data from a longitudinal cohort of children in eastern Massachusetts to investigate the extent to which neighborhood-level ChOI in midchildhood was associated with individual-level cardiometabolic outcomes in midchildhood and early adolescence. We hypothesized that children residing in areas with more favorable neighborhood opportunities in midchildhood would have reduced adiposity and better cardiometabolic health outcomes from midchildhood to early adolescence.
Methods
Study Population
Project Viva is an ongoing study of prenatal and perinatal influences on maternal, fetal, and child health.14 Briefly, we recruited eligible pregnant women during their first prenatal appointment between April 1999 and November 2002 from obstetric practices at Atrius Harvard Vanguard Medical Associates in eastern Massachusetts. Mothers provided written informed consent at enrollment and follow-up visits, and children provided verbal assent at the midchildhood and early adolescent visits. The Institutional Review Board of Harvard Pilgrim Health Care approved the project in line with ethical standards established by the Declaration of Helsinki. Of 2128 live singleton births, this study included 1010 participants who provided residential addresses at the midchildhood visit between 2007 and 2011. We further limited our analysis to 743 children with at least 1 outcome measure at either the midchildhood or early adolescent visit (between 2012 and 2016) (Supplemental Fig 5).
Exposure: ChOI
Researchers at the Institute for Child, Youth and Family Policy at Brandeis University developed the ChOI as a summary measure of the quality of neighborhoods children would typically experience everyday across the United States.11,15 Briefly, the index quantifies 29 indicators of neighborhood conditions (drawn from public sources such as the Census Bureau, the National Center for Education Statistics, the US Department of Agriculture, and the Environmental Protection Agency) that matter for children’s healthy development in 3 domains: education, health and environment, and social and economic (Supplemental Table 2).15,16 A standardized z score for each indicator (mean 0; SD 1), as well as a domain-specific and overall ChOI score (range 1–100 U) was calculated for 72 195 census tracts (ie, neighborhoods) for 50 US states and Washington DC at 2 time periods: 2010 and 2015. The scores were also categorized as very low (<20 U), low (20–<40 U), moderate (40–<60 U), high (60–<80 U), or very high (≥80 U) opportunity. The ChOI scores were standardized at the metropolitan, state, and national levels, such that higher scores reflect more favorable neighborhood opportunities relative to other neighborhoods at the metropolitan, state, or national level, respectively. Detailed methods for the construction of these scores are publicly available.16 We geocoded each participant’s residential address obtained in midchildhood (mean 7.9 years; SD 0.8) using ArcGIS (Esri, Redlands, CA) and linked the resultant census tract location for each participant to census tract-level ChOI data for the year 2010 (the year closest to the midchildhood visit).
Outcomes: Adiposity and Cardiometabolic Risk Markers
Adiposity
At the midchildhood (mean 7.9 years; SD 0.8) and early adolescent (mean 13.1 years; SD 0.9) visits, trained research assistants measured weight, standing height, and waist circumference and assessed percentage body fat (%BF) using foot-to-foot bioimpedance (Tanita TBF-300A; Tanita, Arlington Heights, IL) and fat mass and trunk fat mass using whole-body dual radiograph absorptiometry (Hologic model Discovery A; Hologic, Bedford, MA) according to standardized protocols. We calculated the following adiposity indices (all in kilograms of mass divided by height in meters squared): fat mass index and trunk fat mass index.
Cardiometabolic Risk Markers
At the midchildhood and early adolescent visits, trained research assistants measured systolic blood pressure using calibrated automated oscillometric monitors (HEM-907XL; Omron, Bannockburn, IL). Trained technicians also collected fasting blood specimens at both visits; all samples were centrifuged within 24 hours, with plasma aliquots stored at −80°C. We measured fasting glucose, insulin, high-density lipoprotein, cholesterol, triglycerides and C-reactive protein (CRP) according to standard protocols. We calculated insulin resistance using the homeostasis model assessment (HOMA-IR) and log-transformed the values using natural logarithms to normalize the distribution. Subsequently, we calculated a metabolic risk z score for each child as the average of the sum of z scores for waist circumference, HOMA-IR, triglycerides, high-density lipoprotein, cholesterol (inverted), and systolic blood pressure.17
Covariates
Mothers reported their prepregnancy weight, height, smoking history, highest education level, household income, marital status, and their partner’s highest education via questionnaires and interviews at recruitment in early pregnancy. We calculated prepregnancy BMI as self-reported prepregnancy weight divided by height squared. We categorized parental education as having obtained a college degree (yes or no); household income (ie, total income of the mother and family members in the same household) as >$70 000 per year or ≤$70 000 per year; marital status as married and/or cohabiting (yes or no); and smoking history as never smoked, smoked before pregnancy, or smoked during pregnancy. Mothers reported their child’s race or ethnicity, which we categorized as white, Black, Hispanic, Asian, or other. We extracted data on child sex from delivery hospital medical records. We selected these covariates on the basis of previous publications linking ChOI and child health.12,13
Statistical Analyses
We used mixed-effect models to estimate the associations of neighborhood ChOI in midchildhood with adiposity and cardiometabolic risk markers in both midchildhood and early adolescence. We first examined associations for overall and domain-specific ChOI scores (as continuous variables); we used national-level ChOI scores (n = 743 children residing in the United States) for our primary analyses, and state- (n = 687 children residing in MA) and metropolitan-level (n = 653 children residing in the Boston metropolitan area) ChOI scores for secondary analyses. To identify whether the associations may be driven by specific ChOI indicators, we further examined associations for each individual ChOI indicator (as z scores) with the outcomes. For all analyses, we modeled ChOI variables, child age, sex (for outcomes that are not sex specific) and race or ethnicity, biparental educational level, household income, and maternal marital status as fixed effects and included a random intercept and random linear slope for child age to account for repeated outcome measures in the same child. We accounted for clustering of children residing within the same neighborhood by including a random-effect term for census tract. We additionally investigated effect modification by child sex by adding multiplicative interaction terms with ChOI. In sensitivity analyses, we further adjusted for maternal prepregnancy BMI and pregnancy smoking status, factors we have previously found to strongly predict child obesity and metabolic risk in this cohort.18,19 We also conducted inverse probability of censoring weighting analyses to control for potential selection bias due to loss to follow-up between midchildhood and early adolescent visits.20 We performed all analyses using SAS version 9.4 (SAS Institute, Inc, Cary, NC).
Results
Children with ChOI data in midchildhood (versus those without) had parents who were more likely to have a college degree or have a household income >$70 000 per year at enrollment (Supplemental Table 3). On the basis of nationwide distributions of the ChOI, 11.2% (n = 83 children) resided in areas of very low overall opportunity, whereas 55.3% (n = 411 children) resided in areas of very high overall opportunity in midchildhood. Children residing in areas of very high overall opportunity (versus very low) had the highest ChOI scores for the education, health and environment, and social and economic domains, were less likely to be Black or Hispanic, and had lower measures of adiposity and cardiometabolic risk markers in midchildhood and early adolescence. They also had parents with higher socioeconomic backgrounds and had mothers with lower prepregnancy BMI and who were less likely to smoke during pregnancy (Table 1).
TABLE 1.
Individual and Family Characteristics of Children in Project Viva According to Categories of Overall ChOI
| Overall, N = 743 | Overall ChOI Categories | |||||
|---|---|---|---|---|---|---|
| Very Low (<20 U), n = 83 (11.2%) | Low (20–<40 U), n = 34 (4.6%) | Moderate (40–<60 U), n = 65 (8.8%) | High (60–<80 U), n = 150 (20.2%) | Very High (≥80 U), n = 411 (55.3%) | ||
| Domain-specific ChOI scores, U, mean (SD) | ||||||
| Education | 74.1 (26.9) | 17.2 (10.0) | 38.4 (14.5) | 52.1 (18.4) | 77.8 (11.6) | 90.6 (7.0) |
| Health and environment | 77.7 (22.7) | 41.0 (21.6) | 43.6 (22.9) | 67.8 (22.1) | 78.2 (17.0) | 89.3 (9.9) |
| Social and economic | 67.4 (27.9) | 8.4 (5.4) | 30.1 (6.0) | 44.8 (8.4) | 64.8 (9.4) | 86.9 (8.6) |
| Mother | ||||||
| Prepregnancy BMI, mean (SD) | 24.7 (5.1) | 27.2 (6.6) | 24.6 (4.5) | 26.2 (5.8) | 24.4 (4.8) | 24.2 (4.6) |
| College degree, n (%) | 520 (70) | 26 (32) | 15 (44) | 29 (45) | 104 (69) | 346 (84) |
| Married or cohabiting, n (%) | 681 (92) | 59 (72) | 28 (82) | 56 (86) | 142 (95) | 396 (97) |
| Smoking status, n (%) | ||||||
| Never smoked | 514 (69) | 61 (74) | 20 (59) | 51 (78) | 103 (69) | 279 (68) |
| Smoked before pregnancy | 156 (21) | 7 (9) | 7 (21) | 10 (15) | 29 (19) | 103 (25) |
| Smoked during pregnancy | 71 (10) | 14 (17) | 7 (21) | 4 (6) | 17 (11) | 29 (7) |
| Household, n (%) | ||||||
| Partner college degree | 467 (69) | 11 (19) | 12 (41) | 22 (42) | 92 (65) | 330 (84) |
| Household income >$70 000 per y at enrollment | 434 (64) | 12 (21) | 7 (27) | 22 (39) | 85 (59) | 308 (79) |
| Child, n (%) | ||||||
| Race or ethnicity | ||||||
| White | 484 (65) | 3 (4) | 9 (26) | 27 (42) | 111 (74) | 334 (81) |
| Black | 120 (16) | 54 (66) | 14 (41) | 23 (35) | 9 (6) | 20 (5) |
| Hispanic | 28 (4) | 10 (12) | 4 (12) | 3 (5) | 5 (3) | 6 (1) |
| Asian | 22 (3) | 1 (1) | 1 (3) | 0 (0) | 6 (4) | 14 (3) |
| Other | 88 (12) | 14 (17) | 6 (18) | 12 (18) | 19 (13) | 37 (9) |
| Female sex | 382 (51) | 39 (47) | 13 (38) | 36 (55) | 71 (47) | 223 (54) |
| Midchildhood measures, mean (SD) | ||||||
| Age, y | 7.9 (0.8) | 8.2 (0.9) | 8.1 (0.8) | 8.1 (0.9) | 7.9 (0.8) | 7.8 (0.8) |
| %BF | 19.1 (6.9) | 22.7 (9.2) | 19.0 (6.9) | 21.6 (8.0) | 19.3 (6.9) | 17.9 (5.8) |
| FMI, kg/m2 | 4.4 (1.8) | 5.1 (2.8) | 4.4 (2.3) | 4.8 (2.2) | 4.4 (1.6) | 4.1 (1.4) |
| TFMI, kg/m2 | 1.5 (0.8) | 1.8 (1.3) | 1.5 (1.0) | 1.6 (1.0) | 1.5 (0.8) | 1.4 (0.6) |
| Log CRP, mg/L | −1.62 (1.66) | −1.12 (1.95) | −1.51 (1.92) | −1.41 (1.67) | −1.53 (1.68) | −1.82 (1.53) |
| Log HOMA-IR, U | 0.36 (0.74) | 0.65 (0.75) | 0.26 (0.96) | 0.51 (0.85) | 0.27 (0.80) | 0.31 (0.67) |
| Metabolic risk score, SD units | 0.00 (0.59) | 0.15 (0.70) | −0.18 (0.62) | 0.13 (0.75) | −0.01 (0.56) | −0.03 (0.54) |
| Early adolescent measures, mean (SD) | ||||||
| Age, y | 13.1 (0.8) | 13.1 (0.7) | 13.2 (1.0) | 13.2 (1.0) | 13.1 (1.0) | 13.0 (0.8) |
| %BF | 21.7 (10.0) | 26.1 (13.0) | 20.6 (10.7) | 24.5 (10.6) | 21.3 (8.4) | 20.6 (9.3) |
| FMI, kg/m2 | 6.3 (2.9) | 7.5 (4.3) | 6.3 (3.2) | 6.6 (3.5) | 6.4 (2.4) | 5.9 (2.5) |
| TFMI, kg/m2 | 2.4 (1.4) | 2.9 (2.0) | 2.3 (1.4) | 2.6 (1.7) | 2.5 (1.2) | 2.2 (1.2) |
| Log CRP, mg/L | −1.04 (1.16) | −0.60 (1.35) | −0.99 (1.20) | −0.93 (1.29) | −1.10 (1.08) | −1.15 (1.11) |
| Log HOMA-IR, U | 0.97 (0.60) | 1.16 (0.67) | 0.99 (0.54) | 1.05 (0.72) | 1.01 (0.51) | 0.89 (0.59) |
| Metabolic risk score, SD units | −0.02 (0.60) | 0.13 (0.65) | −0.04 (0.57) | −0.03 (0.56) | 0.03 (0.59) | −0.07 (0.60) |
FMI, fat mass index; TFMI, trunk fat mass index.
Associations for Overall and Domain-Specific ChOI Scores
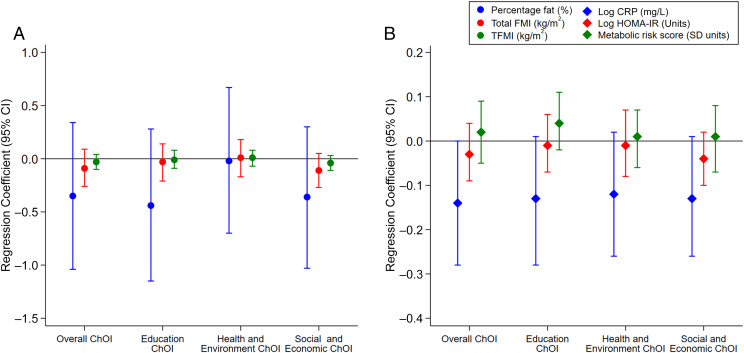
After adjusting for individual and family sociodemographics, no significant associations of ChOI with adiposity were observed (Fig 1A). Children who resided in areas with higher overall opportunity (per 25-U increase in ChOI score) had persistently lower levels of CRP from midchildhood to early adolescence (β = .14 mg/L; 95% confidence interval [CI], −.28 to .00). Similar associations with CRP were noted for children who resided in areas with higher education opportunity (β = −.13 mg/L; 95% CI, −.28 to .01), higher health and environment opportunity (β = −.12 mg/L; 95% CI, −.26 to .02), and higher social and economic opportunity (β = −.13 mg/L; 95% CI, −.26 to .01) (Fig 1B). Overall ChOI also significantly interacted with child sex in its association with HOMA-IR; the relationship of higher ChOI scores with lower HOMA-IR from midchildhood to early adolescence was more pronounced in girls (β = −.08 U; 95% CI −.18 to .02) than in boys (β = .00 U; 95% CI −.09 to .09) but both results were not significant. The patterns of associations were similar when using state- and metropolitan-level ChOI scores, albeit with wider 95% CIs due to smaller sample sizes (Supplemental Figs 6 and 7).
FIGURE 1.
A and B, Associations of national-level overall and domain-specific ChOI scores in midchildhood with adiposity (A) and cardiometabolic risk markers (B) from midchildhood to early adolescence. Effect estimates reflect a per 25-U increase in ChOI scores. All models are adjusted for child sex (for outcomes that are not sex specific), child race or ethnicity, biparental educational level, household income, and maternal marital status. FMI, fat mass index; TFMI, trunk fat mass index.
Associations for Each Indicator in the Education Domain
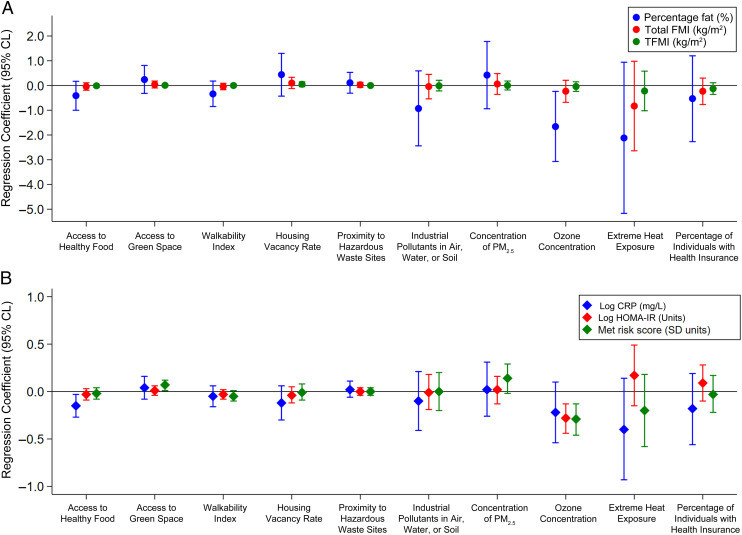
Children who resided in areas with higher rates of adult college attainment or college enrollment had persistently lower %BF, CRP, and HOMA-IR from midchildhood to early adolescence. Additionally, those who resided in areas with a greater number of high-quality early childhood education centers had persistently lower %BF, CRP, and metabolic risk z scores from midchildhood to early adolescence (Fig 2).
FIGURE 2.
A and B, Associations of each indicator in the education domain with adiposity (A) and cardiometabolic risk markers (B) from midchildhood to early adolescence. Effect estimates reflect a per SD-unit increase in each indicator. All models are adjusted for child sex (for outcomes that are not sex specific), child race or ethnicity, biparental educational level, household income, and maternal marital status. AP, advanced placement; ECE, early childhood education center; FMI, fat mass index; TFMI, trunk fat mass index.
Associations for Each Indicator in the Health and Environment Domain
Children who resided in areas with lower ozone concentrations had persistently lower %BF, HOMA-IR, and metabolic risk z scores from midchildhood to early adolescence. Also, those who resided in areas with greater access to healthy food had persistently lower CRP from midchildhood to early adolescence (Fig 3).
FIGURE 3.
A and B, Associations of each indicator in the health and environment domain with adiposity (A) and cardiometabolic risk markers (B) from midchildhood to early adolescence. Effect estimates reflect a per SD-unit increase in each indicator. All models are adjusted for child sex (for outcomes that are not sex specific), child race or ethnicity, biparental educational level, household income, and maternal marital status. FMI, fat mass index; PM2.5, particulate matter less than 2.5 microns; TFMI, trunk fat mass index.
Associations for Each Indicator in the Social and Economic Domain
Children who resided in areas with greater proximity to employment had persistently lower HOMA-IR and metabolic risk scores from midchildhood to early adolescence (Fig 4).
FIGURE 4.
A and B, Associations of each indicator in the social and economic domain with adiposity (A) and cardiometabolic risk markers (B) from midchildhood to early adolescence. Effect estimates reflect a per SD-unit increase in each indicator. All models are adjusted for child sex (for outcomes that are not sex specific), child race or ethnicity, biparental educational level, household income, and maternal marital status. FMI, fat mass index; TFMI, trunk fat mass index.
Sensitivity Analyses
Additional adjustment for prepregnancy BMI and pregnancy smoking status, as well as inverse probability of censoring weighting analyses, revealed no appreciable changes in the associations of ChOI in midchildhood with adiposity and cardiometabolic risk markers from midchildhood to early adolescence (Supplemental Tables 4 and 5).
Discussion
We found that children who resided in neighborhoods with more favorable opportunities (ie, higher ChOI scores) in midchildhood had persistently lower levels of inflammation in midchildhood and early adolescence. These associations were independent of individual and family sociodemographics, such as biparental education, maternal marital status, household income, and child race or ethnicity. In addition, we noted that certain indicators of neighborhood opportunity (ie, adult college attainment rate, college enrollment rate, number of high-quality childhood education centers, access to healthy food, ozone concentration, and proximity to employment in midchildhood), but not others, were associated with adiposity and specific cardiometabolic risk markers from midchildhood to early adolescence.
In previous cross-sectional studies among subjects aged <18 years, associations between ChOI and acute health care use and stress physiology were reported.12,13 Acevedo-Garcia et al11 reported strong correlations between overall ChOI (and each of its 3 domains) and birth outcomes (preterm birth and low birth weight). Authors of past studies have also reported associations of other indices that tap into specific ChOI components with birth outcomes and language development in children.1,21 To date, it remains unclear whether the ChOI is predictive of cardiometabolic outcomes in adolescence, an important question given the high and increasing prevalence of morbidity from these conditions.22,23 Thus, in our study, we make an important contribution to the extant literature by providing evidence of the prospective relationship between neighborhood-level ChOI and individual-level cardiometabolic outcomes from midchildhood to adolescence.
In previous research examining the cardiometabolic health consequences of exposure to neighborhood environments, researchers have primarily used neighborhood indices that only represent aspects of socioeconomic disadvantage (eg, low household income and unemployment).1,8 Recent findings from the New England Family Study showed that residing in low socioeconomic status neighborhoods at birth or childhood (∼7 years) conferred a larger risk for increased adiposity and blood pressure in adulthood.24,25 Emerging evidence has also revealed the impact of other components of neighborhood environment on child health outcomes. For example, Singh et al26 reported 20% to 45% higher odds of overweight and obesity for children who resided in neighborhoods that lacked access to amenities, sidewalks, walking paths, parks, or playgrounds. Children who have greater access to parks and open spaces are also more likely to engage in physical activity, which, in turn, may bring about greater health benefits.27,28 Magnuson et al29 documented positive relationships between access to high-quality early childhood educational opportunities and child development. More recently, Karra et al30 also reported associations between improved access to primary health care services and reduced child mortality. Taken together, in our study, we broaden the scope of this research by identifying more novel features of neighborhood conditions (such as number of high-quality childhood education centers, access to healthy food, and ozone concentration) that showed associations with adiposity and cardiometabolic risk measures from midchildhood to early adolescence.
A possible explanation for the association between disadvantaged neighborhoods and adverse health outcomes lies in the health-compromising behaviors that are inextricably linked to the lack of resources or amenities in such neighborhoods.31,32 In investigative studies of adults residing in neighborhoods with better access to health-promoting resources (ie, nutrition counseling and behavioral knowledge), researchers found that people adopted more positive behaviors (eg, more physical activity, healthier dietary patterns, and a less stressful lifestyle) that could reduce the risk of developing an adverse cardiometabolic profile later in life.33 Further studies are warranted to replicate these findings and to evaluate the likely mechanisms.
We also observed sex differences in these associations, whereby the relationship of ChOI with insulin resistance from midchildhood to early adolescence was more pronounced in girls than in boys. The underlying mechanism may involve sex hormones, which are known to have important effects on insulin resistance in adolescence.34 However, these sex differences should be interpreted with caution, given that children are often more insulin-resistant during pubertal development in adolescence, and girls are typically further along in puberty compared with boys.35 Further studies are warranted to understand these sex differences.
Previous individual-level and school-based interventions involving nutrition counseling and structured physical activity sessions have shown promise in effectively reducing adiposity and other cardiometabolic risk markers in children with obesity.36,37 The value of ChOI predicting later cardiometabolic health outcomes extends the scope of pediatric patient care beyond these individual-level factors. Our findings suggest that the ChOI could be a potentially useful index to target high-risk children and even tailor interventions for children at risk for developing adverse cardiometabolic health outcomes by additionally addressing the disparate contexts of the neighborhood where they reside. Moreover, the ChOI could be used to guide place-based initiatives, such as neighborhood development projects, that aim to improve access to essential resources and provide families with the environments needed to support healthy child development.38,39 More research is needed to clarify whether such initiatives that alter specific components of neighborhood opportunity would be effective in improving later cardiometabolic health.
Strengths of our study include its prospective study design and wide range of cardiometabolic outcomes measured in midchildhood and early adolescence by highly trained staff using standardized protocols. We also assessed neighborhood opportunity in midchildhood, a period when children were unlikely to have selected their place of residence. This reduces the likelihood of self-selection and resulting reverse causality (ie, choosing a place of residence on the basis of predisposition to certain health behaviors), which is a common obstacle in many studies of neighborhood research.40,41
Our study is not without limitations. First, the ChOI was limited to components for which nationally representative data were available. Certain factors, such as crime and exposure to neighborhood violence, which may also be important to child health, were not included because of a lack of comparable neighborhood-level data across the United States.16 Nevertheless, all indicators had been vetted for their relevance to child health and development. Second, despite having residential addresses at earlier study visits (birth, infancy, and early childhood), we were only able to examine neighborhood opportunity in midchildhood. This was because the ChOI was derived by using census and survey data between 2008 and 2012, which corresponded closely with the midchildhood visit period in our study. Further studies are warranted to develop similar measures of neighborhood opportunity at earlier time points and to test sensitive periods of exposure to neighborhood opportunity throughout the life course. Third, we used census tracts as a marker of exposure, which may not capture the relevant area where children spend time. Fourth, we investigated several ChOI indicators and cardiometabolic outcomes, therefore increasing the risk of false-positive results. We chose not to adjust for multiple comparisons because we had based the significance of our findings on the strength and consistency of the associations observed across related cardiometabolic outcomes.42 Fifth, we were unable to link ChOI data to participants who did not attend the midchildhood visit. Differences between children included and excluded in this study might have led to selection bias, but we adjusted for this to a certain degree by conducting inverse probability of censoring weighting analyses, which showed no appreciable changes from our main findings. Lastly, Project Viva is predominantly composed of participants from higher socioeconomic backgrounds; at recruitment, ∼70% were college educated and 64% reported household incomes >$70 000 per year, which explains the large proportion of children (75.5%) with high or very high ChOI scores. The limited variation of ChOI in our sample might explain the weak associations between ChOI and cardiometabolic outcomes. Nevertheless, our sample also included 117 (15.8%) children residing in neighborhoods with very low and low ChOI scores. Therefore, we believe there is sufficient variation in our exposure. Our study findings also may not be generalizable to other ethnic groups and populations from different settings because many of our participants were white.
Conclusions
Our findings suggest that more favorable neighborhood opportunities in midchildhood predict better cardiometabolic health from midchildhood to early adolescence. These observations appear to be driven by specific indicators within the education (ie, adult college attainment rate, college enrollment rate, number of high-quality childhood education centers), health and environment (ie, access to healthy food and ozone concentration), and social and economic (ie, proximity to job employment) domains. More research is needed to clarify whether initiatives that alter specific components of neighborhood opportunity would be effective in improving later cardiometabolic health.
Glossary
- %BF
percentage body fat
- CI
confidence interval
- ChOI
Child Opportunity Index
- CRP
C-reactive protein
- HOMA-IR
homeostasis model assessment
Footnotes
Dr Aris conceived and conceptualized the study, drafted the initial manuscript, interpreted the results, and reviewed and revised the manuscript; Ms Rifas-Shiman conducted the analyses and reviewed and revised the manuscript; Drs Jimenez, Li, Hivert, Oken, and James interpreted the results and critically reviewed the manuscript for important intellectual content; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Project Viva is supported by the National Institutes of Health (R01 HD0345568, UG3 OD023286). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Buka SL, Brennan RT, Rich-Edwards JW, Raudenbush SW, Earls F. Neighborhood support and the birth weight of urban infants. Am J Epidemiol. 2003;157(1):1–8 [DOI] [PubMed] [Google Scholar]
- 2.Caughy MO, Nettles SM, O’Campo PJ. The effect of residential neighborhood on child behavior problems in first grade. Am J Community Psychol. 2008;42(1–2):39–50 [DOI] [PubMed] [Google Scholar]
- 3.Sellström E, Bremberg S. The significance of neighbourhood context to child and adolescent health and well-being: a systematic review of multilevel studies. Scand J Public Health. 2006;34(5):544–554 [DOI] [PubMed] [Google Scholar]
- 4.Christian H, Zubrick SR, Foster S, et al. The influence of the neighborhood physical environment on early child health and development: a review and call for research. Health Place. 2015;33:25–36 [DOI] [PubMed] [Google Scholar]
- 5.Krieger N. Epidemiology and the web of causation: has anyone seen the spider? Soc Sci Med. 1994;39(7):887–903 [DOI] [PubMed] [Google Scholar]
- 6.Pillas D, Marmot M, Naicker K, Goldblatt P, Morrison J, Pikhart H. Social inequalities in early childhood health and development: a European-wide systematic review. Pediatr Res. 2014;76(5):418–424 [DOI] [PubMed] [Google Scholar]
- 7.Macintyre S, Ellaway A, Cummins S. Place effects on health: how can we conceptualise, operationalise and measure them? Soc Sci Med. 2002;55(1):125–139 [DOI] [PubMed] [Google Scholar]
- 8.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: the Public Health Disparities Geocoding Project (US). J Epidemiol Community Health. 2003;57(3):186–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slopen N, Non A, Williams DR, Roberts AL, Albert MA. Childhood adversity, adult neighborhood context, and cumulative biological risk for chronic diseases in adulthood. Psychosom Med. 2014;76(7):481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickett KE, Pearl M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: a critical review. J Epidemiol Community Health. 2001;55(2):111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acevedo-Garcia D, McArdle N, Hardy EF, et al. The child opportunity index: improving collaboration between community development and public health. Health Aff (Millwood). 2014;33(11):1948–1957 [DOI] [PubMed] [Google Scholar]
- 12.Kersten EE, Adler NE, Gottlieb L, et al. Neighborhood child opportunity and individual-level pediatric acute care use and diagnoses. Pediatrics. 2018;141(5):e20172309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roubinov DS, Hagan MJ, Boyce WT, Adler NE, Bush NR. Family socioeconomic status, cortisol, and physical health in early childhood: the role of advantageous neighborhood characteristics. Psychosom Med. 2018;80(5):492–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oken E, Baccarelli AA, Gold DR, et al. Cohort profile: project viva. Int J Epidemiol. 2015;44(1):37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acevedo-Garcia D, Noelke C, McArdle N, et al. Racial and ethnic inequities in children’s neighborhoods: evidence from the new child opportunity index 2.0. Health Aff (Millwood). 2020;39(10):1693–1701 [DOI] [PubMed] [Google Scholar]
- 16.Noelke C, McArdle N, Baek M, et al. How we built it: the nuts and bolts of constructing the Child Opportunity Index 2.0. 2020. Available at: www.diversitydatakids.org/research-library/research-brief/how-we-built-it. Accessed February 6, 2020
- 17.Eisenmann JC, Laurson KR, DuBose KD, Smith BK, Donnelly JE. Construct validity of a continuous metabolic syndrome score in children. Diabetol Metab Syndr. 2010;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oken E, Huh SY, Taveras EM, Rich-Edwards JW, Gillman MW. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res. 2005;13(11):2021–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perng W, Gillman MW, Mantzoros CS, Oken E. A prospective study of maternal prenatal weight and offspring cardiometabolic health in midchildhood. Ann Epidemiol. 2014;24(11):793–800.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–625 [DOI] [PubMed] [Google Scholar]
- 21.Sampson RJ, Sharkey P, Raudenbush SW. Durable effects of concentrated disadvantage on verbal ability among African-American children. Proc Natl Acad Sci USA. 2008;105(3):845–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kit BK, Kuklina E, Carroll MD, Ostchega Y, Freedman DS, Ogden CL. Prevalence of and trends in dyslipidemia and blood pressure among US children and adolescents, 1999–2012. JAMA Pediatr. 2015;169(3):272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315(21):2292–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilman SE, Huang YT, Jimenez MP, et al. Early life disadvantage and adult adiposity: tests of sensitive periods during childhood and behavioural mediation in adulthood. Int J Epidemiol. 2019;48(1):98–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jimenez MP, Wellenius GA, Subramanian SV, et al. Longitudinal associations of neighborhood socioeconomic status with cardiovascular risk factors: a 46-year follow-up study. Soc Sci Med. 2019;241:112574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh GK, Siahpush M, Kogan MD. Neighborhood socioeconomic conditions, built environments, and childhood obesity. Health Aff (Millwood). 2010;29(3):503–512 [DOI] [PubMed] [Google Scholar]
- 27.Babey SH, Hastert TA, Yu H, Brown ER. Physical activity among adolescents. When do parks matter? Am J Prev Med. 2008;34(4):345–348 [DOI] [PubMed] [Google Scholar]
- 28.Lovasi GS, Schwartz-Soicher O, Quinn JW, et al. Neighborhood safety and green space as predictors of obesity among preschool children from low-income families in New York City. Prev Med. 2013;57(3):189–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magnuson KA, Meyers MK, Ruhm CJ, Waldfogel J. Inequality in preschool education and school readiness. Am Educ Res J. 2004;41(1):115–157 [Google Scholar]
- 30.Karra M, Fink G, Canning D. Facility distance and child mortality: a multi-country study of health facility access, service utilization, and child health outcomes. Int J Epidemiol. 2017;46(3):817–826 [DOI] [PubMed] [Google Scholar]
- 31.Hanson MD, Chen E. Socioeconomic status and health behaviors in adolescence: a review of the literature. J Behav Med. 2007;30(3):263–285 [DOI] [PubMed] [Google Scholar]
- 32.Robinette JW, Charles ST, Almeida DM, Gruenewald TL. Neighborhood features and physiological risk: an examination of allostatic load. Health Place. 2016;41:110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Odgers CL, Moffitt TE, Tach LM, et al. The protective effects of neighborhood collective efficacy on British children growing up in deprivation: a developmental analysis. Dev Psychol. 2009;45(4):942–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agirbasli M, Agaoglu NB, Orak N, et al. Sex hormones, insulin resistance and high-density lipoprotein cholesterol levels in children. Horm Res Paediatr. 2010;73(3):166–174 [DOI] [PubMed] [Google Scholar]
- 35.Levy-Marchal C, Arslanian S, Cutfield W, et al.; ESPE-LWPES-ISPAD-APPES-APEG-SLEP-JSPE; Insulin Resistance in Children Consensus Conference Group . Insulin resistance in children: consensus, perspective, and future directions. J Clin Endocrinol Metab. 2010;95(12):5189–5198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sobol-Goldberg S, Rabinowitz J, Gross R. School-based obesity prevention programs: a meta-analysis of randomized controlled trials. Obesity (Silver Spring). 2013;21(12):2422–2428 [DOI] [PubMed] [Google Scholar]
- 37.Foster GD, Linder B, Baranowski T, et al.; HEALTHY Study Group . A school-based intervention for diabetes risk reduction. N Engl J Med. 2010;363(5):443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heberle LC, McReynolds B, Sizemore S, Schilling J. HUD’s sustainable communities initiative: an emerging model of place-based federal policy and collaborative capacity building. Cityscape. 2017;19(3):9–38 [Google Scholar]
- 39.Tegeler P, Haberle M, Gayles E. Affirmatively furthering fair housing in HUD housing programs: a first term report card. Journal of Affordable Housing and Community Development Law. 2013;22:27–60 [Google Scholar]
- 40.Arcaya MC, Subramanian SV, Rhodes JE, Waters MC. Role of health in predicting moves to poor neighborhoods among Hurricane Katrina survivors. Proc Natl Acad Sci USA. 2014;111(46):16246–16253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diez Roux AV, Mair C. Neighborhoods and health. Ann NY Acad Sci. 2010;1186:125–145 [DOI] [PubMed] [Google Scholar]
- 42.Streiner DL. Best (but oft-forgotten) practices: the multiple problems of multiplicity-whether and how to correct for many statistical tests. Am J Clin Nutr. 2015;102(4):721–728 [DOI] [PubMed] [Google Scholar]