Abstract
Purpose:
To investigate the metabolic pathways of triapine in primary cultures of human hepatocytes and human hepatic subcellular fractions; to investigate interactions of triapine with tenofovir and emtricitabine; and to evaluate triapine as a perpetrator of drug interactions. The results will better inform future clinical studies of triapine, a radiation sensitizer currently being studied in a phase III study.
Methods:
Triapine was incubated with human hepatocytes and subcellular fractions in the presence of a number of inhibitors of drug metabolizing enzymes. Triapine depletion was monitored by LC-MS/MS. Tenofovir and emtricitabine were co-incubated with triapine in primary cultures of human hepatocytes. Triapine was incubated with a CYP probe cocktail and human liver microsomes, followed by LC-MS/MS monitoring of CYP specific metabolite formation.
Results:
Triapine was not metabolized by FMO, AO/XO, MAO-A/B, or NAT-1/2, but was metabolized by CYP450s. CYP1A2 accounted for most of the depletion of triapine. Tenofovir and emtricitabine did not alter triapine depletion. Triapine reduced CYP1A2 activity and increased CYP2C19 activity.
Conclusion:
CYP1A2 metabolism is the major metabolic pathway for triapine. Triapine may be evaluated in cancer patients in the setting of HIV with emtricitabine or tenofovir treatment. Confirmatory clinical trials may further define the in vivo triapine metabolic fate and quantify any drug-drug interactions.
Keywords: triapine, metabolism, drug-drug interaction, CYP450, metabolic pathway
Introduction
Triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone, 3-AP, Triapine®) belongs to a class of α-N-heterocyclic thiosemicarbazones that inhibits the catalytic activity of ribonucleotide reductase (RNR). [1,2] RNR converts ribonucleotide diphosphates into deoxyribonucleotide diphosphates, an important prerequisite for DNA synthesis as its inhibition terminates DNA base chain elongation [3]. RNR is frequently overexpressed in cancer cells and for this reason, it has been an attractive target for anticancer therapy. [4,5] Further, triapine when given concomitantly with cisplatin and radiation therapy has resulted in improved clinical outcomes in women with advanced stage cervical cancer [6].
Triapine has been studied in more than 20 phase I and phase II clinical studies1, and recently, a phase III study was started in stage IB2, II, or IIIB-IVA cervical cancer or stage II-IVA vaginal cancer in combination with radiation and cisplatin (ClinicalTrials.gov Identifier: NCT02466971). Following oral administration in humans, triapine reaches a Cmax of 5.0 ± 3.8 μM (~1000 ng/mL) in 1.9 ± 1.4 h [7]. While triapine is effective against advanced leukemias [8] and against gynecologic cancers when combined with radiation [9], it is largely ineffective against solid tumors [10–15]. It has been noted in previous reports that rapid metabolism and excretion may be responsible for its lack of clinical activity in solid tumor [16]. Yet, there is minimal information on the enzymes involved in the biotransformation of triapine. Recently, Pelivan et al reported a dehydrogenated metabolic oxidation product of triapine, as well as amino hydroxylated metabolites of both the terminal amino and the pyridine amino function in human liver microsomes. Mouse studies resulted in detection of three additional, pyridine ring hydroxylated metabolites in serum, kidney, liver, and urine, as well as a oxidatively desulfurated metabolite, and two triapine N-glucuronide metabolites in mouse urine [17]. In humans, the total body clearance of triapine is 57.5 ± 25 L/h following intravenous administration, which is close to the liver blood flow [18,19]. Since urinary excretion of triapine is minimal in humans and as biliary excretion of a molecule of this size is unlikely to occur, metabolism is the major contributor to triapine clearance [20–22]. The precise enzymes involved in triapine metabolism have not been described in the literature.
Triapine is specifically being evaluated in uterine cervix cancer which is considered an acquired immunodeficiency syndrome (AIDS)-defining illness [23]. In preparation for an evaluation of triapine in cancer patients who are also human immunodeficiency virus (HIV)-infected and receive highly effective antiretroviral therapy, the potential for drug-drug interactions with HIV drugs such as tenofovir and emtricitabine needs to be evaluated.
Our study aimed to: (1) investigate the metabolic pathways of triapine in primary cultures of human hepatocytes and human subcellular fractions; (2) investigate the potential drug-drug interaction between tenofovir and emtricitabine, and triapine; and (3) evaluate the drug interaction potential of triapine through CYP450 enzymes.
Methods
Materials
Triapine was obtained from Selleckchem (Houston, TX), tenofovir was generously donated by Dr. Lisa Rohan (University of Pittsburgh). Methimazole, N-benzyl imidazole, chlorogyline, deprenyl hydrochloride, (+) raloxifene hydrochloride, carnitine acetyltransferase, acetyl DL-carnitine, acetyl coA, triethanolamine and allopurinol were obtained from Sigma Aldrich (St Louis, MO). Human liver microsomes, human liver mitochondria, and human liver cytosol with high aldehyde oxidase and xanthine oxidase activity were obtained from Xenotech (Kansas City, KS). Human liver cytosol was obtained from Corning (Corning, NY). Plated primary human hepatocytes were obtained from the Department of Surgery at the University of Pittsburgh. (Suppl.Table 1). Furafylline, phenyl piperidinyl propane, montelukast, sulfaphenazone, tranylcypromine hydrochloride, emtricitabine, disulfiram, and 4-methyl pyrazole were obtained from Cayman Chemical (Ann Arbor, MI). [2H6]-tenofovir and [2H3, 15N]-emtricitabine were purchased from Alsachim (Illkirch-Graffenstaden, France). 3-Benzyl nirvanol was purchased from Santa Cruz Biotechnology (Dallas, TX). Quinidine was obtained from Alfa Aesar (Tewksbury, MA). All samples generated were stored at −20 °C or lower until further analysis.
Triapine metabolism in human liver microsomes
Human liver microsomes (1.1 mg/mL) were incubated with MgCl2 (10 mM), EDTA (1.15 mM) in 0.1 M phosphate buffer pH 7.4. For CYP-mediated reactions, triapine (6 μM) was added followed by preincubation for 5 min at 37 °C. The reaction was initiated by the addition of NADPH (2 mM). For FMO mediated reactions in phosphate buffer pH 8.4, following addition of NADPH (2 mM) the solution was preincubated at 37 °C for 5 min. The reaction was subsequently initiated by addition of triapine (6 μM). The specific inhibitors, N-benzyl imidazole for CYP (1 mM) or methimazole (200 μM) for FMO, were added before the preincubation step. The total volume of the reaction was 500 μL. Aliquots (50 μL) were taken at 5, 20, 40, 80, and 120 min. The reactions were stopped by addition of 100 μL of methanol.
Triapine metabolism in human hepatocytes
Hepatocytes were plated at 1–1.5 million cells/well in six-well plates and replenished with fresh media every day. Cells were allowed to stabilize for 24 h prior to treatment. Hepatocytes were pretreated with specific enzyme inhibitors: N-benzyl imidazole (1 mM), methimazole (200 μM), raloxifene hydrochloride (100 μM), allopurinol (100 μM), chlorgyline (0.5 μM), deprenyl hydrochloride (0.5 μM) for 30 min followed by treatment with triapine in presence of these inhibitors for 48 h, and sampling (cell scrapings + media) at 0, 2, 6, 24, and 48 h. Control plates for triapine metabolism were also processed simultaneously.
Triapine metabolism by CYP450 isoforms in human liver microsomes
For CYP isoform identification, the same procedure as noted above for human liver microsomes was followed. The CYP isoform specific inhibitors used were: furafylline (20 μM) for 1A2 [24], tranylcypromine (0.5 μM) for 2A6, 2-phenyl-2-(1-piperidinyl)propane (PPP) (30 μM) for 2B6, montelukast (0.5 μM) for 2C8, sulfaphenazone (5 μM) for 2C9, (+) 3-benzyl nirvanol (0.3 μM) for 2C19, quinidine (2 μM) for 2D6, 4-methylpyrazole (10 μM) for 2E1. For PPP, the preincubation was for 30 min followed by addition of NADPH. For all other inhibitors, the preincubation time was 10 min.
Triapine metabolism by glucuronosyltransferases in human liver microsomes
The incubation for glucuronide metabolism was adapted from a previously published approach to allow for a 500 μL incubation volume [25]. The incubation mixture contained 0.1 M phosphate buffer (pH 7.4), 0.5 mg/mL microsomal protein concentration, 25 μg/mL alamethicin, 10 mM MgCl2, and 1.2 μg/mL triapine. The reaction (N=3) was started with the addition of 2 mM UDPGA. At 5, 20, 40, 80, and 120 minutes, 100 μL aliquots were sampled from the reaction mixture for analysis. SN38 was incubated at 1 μg/mL to monitor SN38 glucuronide generation as positive control.
Triapine metabolism in human liver mitochondria and cytosol with high AO/XO activity
Triapine (6 μM) was incubated with human liver cytosol enriched with aldehyde oxidase (AO) and xanthine oxidase (XO) (0.5 mg/mL) or human liver mitochondria (0.5 mg/mL) for 90 min in the presence and absence of specific enzyme inhibitors: raloxifene hydrochloride (100 μM) for AO, allopurinol (200 μM) for XO, chlorgyline (0.5 μM) for MAO-A and deprenyl (0.5 μM) for MAO-B. Deprenyl and chlorgyline were preincubated for 18 min before the initiation of reaction by addition of triapine. Human liver mitochondria were used for screening monoamine oxidases (MAO)-A/B. The reactions were carried out in 0.1 M phosphate buffer and total reaction volume was 500 μL at 37 °C.
Triapine metabolism by acetyltransferases in human hepatocytes
Primary cultures of human hepatocytes were obtained from the hepatocyte isolation facility at the University of Pittsburgh, see above. After 24 h, hepatocytes were pre-treated with either acetaminophen (2 mM, N-acetyl transferase-2 inhibitor) or disulfiram (50 μM, N-acetyl transferase-1 inhibitor) in duplicates. Control wells were treated with vehicle (0.2% dimethyl sulfoxide (DMSO)) simultaneously. Following 30 minutes of pre-incubation with the inhibitors, the cells were treated with triapine (6 μM) for 48 h with sampling (cell scrapings + media) at 2, 6, and 24 h respectively. The metabolic capacity of cells was assessed by metabolic conversion of testosterone either in presence of rifampin (10 μM) or ketoconazole (10 μM).
Drug interaction between tenofovir and emtricitabine, and triapine
Human hepatocytes were obtained from the University of Pittsburgh hepatocyte isolation facility and plated at 1–1.5 million cells/well in six-well plates. The cells were allowed to stabilize for 24 h followed by treatment with relevant concentrations of tenofovir (0.5 μM), emtricitabine (7.2 μM), or rifampin (10 μM), or ketoconazole (10 μM) for 4 days. Drug solutions were prepared in Williams E media containing cell supplements and dexamethasone and replenished daily. Control wells (no drug treatment) were maintained along with the treatment wells. On the 5th day, 6 μM triapine along with the above drugs was added to the respective wells, and the treatment was continued for 48 h with sampling (cell scrapings + media) at 0, 2, 6, 24, and 48 h. Cells were lysed by ultrasonication (1 min, 50 W).
Impact of triapine on production of CYP specific metabolic products of probe substrates
CYP probe cocktail consisting of phenacetin (5 μM) for CYP1A2, diclofenac (2.5 μM) for CYP2C9, S-mephenytoin (30 μM) for CYP2C19, dextromethorphan (5 μM) for CYP2D6 and midazolam (2.5 μM) for CYP3A4, were incubated with human liver microsomes (pool of 50, mixed gender, Sekisui Xenotech, Kansas City, KS) at a concentration of 0.25 mg/mL, based on a previous report [26]. The reactions were carried out in phosphate buffer (pH 7.4). The reactions were initiated by addition of 1 mM NADPH, and carried out at 37 ºC in open Eppendorf tubes for 15 min. The total reaction volume was 500 μL and the reactions were stopped by freezing on dry-ice/methanol. The reactions were carried out with and without triapine (2 μg/mL, the higher end of clinically expected plasma concentrations).
Bioanalysis
A previously validated LC-MS/MS assay was adapted to quantitate triapine [27]. In brief, 300 μL of acetonitrile with 10% ammonium acetate (pH 6.5) was added to each 100 μL sample containing 10 μL of 0.1 μg/mL isotopic internal standard ([13C3,15N]-triapine). Samples were vortexed for 1 min and then centrifuged at 13,000 × g at room temperature for 10 min. 200 μL of the resulting supernatant was transferred to autosampler vials followed by injection of 5 μL into the LC–MS/MS system. The LC system was an Agilent 1200 SL autosampler and binary pump, a Shodex ODP2 HP–2 B (5 μm, 50 × 2 mm) column, and an isocratic mobile phase. Mobile phase solvent A consisted of acetonitrile/10 mM ammonium acetate pH 6.5 (90/10, v/v), and mobile phase solvent B consisted of water with 10 mM ammonium acetate pH 6.5. Analytes were detected using an API Sciex 4000 mass spectrometer. The total run time was 3 minutes. The assay range was 30–3,000 ng/mL, and calibrators and QCs were prepared in a sample matrix of either hepatocyte media containing 20% human plasma or microsome incubation buffer containing 0.25 mg/mL bovine serum albumin to match hepatocyte or microsome incubation samples, respectively.
The bioanalysis of the metabolites of the CYP probes from the human liver microsomal incubations was performed by LC-MS/MS, based on a previously reported method [26]. Samples were prepared by taking 100 μL and mixing with 50 μL acetonitrile containing 5 ng/mL terfenadine internal standard. The calibration ranges and transitions are detailed as follows: 1-hydroxy midazolam (m/z 342>324) ranged from 1–300 ng/mL, 4-hydroxy diclofenac (m/z 312>266) from 10–3,000 ng/mL and dextrorphan (m/z 258.5>199) from 1–300 ng/mL, 4-hydroxy mephenytoin (m/z 235>150) from 10–3,000 ng/mL, and acetaminophen (m/z 152>110) from 10–3,000 ng/mL. The LC method consisted of Solvent A (acetonitrile, 0.1% formic acid) and Solvent B (water, 0.1% formic acid), pumped through an Phenomenex XB-C18 (2.6 μm, 100 × 2.1 mm) at a constant flow rate of 0.3 mL/min. The gradient changed solvent A from 0% to 50% over 3 min, held until 6 min, followed by a return to initial conditions to allow for re-equilibration for 4 min, with a total run time of 10 min.
Results
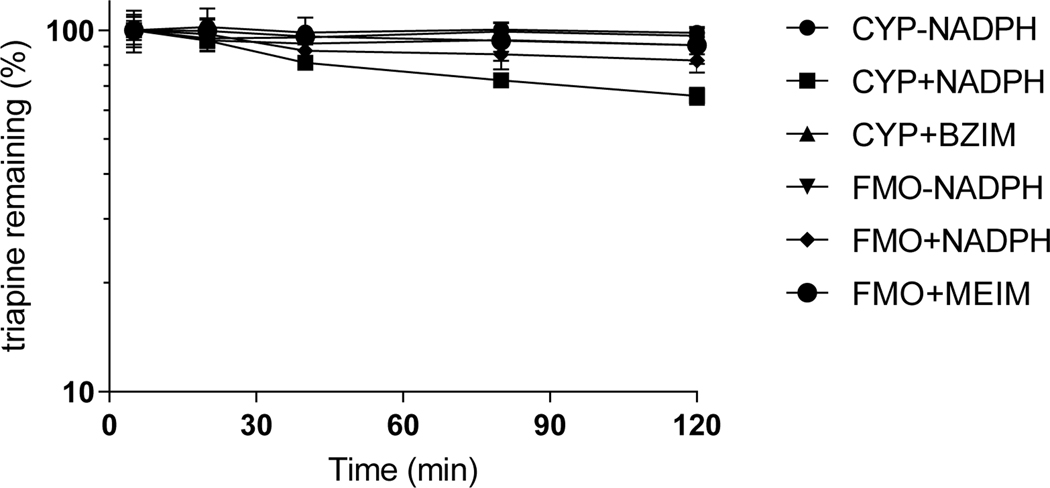
Control incubation without either NADPH or microsomes resulted in >95% of triapine remaining intact after 2 h indicating that triapine was stable under the incubation conditions throughout the duration of the experiment. (Suppl.Table 2). In NADPH-fortified human liver microsomes (pH 7.4), triapine exhibited an estimated half-life of approximately 3 h with depletion of about 35% of the starting concentration after 2h. Minimal depletion of triapine was observed under the same incubation conditions in the presence of the CYP isoform non-specific inhibitor N-benzyl imidazole (Figure 1, Suppl.Table 3). When triapine was incubated with human liver microsomes in phosphate buffer pH 8.4 (boosting FMO enzyme activity [28] 2) fortified with NADPH, 17% of triapine disappeared within 2 h. However, control incubations both without NADPH or in the presence of FMO inhibitor methimazole resulted in depletion of only 9%, indicating that FMO only has a minor contribution to the metabolism of triapine (Figure 1, Suppl.Table 3).
Figure 1.
Triapine depletion in human liver microsomes at pH 7.4 (CYP) or pH 8.4 (FMO) in the presence of CYP inhibitor N-benzyl imidazole (BZIM) or FMO inhibitor methimazole (MEIM) (mean, SD).
Triapine was not appreciably metabolized by human liver microsomal glucuronyltransferases over a 120-minute incubation period with 100.3% triapine remaining (Suppl.Figure 1, Suppl.Table 4).
Triapine did not undergo metabolism over a 90-minute incubation time period in human liver cytosol enriched in AO/XO in the presence or absence of raloxifene-a selective AO inhibitor and allopurinol-a selective XO inhibitor (Suppl.Figure 2, Suppl.Table 5). Similarly, there was no triapine depletion in human liver mitochondria after incubation in the presence or absence of MAO-A and B inhibitors (Suppl.Figure 3, Suppl.Table 6).
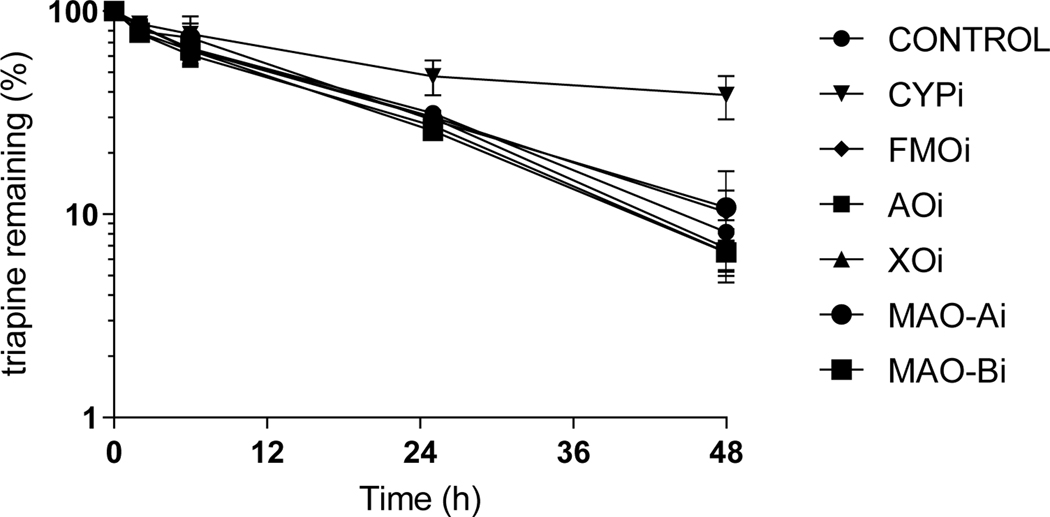
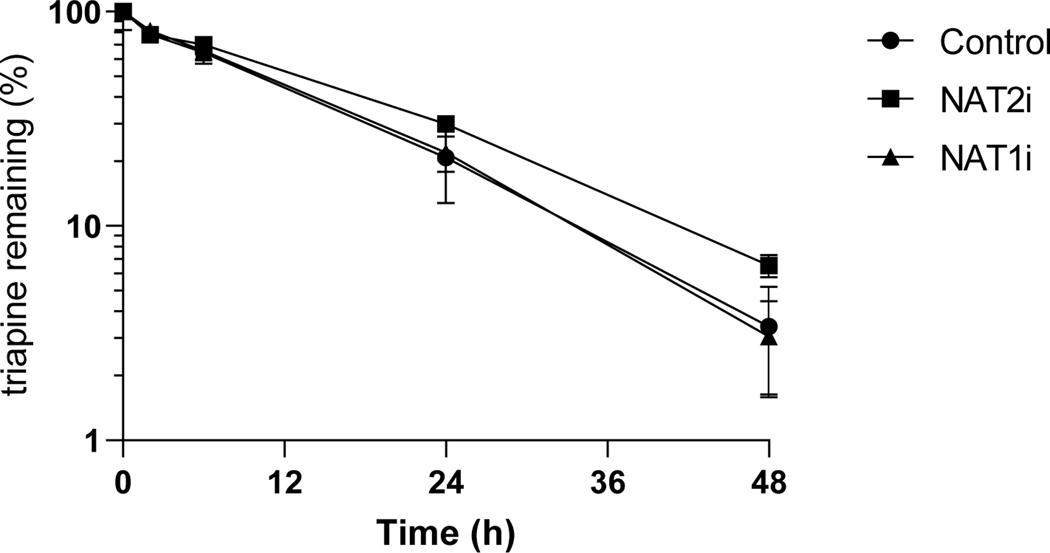
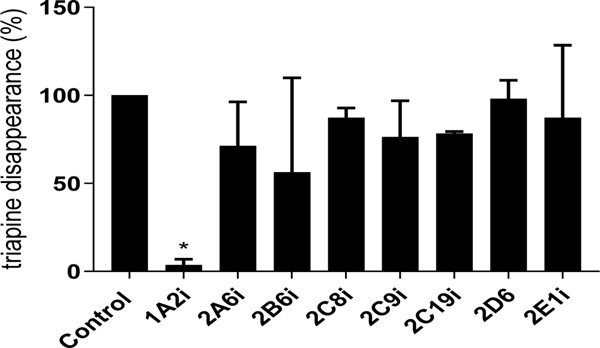
Triapine metabolism studies in hepatocytes in the presence of enzyme inhibitors indicated that triapine metabolism was inhibited by the non-isoform specific CYP inhibitor N-benzyl imidazole. The depletion of triapine in the presence of all other non-CYP enzyme inhibitors previously tested in subcellular fractions was comparable to the control wells containing hepatocytes with no inhibitors (Figure 2, Suppl.Table 7). In the presence of acetylation inhibitors, triapine metabolism was not significantly different from that in the presence of inhibitors (Figure 3, Suppl.Table 8), even when incubated with human cytosol fortified with acetylation regenerating system (data not shown). In screening CYP isoform specific inhibitors, only CYP1A2 inhibitor furafylline was able to completely inhibit triapine depletion in human liver microsomes (Figure 4, Suppl.Table 9).
Figure 2.
Triapine depletion in primary human hepatocytes in presence of enzyme inhibitors of aldehyde oxidase (AO, raloxifene), xanthine oxidase (XO, allopurinol), cytochrome P450 (CYP, N-benzyl imidazole), flavin monooxygenase (FMO, methimazole), or monoamine oxidase (MAO) A (chlorgyline) or B (deprenyl) (mean, SD).
Figure 3.
Triapine depletion in human hepatocytes in the presence of inhibitors of acetylating enzymes NAT-1 (disulfiram) or NAT-2 (acetaminophen).
Figure 4.
Triapine depletion in human liver microsomes in the presence of isoform specific CYP inhibitors (mean, SD). *p<0.05. CYP1A2, furafylline; CYP2A6, tranylcypromine; CYP2B6, 2-phenyl-2-(1-piperidinyl)propane; CYP2C8, Montelukast; CYP2C9, sulfaphenazone; CYP2C19, 3-benzyl nirvanol; CYP2D6, quinidine; CY2E1, 4-methylpyrazole. CYP3A4 specific inhibition was not performed as CYP3A was not involved in triapine metabolism, given the unaltered depletion of triapine by hepatocytes pretreated with rifampin or incubated with ketoconazole, see Figure 5.
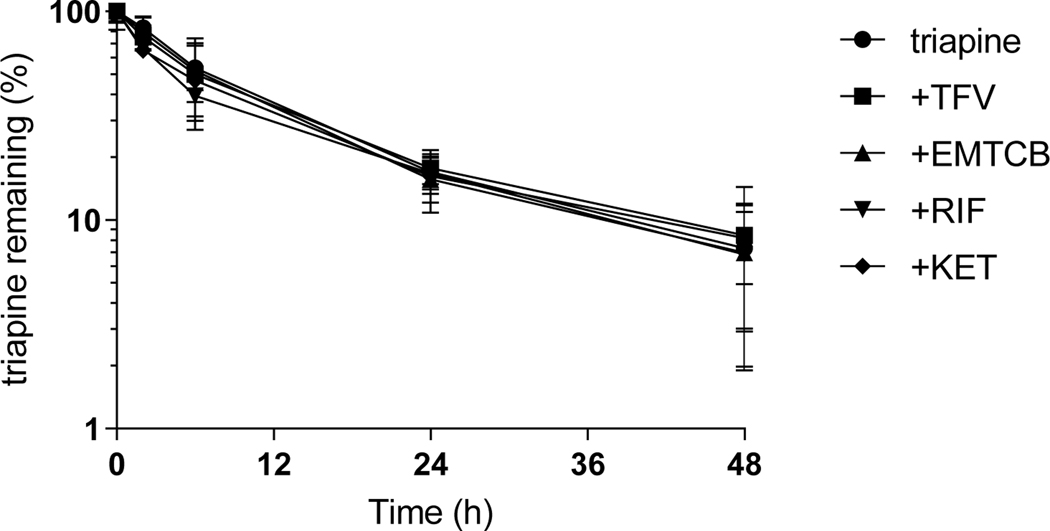
Triapine incubated with human hepatocytes exhibited a mean half-life of 8 hours, which was not impacted by co-incubation with tenofovir and emtricitabine. The AUC0–48h and the %triapine remaining after 48 hours were not significantly different (Figure 5, Suppl.Table 10). Triapine depletion was also unaffected by the presence of CYP3A inducer rifampin or CYP3A inhibitor ketoconazole.
Figure 5.
Interaction between triapine and tenofovir (TVF), emtricitabine (EMTCB), rifampin (RIF), or ketoconazole (KET) in primary human hepatocytes (mean, SD).
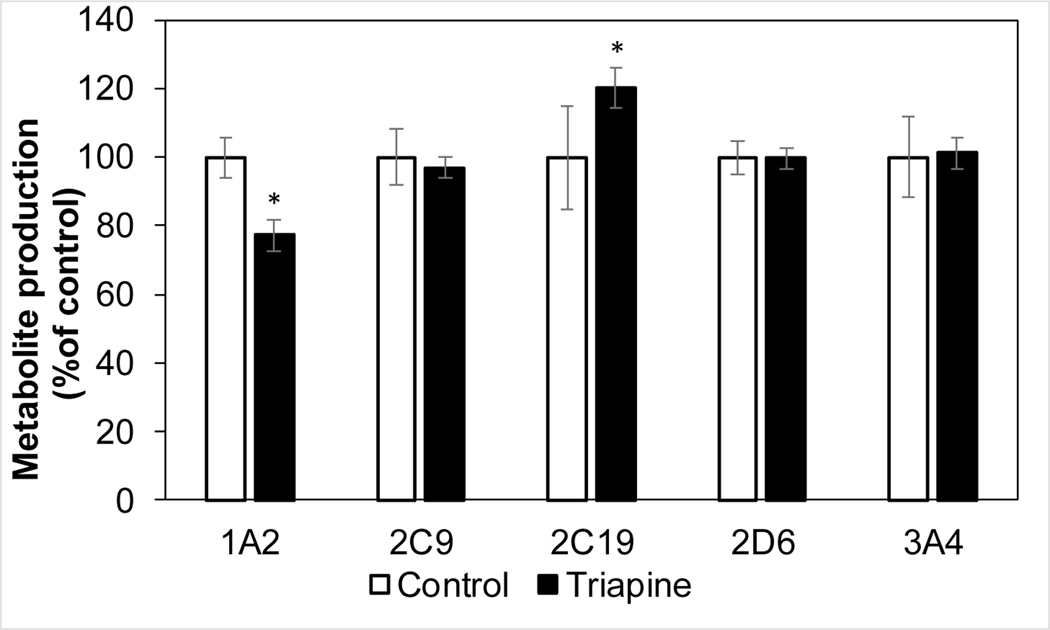
Triapine at clinically relevant concentrations i.e. 6 μM (Cmax of approximately 5.0 μM after intravenous or oral dosing [7]) inhibited metabolism of CYP1A2 probe phenacetin by 23% and appeared to increase the metabolism of CYP2C19 probe S-mephenytoin by 20% (Figure 6).
Figure 6.
Triapine impact on production of CYP specific metabolites of probe substrates in human liver microsomes (mean, SD). *p<0.05
Discussion
Our study aimed to investigate the metabolic pathways of triapine in primary human hepatocytes and human subcellular fractions; to investigate drug-drug interactions between triapine and the anti-HIV agents tenofovir or emtricitabine; and to evaluate triapine as a perpetrator of drug interactions.
While triapine displays a high clearance in humans that is close to the liver blood flow [18,19], human microsomes fortified with NADPH resulted in only 35% depletion of triapine, suggesting that perhaps additional enzymes may be involved in its metabolism or that a substantial portion is eliminated unchanged. One of the major pathways involved in triapine metabolism is a CYP450-related pathway, with the non-specific CYP450 inhibitor N-benzyl imidazole abolishing most of the triapine depletion. Other metabolic pathways were also evaluated. Microsomal studies in the presence of methimazole, an FMO inhibitor, indicated that FMO may have a minor contribution in the metabolism of triapine, which was confirmed in the human hepatocytes. This is consistent with previous reports that observed triapine to produce oxidative metabolites such as dehydrogenated and hydroxylated metabolites in human liver microsomes [17]. Triapine displayed minimal depletion in the presence of AO/XO rich human liver cytosol and in human liver mitochondria indicating that neither AO/XO nor monoamine oxidases (MAOs) are involved in metabolism of triapine. Lastly, hepatocyte studies with NAT-1 and NAT-2 inhibitors indicated acetylation is not a major contributor to triapine metabolism.
While the involvement of CYP450s was evident from the microsomal and hepatocyte studies, it was also clear that CYP3A is not involved in triapine metabolism with depletion of triapine being unaltered after incubation of hepatocytes pretreated with rifampin or incubated with ketoconazole. Furafylline, the isoform specific CYP1A2 inhibitor, completely inhibited the depletion of triapine in human liver microsomes indicating that CYP1A2 was the predominant microsomal isoform responsible for triapine metabolism. Triapine was not metabolized by human liver UDP-glucuronosyltransferases. Previously, Pelivan et al. had detected two triapine N-glucuronide metabolites in the urine but not serum, liver, or kidney of mice treated with triapine [17]. Possible explanations for this discrepancy are the concentrated nature of urine samples, and a possible species difference between mice and human microsomes in their propensity to glucuronidate triapine.The primary route of elimination of tenofovir and emtricitabine is as unchanged drug through the urine [29–32], making them less susceptible as metabolic drug interaction victims. Conversely, tenofovir or emtricitabine did not alter the half-life or the exposure of triapine in hepatocytes. Hence, tenofovir and emtricitabine do not appear to impact the transport of triapine into the hepatocytes nor its metabolic clearance. The absence of a drug interaction in hepatocytes will facilitate the undertaking of clinical trials involving co-administration of triapine with these anti-HIV drugs in the setting of cancer patients with HIV co-infection.
We also evaluated triapine as a perpetrator of CYP450 mediated drug-drug interactions. Triapine had a small inhibitory effect on CYP1A2 activity, likely due to competitive inhibition, as CYP1A2 represents the major metabolic clearance pathway for triapine. Surprisingly, triapine also appeared to cause a slight increase in CYP2C19 activity. Increased CYP450 enzyme activity has been reported before with acetonitrile and acetone stimulating CYP2C9 mediated tolbutamide hydroxylation by 2–3-fold in microsomes [33], quinidine increasing CYP3A4 Vmax for S-warfarin 4’-hydroxylation 2.5-fold and Vmax for R-warfarin 10-hydroxylation 5-fold [34], quinidine increasing CYP3A4 mediated 5-, 5’-, and 10-hydroxylation of diclofenac, piroxicam and R-warfarin, respectively by 5–7-fold [35]. Sorafenib and sunitinib activated midazolam 1’-hydroxylation by CYP3A5 but inhibited that by CYP3A4. [36], and citrate stimulated CYP3A4 catalytic activity [37]. These in vitro observations of increases in Vmax were confirmed in vivo in monkeys where the catalytic capacity of monkey hepatic CYP3A toward diclofenac metabolism was enhanced almost 2-fold by quinidine [38]. The relatively small effect size of triapine on CYP2C19 activity is unlikely to be of clinical significance, especially since most drugs that are substrates for CYP2C19 will have additional metabolic routes in addition to CYP2C19.
Our results will be instrumental in guiding future clinical studies of the metabolism of triapine, to confirm the human metabolic fate of triapine, and to quantify the drug interactions of triapine both as a victim and perpetrator.
Supplementary Material
Acknowledgments
Funding
Support: Grant UM1 CA186690 (NCI-CTEP), R50 CA211241 (NCI), and U24CA247643 (NCI). This project used the UPMC Hillman Cancer Center Cancer Pharmacokinetics and Pharmacodynamics Facility (CPPF) and the Clinical Pharmacokinetics Laboratory in the School of Pharmacy and was supported in part by award P30 CA47904 (NCI).
Footnotes
https://clinicaltrials.gov/ct2/results?cond=&term=triapine&cntry=&state=&city=&dist (accessed October 4th 2019)
https://www.absorption.com/wp-content/uploads/2014/07/Substrate+Specificity+for+FMO-+and+CYPMediated+Reactions+and+Enzyme+Inactivation+in+HLM_+Methyl-p-Tolyl+Sulfide+versus+Benzydamine.pdf (accessed 10/06/2019)
Conflict of Interest
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Moore EC, Sartorelli AC (1984) Inhibition of ribonucleotide reductase by alpha-(N)-heterocyclic carboxaldehyde thiosemicarbazones. Pharmacology & therapeutics 24 (3):439–447 [DOI] [PubMed] [Google Scholar]
- 2.Yu Y, Kalinowski DS, Kovacevic Z, Siafakas AR, Jansson PJ, Stefani C, Lovejoy DB, Sharpe PC, Bernhardt PV, Richardson DR (2009) Thiosemicarbazones from the old to new: iron chelators that are more than just ribonucleotide reductase inhibitors. Journal of medicinal chemistry 52 (17):5271–5294. doi: 10.1021/jm900552r [DOI] [PubMed] [Google Scholar]
- 3.Thelander L, Reichard P (1979) Reduction of ribonucleotides. Annu Rev Biochem 48:133–158. doi: 10.1146/annurev.bi.48.070179.001025 [DOI] [PubMed] [Google Scholar]
- 4.Zhou BS, Tsai P, Ker R, Tsai J, Ho R, Yu J, Shih J, Yen Y (1998) Overexpression of transfected human ribonucleotide reductase M2 subunit in human cancer cells enhances their invasive potential. Clinical & experimental metastasis 16 (1):43–49 [DOI] [PubMed] [Google Scholar]
- 5.Karp JE, Giles FJ, Gojo I, Morris L, Greer J, Johnson B, Thein M, Sznol M, Low J (2008) A phase I study ofthe novel ribonucleotide reductase inhibitor 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, Triapine) in combination with the nucleoside analog fludarabine for patients with refractory acute leukemias and aggressive myeloproliferative disorders. Leukemia research 32 (1):71–77. doi: 10.1016/j.leukres.2007.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunos CA, Waggoner S, von Gruenigen V, Eldermire E, Pink J, Dowlati A, Kinsella TJ (2010) Phase I trial of pelvic radiation, weekly cisplatin, and 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) for locally advanced cervical cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 16 (4):1298–1306. doi: 10.1158/1078-0432.CCR-09-2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao J, Synold TW, Morgan RJ Jr., Kunos C, Longmate J, Lenz HJ, Lim D, Shibata S, Chung V, Stoller RG, Belani CP, Gandara DR, McNamara M, Gitlitz BJ, Lau DH, Ramalingam SS, Davies A, Espinoza-Delgado I, Newman EM, Yen Y (2012) A phase I and pharmacokinetic study of oral 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) in the treatment of advanced-stage solid cancers: a California Cancer Consortium Study. Cancer chemotherapy and pharmacology 69 (3):835–843. doi: 10.1007/s00280-011-1779-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeidner JF, Karp JE, Blackford AL, Smith BD, Gojo I, Gore SD, Levis MJ, Carraway HE, Greer JM, Ivy SP, Pratz KW, McDevitt MA (2014) A phase II trial of sequential ribonucleotide reductase inhibition in aggressive myeloproliferative neoplasms. Haematologica 99 (4):672–678. doi: 10.3324/haematol.2013.097246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunos CA, Radivoyevitch T, Waggoner S, Debernardo R, Zanotti K, Resnick K, Fusco N, Adams R, Redline R, Faulhaber P, Dowlati A (2013) Radiochemotherapy plus 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) in advanced-stage cervical and vaginal cancers. Gynecologic oncology 130 (1):75–80. doi: 10.1016/j.ygyno.2013.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traynor AM, Lee JW, Bayer GK, Tate JM, Thomas SP, Mazurczak M, Graham DL, Kolesar JM, Schiller JH (2010) A phase II trial of triapine (NSC# 663249) and gemcitabine as second line treatment of advanced non-small cell lung cancer: Eastern Cooperative Oncology Group Study 1503. Investigational new drugs 28 (1):91–97. doi: 10.1007/s10637-009-9230-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attia S, Kolesar J, Mahoney MR, Pitot HC, Laheru D, Heun J, Huang W, Eickhoff J, Erlichman C, Holen KD (2008) A phase 2 consortium (P2C) trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) for advanced adenocarcinoma of the pancreas. Investigational new drugs 26 (4):369–379. doi: 10.1007/s10637-008-9123-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackenzie MJ, Saltman D, Hirte H, Low J, Johnson C, Pond G, Moore MJ (2007) A Phase II study of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) and gemcitabine in advanced pancreatic carcinoma. A trial of the Princess Margaret hospital Phase II consortium. Investigational new drugs 25 (6):553–558. doi: 10.1007/s10637-007-9066-3 [DOI] [PubMed] [Google Scholar]
- 13.Knox JJ, Hotte SJ, Kollmannsberger C, Winquist E, Fisher B, Eisenhauer EA (2007) Phase II study of Triapine in patients with metastatic renal cell carcinoma: a trial of the National Cancer Institute of Canada Clinical Trials Group (NCIC IND.161). Investigational new drugs 25 (5):471–477. doi: 10.1007/s10637-007-9044-9 [DOI] [PubMed] [Google Scholar]
- 14.Ma B, Goh BC, Tan EH, Lam KC, Soo R, Leong SS, Wang LZ, Mo F, Chan AT, Zee B, Mok T (2008) A multicenter phase II trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, Triapine) and gemcitabine in advanced non-small-cell lung cancer with pharmacokinetic evaluation using peripheral blood mononuclear cells. Investigational new drugs 26 (2):169–173. doi: 10.1007/s10637-007-9085-0 [DOI] [PubMed] [Google Scholar]
- 15.Nutting CM, van Herpen CM, Miah AB, Bhide SA, Machiels JP, Buter J, Kelly C, de Raucourt D, Harrington KJ (2009) Phase II study of 3-AP Triapine in patients with recurrent or metastatic head and neck squamous cell carcinoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 20 (7):1275–1279. doi: 10.1093/annonc/mdn775 [DOI] [PubMed] [Google Scholar]
- 16.Pelivan K, Frensemeier LM, Karst U, Koellensperger G, Heffeter P, Keppler BK, Kowol CR (2018) Comparison of metabolic pathways of different alpha-N-heterocyclic thiosemicarbazones. Analytical and bioanalytical chemistry 410 (9):2343–2361. doi: 10.1007/s00216-018-0889-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelivan K, Frensemeier L, Karst U, Koellensperger G, Bielec B, Hager S, Heffeter P, Keppler BK, Kowol CR (2017) Understanding the metabolism of the anticancer drug Triapine: electrochemical oxidation, microsomal incubation and in vivo analysis using LC-HRMS. Analyst 142 (17):3165–3176. doi: 10.1039/c7an00902j [DOI] [PubMed] [Google Scholar]
- 18.Kunos CA, Chu E, Beumer JH, Sznol M, Ivy SP (2017) Phase I trial of daily triapine in combination with cisplatin chemotherapy for advanced-stage malignancies. Cancer chemotherapy and pharmacology 79 (1):201–207. doi: 10.1007/s00280-016-3200-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verbeeck RK (2008) Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. European journal of clinical pharmacology 64 (12):1147–1161. doi: 10.1007/s00228-008-0553-z [DOI] [PubMed] [Google Scholar]
- 20.Feun L, Modiano M, Lee K, Mao J, Marini A, Savaraj N, Plezia P, Almassian B, Colacino E, Fischer J, MacDonald S (2002) Phase I and pharmacokinetic study of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) using a single intravenous dose schedule. Cancer chemotherapy and pharmacology 50 (3):223–229. doi: 10.1007/s00280-002-0480-0 [DOI] [PubMed] [Google Scholar]
- 21.Murren J, Modiano M, Clairmont C, Lambert P, Savaraj N, Doyle T, Sznol M (2003) Phase I and pharmacokinetic study of triapine, a potent ribonucleotide reductase inhibitor, administered daily for five days in patients with advanced solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research 9 (11):4092–4100 [PubMed] [Google Scholar]
- 22.Yang X, Gandhi YA, Duignan DB, Morris ME (2009) Prediction of biliary excretion in rats and humans using molecular weight and quantitative structure-pharmacokinetic relationships. The AAPS journal 11 (3):511–525. doi: 10.1208/s12248-009-9124-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maiman M, Fruchter RG, Clark M, Arrastia CD, Matthews R, Gates EJ (1997) Cervical cancer as an AIDS-defining illness. Obstet Gynecol 89 (1):76–80 [DOI] [PubMed] [Google Scholar]
- 24.Khojasteh SC, Prabhu S, Kenny JR, Halladay JS, Lu AY (2011) Chemical inhibitors of cytochrome P450 isoforms in human liver microsomes: a re-evaluation of P450 isoform selectivity. European journal of drug metabolism and pharmacokinetics 36 (1):1–16. doi: 10.1007/s13318-011-0024-2 [DOI] [PubMed] [Google Scholar]
- 25.Kiesel BF, Parise RA, Guo J, Huryn DM, Johnston PA, Colombo R, Sen M, Grandis JR, Beumer JH, Eiseman JL (2016) Toxicity, pharmacokinetics and metabolism of a novel inhibitor of IL-6-induced STAT3 activation. Cancer chemotherapy and pharmacology 78 (6):1225–1235. doi: 10.1007/s00280-016-3181-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pillai VC, Strom SC, Caritis SN, Venkataramanan R (2013) A sensitive and specific CYP cocktail assay for the simultaneous assessment of human cytochrome P450 activities in primary cultures of human hepatocytes using LC-MS/MS. Journal of pharmaceutical and biomedical analysis 74:126–132. doi: 10.1016/j.jpba.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumoto J, Kiesel BF, Parise RA, Guo J, Taylor S, Huang M, Eiseman JL, Ivy SP, Kunos C, Chu E, Beumer JH (2017) LC-MS/MS assay for the quantitation of the ribonucleotide reductase inhibitor triapine in human plasma. Journal of pharmaceutical and biomedical analysis 146:154–160. doi: 10.1016/j.jpba.2017.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casarett and Doull’s Toxicology: The Basic Science of Poisons (2008). 7th edition edn. McGraw-Hill, New York. doi:DOI: 10.1036/0071470514 [DOI] [Google Scholar]
- 29.DeChristoforo R, Penzak SR (2004) Tenofovir: a nucleotide analogue reverse-transcriptase inhibitor for treatment of HIV infection. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists 61 (1):86–98; quiz 99–100 [DOI] [PubMed] [Google Scholar]
- 30. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021752s005lbl.pdf.
- 31.Blum MR, Chittick GE, Begley JA, Zong J (2007) Steady-state pharmacokinetics of emtricitabine and tenofovir disoproxil fumarate administered alone and in combination in healthy volunteers. Journal of clinical pharmacology 47 (6):751–759. doi: 10.1177/0091270007300951 [DOI] [PubMed] [Google Scholar]
- 32.Bang LM, Scott LJ (2003) Emtricitabine: an antiretroviral agent for HIV infection. Drugs 63 (22):2413–2424; discussion 2425–2416 [DOI] [PubMed] [Google Scholar]
- 33.Palamanda J, Feng WW, Lin CC, Nomeir AA (2000) Stimulation of tolbutamide hydroxylation by acetone and acetonitrile in human liver microsomes and in a cytochrome P-450 2C9-reconstituted system. Drug metabolism and disposition: the biological fate of chemicals 28 (1):38–43 [PubMed] [Google Scholar]
- 34.Ngui JS, Chen Q, Shou M, Wang RW, Stearns RA, Baillie TA, Tang W (2001) In vitro stimulation of warfarin metabolism by quinidine: increases in the formation of 4’- and 10-hydroxywarfarin. Drug metabolism and disposition: the biological fate of chemicals 29 (6):877–886 [PubMed] [Google Scholar]
- 35.Zhang Z, Li Y, Shou M, Zhang Y, Ngui JS, Stearns RA, Evans DC, Baillie TA, Tang W (2004) Influence of different recombinant systems on the cooperativity exhibited by cytochrome P4503A4. Xenobiotica; the fate of foreign compounds in biological systems 34 (5):473–486. doi: 10.1080/00498250410001691271 [DOI] [PubMed] [Google Scholar]
- 36.Sugiyama M, Fujita K-I, Murayama N, Akiyama Y, Yamazaki H, Sasaki Y (2011) Sorafenib and Sunitinib, Two Anticancer Drugs, Inhibit CYP3A4-Mediated and Activate CY3A5-Mediated Midazolam 1′-Hydroxylation. Drug Metabolism and Disposition 39 (5):757–762. doi: 10.1124/dmd.110.037853 [DOI] [PubMed] [Google Scholar]
- 37.Sevrioukova IF, Poulos TL (2015) Anion-Dependent Stimulation of CYP3A4 Monooxygenase. Biochemistry 54 (26):4083–4096. doi: 10.1021/acs.biochem.5b00510 [DOI] [PubMed] [Google Scholar]
- 38.Tang W, Stearns RA, Kwei GY, Iliff SA, Miller RR, Egan MA, Yu NX, Dean DC, Kumar S, Shou M, Lin JH, Baillie TA (1999) Interaction of diclofenac and quinidine in monkeys: stimulation of diclofenac metabolism. The Journal of pharmacology and experimental therapeutics 291 (3):1068–1074 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.