Abstract
Context:
There is scarcity of data on thyroid function abnormality in COVID-19 patients in world literature.
Aims:
The objective of this study was to assess thyroid function tests in hospitalized patients of COVID-19.
Settings and Design:
Sixty (60) patients with COVID-19 detected by RT-PCR admitted in General Medicine isolation ward and COVID block of a tertiary care teaching hospital were selected by semi-purposive sampling.
Materials and Methods:
These patients were assessed for thyroid function tests, including total T3, free T3, total T4, free T4, TSH and anti-TPO antibody along with other baseline investigations. Patients with pre-existing thyroid–related ailments, those on levothyroxine or anti-thyroid drugs or other drugs known to interfere with the results were excluded.
Results:
There were 43.3% patients in mild, 26.7% in moderate, and 30% in severe category, according to local COVID-19 severity classification protocol. 35% patients had one or more abnormality in the thyroid function, low TSH being the most common (18.33%). 9.1% patients had characteristic pattern of thyroiditis. In most of the others thyroid function did not match any typical pattern. There was no significant difference in any of the parameters of the thyroid function test between mild, moderate, and severe groups.
Conclusion:
Thyroid function may be abnormal in all categories of patients during COVID-19 infection, even in absence of pre-existing thyroid ailments. Although low TSH is the commonest abnormality and typical pattern of thyroiditis can be seen in a subsection of patients, in majority of the patients, thyroid function abnormality does not follow any characteristic pattern and likely represents a combination of thyroiditis and sick euthyroid syndrome in different points of its spectrum.
Keywords: COVID-19, COVID-19 and thyroid, thyroid function
INTRODUCTION
COVID-19, a disease caused by SARS-CoV-2 infection, has affected more than 36 million people worldwide and has been described, quite aptly, as “pandemic” by the World Health Organization (WHO).[1] Although predominantly a respiratory pathogen, the virus may also alter the functions of other organs of the body, for example, the heart,[2] kidneys,[3] etc. Alteration of the thyroid gland function in COVID-19 has also been described, although data is limited. Whether the abnormalities of thyroid function associated with COVID-19 can be explained with that due to Sick Euthyroid Syndrome has also remained elusive. This scarcity of data and in the face of rapidly changing scenario has also been acknowledged in recommendations by the professional bodies.[4,5] With this background, our aim of the study was to formally assess the thyroid function in patients with COVID-19 admitted with varying degree of severity in a tertiary care teaching hospital. We also aimed to look at whether any specific pattern of thyroid dysfunction in COVID-19 patients can be established.
MATERIALS AND METHODS
Methodology
The present work was a single-centre, hospital-based study, conducted in a tertiary care teaching hospital between August 1 and September 30, 2020 (2 months). The project was approved by the Institutional Ethical Committee. During the course of the study, the status of the hospital changed from Level 2 (eligible to treat symptomatic patients with suspicion of COVID-19, but after confirmation of the diagnosis, the patients are to be transferred to Designated COVID Care Centre) to Level 4 (eligible to treat seriously symptomatic patients suffering from COVID-19). The sample size was sixty (60). Patients admitted in General Medicine isolation ward and subsequently diagnosed as COVID-19 by reverse transcriptase-polymerase chain reaction (RT-PCR) from oral and nasopharyngeal swab, and patients admitted directly to COVID block after diagnosis were selected by semi-purposive sampling. A “confirmed case” was defined as “A person with laboratory confirmation of COVID-19 infection by RT-PCR, irrespective of clinical signs and symptoms.” Written, informed consent was taken from all of the patients. The blood samples were drawn at admission, before starting definitive treatment and were tested at our central laboratory and department of Biochemistry. “Mild disease” was defined as fever with malaise or mild cough, but no shortness of breath. “Moderate disease” was defined in adults as presence of dyspnoea with respiratory rate more than 24/min or SpO2 between 90 and 94% in room air, pneumonia not fulfilling the criteria of “severe” disease, or presence of altered liver or renal function tests. Severe disease was defined as presence of severe dyspnoea with respiratory rate more than 30/min or SpO2 less than 90% in room air, presence of ARDS, severe sepsis or septic shock.[6,7] Total T3, Free T3, Total T4, Free T4, TSH, anti-TPO antibody and Ferritin were tested by chemiluminescent detection in ADVIA Centaur XP immunoassay systems, Siemens-healthineers, Germany. The ADVIA Centaur TSH3-Ultra assay is a third-generation assay that employs anti-FITC monoclonal antibody covalently bound to paramagnetic particles, an FITC labeled anti-TSH capture mAb, a tracer consisting of acridium ester, an anti-TSH mAb conjugated to bovine serum albumin for Chemiluminescent detection. The reference interval for TSH in Adult population is 0.35-4.6 μIU/ml. Analytical measuring range of serum TSH concentrations from 0.008 to 150 μIU/ml. Coefficient of variation for TSH is 1.3. Internal Quality Control lies within ± 1 SD & Bio-Rad EQAS Z-score is <1.0. Serum Free T4 was detected by a competitive immunoassay in CLIA platform where reference interval is 0.89-1.80 ng/dl and overall CV is 4.16%. Daily Internal Quality Control & External Quality Assurance Schemes are within acceptable limit. Serum FT3 is also by competitive immunoassay in CLIA platform, CV% is 4.05, reference interval is 2.3-4.2 pg/ml. Reference range of T4 is 4.6-11.5 μg/dl & T3 is 0.81-1.87 ng/ml and CV% 5.55 and 3.44, respectively. Serum Ferritin was estimated by two-site immunoassay using direct chemiluminometric technology in the above-mentioned instrument. Reference intervals are in adult male 28-397 ng/dl & in adult female 5-159 ng/dl. CV% of the assay is 3.7. D-dimer was tested from citrated plasma in Stago-STA Compact Max Coagulometer, Diagnostica Stago, USA. Reference Interval for D-dimer <0.50 μg/ml for healthy subjects. Precision in CV% being 7.3 and no hook effect is detected upto 500 μg/ml. INCLUSION CRITERIA: Confirmed cases of COVID 19 (RT-PCR) patients admitted in General Medicine isolation ward, and COVID block aged 18 years or older. EXCLUSION CRITERIA: Known patients of thyroid disorder and on thyroxin or anti–thyroid drugs, patients on drugs known to cause abnormality in thyroid function, for example, Amiodarone, Lithium, glucocorticoids or had received radioiodine in the last 6 months, patients who had received glucocorticoids prior to coming to our hospital, HIV infected patients, diagnosed patients with acute or chronic liver disease, known diabetic patients and on anti-diabetic medications.
Statistical analysis
Data was entered in Microsoft excel spread sheet and analyzed using RStudio Version 1.3.1056. As most of the data was not normally distributed, the total number (proportions) of categorical variables and median (first quarter, third quarter) of the continuous variables were used. Kruskal--Wallis and ANOVA test were used to determine the difference among more than three groups. Freeman Halton's extension of Fisher's test was used for the categorical variables instead of Chi-square test since the expected frequencies were less than 5. Differences were considered statistically significant when the P value was <0.05.
RESULTS
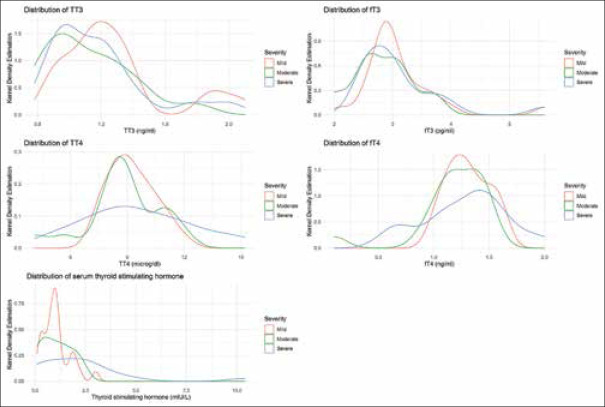
Among 60 patients, there were 26 patients (43.3%) in mild group, 16 patients (26.7%) in moderate group, and 18 patients (30%) in severe group. Of the total 60 patients, 21 patients (35%) showed one or more abnormality in thyroid function. The commonest abnormality in thyroid function test was low TSH, found in 11 patients (18.33%). The distribution of altered thyroid function parameters is given in Table 1. Among the eleven (11) patients with low TSH values, one pt (9.1%) had elevated total and free T3 and T4 values, a pattern typically suggestive of thyroiditis. Another two patients had elevated total T4 only with freeT4, total T3, and freeT3 remaining within normal range. When we tried to look at whether there is any association with alteration of any of the thyroid function parameter with severity of the disease, it was established with freeT4 only (P = 0.009) [Table 2]. To decipher whether there was any difference between any of the parameters of thyroid function test among the mild, moderate, and severe patient groups, we used ANOVA for total T4, as the data was normally distributed and Kruskal-Wallis for the rest. However, none of the parameters differed significantly among the three groups [Table 3]. Figure 1 shows a Kernel Density estimation of distribution of different thyroid function parameter values among mild, moderate, and severe group of patients. Nine (9) out of sixty (60) patients, that is, 15% were anti-TPO antibody positive. There was no significant difference in d-dimer (0.6 mcg/ml and 0.62 mcg/ml, P = 0.64) or ferritin (154.2 ng/dl and 182.4 ng/dl, P = 0.55) values between mild and moderate group of COVID-19 patients [Table 4].
Table 1.
Distribution of different thyroid function test values in three groups (elevated, normal, low) along with their percentage within brackets. n=60
| Levels | TSH (0.35-4.6 mIU/ml) | Free T3 (2.3-4.2 pg/ml) | Total T3 (0.81-1.87 ng/ml) | Free T4 (0.89-1.8 ng/dl) | Total T4 (4.6-11.5 mcg/dl) |
|---|---|---|---|---|---|
| Elevated | 2 (3.33%) | 2 (3.33%) | 4 (6.67%) | 1 (1.67%) | 6 (10%) |
| Normal | 47 (78.33%) | 55 (91.67%) | 54 (90%) | 54 (90%) | 53 (88.33%) |
| Low | 11 (18.33%) | 3 (5%) | 2 (3.33%) | 5 (8.33%) | 1 (1.67%) |
Table 2.
Distribution of freeT4 values in three groups (elevated, normal, low) according to disease severity of COVID-19. n=60. Statistical significance was carried out using Freeman-Halton extension of Fisher’s exact test
| Free T4 (ng/dl) | Mild | Moderate | Severe | P |
|---|---|---|---|---|
| Elevated | 0 (0.0%) | 0 (0.0%) | 1 (100.0%) | 0.009 |
| Normal | 26 (48.1%) | 15 (27.8%) | 13 (24.1%) | |
| Low | 0 (0.0%) | 1 (20.0%) | 4 (80.0%) |
Table 3.
Comparison of TSH, freeT4, total T4, freeT3, total T3 in mild, moderate and severe group of COVID-19 patients. The median and interquartile range (IQR) values were used for continuous variables. Statistical significance test was carried out using Kruskal-Wallis test for all except for Total T4 for which ANOVA was used
| TSH | Free T4 | Total T4 | Free T3 | Total T3 | |
|---|---|---|---|---|---|
| Mild (n=26) | 1[0.7,1.175] | 1.3[1.2,1.475] | 9.05[8.5,10.1] | 2.9[2.8,3.275] | 1.215[1.1,1.347] |
| Moderate (n=16) | 1[0.375,1.575] | 1.2[1.1,1.4] | 8.75[8.15,9.825] | 2.8[2.575,3.1] | 1.05[0.935,1.25] |
| Severe (n=18) | 1.95[0.625,2.875] | 1.35[1.1,1.5] | 9.05[7.675,11.2] | 2.9[2.625,3.25] | 1.135[0.9725,1.245] |
| P | 0.098 | 0.507 | 0.534 | 0.413 | 0.258 |
Figure 1.
Kernel Density estimation of distribution of different thyroid function parameter values among mild, moderate and severe group of patients
Table 4.
Median and interquartile range (IQR) of D-Dimer and Ferritin levels with corresponding P values. n=19. Kruskal Wallis test was used for significance
| Severity | D-Dimer (mcg/ml) | Ferritin (ng/dl) |
|---|---|---|
| Mild | 0.60 (0.44, 1.08) | 154.20 (58.62, 334.82) |
| Moderate | 0.62 (0.44, 2.06) | 182.40 (56.25, 210.35) |
| P | 0.6419 | 0.5541 |
DISCUSSION
This study was conceived in second half of July 2020, because of lack of properly conducted, published background data then. We excluded diagnosed patients of acute liver disease, as it is associated with an initial rise in Total T3 and T4 levels, these levels become subnormal with progression to liver failure. Similarly, we excluded diagnosed patients with HIV as it also causes alteration of thyroid function. In early stage of HIV infection, T3 and T4 levels rise, although there is weight loss. T3 levels fall with progression to AIDS but TSH usually remains normal. We excluded patients with known diabetes as we also wanted to assess abnormalities of glycemia in hospitalized patients with COVID-19. The data on abnormalities of thyroid function only are being presented here.
Any acute, severe illness can cause abnormalities of circulating TSH or thyroid hormone levels in absence of underlying thyroid disease, a condition known as Sick Euthyroid Syndrome (SES). The most common pattern of SES, as is known, is decrease in total and unbound T3 levels (low T3 syndrome). Very sick person may exhibit a dramatic fall in total T4 and T3 levels (low T4 syndrome) and subnormal TSH is seen in 10% of and increased TSH is observed in 5% patients.[8] In our study, among the eleven (11) patients with low TSH, eight (8) did not have any other abnormality accompanying. So, this pattern cannot be completely explained by sick euthyroid syndrome.
Thyroid function was shown to be significantly altered in many patients with the Severe Acute Respiratory Syndrome (SARS) that had affected more than 8,000 patients over many countries of Asia and rest of the world in 2002-2003.[9] The major alteration was decrease in T3 and T4 in many patients. An association with severity of disease with magnitude of decline in T3 and T4 was also described. A histological study of thyroid gland of patients with SARS showed extensive injury to the thyroid follicular cells as well as the parafollicular cells.[10]
The novel coronavirus or the SARS-CoV-2 that is responsible for the current pandemic, although belong to the same family of Coronaviridae, has got differences in the clinical spectrum from the previous ones like SARS and MERS. To our knowledge, there is only one published study which had formally assessed the thyroid function abnormalities seen in hospitalized patients with COVID-19 prospectively, prior to this publication.[11] In that study, about 15% patients, all admitted in critical care setting, had thyroid function abnormalities suggestive of thyrotoxicosis with low TSH and normal or slightly elevated thyroxine levels. There is another retrospective analysis of 50 patients and a comparison with healthy volunteers, which had shown decreased TSH in about 58% patients with COVID-19. The same study has also reported an inverse correlation of TSH and total T3 values with severity of COVID-19.[12] There is also a case report showing features of subacute thyroiditis in an 18-year-old woman with COVID-19.[13] Our study nearly corroborates with the Italian study in that the commonest abnormality, low TSH was found in 18.8% patients. Of these, one patient (9.1%) had elevated total and free T3 and T4 values, typical of thyroiditis. Other two patients had elevated total T4 only, in the rest eight, low TSH was the sole abnormality. So, although a form of thyrotoxicosis was evident in these patients, no hard pattern of thyroid dysfunction could be established in most of the patients. It might be because of a coexistence of subacute thyroiditis and sick-euthyroid syndrome giving rise to a mixed pattern. Fifteen percent (15%) of our patients were anti-TPO antibody positive. This means there was no higher prevalence of thyroid autoimmunity in our COVID-19 patients, as it is known that anti-TPO antibody can be present in general population in 8--27%.[14] This is also an important observation as there has always been a concern whether individuals having thyroid autoimmunity are more likely to be affected by COVID-19 with lack of convincing data to answer.
On our assay of D-dimer and ferritin, no significant difference was found between mild and moderate group of patients. We do recognize, however, that these were done in very small patient population due to logistic issues and we do not have data for severe group of patients.
This is one of the initial studies of formal thyroid function assessment in COVID-19 in the world and the first from this country. Our study included patients from different clinical scenarios namely mild, moderate, and severe depending upon the local classification protocol. The relatively small sample size is one of the limitations of our study. Other limitations are possibility of selection bias because of this being a tertiary care referral hospital and lack of complete data of all of our patients, especially in the initial days of sampling because we had to transfer the patients to other hospital as per government regulations. This has specifically led to the low number of d-dimer and ferritin values.
CONCLUSION
Some form of alteration of thyroid function is seen in about one third of patients during COVID-19 irrespective of severity of the disease. Low TSH is the most common abnormality seen in 18.3% of patients. It is mostly seen in isolation, but may also be associated with elevated total and/or free T3 or T4 suggestive of subacute thyroiditis. Although a typical pattern is often not found, the overall picture likely represents a combination of thyroiditis and sick euthyroid syndrome in different points of its spectrum. Thyroid autoimmunity is not more common among COVID-19 patients than that found in general population.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors would like to acknowledge the contribution of Professor Dr Saibal Kumar Mukherjee, Principal, NRS Medical College and Hospital, Kolkata, Professor Dr Karabi Baral, Medical Superintendent cum Vice-Principal, NRS Medical College and Hospital, Kolkata and Professor Dr Soma Gupta, Dean of Students' Affairs, NRS Medical College and Hospital, Kolkata towards the completion of the work.
REFERENCES
- 1.World Health Organization-www.who.int [Internet] Geneva: Coronavirus disease (COVID-19)Weekly Epidemiological Update and Weekly Operational Update; [[Last accessed 2020 Oct 10]]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 . [Google Scholar]
- 2.Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zahid U, Ramachandran P, Spitalewitz S, Alasadi L, Chakraborti A, Azhar M, et al. Acute kidney injury in COVID-19 patients: An inner city hospital experience and policy implications. Am J Nephrol. 2020;51:786–96. doi: 10.1159/000511160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.BTA/SFE statement regarding issues specific to thyroid dysfunction during the COVID -19 pandemic. [[cited 2020 Mar 25]]. Available from: www.british-thyroid-association.org .
- 5.Rajput R, Agarwal A, Ganie MA, Bal CS, Sharma DC, Seshadri K, et al. Coronavirus disease 2019 and thyroid disease: Position statement of Indian Thyroid Society. Thyroid Res Pract. 2020;17:4–6. [Google Scholar]
- 6.Government of West Bengal, Department of Health and Family Welfare- www.wbhealth.gov.in [Internet].“Management protocol for COVID-19, second edition”. Available from: https://www.wbhealth.gov.in/uploaded_files/corona/Management_Protocol_for_COVID-19_-_WB_2nd_Edition.pdf .
- 7.Government of India, Ministry of Health and Family Welfare, Director General of Health Services, (EMR division)Version 3-www.mohfw.gov.in[Internet] “Clinical Management Protocol: COVID-19”. [[Last accessed on 2020 Jun 13]]. Available from: http://www.clinicalestablishments.gov.in/WriteReadData/2801.pdf .
- 8.Jameson JL, Mandel SJ, Weetman AP. Hypothyroidism. In: Larry Jameson J, Dennis L Kasper, Dan L Longo, Anthony S Fauci, Stephen L Hauser, Loscalzo J, editors. Harrison's Principles of Internal Medicine. 20th ed. New York: McGraw-Hill; 2018. p. 2709. [Google Scholar]
- 9.World Health Organization-www.who.int [Internet] Geneva: International travel and health; Available from: https://www.who.int/ith/diseases/sars/en/ [Google Scholar]
- 10.Wei L, Sun S, Xu CH, Zhang J, Xu Y, Zhu H, et al. Pathology of the thyroid in severe acute respiratory syndrome. Hum Pathol. 2007;38:95–102. doi: 10.1016/j.humpath.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller I, Cannavaro D, Dazzi D, Covelli D, Mantovani G, Muscatello A, et al. SARS-CoV-2 related atypical thyroiditis. Lancet. 2020;8:739–41. doi: 10.1016/S2213-8587(20)30266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen M, Zhou W, Xu W. Thyroid function analysis in 50 patients with COVID-19: A Retrospective study? Thyroid. 2020 doi: 10.1089/thy.2020.0363. doi: 10.1089/thy.2020.0363. [DOI] [PubMed] [Google Scholar]
- 13.Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis after SARS-COV-2 infection. J Clin Endocrinol Metab. 2020;105:dgaa276. doi: 10.1210/clinem/dgaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salvatore D, Cohen R, Kopp PA, Larsen PR. Thyroid pathophysiology and diagnostic evaluation. In: Melmed S, Auchus RJ, Goldfine AB, Koenig RJ, Rosen CJ, editors. Williams Textbook of Endocrinology. 14th ed. Elsevier; 2020. p. 361. [Google Scholar]