Abstract
Previous studies in MRL +/+ mice suggest involvement of oxidative stress (OS) in trichloroethene (TCE)-mediated autoimmunity. However, molecular mechanisms underlying the autoimmunity remain to be fully elucidated. Even though toll-like receptors (TLRs) and Nuclear factor (erythroid-derived 2)-like2 (Nrf2) pathways are implicated in autoimmune diseases (ADs), interplay of OS, TLR and Nrf2 in TCE-mediated autoimmune response remains unexplored. This study was, therefore, undertaken to clearly establish a link among OS, TLR4 and Nrf2 pathways in TCE-induced autoimmunity. Groups of female MRL +/+ mice were treated with TCE, sulforaphane (SFN, an antioxidant) or TCE + SFN (TCE, 10 mmol/kg, i.p., every 4th day; SFN, 8 mg/kg, i.p., every other day) for 6 weeks. TCE exposure led to greater formation of serum 4-hydroxynonenal (HNE)-protein adducts, HNE-specific circulating immune complexes (CICs) and protein carbonyls which were associated with significant increases in serum antinuclear antibodies (ANAs). Moreover, incubation of splenocytes from TCE-treated mice with HNE-modified proteins resulted in enhanced splenocyte proliferation and cytokine release evidenced by increased expression of cyclin D3, Cyclin-dependent kinase 6 (CDK6) and phospho-pRb as well as increased release of IL-6, TNF-α and INF-γ. More importantly, TCE exposure resulted in increased expression of TLR4, MyD88, IRAK4, NF-kB and reduced expression of Nrf2 and HO-1 in the spleen. Remarkably, SFN supplementation not only attenuated TCE-induced OS, upregulation in TLR4 and NF-kB signaling and down-regulation of Nrf2, but also ANA levels. These results, in addition to providing further support to a role of OS, also suggest that an interplay among OS, TLR4 and Nrf2 pathways contributes to TCE-mediated autoimmune response. Attenuation of TCE-mediated autoimmunity by SFN provides an avenue for preventive and/or therapeutic strategies for ADs involving OS.
Keywords: Trichloroethene, Autoimmunity, Oxidative stress, Toll-like receptor 4, Nrf2, 4-Hydroxynonenal-protein adducts
1. Introduction
Autoimmune diseases (ADs), the complex, chronic, and occasionally life-threatening medical disorders, are thought to impact 3–5% of the population in industrialized countries with rising rates (Dahlgren et al., 2007; Dinse et al., 2020; Lerner et al., 2015; Roberts and Erdei, 2020; Scherlinger et al., 2020). Accumulated epidemiological data suggest continued increases in both incidence and prevalence of ADs, and environmental factors, including chemicals such as trichloroethene (TCE) are implicated to drive these phenomena (Dinse et al., 2020; Khan and Wang, 2018, 2020; Lerner et al., 2015; Roberts and Erdei, 2020; Scherlinger et al., 2020; Wang et al., 2007, 2008). Therefore, there is an urgent need to extensively study the contribution and potential mechanisms of chemical-induced autoimmunity leading to ADs. TCE, a common environmental contaminant and a widely used industrial solvent, is involved in the development of ADs such as system lupus erythematosus (SLE), systemic sclerosis and autoimmune hepatitis, both in human and animal studies (Khan et al., 1995, 2001; Khan and Wang, 2018; Kilburn and Warshaw, 1992; Wang et al., 2007, 2012, 2019a). Previous findings using lupus-prone MRL+/+ mice support that TCE exposure causes cellular, immunological and molecular changes, including oxidative stress-related pathways, that play a role in TCE-mediated autoimmune response (Khan et al., 2001; Khan and Wang, 2018; Wang et al., 2007, 2012, 2019b). However, specific molecular mechanisms in the pathogenesis of TCE-mediated autoimmunity need to be fully elucidated.
Environmental factors play a vital role in the steady rise in the frequency of ADs (Dinse et al., 2020; Khan and Wang, 2018; Lerner et al., 2015; Roberts and Erdei, 2020; Scherlinger et al., 2020). Within the processes of these environmental factors, particularly through oxidative stress-mediated mechanisms, pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) are speculated to be involved in the environmental factor-accelerated ADs (Asadzadeh Manjili et al., 2020; Engelmann et al., 2020; Gill et al., 2010; Li et al., 2019; Peden, 2011; Zheng et al., 2020). These factors induce immune responses through interactions with pattern recognition receptors (PRRs), and toll-like receptors (TLRs) are among the most important PRRs expressed either on the cell surface or in the cytoplasmic vesicles of the immune cells (Asadzadeh Manjili et al., 2020; Engelmann et al., 2020; Fitzgerald and Kagan, 2020; Gill et al., 2010). It is important to point out that the concept of DAMP may represent a link among some environmental factors such as environmental chemical exposure, oxidative stress and ADs (Asadzadeh Manjili et al., 2020; Engelmann et al., 2020; Gill et al., 2010; Kadl et al., 2011; Li et al., 2019; Peden, 2011). Indeed, TLR4, an extensively studied TLR family member, plays a key role in the recognition of DAMPs and/or PAMPs, initiation of immune response and process of autoimmune disorders (Asadzadeh Manjili et al., 2020; Gill et al., 2010; Lim et al., 2014; Liu et al., 2014). Even though TLR pathways, specifically TLR4 signaling, have been implicated in the pathogenesis of ADs and considered as a potential therapeutic target for prevention or/and treatment of the diseases, the role of TLR4 in TCE-mediated autoimmune response remains unexplored.
Oxidative stress is increased in various ADs, including SLE, and shows a good relationship with disease activity and organ damage, suggesting its role in the pathogenesis of ADs (Bona et al., 2020; da Fonseca et al., 2019; Khan and Wang, 2018; Perl, 2013; Smallwood et al., 2018; Wang et al., 2010). Although the mechanisms by which oxidative stress participates in the pathogenesis of ADs are not fully understood, several hypotheses such as oxidative modification of selfantigens (endogenous macromolecules), abnormal activation of cell-death signaling, as well as dysregulation of oxidative stress-responsive pathways have been proposed (Khan and Wang, 2018; Perl, 2013; Smallwood et al., 2018; Wang et al., 2010, 2019a, 2019b). Recently, the most exciting trend concerning the response to oxidative stress has been elucidation of the signaling pathways by which such responses are regulated. Central to the understanding of such regulation is the activation of nuclear factor erythroid 2-related factor 2 (Nrf2) and its interaction with Kelch-like erythroid cell-derived protein with CNC homology (ECH)-associated protein 1 (Keap1) (Baird and Yamamoto, 2020; Banerjee et al., 2020; Smallwood et al., 2018). Nrf2, a master of redox switch in turning on cell signaling pathways, is involved in the regulation of about 1% of human genes via antioxidant response element (ARE) (Baird and Yamamoto, 2020; Kavian et al., 2018; Miller et al., 2020; Smallwood et al., 2018). Nrf2 is now considered as a drug target for various ADs in which oxidative stress and inflammation underlie the disease pathogenesis (Smallwood et al., 2018; Wheeler et al., 2020). Therefore, it would also be interesting to investigate the role of Nrf2 in the pathogenesis as well as potential therapeutic targeting of TCE-mediated autoimmunity.
Our previous studies in MRL+/+ mice clearly support an involvement of oxidative stress in TCE-mediated autoimmunity (Khan et al., 2001; Khan and Wang, 2018; Wang et al., 2007, 2012, 2019a). However, a thorough evaluation of molecular mechanisms underlying the autoimmunity, particularly the role and interaction of oxidative stress-responsive redox signaling pathways including TLR signaling, Nrf2 and NF-κB remain to be fully elucidated. Therefore, in this study, we have attempted to evaluate the interplay and potential of oxidative stress with TLR signaling and Nrf2 pathways in the pathogenesis of TCE-mediated autoimmunity. To our knowledge, this is the first attempt to assess the potential role of TLR signaling pathway in TCE-mediated autoimmunity. The findings in this study, in addition to providing further support to a role of oxidative stress, also suggest that an interplay among oxidative stress, TLR4 signaling, Nrf2 and NF-κB pathways contributes to TCE-mediated autoimmune response.
2. Materials and methods
2.1. Animals and treatments
TCE (purity ≥99%) and sulforaphane (SFN, purity ≥95%) were obtained from Sigma-Aldrich (St. Louis, MO). Five-week old female MRL+/+ (MRL/MpJ) mice were purchased from Jackson Laboratory (Bar Harber, ME). MRL+/+ mice were chosen for our studies as in these mice autoantibodies appear several months after their birth, and disease (SLE) develops spontaneously late in their second year of life, which provides an ideal window of time to investigate the potential of agents such as TCE in inducing/exacerbating ADs (Khan et al., 1995, 2001; Wang et al., 2012, 2019a). Female animals were used in this study because of higher prevalence of ADs in women than in men (Christou et al., 2019; Wang et al., 2010, 2016). The mice were housed at the University of Texas Medical Branch (UTMB) animal house facility and kept under a 12 h light/dark cycle at ~22 °C, 50–60% relative humidity, and were provided standard lab chow and drinking water ad libitum. All experiments were performed in accordance with the guidelines of the National Institutes of Health (NIH) and were approved by the Institutional Animal Care and Use Committee (IACUC) of UTMB. The animals were randomly divided into 4 groups (6 mice in each group) that included control, TCE, SFN or TCE + SFN treatment groups. Groups of mice were exposed to TCE, SFN or TCE + SFN (TCE, 10 mmol/kg in corn oil, ip, every 4th day; SFN, 8 mg/kg in corn oil, ip, every other day) for 6 weeks, whereas controls were given an equal volume of corn oil only (Banerjee et al., 2020; Khan et al., 1995, 2001; Wang et al., 2019a). The details of dose and duration of TCE and SFN exposure are described in previous studies (Banerjee et al., 2020; Guerrero-Beltrán et al., 2012; Khan et al., 1995, 2001; Wang et al., 2015, 2019a). After 6 weeks of TCE, SFN or TCE + SFN treatments, the mice were euthanized under ketamine/xylazine, blood was withdrawn through posterior vena cava, and sera obtained following blood clotting and centrifugation were divided into small aliquots and stored at −80 °C for further analysis. Major organs from the animals were immediately collected, and portions were snap-frozen and stored at −80 °C for subsequent studies. At the same time, taking a portion of spleen, the splenocytes were isolated for various evaluations (Wang et al., 2015, 2019b).
2.2. Quantitation of serum anti-nuclear antibodies
Serum anti-nuclear antibodies (ANA) were determined by using mouse-specific ELISA kit (Alpha Diagnostic Int'l, San Antonio, TX) according to the manufacturer's protocol as described in our earlier reports (Khan et al., 1995; Wang et al., 2007, 2012).
2.3. Serum protein carbonyl determination
Serum protein carbonyl content was determined using a Protein Carbonyl Assay Kit (Cayman Chemical, Ann Arbor, MI) by following the manufacturer's instructions.
2.4. Determination of serum lipid-derived reactive aldehyde (LDRAs)-protein adducts
To quantitate the formation of LDRAs, serum hydroxynonenal (HNE)-protein adducts were determined by using a quantitative competitive ELISA assay as reported in our previous studies (Khan et al., 2002; Wang et al., 2010, 2012, 2015).
2.5. Analysis of HNE-specific circulating immune complexes (CICs) in the serum
The level of HNE-specific CICs in the serum was analyzed using specific ELISAs established in our laboratory (Wang et al., 2016).
2.6. Cell culture and ex vivo studies
Splenocytes isolated from spleens of control, TCE, SFN or TCE + SFN treated mice were plated into 24-well flat-bottom plates at 2 × 106/well in a total volume of 1 ml. Mouse serum albumin (MSA, 10 μg/ml; Sigma) or HNE-MSA adducts (10 μg/ml) were added into culture plates to stimulate lymphocytes and incubated at 37 °C with 5% CO2 (Wang et al., 2008, 2011, 2012). After incubation for 48 h, the splenocytes from each well were harvested, and total cell protein was extracted for the determination of cyclin D3, Cyclin-dependent kinase 6 (CDK6) and phosphorylated retinoblastoma protein (pRb) by using Western blot analysis (Wang et al., 2011, 2019a). In the culture supernatants, the release of IL-6, TNF-α and INF-γ was quantitated using specific Quantikine ELISA kits according to manufacturer's instructions (R&D Systems, Inc., Minneapolis, MN).
2.7. Protein expression of TLR4, MyD88, IRAK4, Nrf2, HO-1 and NF-κB in the spleen
Splenic nuclear proteins were extracted using NE-PER nuclear extraction reagents (Thermo Fisher Scientific, Waltham, MA) according to manufacturer's instructions and total protein from spleen tissues was isolated using T-PER tissue protein extraction reagent (Thermo Fisher Scientific) following the manufacturer's instructions (Banerjee et al., 2020; Wang et al., 2011, 2019a, 2019b). The protein expression of Nrf2 and NF-κB was determined in nuclear fraction, whereas TLR4, myeloid differentiation primary response 88 (MyD88), interleukin-1 receptor-associated kinase 4 (IRAK4), and heme oxygenase-1 (HO-1) expression was evaluated in the whole tissue proteins using Western blot as reported earlier and quantification of blot signals was done by densitometry and normalized using β-actin expression (Wang et al., 2011, 2019a).
2.8. Statistical analyses
All values are presented as means ± SD. Data were subjected to statistical evaluation using one-way ANOVA followed by Tukey-Kramer multiple comparisons test (GraphPad Prism software, La Jolla, CA). The values of p < 0.05 were considered as statistically significant.
3. Results
3.1. TCE-mediated autoimmune response and its attenuation by antioxidant SFN
In the previous studies, our laboratory and other research groups have established a link between TCE exposure and autoimmunity in MRL+/+ mice (Cai et al., 2008; Griffin et al., 2000; Khan et al., 1995, 2001; Wang et al., 2007, 2009, 2012, 2013). To further verify the role of TCE in promoting an autoimmune response, we determined serum levels of ANA, an established biomarker of autoimmunity (Dinse et al., 2020; Khan et al., 1995, 2001; Wang et al., 2019b). As shown in Fig. 1, TCE exposure led to increased formation of ANA in comparison to controls, which is consistent with our earlier reports (Cai et al., 2008; Khan et al., 1995, 2001; Wang et al., 2007, 2012, 2013). Interestingly, SFN supplementation effectively attenuated TCE-mediated increases in ANA production, suggesting a protective effect of SFN in TCE-induced autoimmunity and further supporting the role of oxidative stress.
Fig. 1.
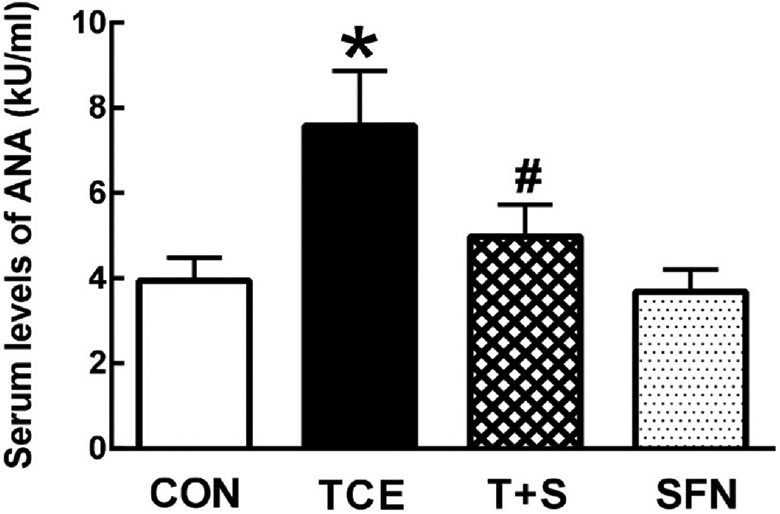
TCE exposure accelerated autoimmune response in MRL+/+ mice which was attenuated by SFN. Female mice were treated with TCE, SFN or TCE + SFN for 6 weeks, and serum ANA levels were determined using mouse-specific ELISA. The results are expressed as means ± SD (n = 6). * p < 0.05 vs. controls, # p < 0.05 vs. TCE-treated mice.
3.2. TCE exposure led to increased oxidative stress which was attenuated by SFN
Increasing evidence supports a critical role of oxidative stress in the pathogenesis of a variety of ADs (Bona et al., 2020; Khan and Wang, 2018; Wang et al., 2010, 2016). In fact, our earlier studies suggest an involvement of oxidative stress in TCE-mediated autoimmunity (Khan et al., 2001; Wang et al., 2007, 2009, 2012, 2013). To further support the role of oxidative stress in the pathogenesis of autoimmunity, we exposed MRL+/+ mice to TCE with or without antioxidant SFN and analyzed markers of oxidative stress. TCE exposure led to significantly increased serum carbonylated proteins (protein carbonyls) and HNE-protein adducts, suggesting increased oxidative modification of proteins (Fig. 2). Furthermore, TCE exposure also resulted in increased formation of serum HNE-specific CIC, supporting an immunogenic potential of HNE-protein adducts that could contribute to TCE-mediated autoimmune response. Remarkably, the elevated levels of both oxidatively modified proteins and HNE-specific CIC were significantly attenuated by SFN supplementation (Fig. 2).
Fig. 2.
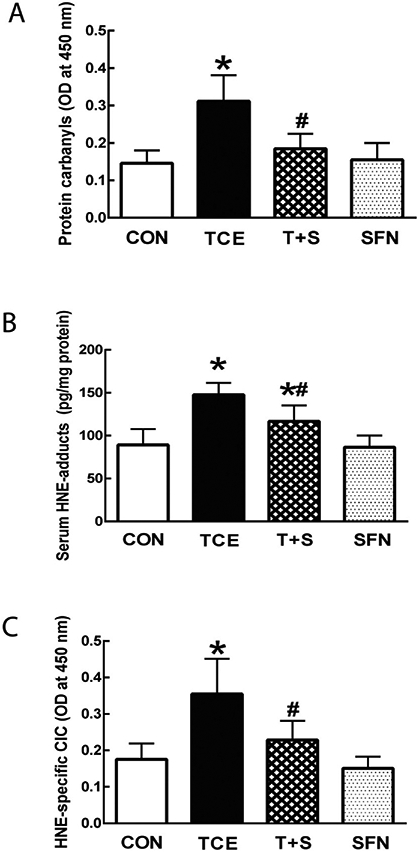
TCE exposure led to increased oxidative stress. Serum levels of protein carbonyls (A), HNE-protein adducts (B) and HNE-specific CICs (C) in MRL+/+ mice treated with TCE, SFN or TCE + SFN for 6 weeks. The data are presented as means ± SD (n = 6). * p < 0.05 vs. respective controls, # p < 0.05 vs. TCE-treated mice.
3.3. HNE-protein adducts play an important role in stimulating TCE-accelerated inflammatory/autoimmune response
Recent reports suggest that oxidatively modified proteins can trigger an autoimmune response and also show striking correlation with the disease activity and organ damage in ADs (Carubbi et al., 2019; Perl, 2013; Wang et al., 2012; Yang et al., 2018). As mentioned earlier in the results, TCE exposure promoted autoimmune response in MRL+/+ mice which was associated with increased formation of HNE-protein adducts as well as HNE-specific CIC. Moreover, we also observed that TCE exposure leads to significant increases in spleen weight (data not shown). Therefore, to further establish the contribution of oxidative stress, particularly contribution of oxidatively modified proteins in TCE-mediated autoimmunity, we conducted ex vivo studies by treating splenocytes from control, TCE or TCE + SFN treated mice with HNE-protein adducts and analyzed markers of cell proliferation and inflammation. As shown in Fig. 3, incubation of splenocytes with HNE-MSA adducts significantly enhanced the protein expression of cyclin D3, CDK6 and phospho-pRb in the splenocytes from TCE-treated mice in comparison with controls, suggesting that HNE-modified proteins can lead to increased cellular proliferation. Remarkably, elevated expression of these proteins was less pronounced in the splenocytes from the mice exposed to TCE + SFN compared with the mice treated with TCE only (Fig. 3). Furthermore, incubation of splenocytes from TCE-treated mice with HNE-MSA also promoted IL-6, TNF-α and INF-γ release (Fig. 4), which was less pronounced from splenocytes of mice also treated with SFN along with TCE (Fig 1). Together, these results provide compelling evidence to support that oxidative modification of proteins could play a critical role in initiating an inflammatory/autoimmune response.
Fig. 3.
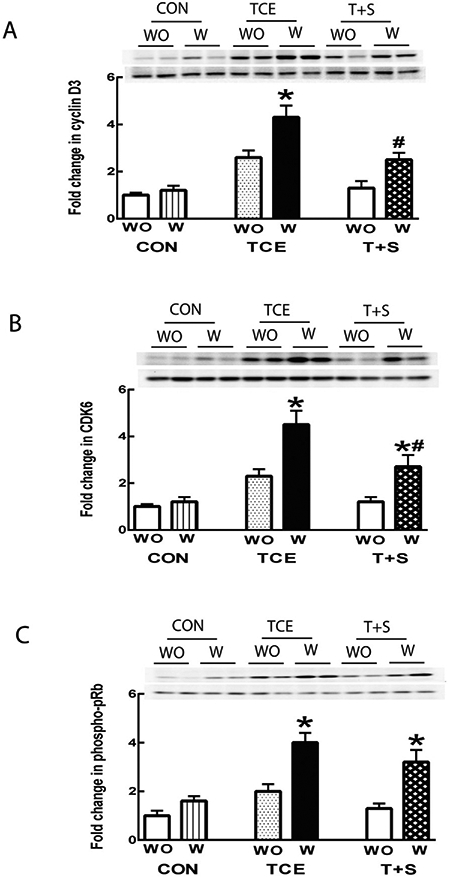
HNE-protein adducts promoted proliferation of splenocytes isolated from TCE-treated mice. Representative Western blot for cyclin D3 (A), CDK6 (B) and phospho-pRb (C) in the splenocytes following 48 h incubation with or without HNE-protein adducts. The data are presented as means ± SD (n = 4). * p < 0.05 vs. respective splenocytes without treatment with HNE-protein adducts, # p < 0.05 vs. the splenocytes from TCE-treated mice incubated with HNE-protein adducts. Note: WO: Without treatment with HNE-protein adducts; W: treated with HNE-protein adducts.
Fig. 4.
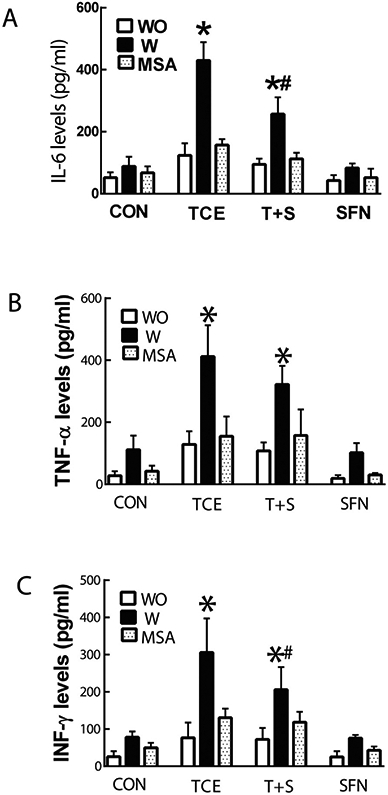
HNE-protein adducts increase cytokine release from splenocytes of TCE-treated mice. Cytokine levels of IL-6 (A), TNF-α (B) and INF-γ (C) in the culture supernatants of splenocytes with (W) or without (WO) the presence of HNE-protein adducts. The data are presented as means ± SD (n = 4). * p < 0.05 vs. respective splenocytes without treatment with HNE-protein adducts, # p < 0.05 vs. splenocytes incubated with HNE-protein adducts from TCE-treated mice. Note: WO: Without treatment with HNE-protein adducts; W: treated with HNE-protein adducts.
3.4. TCE induces dysregulation of TLR4 signaling
TLR pathways, particularly TLR4 signaling, have been involved in the pathogenesis of ADs, and also considered as a potential therapeutic target for prevention and/or treatment of inflammatory diseases, including ADs (Asadzadeh Manjili et al., 2020; Engelmann et al., 2020; Fitzgerald and Kagan, 2020; Liu et al., 2014). Recent studies indicate that oxidative stress, especially oxidatively modified proteins, contributes to inflammation via activating TLR pathways and TLR family has a critical link between oxidative stress and inflammatory diseases (Carubbi et al., 2019; Gill et al., 2010; Kadl et al., 2011; Li et al., 2019). It was, therefore, critically important to explore the contribution of TLR4 signaling in TCE-mediated autoimmune response. As shown in Fig. 5, TCE exposure in MRL+/+ mice led to significantly increased protein expression of TLR4, MyD88 and IRAK-4, suggesting an upregulation of TLR4 signaling. Interestingly, co-exposure with Nrf2 activator SFN clearly attenuated the TCE-mediated enhanced expression of TLR4, MyD88 and IRAK-4, indicating that SFN ameliorates TCE-mediated dysregulation of TLR4 signaling pathway, and thus disrupts the cross-talk between TLR4 and Nrf2.
Fig. 5.
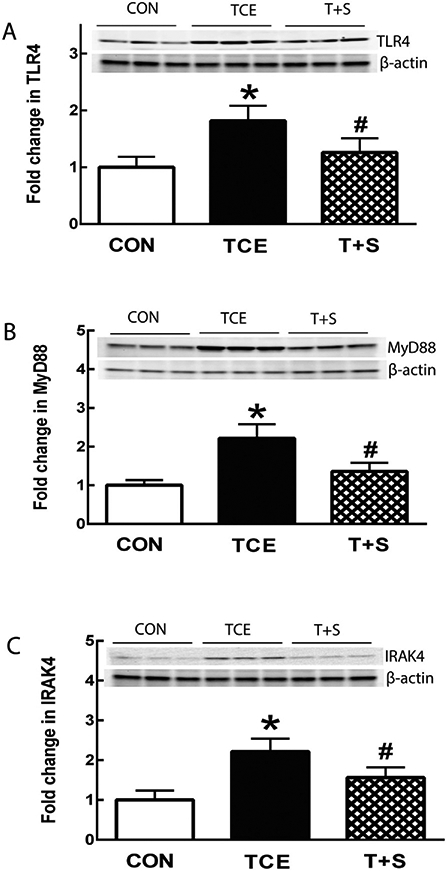
TCE exposure resulted in dysregulation of TLR4 signaling in the spleens of MRL+/+ mice, which was ameliorated by SFN supplementation. Representative Western blot for TLR4 (A), MyD88 (B) and IRAK4 (C) in the spleens of MRL+/+ mice following TCE or TCE + SFN exposure for 6 weeks. The results are expressed as means ± SD (n = 6). * p < 0.05 vs. controls, # p < 0.05 vs. TCE-treated mice.
3.5. TCE exposure results in systemic impairment of Nrf2 pathway which is improved by SFN supplementation
Nrf2 acts a critical oxidative stress sensor and a key antioxidant system regulator (Banerjee et al., 2020; Kavian et al., 2018; Smallwood et al., 2018; Zheng et al., 2020). Increasing evidence supports a link between Nrf2 and TLR family in disease pathogenesis (Chen et al., 2016; Ingram et al., 2019; Yan et al., 2018). Since we observed oxidative modification and upregulation of TLR4 signaling following TCE treatment in this study, it was logical to evaluate Nrf2 and its target genes in response to TCE exposure. As shown in Fig. 6, TCE exposure led to dramatically reduced expression of both Nrf2 and HO-1 in the spleens of MRL+/+ mice, suggesting an impairment of Nrf2 pathways following TCE exposure. More importantly, SFN supplementation clearly ameliorated the TCE-induced reduction in both Nrf2 and HO-1 (Fig. 6).
Fig. 6.
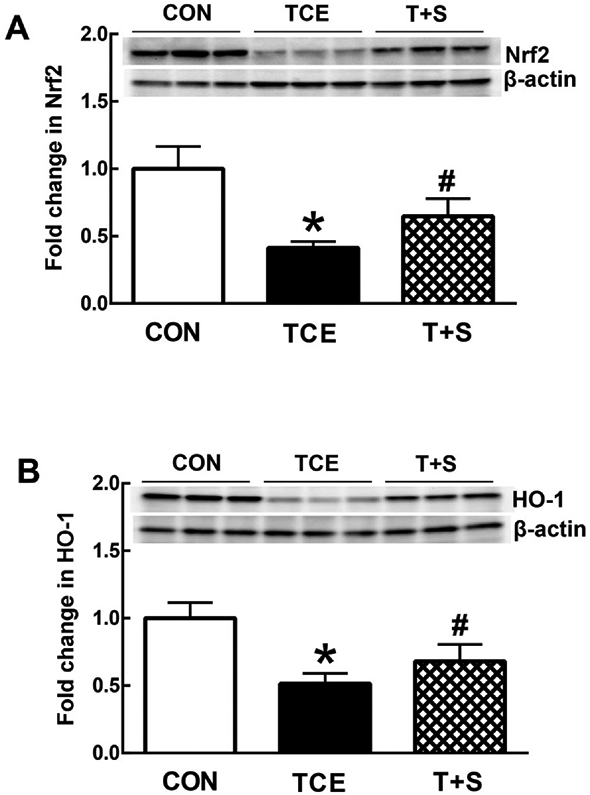
TCE exposure in MRL+/+ mice caused impairment in Nrf2 pathway, which was improved by SFN. Representative Western blot for Nrf2 (A) and HO-1 (B) in the spleens of MRL+/+ mice following treatment with TCE or TCE + SFN for 6 weeks. The data are presented as means ± SD (n = 6). * p < 0.05 vs. controls, # p < 0.05 vs. TCE-treated mice.
3.6. SFN supplementation ameliorates TCE-induced NF-κB activation
NF-κB, an extensively studied transcription factor, has been implicated in ADs with its role in the transcription of a number of genes (Herrington et al., 2016; Miraghazadeh and Cook, 2018; Sivandzade et al., 2019; Sun et al., 2013). It regulates cell response to oxidative stress and inflammation via interplay with Nrf2 (Ganesh Yerra et al., 2013; Sivandzade et al., 2019; Smallwood et al., 2018). It is also the main target transcription factor of TLR4 signaling (Chen et al., 2016; Fitzgerald and Kagan, 2020; Liu et al., 2014). Therefore, we analyzed its activation in the spleen following TCE exposure. Consistent with our previous findings in the livers (Banerjee et al., 2020; Wang et al., 2019b), TCE exposure also dramatically increased NF-κB (p65) activation in the spleens of MRL+/+ mice, and SFN supplementation remarkably attenuated the NF-κB activation mediated by TCE exposure. (See Fig. 7.)
Fig. 7.
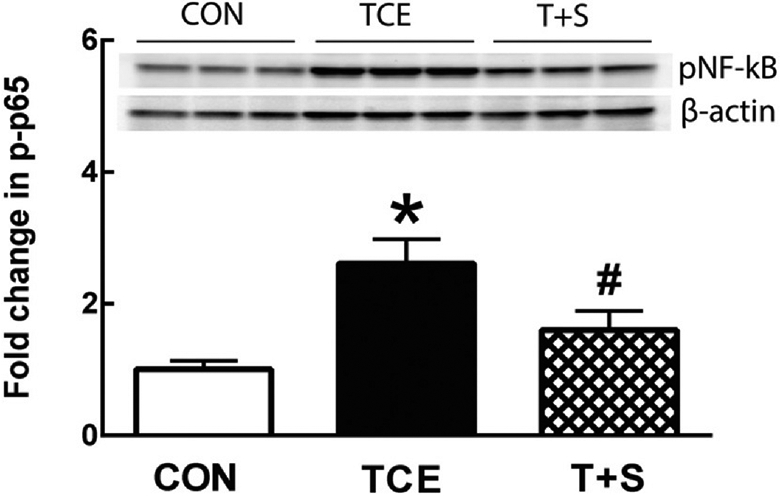
TCE treatment led to increased NF-κB (p65) expression in MRL+/+ mice, which was alleviated SFN supplementation. Representative Western blot for p-NF-κB (p65) in the spleens of MRL+/+ mice following treatment with TCE or TCE + SFN for 6 weeks. The data are presented as means ± SD (n = 6). * p < 0.05 vs. controls, # p < 0.05 vs. TCE-treated mice.
4. Discussion
Oxidative stress has drawn attention in recent years for its critical roles in the pathogenesis of chronic diseases, especially ADs (Bona et al., 2020; Khan and Wang, 2018; Smallwood et al., 2018; Wang et al., 2010). Previous studies in our laboratory have established a link among TCE exposure, oxidative stress and autoimmunity (Khan et al., 2001; Wang et al., 2007, 2012, 2015, 2019a, 2019b). However, the exact molecular mechanisms through which oxidative stress may contribute to the initiation and progression of inflammation/autoimmunity need to be thoroughly established. Although TLR signaling and Nrf2 pathways have been implicated in ADs, their roles and interplay of oxidative stress, TLR4 and Nrf2 in TCE-mediated autoimmune response remain unexplored. In this study, therefore, we exposed female MRL+/+ mice to TCE along with SFN, an antioxidant and well-known Nrf-2 activator, and determined the markers of oxidative stress, inflammation/autoimmunity, and TLR4, Nrf2/NF-κB pathways. Our findings clearly suggest a role of oxidative stress in initiation and perpetuation of autoimmunity via oxidative modification and dysregulation of oxidative stress-responsive signaling pathways.
To unravel the molecular mechanisms contributing to the disease pathogenesis, we first verified the association between TCE exposure and autoimmunity in this study. Consistent with our earlier reports (Cai et al., 2008; Khan et al., 1995, 2001; Wang et al., 2009, 2015, 2019b), TCE exposure promoted autoimmunity in MRL+/+ mice based on increased serum ANA levels. Importantly, antioxidant SFN supplementation attenuated TCE-mediated autoimmune response, providing further support to the role of oxidative stress in the disease pathogenesis.
Generation of reactive oxygen/nitrogen species (RONS) and imbalances in the antioxidant defense system are common toxic mechanism for many compounds (Khan and Wang, 2018, 2020; Peden, 2011; Zheng et al., 2020). Oxidative stress can trigger direct modification of certain proteins, or alternatively, modification of macromolecules by LPDAs such as HNE or MDA generated as a consequence of increased lipid peroxidation (Carubbi et al., 2019; Khan et al., 2001; Wang et al., 2007, 2008). In this study, TCE exposure enhanced protein carbonylation and led to increased HNE-protein adduct formation. More importantly, TCE-induced formation of HNE-specific CICs was associated with increased serum levels of ANA, which not only shows the immunogenic characteristics of oxidatively modified proteins, but also suggests a potential contribution of oxidative stress in the pathogenesis of TCE-mediated autoimmunity.
Since TCE exposure resulted in increased HNE-adducts and HNE-specific CICs, it was necessary to further evaluate the potential of HNE-modified proteins in inducing an inflammatory/autoimmune response. Our ex vivo studies showed remarkable increases in cyclin D3, CDK6 and phospho-pRb, suggesting that HNE-modified proteins can promote proliferation/activation of splenocytes, including T and B cells, in TCE-treated mice. Moreover, incubation of spleocytes from TCE-treated mice with HNE-protein adducts lead to greater generation of proinflammatory cytokines including IL-6, TNF-α and INF-γ. It is known that IL-6 and TNF-α promote the activation and differentiation of immune cells implicated in autoimmune response and also play a critical role in B cell hyperactivation (Bona et al., 2020; Lim et al., 2014; Yang et al., 2018). Also, INF-γ which is mainly secreted by Th1 cells, is a key cytokine involved in autoimmune pathogenesis via multiple signaling pathways including TLR4/NF-κB pathways (Barrat et al., 2019; Liu et al., 2014; Yang et al., 2018). Our findings thus clearly suggest a role for oxidatively modified proteins in inflammation/autoimmunity, and also support their contribution to the pathogenesis of inflammation/autoimmunity via Th1 cell activation.
Beside initiating and promoting autoimmune response by serving as neoantigens, oxidatively modified proteins can also activate DAMPs and/or PAMPs of immune system, thereby triggering inflammation/autoimmunity responses being recognized by PRRs such as TLR (Carubbi et al., 2019; Kadl et al., 2011; Sutti et al., 2014). TLR 4, an extensively studied TLR family member, plays a key role in the recognition of DAMPs and/or PAMPs, initiation of immune response and progression of autoimmune disorders (Asadzadeh Manjili et al., 2020; Gill et al., 2010; Lim et al., 2014; Liu et al., 2014). One of the most distinctive features of TLR4 is that it is expressed in non-specialist types of immunocytes, yet it is the central component of the immune system, responding to a plethora of ligands (exogenous and endogenous) (Garcia et al., 2020; Gill et al., 2010). As a link for oxidative stress and inflammation/autoimmunity, TLR4 responds to DAMPs or PAMPs such as oxidatively modified proteins using MyD88 adaptor pathway, and the adaptor protein MyD88 could interact with IRAK4 leading to activation of transcription factors including NF-κB (Asadzadeh Manjili et al., 2020; Chen et al., 2016; Gill et al., 2010; Liu et al., 2014). Here, for the first time, we provide evidence that TCE exposure causes remarkable increases in the expression of TLR4 as well as MyD88 and IRAK4 along with increased autoimmune response, suggesting potential role of TLR4 pathways in TCE-promoted autoimmunity. Furthermore, SFN, an antioxidant and Nrf2 activator, clearly ameliorated these changes, further suggesting the interactions and roles of oxidative stress, TLR4 and Nrf2 signaling pathways in promoting autoimmunity.
Antioxidant defenses are crucial in countering excessive RONS production and influence the disease outcome including ADs (Kavian et al., 2018; Miller et al., 2020; van Horssen et al., 2010; Wang et al., 2010, 2016). Nrf2 is a key redox-sensitive transcription factor which regulates expression of hundreds of antioxidant proteins including NQO1, HO-1 and glutathione synthesis, and plays a central role in cellular defense against oxidative stress (Baird and Yamamoto, 2020; Kavian et al., 2018; van Horssen et al., 2010; Wang et al., 2019b). Indeed, both decreased levels of antioxidant enzyme and/or non-enzyme antioxidants and dysregulated Nrf2 pathways are reported in AD patients and animal studies (Kavian et al., 2018; Khan and Wang, 2018; Miller et al., 2020; Wang et al., 2010, 2016). In view of increased oxidative stress and upregulated TLR4 signaling pathway in this study, it was logical to assess the redox state following TCE and TCE + SFN exposure. As evident from the results, TCE exposure led to decreases in Nrf2 as well as HO-1 in the spleens. Importantly, SFN remarkably attenuated the decreases in Nrf2 and HO-1 and was associated with improved autoimmune response following TCE exposure, providing support to a compromised defense mechanism of Nrf2 pathway to oxidative stress following TCE exposure.
NK-κB is a well-known proinflammatory mediator, immune tolerance mediator and a key lymphocyte regulator (Herrington et al., 2016; Miraghazadeh and Cook, 2018; Sun et al., 2013). While TRL4 can activate NK-κB signaling via activating IRLA4, recent studies have indicated that oxidative stress activates NK-κB through multiple pathways and activated NK-κB can suppress Nrf2 by competing for the transcriptional co-activator CREB-binding protein-p300 complex (Ganesh Yerra et al., 2013; Gill et al., 2010; Sivandzade et al., 2019; Sun et al., 2013). Thus, NK-κB may play a role in the ADs serving as a key link for these signaling pathways. In this study, TCE exposure led to increased activation of NK-κB (p65) and was associated with the elevation of autoimmune response accompanied by enhanced oxidative stress, upregulated TLR signaling, impaired Nrf2 signaling pathways, as well as activated splenocytes and increased release of proinflammatory cytokines. These findings support the contribution of NK-κB in concert with oxidative stress, TLR and Nrf2 signaling pathways in the pathogenesis of TCE-mediated autoimmune response.
ADs are complex medical disorders, and mechanisms of ADs are complicated that involves multiple signaling pathways (Roberts and Erdei, 2020; Scherlinger et al., 2020; Theofilopoulos et al., 2017; Wang et al., 2010, 2016). In this study, we investigated the mechanisms of TCE-mediated autoimmunity by evaluating the interplay and roles of oxidative stress and TLR4, Nrf2-/NF-κB-signaling pathways. Our findings demonstrate that TCE exposure leads to oxidative stress and oxidative modification of proteins which influence the TLR4 signaling and Nrf2/NF-κB pathways, causing autoreactive cell proliferation, cytokine generation and loss of immune tolerance, ultimately resulting in an autoimmune response (Fig. 8). Attenuation of TCE-induced autoimmunity by SFN provides an avenue for preventive and/or therapeutic strategy for ADs. Further studies are needed to verify the contribution of specific signaling pathways and identify novel therapeutic targets of ADs by knocking out specific genes such as Nrf2, TLR4 and TRAK4, and/or inhibiting/activating specific proteins.
Fig. 8.
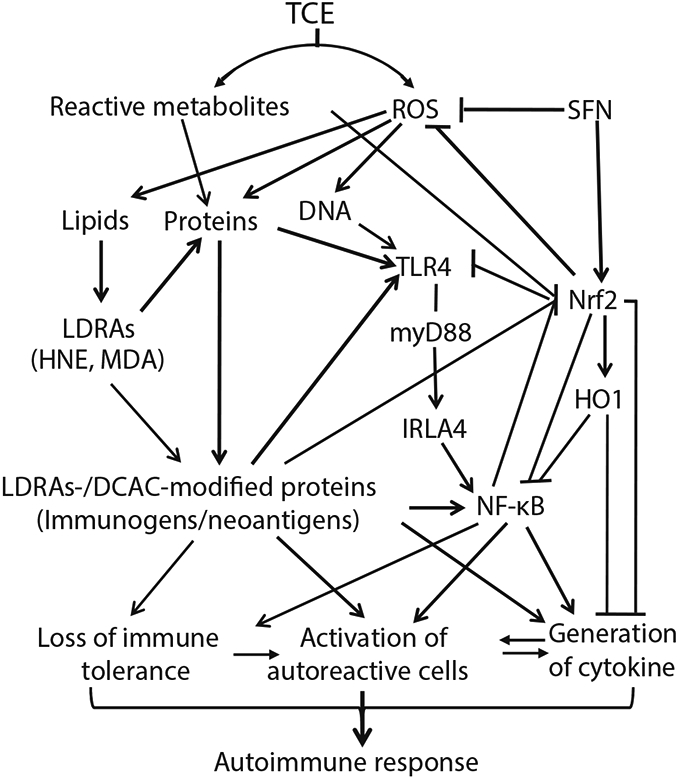
Schematic presentation of our hypothesis. TCE exposure causes oxidative stress and oxidative modification of proteins which interact with TLR signaling and Nrf2-NF-κB pathways, and lead to elevated lymphocyte proliferation, cytokine generation and loss of immune tolerance, eventually resulting to autoimmunity in MRL+/+ mice. Improvement in oxidative stress, dysregulation of TLR signaling and impairment of Nrf2-NF-κB pathway following SFN supplementation attenuates increased activation of lymphocytes and release of cytokines, ultimately leading to alleviation of inflammation/autoimmunity mediated by TCE exposure.
Acknowledgements
This work is supported by Grants ES016302 and ES026887 from the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
Footnotes
Declaration of Competing Interest
The authors declare that no competing interests exist.
References
- Asadzadeh Manjili F, Yousefi-Ahmadipour A, Kazemi Arababadi M, 2020. The roles played by TLR4 in the pathogenesis of multiple sclerosis; a systematic review article. Immunol. Lett 220, 63–70. [DOI] [PubMed] [Google Scholar]
- Baird L, Yamamoto M, 2020. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol 40, e0009–e0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee N, Wang H, Wang G, Khan MF, 2020. Enhancing the Nrf2 antioxidant signaling provides protection against trichloroethene-mediated inflammation and autoimmune response. Toxicol. Sci 175, 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrat FJ, Crow MK, Ivashkiv LB, 2019. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol 20, 1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bona N, Pezzarini E, Balbi B, Daniele SM, Rossi MF, Monje AL, Basiglio CL, Pelusa HF, Arriaga SMM, 2020. Oxidative stress, inflammation and disease activity biomarkers in lupus nephropathy. Lupus 29, 311–323. [DOI] [PubMed] [Google Scholar]
- Cai P, Konig R, Boor PJ, Kondraganti S, Kaphalia BS, Khan MF, Ansari GA, 2008. Chronic exposure to trichloroethene causes early onset of SLE-like disease in female MRL+/+ mice. Toxicol. Appl. Pharmacol 228, 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carubbi F, Alunno A, Gerli R, Giacomelli R, 2019. Post-translational modifications of proteins: novel insights in the autoimmune response in rheumatoid arthritis. Cells 8, 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yan L, Guo Z, Chen Z, Chen Y, Li M, Huang C, Zhang X, Chen L, 2016. Adipose-derived mesenchymal stem cells promote the survival of fat grafts via crosstalk between the Nrf2 and TLR4 pathways. Cell Death Dis. 7, e2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou EAA, Banos A, Kosmara D, Bertsias GK, Boumpas DT, 2019. Sexual dimorphism in SLE: above and beyond sex hormones. Lupus 28, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Fonseca LJS, Nunes-Souza V, Goulart MOF, Rabelo LA, 2019. Oxidative stress in rheumatoid arthritis: what the future might hold regarding novel biomarkers and add-on therapies. Oxidative Med. Cell. Longev 2019, 7536805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren J, Takhar H, Anderson-Mahoney P, Kotlerman J, Tarr J, Warshaw R, 2007. Cluster of systemic lupus erythematosus (SLE) associated with an oil field waste site: a cross sectional study. Environ. Health 6, 8–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinse GE, Parks CG, Weinberg CR, Co CA, Wilkerson J, Zeldin DC, Chan EKL, Miller FW, 2020. Increasing prevalence of antinuclear antibodies in the United States. Arthritis Rheum. 72, 1026–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann C, Sheikh M, Sharma S, Kondo T, Loeffler-Wirth H, Zheng YB, Novelli S, Hall A, Kerbert AJC, Macnaughtan J, Mookerjee R, Habtesion A, Davies N, Ali T, Gupta S, Andreola F, Jalan R, 2020. Toll-like receptor 4 is a therapeutic target for provention and treatment of liver failure. J. Hepatol 73, 102–112. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Kagan JC, 2020. Toll-like receptors and the control of immunity. Cell 180, 1044–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh Yerra V, Negi G, Sharma SS, Kumar A, 2013. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol. 1, 394–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MM, Goicoechea C, Molina-Álvarez M, Pascual D, 2020. Toll-like receptor 4: a promising crossroads in the diagnosis and treatment of several pathologies. Eur. J. Pharmacol 874, 172975. [DOI] [PubMed] [Google Scholar]
- Gill R, Tsung A, Billiar T, 2010. Linking oxidative stress to inflammation: toll-like receptors. Free Radic. Biol. Med 48, 1121–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JM, Blossom SJ, Jackson SK, Gilbert KM, Pumford NR, 2000. Trichloroethylene accelerates an autoimmune response by Th1 T cell activation in MRL+/+ mice. Immunopharmacology 46, 123–137. [DOI] [PubMed] [Google Scholar]
- Guerrero-Beltrán CE, Calderón-Oliver M, Pedraza-Chaverri J, Chirino YI, 2012. Protective effect of sulforaphane against oxidative stress: recent advances. Exp. Toxicol. Pathol 64, 503. [DOI] [PubMed] [Google Scholar]
- Herrington FD, Carmody RJ, Goodyear CS, 2016. Modulation of NF-κB signaling as a therapeutic target in autoimmunity. J. Biomol. Screen 21, 223–242. [DOI] [PubMed] [Google Scholar]
- Ingram S, Mengozzi M, Sacre S, Mullen L, Ghezzi P, 2019. Differential induction of nuclear factor-like 2 signature genes with toll-like receptor stimulation. Free Radic. Biol. Med 135, 245–250. [DOI] [PubMed] [Google Scholar]
- Kadl A, Sharma PR, Chen W, Agrawal R, Meher AK, Rudraiah S, Grubbs N, Sharma R, Leitinger N, 2011. Oxidized phospholipid-induced inflammation is mediated by toll-like receptor 2. Free Radic. Biol. Med 51, 1903–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavian N, Mehlal S, Jeljeli M, Saidu NEB, Nicco C, Cerles O, Chouzenoux S, Cauvet A, Camus C, Ait-Djoudi M, Chéreau C, Kerdine-Römer S, Allanore Y, Batteux F, 2018. The Nrf2-antioxidant response element signaling pathway controls fibrosis and autoimmunity in scleroderma. Front. Immunol 9, 1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MF, Wang G, 2018. Environmental agents, oxidative stress and autoimmunity. Curr. Opin. Toxicol 7, 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MF, Wang H, 2020. Environmental exposures and autoimmune diseases: contribution of gut microbiome. Front. Immunol 10, 3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MF, Kaphalia BS, Prabhakar BS, Kanz MF, Ansari GA, 1995. Trichloroethene-induced autoimmune response in female MRL+/+ mice. Toxicol. Appl. Pharmacol 134, 155–160. [DOI] [PubMed] [Google Scholar]
- Khan MF, Wu X, Ansari GA, 2001. Anti-malondialdehyde antibodies in MRL+/+ mice treated with trichloroethene and dichloroacetyl chloride: possible role of lipid peroxidation in autoimmunity. Toxicol. Appl. Pharmacol 170, 88–92. [DOI] [PubMed] [Google Scholar]
- Khan MF, Wu X, Tipnis UR, Ansari GA, Boor PJ, 2002. Protein adducts of malondialdehyde and 4-hydroxynonenal in livers of iron loaded rats: quantitation and localization. Toxicology 173, 193–201. [PubMed] [Google Scholar]
- Kilburn KH, Warshaw RH, 1992. Prevalence of symptoms of systemic lupus erythematosus (SLE) and of fluorescent antinuclear antibodies associated with chronic exposure to trichloroethylene and other chemicals in well water. Environ. Res 57, 1–9. [DOI] [PubMed] [Google Scholar]
- Lerner A, Jeremias P, Matthias T, 2015. The world incidence and prevalence of autoimmune diseases is increasing. Int. J. Celiac. Dis 3, 151–155. [Google Scholar]
- Li Y, Deng SL, Lian ZX, Yu K, 2019. Roles of toll-like receptors in Nitroxidative stress in mammals. Cells 8, 576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H, Kim YU, Sun H, Lee JH, Reynolds JM, Hanabuchi S, Wu H, Teng BB, Chung Y, 2014. Proatherogenic conditions promote autoimmune T helper 17 cell responses in vivo. Immunity 40, 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yin H, Zhao M, Lu Q, 2014. TLR2 and TLR4 in autoimmune diseases: a comprehensive review. Clin. Rev. Allergy Immunol 47, 136–147. [DOI] [PubMed] [Google Scholar]
- Miller WP, Sunilkumar S, Giordano JF, Toro AL, Barber AJ, Dennis MD, 2020. The stress response protein REDD1 promotes diabetes-induced oxidative stress in the retina by Keap1-independent Nrf2 degradation. J. Biol. Chem 295, 7350–7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraghazadeh B, Cook MC, 2018. Nuclear factor-kappaB in autoimmunity: man and mouse. Front. Immunol 9, 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden DB, 2011. The role of oxidative stress and innate immunity in O(3) and endotoxin-induced human allergic airway disease. Immunol. Rev 242, 91–105. [DOI] [PubMed] [Google Scholar]
- Perl A, 2013. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat. Rev. Rheumatol 9, 674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MH, Erdei E, 2020. Comparative United States autoimmune disease rates for 2010–2016 by sex, geographic region, and race. Autoimmun. Rev 19, 102423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherlinger M, Mertz P, Sagez F, Meyer A, Felten R, Chatelus E, Javier RM, Sordet C, Martin T, Korganow AS, Guffroy A, Poindron V, Richez C, Truchetet ME, Blanco P, Schaeverbeke T, Sibilia J, Devillers H, Arnaud L, 2020. Worldwide trends in all-cause mortality of auto-immune systemic diseases between 2001 and 2014. Autoimmun. Rev 19, 102531. [DOI] [PubMed] [Google Scholar]
- Sivandzade F, Prasad S, Bhalerao A, Cucullo L, 2019. NRF2 and NF-κB interplay in cerebrovascular and neurodegenerative disorders: molecular mechanisms and possible therapeutic approaches. Redox Biol. 21, 101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood MJ, Nissim A, Knight AR, Whiteman M, Haigh R, Winyard PG, 2018. Oxidative stress in autoimmune rheumatic diseases. Free Radic. Biol. Med 125, 3–14. [DOI] [PubMed] [Google Scholar]
- Sun SC, Chang JH, Jin J, 2013. Regulation of nuclear factor-κB in autoimmunity. Trends Immunol. 34, 282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutti S, Jindal A, Locatelli I, Vacchiano M, Gigliotti L, Bozzola C, Albano E, 2014. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology 59, 886–897. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos AN, Kono DH, Baccala R, 2017. The multiple pathways to autoimmunity. Nat. Immunol 18, 716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Horssen J, Drexhage JA, Flor T, Gerritsen W, van der Valk P, de Vries HE, 2010. Nrf2 and DJ1 are consistently upregulated in inflammatory multiple sclerosis lesions. Free Radic. Biol. Med 49, 1283–1289. [DOI] [PubMed] [Google Scholar]
- Wang G, Cai P, Ansari GA, Khan MF, 2007. Oxidative and nitrosative stress in trichloroethene-mediated autoimmune response. Toxicology 229, 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, König R, Ansari GAS, Khan MF, 2008. Lipid peroxidation-derived aldehyde-protein adducts contribute to trichloroethene-mediated autoimmunity via activation of CD4+ T cells. Free Radic. Biol. Med 44, 1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Wang J, Ma H, Khan MF, 2009. Increased nitration and carbonylation of proteins in MRL+/+ mice exposed to trichloroethene: potential role of protein oxidation in autoimmunity. Toxicol. Appl. Pharmacol 237, 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Pierangeli SS, Papalardo E, Ansari GA, Khan MF, 2010. Markers of oxidative and nitrosative stress in systemic lupus erythematosus: correlation with disease activity. Arthritis Rheum. 62, 2064–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Wang J, Fan X, Ansari GA, Khan MF, 2012. Protein adducts of malondialdehyde and 4-hydroxynonenal contribute to trichloroethene-mediated autoimmunity via activating Th17 cells: dose- and time-response studies in female MRL+/+ mice. Toxicology 292, 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Wang J, Ma H, Ansari GA, Khan MF, 2013. N-acetylcysteine protects against trichloroethene-mediated autoimmunity by attenuating oxidative stress. Toxicol. Appl. Pharmacol 273, 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Wakamiya M, Wang J, Ansari GA, Firoze Khan M, 2015. iNOS null MRL+/+ mice show attenuation of trichloroethene-mediated autoimmunity: contribution of reactive nitrogen species and lipid-derived reactive aldehydes. Free Radic. Biol. Med 89, 770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Pierangeli SS, Willis R, Gonzalez EB, Petri M, Khan MF, 2016. Significance of lipid-derived reactive aldehyde-specific immune complexes in systemic lupus erythematosus. PLoS One 11, e0164739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Ma H, Wang J, Khan MF, 2019a. Contribution of poly(ADP-ribose)polymerase-1 activation and apoptosis in trichloroethene-mediated autoimmunity. Toxicol. Appl. Pharmacol 362, 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Wakamiya M, Wang J, Ansari GAS, Khan MF, 2019b. Cytochrome P450 2E1-deficient MRL+/+ mice are less susceptible to trichloroethene-mediated autoimmunity: involvement of oxidative stress-responsive signaling pathways. Free Radic. Biol. Med 143, 324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang G, Ma H, Khan MF, 2011. Enhanced expression of cyclins and cyclindependent kinases in aniline-induced cell proliferation in rat spleen. Toxicol. Appl. Pharmacol 250, 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MA, Clark IC, Tjon EC, Li Z, Zandee SEJ, Couturier CP, Watson BR, Scalisi G, Alkwai S, Rothhammer V, Rotem A, Heyman JA, Thaploo S, Sanmarco LM, Ragoussis J, Weitz DA, Petrecca K, Moffitt JR, Becher B, Antel JP, Prat A, Quintana FJ, 2020. MAFG-driven astrocytes promote CNS inflammation. Nature 578, 593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Li J, Zhang L, Sun Y, Jiang J, Huang Y, Xu H, Jiang H, Hu R, 2018. Nrf2 protects against acute lung injury and inflammation by modulating TLR4 and Akt signaling. Free Radic. Biol. Med 121, 78–85. [DOI] [PubMed] [Google Scholar]
- Yang ML, Doyle HA, Clarke SG, Herold KC, Mamula MJ, 2018. Oxidative modifications in tissue pathology and autoimmune disease. Antioxid. Redox Signal 29, 1415–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Gonçalves FM, Abiko Y, Li H, Kumagai Y, Aschner M, 2020. Redox toxicology of environmental chemicals causing oxidative stress. Redox Biol. 34, 101475. [DOI] [PMC free article] [PubMed] [Google Scholar]


