Abstract
Previous research has confirmed strong associations between periodontitis and diabetes mellitus (DM), supporting DM as a risk factor for periodontal disease and suggesting a bidirectional relationship. Causal relationships have not been confirmed.
Aim:
The aim of this paper is to review the most current evidence of the nature of this relationship and examine whether non-surgical periodontal therapy (NSPT) significantly lowers glycemic (HbA1c) control.
Methods:
The PICO question was, “For individuals with type 2 diabetes mellitus (T2DM) and periodontitis, will non-surgical periodontal therapy (NSPT), as compared to no treatment, improve the individual’s glycemic control as measured by HbA1c.” Only systematic reviews (SRs) with or without a meta-analysis (MA) of randomized controlled trials (RCTs) or umbrella reviews of SRs and MAs of RCTs published in the English language between 2007 and 2019 were included. Several databases were searched as per their protocols. Quality assessments were conducted by both authors using the PRISMA checklist. The Bradford Hill criteria were used to determine evidence for causality.
Results:
Of 54 records retrieved, after elimination of duplicates and studies not meeting inclusion criteria, 5 SRs/MAs and 3 umbrella reviews of SRs/MAs were selected. All 5 SRs/MAs reported reductions in HbA1c levels 3 months following NSPT, but effect sizes were small and 2 were not statistically significant. The 3 umbrella reviews consistently reported small reductions in HbA1c, but high levels of heterogeneity and moderate to high risk of bias. The Bradford Hill criteria failed to support a causal relationship between periodontitis and T2DM.
Conclusions:
Whether NSPT compared with no treatment in persons with T2DM improves the individual HbA1c remains unclear as does the exact nature of the relationship between periodontitis and T2DM.
Keywords: diabetes mellitus, meta-analysis, non-surgical periodontal treatment or therapy, periodontal disease, periodontitis, systematic reviews, type 2 diabetes (T2DM)
Abstract
La recherche précédente a confirmé de fortes associations entre la maladie parodontale et le diabète sucré (DS), appuyant le DS comme facteur de risque de la maladie parodontale, et la suggestion d’un lien bidirectionnel. Des associations causales n’ont pas été confirmées.
Objectif :
Le présent document vise à examiner les données probantes les plus actuelles pour examiner la nature de ce lien et déterminer si la thérapie parodontale non chirurgicale (TPNC) diminue considérablement la régulation glycémique (HbA1c).
Méthodologie :
La question PICO était : « Les personnes souffrant de diabète sucré de type 2 (DST2) et de parodontite verront-elles une amélioration de leur régulation glycémique, telle que mesurée par la HbA1c, s’ils ont une thérapie parodontale non chirurgicale (TPNC) par rapport à ne pas obtenir de traitement? » Seules les revues systématiques (RS) avec méta-analyses (MA) d’essais contrôlés randomisés (ECR) ou des examens-cadres de RS/MA d’ECR publiés en anglais entre 2007 et 2019 ont été inclus. Les recherches ont été effectuées dans plusieurs banques de données selon leurs protocoles respectifs. Des évaluations de la qualité ont été effectuées par les 2 auteures au moyen de la liste de vérification PRISMA. Les critères de Bradford Hill ont été utilisés pour déterminer les preuves de causalité.
Résultats :
Parmi les 54 dossiers repérés après l’élimination des doubles et des études qui ne répondaient pas aux critères d’inclusion, 5 RS/MA et 3 examens-cadres de RS/MA ont été sélectionnés. Les 5 RS/MA ont tous indiqué des réductions dans les niveaux de la HbA1c, 3 mois après la TPNC, mais l’ampleur de l’effet était faible et 2 d’entre elles n’étaient pas statistiquement significatives. Les 3 examens-cadres ont indiqué de façon uniforme de petites réductions dans la HbA1c, malgré des taux élevés d’hétérogénéité et un risque de biais de modéré à élevé . Les critères de Bradford Hill n’ont pas réussi à appuyer une relation causale entre la parodontite et le DST2.
Conclusions :
Il n’est toujours pas clair si la TPNC chez les personnes souffrant de DST2 améliore leur HbA1c, par rapport à ne recevoir aucun traitement, comme demeure inconnue la nature exacte du lien entre la parodontite et le DST2.
CANADIAN DENTAL HYGIENISTS ASSOCIATION POSITION STATEMENT.
The Canadian Dental Hygienists Association acknowledges that there are very well-established associations, including a bidirectional relationship, between periodontal disease and diabetes mellitus. However, there is insufficient evidence at this time of a causal relationship. There is weak evidence that non-surgical periodontal treatment improves glycemic control.
INTRODUCTION
Diabetes mellitus (DM) is a chronic disease affecting individuals of all ages. It is a serious condition characterized by hyperglycemia when poorly controlled and can lead to a series of complications including cardiovascular disease, renal failure, neuropathies, vision loss, and amputation of limbs.1, 2 Type 2 diabetes mellitus (T2DM) is the most prevalent form, comprising 90% of cases, and is considered a metabolic disorder in which either the ability of the pancreas to produce enough insulin is impaired, or the body is unable to use the insulin produced. Type 1 diabetes, on the other hand, is classified as an autoimmune disease rather than a metabolic disorder, in which insulin-producing cells have been destroyed and the body is no longer able to produce insulin.1 According to the most recent surveillance data published by the Public Health Agency of Canada (PHAC), approximately 3 million Canadians, 8.1% of the population, were living with diabetes in 2014.1 This represents a 37.3% increase in prevalence over a 10 year period, placing a significant economic burden on the Canadian health care system. Also noteworthy is that the incidence of diabetes has been shown to increase with age.1
There has been a longstanding, well-established relationship between periodontal disease and diabetes. Diabetes is currently recognized as one of only 2 true risk factors for periodontal disease (along with smoking) and has been incorporated as part of the “grading” component of the new classification of periodontal diseases by the American Academy of Periodontology (AAP) and the European Federation of Periodontology (EFP).3,4 In addition to the evidence supporting diabetes as a risk factor for periodontal disease, there have been numerous reports of a bidirectional relationship between the 2 diseases, suggesting that periodontitis may be a complication of diabetes and have an adverse effect on glycemic control by raising blood glucose levels.5-8 The relationship between periodontal disease and diabetes is complex as diabetes is a metabolic disorder that is interrelated with a cluster of conditions collectively referred to as metabolic syndrome. This syndrome includes excess body fat, particularly around the waist and abdomen, elevated levels of plasma glucose and triglycerides, as well as hypertension and reduced levels of high-density lipoproteins.6 There is growing evidence that periodontitis may also be associated with obesity, thus potentially placing periodontal disease within the metabolic syndrome cluster.6 It is difficult to establish if these inter-related associations are related to causality. Findings from an expert panel at the 2013 joint EFP/AAP workshop on “Periodontitis and Systemic Diseases” concluded that “reported associations do not imply causality and the establishment of causality would require new studies that fulfil the Bradford Hill or equivalent criteria.”9
The Canadian Dental Hygienists Association (CDHA) last published a position paper on the relationship between periodontal disease and diabetes in 2006,10 making this update of the evidence long overdue. This current paper is the fourth in a series on oral–systemic linkages; the first 3 explored whether the evidence of previously established relationships between periodontal disease and cardiovascular disease, adverse pregnancy outcomes, and respiratory diseases, respectively, has evolved to one of causality.11-13 To establish the nature of the current relationship between diabetes and periodontal disease, this paper will once again analyse the highest levels of evidence available in the form of systematic reviews and meta-analyses of randomized controlled trials (RCTs) by applying the Bradford Hill criteria14 for causation. A discussion of the difference between association and causality can be found in previous papers in this series.11-13
In applying the Bradford Hill criteria (Appendix A), the focus of the experimental evidence will be on whether the treatment of periodontitis has an actual impact on lowering the severity of type 2 diabetes (T2DM). The PICO question for this umbrella review specifies non-surgical periodontal therapy (NSPT) as the sole intervention, thereby excluding studies using other types of interventions such as NSPT combined with antibiotics or antimicrobials, or surgery. The reason for their exclusion is that a plethora of systematic reviews and meta-analyses have consistently reported no improvements in glycemic control with these adjunctive therapies either individually or in combination with NSPT.15-19
The aim of this position paper is to review and analyse the most current evidence available to determine the nature of the relationship between periodontal disease and diabetes and if NSPT consistently has a significant effect on glycemic control.
METHODOLOGY
The overarching PICO question explored in this series of position papers was customized in this paper for type 2 diabetes. “For individuals with type 2 diabetes mellitus (T2DM) and periodontitis (Population), will non-surgical periodontal therapy (NSPT) (Intervention), as compared to no treatment (Comparison group), improve the person’s glycemic control as measured by HbA1c (Outcome).”
Eligibility criteria
Both authors independently searched the literature limiting the search to systematic reviews (SRs) with or without meta-analyses (MAs) of intervention studies using the inclusion and exclusion criteria identified in Table 1. SRs and MAs of observational studies were excluded, as were SRs and MAs published prior to 2007, which may have been included in CDHA’s previous position paper on this subject.
Table 1.
Inclusion and exclusion criteria
|
Inclusion criteria |
Exclusion criteria |
|
Published between 2007 and 2019 |
Published before 2007 |
|
English language |
Languages other than English |
|
Systematic reviews (SRs) with or without meta-analyses (MAs) of RCTs, umbrella reviews of SRs/MAs of RCTs |
Abstracts, posters, conference proceedings, editorials or commentaries, duplicate studies, narrative reviews, RCTs, observational studies/both cohort and case–control and systematic reviews of observational studies and/or case-control studies |
|
Baseline and outcome measurement of HbA1c |
|
|
Studies involving humans |
Animal studies (in vivo, ex vivo) and in vitro studies |
Search strategy
(a) Databases searched were PubMed, MEDLINE, EbscoHost, CINAHL, Scopus, Cochrane Registry of Systematic Reviews, and Clinical Trials Registry (clinicaltrials.gov). Additionally, bibliographies of retrieved articles were hand searched for further relevant systematic reviews and meta-analyses and added when appropriate.
(b) Keywords used for each search were as follows: periodontal disease; periodontitis; non-surgical periodontal treatment or therapy; AND diabetes mellitus; type 2 diabetes (T2DM); AND systematic reviews; meta-analysis.
(c) Multiple searches (limited to publications after 2007 and in the English language) were conducted according to the conventions of each particular database. Within the same database, multiple strategies were used. For example, searches within PubMed were as follows:
(periodontal disease OR periodontitis OR non-surgical periodontal therapy OR NSPT) AND (diabetes mellitus or type 2 diabetes mellitus OR T2DM) AND (systematic reviews OR meta-analysis)
(periodontal disease OR periodontitis OR non-surgical periodontal therapy OR NSPT) AND (diabetes mellitus or type 2 diabetes mellitus OR T2DM). This search was then limited by applying the filter “Article Type,” which provides check-off boxes including one for systematic review and another for meta-analysis.
Study selection
Both authors independently screened the titles and abstracts of all articles retrieved by the search using the inclusion criteria and then discussed their choices to reach consensus regarding their suitability for full-text reading. Both authors independently reviewed the selected full-text articles and reached consensus on their inclusion or exclusion.
Quality assessment
The methodological quality of the selected SRs and MAs was assessed blindly by both authors using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist tool.20 Scores of the 2 authors were compared and discussed where inconsistencies occurred to reach consensus. Umbrella reviews were not assessed by PRISMA since not all categories applied.
Data extracted
Information was extracted from each selected SR and MA, compiled, and presented in table format: year published; number of RCTs included; country of origin; methods used for assessing risk of bias; heterogeneity; outcomes measured; and conclusions of the findings.
RESULTS
A total of 54 records were retrieved from database searches and a review of references within publications identified. After eliminating duplicates, critical appraisals of studies, and articles that did not meet the inclusion criteria, 8 studies15,19,21-26 remained eligible for review, 5 of which included MAs15,19,21-23 and 3 of which were umbrella reviews of MAs.24-26 A flow diagram (Figure 1) illustrates the selection process, and Appendix B reports the reasons for eliminating full-text articles that did not meet the inclusion criteria. Characteristics and results of the quality appraisal of the 5 included SRs/MAs and 3 umbrella reviews are shown in Table 2. Based on the PRISMA checklist’s 27 items, scores ranged from 23 to 26 for the SRs/MAs thus classifying them as high quality. Agreement between the 2 independent evaluators was close to 100% with only 2 scores being off by 1 point. Four (4) of the five (5) SRs/MAs reported high levels of heterogeneity in their included studies and all reported moderate to high risks of bias. All SRs/MAs reported reductions in HbA1c levels following NSPT at 3 months, although effect sizes were small and 2 of the 5 were not statistically significant.21,22 The 3 umbrella reviews were consistent in findings of high levels of heterogeneity and moderate to high risk of bias reported by the included systematic review authors. All results were similar in that mean differences in HbA1c levels were small and, in some cases, not statistically significant at 3 months following NSPT. The 5 SRs/MAs included in our umbrella review were also included in the Botero umbrella review24 ; PRISMA scores were almost identical in the 2 reviews.
Figure 1.
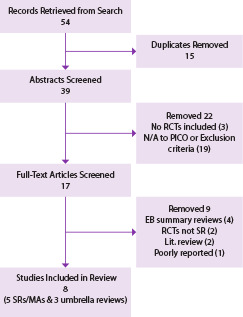
Diabetes search flow diagram
DISCUSSION
The purpose of this position paper is to review and analyse the most current evidence available to determine the nature of the relationship between periodontal disease and diabetes. Although a plethora of evidence confirms that periodontal disease and diabetes mellitus are independently associated,27 whether this relationship is due to common risk factors or to a causal link is unknown.28,29 A bidirectional relationship has been well established for over 20 years.7 Indeed, it has been shown that individuals with diabetes who have periodontitis have poorer glycemic control than those without periodontitis. Also, individuals with diabetes are more susceptible to developing periodontitis, thus it has been determined a risk factor for periodontitis.
One possible explanation for this association is that diabetes may directly influence the oral microbiome, resulting in a state of dysbiosis.2,29 However, the explanation most studied involves the inflammatory pathway.28,30 Inflammatory markers have been shown repeatedly to be elevated in the presence of these 2 comorbidities.28 Studies have suggested that periodontal therapy can have a positive effect on glycated hemoglobin levels in the blood (HbA1c) by reducing the periodontal inflammatory load.
Results from these studies are inconclusive for several reasons that became evident during the search process for this position paper. Search criteria were restricted to only those systematic reviews that evaluated RCTs in which the primary intervention was NSPT and HbA1c was the primary outcome. Most retrieved studies had a mixture of interventions and ,outcome measures that were not considered separately in their meta-analyses, making it impossible to determine the effect of NSPT on HbA1c alone, thus they were not included in this review. Some of the SRs and MAs that were selected for inclusion did, however, have antibiotics as an intervention, but the authors had conducted separate MAs reporting results for NSPT separately.
Findings from the 5 SRs/MAs were relatively consistent in that they all found improvements, albeit small, in HbA1c levels up to 3 months following NSPT. Average mean reductions in HbA1c percentages over the 5 SR/MAs was 0.37, ranging from 0.26 to 0.65. Two of the SRs/MAs reported 6-month outcomes but neither had significant changes in glycated hemoglobin levels.15,19 This finding was also described by the authors of the 3 umbrella reviews.24,25,26 From a clinical perspective, this finding provides strong evidence in support of 3-month periodontal maintenance appointments for those with diabetes, yet none of the review authors made mention of this.
Despite the small effect sizes reported in these studies, the authors discussed the potential clinical significance of these small changes, indicating that they may still be of benefit to the patient. Li et al.22 pointed out that Lakschevitz and colleagues31 reported that a 1% reduction in HbA1c levels means a potential 35% decrease in diabetic complications. Furthermore, the same authors estimated a 0.2% reduction in A1c values could decrease mortality by 10%. If this is the case, then it seems that even small reductions could have a positive impact on the health of the patient.
Li et al.22 raised a very significant issue, that of the difference in results between studies with small numbers of participants and those with large numbers of participants. They conducted 3 separate MAs comparing the results from 6 studies with small sample sizes (N < 80) with those from 4 large sample studies (N > 80) and then finally the pooled results from both small and large studies. The results were interesting as the small sample studies had –0.46% mean reduction in HbA1c, which was statistically significant, while the large sample studies had only –0.01% reduction in HbA1c, which was non-significant. Surprisingly, when both small and large studies were combined, there was an overall mean reduction of –0.27%, which was statistically significant despite the total “n” for the large studies being 3 times the total “n” for the small studies, suggesting that these results may not reflect actual outcomes. The authors suggested that smaller samples have larger variance and thus could be more likely to overestimate the effect sizes compared with larger trials. This certainly supports the necessity for larger scale clinical trials.
Interestingly, the largest of the RCTs included in the Li et al.22 SR/MA was a US National Institute of Dental and Craniofacial Research (NIDCR)-supported study with a total of 514 participants (257 in each study arm) that was expected to be a landmark study providing more definitive results than the smaller sample studies.32,33
Table 2.
Characteristics of included studies and their quality appraisal results
|
Author, year, and country |
Study type |
No. & type of studies |
Outcomes measured |
Risk of bias measure |
Heterogeneity |
Prisma score |
Conclusions |
|
|
1 |
Corbella S et al.19 2013 (Italy) |
SR/MA |
15 total RCTs Only 8 NSPT vs no tx control Study range: 2001–2012 |
HbA1c FPG |
Cochrane Handbook Risk of bias: High (3) Low (5) |
Signif. I2 = 50% |
24/27 |
Baseline to 3 mos. HbA1c mean diff. (–0.38%) p = 0.01 Mean diff. at 6 mos. (–0.31%) p = 0.15 Authors’ conclusion: NSPT might be effective in metabolic control but significance is questionable & needs further investigation. |
|
2 |
Jain A et al.21 2019 (India) |
SR/MA |
6 RCTs NSPT vs no tx control Study range: 2011–2014 |
HbA1c |
Cochrane Handbook Risk of bias: High for detection & performance Unclear for selection bias |
Signif. I2 = 84% |
24/27 |
Baseline to 3 mos. HbA1c mean diff. (–0.26) but not statistically significant Authors’ conclusion: NSPT showed a modest trend in reducing HbA1c and should be included as part of the medical regime for patients with diabetes. |
|
3 |
Li Q, et al.22 2015 (China) |
MA |
9 RCTs Study range: 2005 (1) 2008 (1) 2012–2014 |
HbA1c |
Cochrane Handbook Risk of bias: High (3) Low (6) |
Moderate I2 = 41.7b |
23/27 |
Baseline to 3 mos. HbA1c mean diff. (pooled) (–0.27%) p = 0.0007 HbA1c mean diff. (large studies only) (–0.014%) p = 0.87 Authors’ conclusion: The moderate reduction in HbA1c after NSPT is consistent with previous SRs, however more large scale and higher quality studies are required. |
|
4 |
Sgolastra FG et al.23 2013 (Italy) |
MA |
5 RCTs Study range: 2005–2008 (2) 2012 (3) |
HbA1c |
Consort Risk of bias: High (3) Low (2) |
None I2 = 0% |
25/27 |
Baseline to 3 mos. HbA1c mean diff. 0.65% (p = 00001) Authors’ conclusion: Results seem to support improvements in glycemic control, however future studies needed to confirm results. |
|
5 |
Simpson TC et al.15 2015 (UK) |
Cochrane SR/MA |
35 RCTs (14 studies NSPT vs no tx) |
HbA1c |
Cochrane Handbook & Evidence Grade Risk of bias: High (29) Low (2) Unclear (4) GRADE Low |
Moderate I2 = 53% |
26/27 |
MAs for 14 studies (HbA1c after NSPT vs no tx) Largest # of participants (1499) compared to the other 4 reviews. Baseline to 3 mos. HbA1c mean diff. –0.29% (p = 0.003) Baseline to 6 mos. HbA1c mean diff. 0.02% Note: 2 subgroups (SRP [8] and SRP + antimicrobials [7]) No significant diff. between groups (p = 0.25) Authors’ conclusion: Low-quality evidence that SRP improves glycemic control in people with diabetes. |
|
6 |
Botero JE et al.24 2016 (Colombia) |
Umbrella review |
13 SR/MAs |
HbA1c |
PRISMA & AMSTAR high quality (8) moderate quality (5) Risk of bias: High or unclear for the majority of studies |
High (Range 0% to 89%) |
N/A |
Range in reduction of HbA1c for all studies was (0.23 to 1.03) Authors’ conclusion: Highly heterogenous studies with small sample sizes suggest NSPT could help improve glycemic control at 3 mos. |
|
7 |
Faggion CM et al.25 2016 (Germany) |
Umbrella review |
11 SR/MAs |
HbA1c |
AMSTAR & OQAQ Moderate quality (score range 5 to 9) |
High |
N/A |
Mean reduction of HbA1c for all studies was 0.47 (range 0.24 to 1.03) Authors’ conclusion: Findings do not support that NSPT improves glycemic control |
|
8 |
Hasuike A et al.26 2017 (Japan) |
Umbrella review |
13 MAs within 9 SRs |
HbA1c |
AMSTAR Not high quality |
High I2 > 40% |
N/A |
Range in reduction of HbA1c for all studies was (–0.93 to 0.13) Authors’ conclusion: Significant diff. but effect size small & studies not of high quality. |
Results of this multicentre RCT by Engebretson and colleagues were controversial as the HbA1c values actually increased in the intervention group by 0.17% at 6 months with no significant differences with the control group.32 The authors concluded NSPT did not improve glycemic control in individuals with DM,32 leading researchers to believe that perhaps this is the case. This study would have had a large impact on Li et al.’s22 subgroup MA comparing the smaller and larger sampled studies. However, in a follow-up critical review of the Engebretson et al.32 study, Borgnakke along with multiple other experts in the field,33 published a critical review reporting some serious concerns with this study. They presented a very thorough critique of their findings, reporting 3 major methodological flaws: 1) non-compliance with the study’s eligibility criteria related to baseline HbA1c values, finding that over 60% of both test and control groups at baseline had HbA1c levels below 8.0% placing these participants closer to the margin of good glycemic control at the beginning of the study; 2) periodontal treatment received by the participants in the intervention group failed to reach acceptable standards, thus increasing the likelihood of inaccurate results; and 3) the level of obesity in the treatment group was high (mean BMI 34.7 [±7.5] kg/m 2 ), which would have affected the anti-inflammatory outcome of the periodontal treatment.33 The major concern expressed by the authors of this critical review was that the findings of this study have been taken out of context and considered to “prove” there is no effect of NSPT on HbA1c.33 They strongly stress “this is an unsafe and incorrect conclusion and dangerously misleading to the profession, the public, and other stakeholders, such as policy makers, health plan managers, and insurance companies.”33
Although the overall results of this umbrella review showed significant reductions in HbA1c, ranging on average from 0.26% to 0.65% at 3 months, the small effect sizes were disappointing. Poor quality studies with small sample sizes, mixed interventions with mixed populations, high risk of bias, and heterogeneity most likely affected the results of the studies analysed. Perhaps another explanation for these results could be that NSPT is designed to lower inflammation in the mouth. Although it may have a small impact on the overall glycemic control of the client with diabetes, there are several other factors that may have a much stronger influence in lowering glycated hemoglobin values. Factors such as diet, BMI, smoking, and the impact of medications were not included in these studies nor were they controlled for. Table 3 provides a summary of these shortcomings. In a recent consensus report of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology,27 it was suggested that the magnitude of the short-term reductions in HbA1c over a 3-month period following NSPT would be comparable to adding a second diabetic medication. If this is the case, then perhaps even these small effect sizes can be meaningful.
Table 3.
Summary of issues identified by authors of systematic reviews of RCTs
|
Using the Bradford Hill criteria for causation to determine whether a causal relationship exists between periodontitis and diabetes, it is clear that several criteria have not yet been satisfied. In examining the “strength of association,” low to moderate evidence was presented in all 5 SRs/MAs and 3 umbrella reviews that NSPT could have a positive effect on glycemic control by lowering HbA1c values, albeit very small, over a 3-month period. However, no evidence was found to support sustainability of the reduction at 6 months. Furthermore, smaller sample size studies tended to demonstrate some positive effects (although not all statistically significant) in lowering HbA1c while those with larger study populations did not produce significant results. Several authors, including those of 2 of the 3 umbrella reviews, indicated studies were generally of poor quality. “Consistency” has not been met as there were inconsistencies in findings particularly between small and large sample size studies. Similarly, the criterion of “specificity,” which requires that a factor influences a specific outcome, and that the more specific an association between a factor and an effect, the greater the probability that it is causal, was not demonstrated. While this was the case in the small sample size studies, it was not so with the large study population studies, but those studies may have had methodological flaws.
The criterion of “temporality,” which requires that the disease (periodontitis) precede the outcome (diabetes), has not been established, definitely weakening the cause-and-effect hypothesis. Some amount of temporality was established, however, by sustaining improvements in glycemic control over a period of 3 months, but those improvements were small and, in some cases, not statistically significant. Studies included in this review have not demonstrated a “dose–response” outcome when comparing results with various magnitudes of periodontitis, showing that those with more severe periodontal disease would be at greater risk for diabetes. However, studies have shown the reverse, where those with more uncontrolled HbA1c have a higher risk for periodontitis.34
The criterion of “biological plausibility” has been met, as numerous prior studies including animal studies have supported the concept that hyperglycemic conditions such as diabetes induce advanced glycation end products (AGEs) in the periodontal tissues. Numerous studies have provided strong evidence that glycemic control is worsened in the presence of periodontitis. In addition, diabetic complications have been shown to be more severe in patients with periodontitis than those without periodontitis.28 The criterion of “coherence” also has been previously met as numerous biological and human studies have well established that a relationship exists between periodontal disease and diabetes.
Although numerous “experiments” (RCTs) to determine if periodontal therapy can play a role in improving glycemic control have been conducted and evaluated in these 5 well-conducted SRs/MAs and 3 umbrella reviews, results have been mixed. Several smaller studies have shown reductions in HbA1c following NSPT, but their clinical significance is unclear, particularly when some larger studies have shown no effect. “Analogy,” although the weakest criterion, was not explored in this review. Thus, of the 9 criteria, only 2 (biological plausibility and coherence) can be said to have been fulfilled. Table 4 summarizes these results.
Based on this analysis, it is concluded that there is not sufficient evidence to support a causal relationship between periodontal disease and diabetes.
Table 4.
Bradford Hill criteria results
|
Bradford Hill criterion |
Met |
Partially met |
Not met |
|
Strength of association |
X |
||
|
Consistency |
X |
||
|
Specificity |
X |
||
|
Temporality |
X |
X |
|
|
Dose–response |
X |
||
|
Biological plausibility |
X |
||
|
Coherence |
X |
||
|
Experiment |
X |
X |
|
|
Analogy |
N/A |
N/A |
N/A |
CONCLUSION
Based on findings from the analysis of the 5 SRs/MAs as well as the 3 umbrella reviews investigated, the answer to the PICO question “For individuals with type 2 diabetes mellitus and periodontitis, will non-surgical periodontal therapy (NSPT), as compared to no treatment, improve the person’s glycemic control as measured by HbA1c?” is “unclear.” Current evidence is inconsistent in that larger multicentre clinical trials have not shown significant reductions in HbA1c, while several smaller RCTs have reported small reductions in HbA1c following NSPT. Additionally, results from these smaller studies have not all been statistically significant. Furthermore, both the SRs/MAs and umbrella reviews reported high levels of heterogeneity and risk of bias, thus classifying them as low-quality studies. All authors have recommended that larger scale, better designed clinical trials be conducted to address the numerous shortcomings described in this review. Ultimately, it is important to recognize that the intent of performing NSPT is not to cure diabetes or to improve HbA1c levels, but rather to improve periodontal health. Thus, some of the positive results found in the smaller studies could be spurious at best.
Although a causal relationship has not been established by this umbrella review, the association between periodontal disease and diabetes is strong. The existence of a 2-way street has clearly been demonstrated in past studies, and diabetes is certainly considered a risk factor for periodontitis. The best explanation for the comorbidity of periodontitis and diabetes appears to be through the inflammatory pathway.28,30 Given that diabetes is now a part of the new classification of periodontal diseases and has an impact on the clinician’s determination of a case definition, it will be important to closely monitor the patient’s HbA1c levels and ensure that NSPT is delivered on a consistent basis for those whose A1c levels are greater than 7. The one take-away from the results of this current investigation is that periodontal maintenance must occur at intervals no greater than 3 months since there were no significant effects on glycemic control at 6 months. With the growing prevalence of type 2 diabetes over the past 10 years, now affecting over 8% of Canadians, the implications for Canada’s health care system of not providing consistent NSPT to this population are clear. Dental hygienists can play a pivotal role in reducing significant health care costs by screening their clients, identifying those who may potentially have diabetes unknowingly, providing NSPT to reduce the inflammatory load, and ensuring these clients receive the necessary education and health promotion information to potentially reduce the serious effects of these comorbidities.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest.
APPENDIX
Acknowledgments
This position paper was funded by the Canadian Dental Hygienists Association. Both authors received an honorarium for this work. We wish to thank the CDHA steering committee for their valuable input and guidance throughout the development of this paper.
Footnotes
CDHA Research Agenda categories: risk assessment and management; capacity building of the profession
REFERENCES
- 1.Public Health Agency of Canada (PHAC).Diabetes in Canada: Highlights from the Canadian Chronic Disease Surveillance System. Cat.: HP35-94/2017E-PDF | ISBN: 978-0-660-23635-3 | Pub.: 170260
- 2. Borgnakke WS, Ylöstalo PV, Taylor GW, Genco RJ Effect of periodontal disease on diabetes: Systematic review of epidemiological observational evidence J Periodontol 2013;84(4 Suppl):S135–S152 [DOI] [PubMed] [Google Scholar]
- 3. Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, et al. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification J Periodontol 2018;89(Suppl 1):S1–S8 [DOI] [PubMed] [Google Scholar]
- 4. Tonetti MS, Greenwell H, Kornman KS Staging and grading of periodontitis: Framework and proposal of a new classification and case definition J Periodontol 2018;89(Suppl 1):S159–S172 [DOI] [PubMed] [Google Scholar]
- 5. Genco RJ, Graziani F, Hasturk H Effects of periodontal disease on glycemic control, complications, and incidence of diabetes mellitus Periodontology 2000 2020;83:59–65 [DOI] [PubMed] [Google Scholar]
- 6. Winning L, Linden GJ Periodontitis and systemic disease: Association or causality? Curr Oral Health Rep 2017;4:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taylor GW Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiological perspective Ann Periodontol 2001;6(1):99–112 [DOI] [PubMed] [Google Scholar]
- 8. Borgnakke WS IDF Diabetes Atlas: Diabetes and oral health—A two-way relationship of clinical importance Diabetes Res Clin Pract 2019;157:107839 [DOI] [PubMed] [Google Scholar]
- 9. Linden GJ, Herzberg MC, Working Grp 4 Joint EFP/AAP Periodontitis and systemic diseases: A record of discussions of working group 4 of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases J Clin Periodontol 2013;40:S20–S23 [DOI] [PubMed] [Google Scholar]
- 10. Lux J Review of the oral disease–systemic disease link. Part 1: Heart disease, diabetes Can J Dent Hyg 2006;40(6):288–302 [Google Scholar]
- 11. Lavigne SE, Forrest JL An umbrella review of systematic reviews of the evidence of a causal relationship between periodontal disease and cardiovascular diseases: Position paper from the Canadian Dental Hygienists Association Can J Dent Hyg 2020;54(1):32–41 [PMC free article] [PubMed] [Google Scholar]
- 12. Lavigne SE, Forrest JL An umbrella review of systematic reviews of the evidence of a causal relationship between periodontal disease and adverse pregnancy outcomes: Position paper from the Canadian Dental Hygienists Association Can J Dent Hyg 2020;54(2):92–100 [PMC free article] [PubMed] [Google Scholar]
- 13. Lavigne SE, Forrest JL An umbrella review of systematic reviews of the state of the evidence of a causal relationship between periodontal microbes and respiratory diseases: Position paper from the Canadian Dental Hygienists Association Can J Dent Hyg 2020;54(3):144–155 [PMC free article] [PubMed] [Google Scholar]
- 14. Hill AB The environment and disease: Association or causation? Proc Royal Soc Med 1965;58:295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simpson TC, Weldon JC, Worthington HV, Needleman I, Wild SH, Moles DR, et al. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus (Review) Cochrane Database Syst Rev 2015;11:Art No CD004714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Engebretson S, Kocher T Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta-analysis J Clin Periodontol 2013;40(Suppl 14):S153–S163 [DOI] [PubMed] [Google Scholar]
- 17. Teeuw WJ, Gerdes VEA, Loos BG Effect of periodontal treatment on glycemic control of diabetic patients Diabetes Care 2010;33(2):421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang T-F, Jen I-A, Chou C, Lei Y-P Effects of periodontal therapy on metabolic control in patients with type 2 diabetes mellitus and periodontal disease: A meta-analysis Medicine (Baltimore) 2014;93(28):e292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Corbella S, Francetti L, Taschieri S, De Siena F, Del Fabbro M Effect of periodontal treatment on glycemic control of patients with diabetes: A systematic review and meta-analysis J Diabetes Invest 2013;4(5):502–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, The PRISMA-DTA Group Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA statement JAMA 2018;319(4):388–396doi: 10 1001/jama 2017 19163 [DOI] [PubMed] [Google Scholar]
- 21. Jain A, Gupta J, Bansal D, Sood S, Gupta S, Jain A Effect of scaling and root planing as monotherapy on glycemic control in patients of type 2 diabetes with chronic periodontitis: A systematic review and meta-analysis J Indian Soc Periodontol 2019;23(4):303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Q, Hao S, Fang J, Xie J, Kong X-H, Yang J-X Effect of non-surgical periodontal treatment on glycemic control of patients with diabetes: A meta-analysis of randomized controlled trials Trials 2015;16:291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sgolastra F, Severino M, Pietropaoli D, Gatto R, Monaco A effectiveness of periodontal treatment to improve metabolic control in patients with chronic periodontitis and type 2 diabetes: A meta-analysis of randomized clinical trials J Periodontol 2013;84(7):958–973 [DOI] [PubMed] [Google Scholar]
- 24. Botero JE, Rodriguez Agudelo-Suarez C Periodontal treatment and glycaemic control in patients with diabetes and periodontitis: an umbrella review Aust Dent J 2016;61(2):134–148 [DOI] [PubMed] [Google Scholar]
- 25. Faggion CM Jr, Cullinan MP, Atieh M An overview of systematic reviews on the effectiveness of periodontal treatment to improve glycaemic control J Periodont Res 2016;51:716–725 [DOI] [PubMed] [Google Scholar]
- 26. Hasuike A, Iguchi S, Suzuki D, Kawano E, Sato S Systematic review and assessment of systematic reviews examining the effect of periodontal treatment on glycemic control in patients with diabetes Med Oral Patol Cir Bucal 2017;22(2):e167–e176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graciani F, et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology J Clin Periodontol 2018;45:138–149 [DOI] [PubMed] [Google Scholar]
- 28. Genco RJ, Sanz M Clinical and public health implications of periodontal and systemic diseases: An overview Periodontol 2000 2020;83:7–13 [DOI] [PubMed] [Google Scholar]
- 29. Polak D, Saunui T, Nishimura F, Shapira L Diabetes as a risk factor for periodontal disease—plausible mechanisms Periodontol 2000 2020;83(1):46–58 [DOI] [PubMed] [Google Scholar]
- 30. Genco RJ, Graciani F, Hasturk H Effects of periodontal disease on glycemic control, complications, and incidence of diabetes mellitus Periodontol 2000 2020;83:59–65 [DOI] [PubMed] [Google Scholar]
- 31. Lakschevitz F, Aboodi G, Tenenbaum H, Glogauer M Diabetes and periodontal diseases: Interplay and links Curr Diabetes Rev 2011;7:433–439 [DOI] [PubMed] [Google Scholar]
- 32. Engebretson SP, Hyman LG, Michalowicz BS, Schoenfeld ER, Gelato MC, Hou W, et al. The effect of non-surgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: A randomized controlled trial JAMA 2013;310(23):2523–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Borgnakke WS, Chapple ILC, Genco RJ, Armitage G, Bartold PM, et al. The multi-center randomized controlled trial (RTC) published by the Journal of the American Medical Association (JAMA) on the effect of periodontal therapy on glycated hemoglobin (HbA1c) has fundamental problems J Evid Base Dent Pract 2014;14:127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Genco RJ, Borgnakke WS Diabetes as a potential risk for periodontitis: association studies Periodontol 2000 2020;83(1):40–45 [DOI] [PubMed] [Google Scholar]
Excluded studies
- 35. Abariga SA, Whitcomb BW Periodontitis and gestational diabetes mellitus: A systematic review and meta-analysis of observational studies BMC Pregnancy Childbirth 2016;16(1):344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Borell LN, Joseph SP Periodontal treatment may control glycemic status among diabetic patients J Evid Base Dent Pract 2011;11:92–94 [DOI] [PubMed] [Google Scholar]
- 37. Boyd L, Giblin L, Chadbourne D Bidirectional relationship between diabetes mellitus and periodontal disease: state of the evidence Can J Dent Hyg 2012;46(2):93–102 [Google Scholar]
- 38. Cao R, Li Q, Wu Q, Yao M, Chen Y, Zhou H Effect of non-surgical periodontal therapy on glycemic control of type 2 diabetes mellitus: a systematic review and Bayesian network meta-analysis BMC Oral Health 2019;19:176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Darré L, Vergnes J-N, Gourdy P, Sixou M Efficacy of periodontal treatment on glycaemic control in diabetic patients: A meta-analysis of interventional studies Diabetes Metab 2008;34:497–506 [DOI] [PubMed] [Google Scholar]
- 40. Grellmann AP, Sfreddo CS, Maier J, Lenzi TL, Zanatta FB Systemic antimicrobials adjuvant to periodontal therapy in diabetic subjects: a meta-analysis J Clin Periodontol 2016;43:250–260 [DOI] [PubMed] [Google Scholar]
- 41. Hsu Y-T, Nair M, Angelov N, Lalla E, Lee C-T Impact of diabetes on clinical periodontal outcomes following non-surgical periodontal therapy J Clin Periodontol 2019;46:206–217 [DOI] [PubMed] [Google Scholar]
- 42. Janket S-J Scaling and root planing (SRP) may improve glycemic control and lipid profile in patients with chronic periodontitis (CP) and type 2 diabetes (DM2) in a specific subgroup: A meta-analysis of randomized clinical trials J Evid Base Dent Pract 2014;14:31–33 [DOI] [PubMed] [Google Scholar]
- 43. Liccardo D, Cannavo A, Spagnuolo G, Ferrara N, Cittadini A, Rengo C, Rengo G Periodontal disease: A risk factor for diabetes and cardiovascular disease Int J Mol Sci 2019;20:1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liew AKC, Punnanithinont N, Lee Y-C, Yang J Effect of non-surgical periodontal treatment on HbA1c: A meta-analysis of randomized controlled trials Aust Dent J 2013;58:350–357 [DOI] [PubMed] [Google Scholar]
- 45. Lima RPE, Belém FV, Guimarães Abreu L, Cunha FA, Cota LOM, Eustáquio da Costa J, Oliveira Costa F Effect of periodontal therapy on serum levels of IL-6 in type 2 diabetics: A systematic review Int J Periodontics Restorative Dent 2019;39(1):e1–e10 [DOI] [PubMed] [Google Scholar]
- 46. Lira Junior R, Santos CMML, Oliveira BH, Fischer RG, Santos APP Effects on HbA1c in diabetic patients of adjunctive use of systemic antibiotics in nonsurgical periodontal treatment: A systematic review J Dent 2017;66:1–7 [DOI] [PubMed] [Google Scholar]
- 47. Madianos Koromantzos PN An update of the evidence on the potential impact of periodontal therapy on diabetes outcomes J Clin Periodontol 2018;45:188–195 [DOI] [PubMed] [Google Scholar]
- 48. Mauri-Obradors E, Jané-Salas E, Sabater-Recolons M, Vinas M, López-López J Effect of nonsurgical periodontal treatment on glycosylated hemoglobin in diabetic patients: a systematic review Odontology 2015;103:301–313 [DOI] [PubMed] [Google Scholar]
- 49. Rodríguez-Medina C, Agudelo-Suárez AA, Botero JE Weak evidence hinders the understanding of the benefits of periodontal therapy on glycemic control in patients with diabetes and periodontitis J Evid Based Dent Pract 2016;16(4):236–238 [DOI] [PubMed] [Google Scholar]
- 50. Mizuno H, Ekuni D, Maruyama T, Kataoka K, Yoneda T, Fukuhara D, et al. The effects of non-surgical periodontal treatment on glycemic control, oxidative stress balance and quality of life in patients with type 2 diabetes: a randomized clinical trial PLoS ONE 2017;12(11):e0188171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Papageorgiou SN, Reichert C, Jäger A, Deschner J Effect of overweight/obesity on response to periodontal treatment: systematic review and a meta-analysis J Clin Periodontol 2015;42:247–261 [DOI] [PubMed] [Google Scholar]
- 52. Rovai ES, Souto MLS, Ganhito JA, Holzhausen M, Chambrone L, Pannuti C Efficacy of local antimicrobials in the non-surgical treatment of patients with periodontitis and diabetes: A systematic review J Periodontol 2016;87:1406–1417 [DOI] [PubMed] [Google Scholar]
- 53. Santos CMML, Lira Junior R, Fischer RG, Santos APP, Oliveira BH Systemic antibiotics in periodontal treatment of diabetic patients: A systematic review PLoS ONE 2015;10(12):e0145262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Souto MLS, Rovai ES, Ganhito JÁ, Holzhausen M, Chambrone L, Pannuti CM Efficacy of systemic antibiotics in nonsurgical periodontal therapy for diabetic subjects: a systematic review and meta-analysis Int Dent J 2018;68:207–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sun Q, Feng M, Zhang M, Zhang Y, Cao M, Bian L, et al. Effects of periodontal treatment on glycemic control in type 2 diabetic patients: A meta-analysis of randomized controlled trials Chinese J Physiol 2014;57(6):305–314 [DOI] [PubMed] [Google Scholar]
- 56. Taylor G A meta-analysis finds periodontal treatment provides a non-significant improvement in glycemic control J Evid Base Dent Pract 2007;7:62–63 [Google Scholar]
- 57. Torumtay G, Kirzioglu FY, Öztürk T, Kale B, Calapoglu M, Orhan H Effects of periodontal treatment on inflammation and oxidative stress markers in patients with metabolic syndrome J Periodont Res 2016;51:489–498 [DOI] [PubMed] [Google Scholar]
- 58. Teeuw WJ, Slot DE, Susanto H, Gerdes VEA, Abbas F, D’Aiuto F, et al. Treatment of periodontitis improves the atherosclerotic profile: a systematic review and meta-analysis J Clin Periodontol 2014;41:70–79 [DOI] [PubMed] [Google Scholar]
- 59. Teshome A, Yitayeh A The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: systematic review and meta-analysis BMC Oral Health 2017;17:31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang X, Huan X, Guo X, Luo X, Wang D The effect of periodontal treatment on hemoglobin A1c levels of diabetic patients: A systematic review and meta-analysis PLoS ONE 2014;9(9):e108412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. D’Aiuto F, Gable D, Syed Z, Allen Y, Wanyonyi K, White S, Gallagher J Evidence summary: The relationship between oral diseases and diabetes Br Dent J 2017;222(12):944–948 [DOI] [PubMed] [Google Scholar]
Cited within the manuscript but excluded from analysis
- 2. Borgnakke WS, Ylöstalo PV, Taylor GW, Genco RJ Effect of periodontal disease on diabetes: Systematic review of epidemiological observational evidence J Periodontol 2013;84(4 Suppl ):S135–S152 [DOI] [PubMed] [Google Scholar]
- 16. Engebretson S, Kocher T Evidence that periodontal treatment improves diabetes outcomes: A systematic review and meta-analysis J Clin Periodontol 2013;40(Suppl 14):S153–S163 [DOI] [PubMed] [Google Scholar]
- 17. Teeuw WJ, Gerdes VEA, Loos BG Effect of periodontal treatment on glycemic control of diabetic patients Diabetes Care 2010;33(2):421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang T-F, Jen I-A, Chou C, Lei Y-P Effects of periodontal therapy on metabolic control in patients with type 2 diabetes mellitus and periodontal disease: A meta-analysis Medicine (Baltimore) 2014;93(28):e292 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


