ABSTRACT
Immune checkpoint inhibitors (ICIs) as monotherapy in different solid tumors showed an early detrimental effect in a subset of patients reflected by the early crossover of the progression-free survival (PFS) curves. Currently, combination therapies with ICIs added to chemotherapy or targeted therapy are expanding the landscape of metastatic solid tumors. We have examined the benefits and risks of adding ICIs to the standard of care (SOC) versus SOC alone.
A search of randomized clinical trials (RCTs) comparing ICIs combinations versus the corresponding SOC in different metastatic tumors according to the PRISMA guidelines was performed. Selected endpoints included PFS, time-to-response (TTR), overall survival (OS), overall response rate (ORR), and ≥ grade 3 adverse events (AEs). Subgroup analyses based on backbone treatment and tumor type were included.
A total of 10536 patients (19 studies) were included (ICIs-arm: 5596 patients; SOC-arm: 4940 patients). Globally, PFS, OS, and ORR results favored ICIs-arm. No differences in terms of TTR were found between arms. ICI-arm was associated with a slight increase of ≥ G3 AEs (relative risk: 1.07). The results in multiple myeloma patients are controversial in favor of ICIs combinations.
Adding ICIs to SOC benefits a greater number of patients, prolonging survival with no early detrimental effect. The toxicity profile is safe, with a mild increase of high-grade manageable AEs.
KEYWORDS: Immune checkpoint inhibitors, chemotherapy, targeted therapy, efficacy, safety
Introduction
Overall survival (OS) remains the gold-standard efficacy endpoint for the development of new treatments in oncology.1 To accelerate its development, other fast endpoints, known as surrogate endpoints of OS, such as progression-free survival (PFS), objective response rate (ORR), or pathological complete response (pCR), have been used in different solid tumors. A good correlation with OS is desired to employ these endpoints.
Immune checkpoint inhibitors (ICIs) targeting programmed death-1/programmed death-ligand 1 (PD-1/PD-L1) or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) have shaken the oncological therapeutic landscape up in the last decade. Despite the great benefit noted with these new drugs, many of the trials comparing ICI as monotherapy with the standard of care (based on chemotherapy) showed an early detrimental effect in a subset of patients. This phenomenon is reflected by the early crossover of the Kaplan–Meier curves representing PFS in several trials. Disease progression and death have been shown to happen at a higher proportion in a subgroup of patients treated with ICI during the first months of treatment.2,3 Interestingly, some trials have failed to show an improvement in terms of median PFS values despite the gain observed for OS, suggesting that those measures could not be as good surrogate markers as previously thought in the case of new ICIs.2
The intrinsic delayed effect, different patterns of evolution, such as pseudo-progression or hyper-progression phenomena as well as long-term shift in the host, could justify the impact in OS without differences in other short-term endpoints.4
Conversely, tumor shrinkage is usually associated with a longer duration of response (DOR) among patients treated with ICIs compared to chemotherapy. Thus, this effect would justify the long-term benefits characteristically attached to these new drugs.2,3,5 Taken into consideration the singularities of ICIs, ORR, complete response (CR) rates or PFS rates at specific time-points (6-months-rate, 12-months-rate) have been shown to better capture the benefit with the use of these therapies.6,7
Despite certain misinterpretations in this issue, it remains unclear whether time-to-response (TTR) could be used as a predictor of response.8,9 The response to ICIs usually occurs in the same timeframe as people treated with chemotherapy, with no clear differences in TTR between arms, limiting its usefulness as an early predictive marker for benefit.2,3
In the last years, combination therapy with ICIs and chemotherapy is increasingly being tested as a synergistic strategy.10–13 Whether PFS and/or TTR could be improved through this combinatory approach, and potentially represent an additional very short-term predictive marker for benefit with this strategy, remains to be elucidated.
Therefore, we decided to perform a meta-analysis considering trials in the metastatic setting comparing ICIs-chemotherapy/targeted therapy combinatory strategy versus the standard of care (chemotherapy schemes or targeted therapy without immunotherapy, as properly indicated) to assess the differences in terms of PFS/TTR between arms. Moreover, to strengthen this analysis, we have also assessed the differences in other efficacy (OS, ORR) and safety (grade 3 or more adverse events (AEs)) endpoints compared to standard of care.
Material and methods
Literature search and inclusion criteria
The literature search was accomplished by August 1, 2020. Two different databases were reviewed: MEDLINE and EMBASE. Only combination strategies including agents targeting PD-1/PD-L1 studied in the metastatic setting (in both solid tumors and myeloma) were included in the search: a) anti-PD-1 antibodies: nivolumab (Opdivo®) and pembrolizumab (Keytruda®), b) anti-PD-L1 antibodies: atezolizumab (Tecentric®), durvalumab (Imfinzi®) and avelumab (Bavencio®). The specific words used during the search, filtered by article type (clinical trial), were: “nivolumab” OR “pembrolizumab” OR “atezolizumab” OR “durvalumab” OR “avelumab” OR “PD-1” OR “PD-L1.” Additionally, the manufacturers’ package inserts for drugs included in the meta-analysis were also analyzed to spot original or different data not reported in published trials.
All randomized clinical trials (RCTs) performed in the metastatic setting of solid tumors and myeloma that compared anti-PD-1/PD-L1 plus chemotherapy or targeted therapy, according to each malignancy, versus the standard of care, involving different chemotherapy/targeted therapy schemes without ICI-based therapy (depending upon the type of tumor), were included. Only the most complete report of the RCTs was included when duplicate publications were identified. The studies had to be carried out in ≥18 years-old humans, in any line of treatment, and be published in English. Those studies not yet published/peer-reviewed were not considered for inclusion in this analysis to assure the highest quality of the available data.
To increase the homogeneity of the selected studies, we decided to exclude RCTs including anti-CTLA-4 antibodies, which are far less used in clinical practice. Neither ICI–ICI combinations were included as they involve potentially different mechanisms of action compared to our selected combinations. Strategies involving sequential administration of drugs (induction treatment followed by maintenance therapy) were not considered either because of the same reasons.
We selected PFS, TTR, OS, and ORR as endpoints for efficacy, as well as ≥ grade 3-AEs as safety endpoints to be compared between arms. Trials that met the following criteria were included in the meta-analysis: randomized phase II or III trials, prospective clinical studies in patients with metastatic solid tumors and myeloma, and trials with at least one of the previous endpoints mentioned above available. Two reviewers (A. C-G. and G.d.V.) independently evaluated studies for eligibility.
Data extraction and clinical endpoints
Two investigators (A. C-G. and G. d. V.) extracted the data individually, discordances were resolved by consensus. Data was reported agreeing to Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines.14 Collected variables included first author’s surname, year of publication, National Clinical Trials (NCT) registry number, type of primary tumor, study phase, number of previous treatment lines received, number of enrolled subjects, criteria used for assessing efficacy (Response Evaluation Criteria In Solid Tumors (RECIST) or others) and safety (National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE)) endpoints, blinding (yes/no), treatment arms, number of patients per treatment arms, and median age. The efficacy endpoints selected for the analysis (hazard ratios (HR) with confidence intervals (CI) for PFS and OS between treatment arms, median time values in each arm for PFS, OS and TTR, and percentages in each arm for ORR and ≥ G3 AEs) were also obtained when available.
Statistical analysis
The assessment of heterogeneity was evaluated using the chi-square, p-value lower 0.05 was considered significant heterogeneity, and quantified with the I2 statistic15 with values lower 40%, 30% to 60%, 50% to 90%, and upper 75% interpreted as low, moderate, high, and extreme levels of heterogeneity. Due to the heterogeneity of the studies included, random effects models were used to estimate the pooled effect: HR for PFS and OS, time‐to‐event outcome data, risk ratio for ORR and ≥ G3 AEs, and mean difference for PFS, OS, and TTR between arm.
The assessment of reporting biases was evaluated using funnel plots and quantified by Egger’s linear regression test.16 The output was obtained using Stata version 16 (https://www.stata.com).
Statistical analysis and figures were performed with the meta17 package of R software version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria)
Subgroup analyses were conducted by the type of treatment combined with ICIs (chemotherapy/targeted therapy) and type primary tumor. As in the overall analysis, the purpose of these subgroup analyses was to compare ICI-based therapy with regard to the corresponding standard of care in different subsets of cancer patients.
Results
Study selection
The studies that met the criteria for the final analysis are shown in the flow chart (Figure 1). A total of 855 studies were reviewed through our screening process for RCTs. We did not include (i) non-clinical trial-type studies or studies involving treatment strategies not considered in our review (ICI-ICI combinations, anti-CTLA-4 antibodies, inadequate control arm) (n = 778) and (ii) early phase I/II or non-RCTs, studies performed in primary tumors (mainly lymphomas) or settings (early disease) different from our previous criteria (n = 58). Nineteen RCTs met the standards for inclusion in the meta-analysis.
Figure 1.
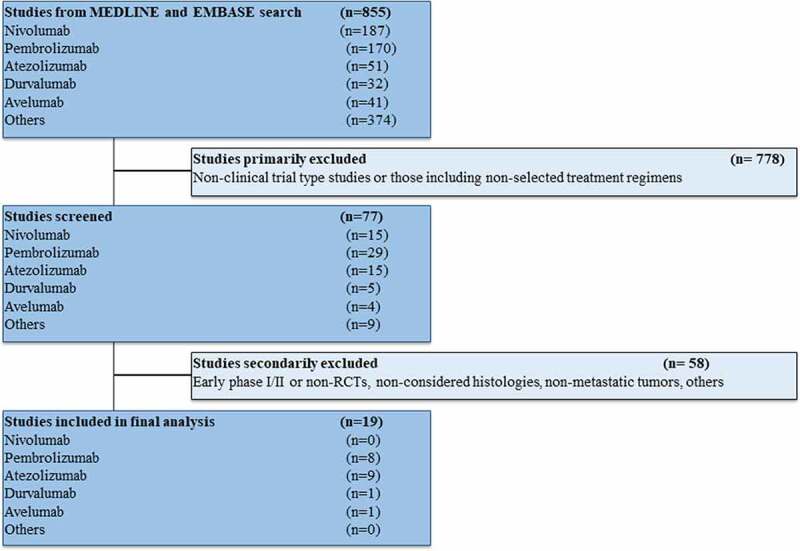
Flow diagram for identification and selection of studies
The baseline characteristics of each trial are displayed in Supplementary Table 1.10–13,18–32 The tumor types included were: non-small cell lung cancer (NSCLC) (five studies), renal-cell carcinoma (RCC) (four studies), small cell lung cancer (SCLC) (two studies), BRAF-mutated melanoma (two studies), myeloma (two studies), head and neck squamous cell carcinoma (HNSCC) (one study), triple-negative breast cancer (TNBC) (one study), hepatocellular carcinoma (one study), and urothelial carcinoma (one study). All trials but one22 were performed in the first-line setting (treatment naïve). All studies had two treatment arms except three,10,24,30 which had three arms (including ICI monotherapy, not considered in our analysis); in addition, two other trials are expected to show results of a third arm (ICI plus chemotherapy combinations) not yet available.13,26 Twelve studies were based on different chemotherapy regimens with or without anti-PD-1/PD-L1 antibodies10–13,18,19,22,23,25,26,28,30 while eight involved targeted therapy;20,21,24,26,27,29,31,32 in one trial, a combination including chemotherapy and targeted therapy (bevacizumab) was tested.26 None of the studies restricted eligible populations according to PD-L1 expression, outcomes collected in this analysis were independent of PD-L1 status.
A total of 10536 patients were available for the meta-analysis: 5596 patients were assigned to the combination therapy with ICIs arm (3515 to chemotherapy plus ICI and 2081 to targeted therapy plus ICI), and 4940 were assigned to the control arm (3022 received chemotherapy and 1918 received targeted therapy). In all trials, efficacy endpoints (PFS, TTR, ORR) were assessed according to the RECIST v1.1 criteria. AEs were evaluated according to CTCAE v4.0 or v4.3. All RCTs were sponsored by pharmaceutical companies.
Efficacy endpoints: PFS and TTR
The PFS results favored ICI-based combinatory therapy. The HR for PFS was improved in those patients treated with this strategy compared to standard of care (HR 0.74; 95% CI 0.67–0.81, p < .0001). Intriguingly, the benefit in terms of this parameter for multiple myeloma patients seems to be less clear (Figure 2a).
Figure 2.
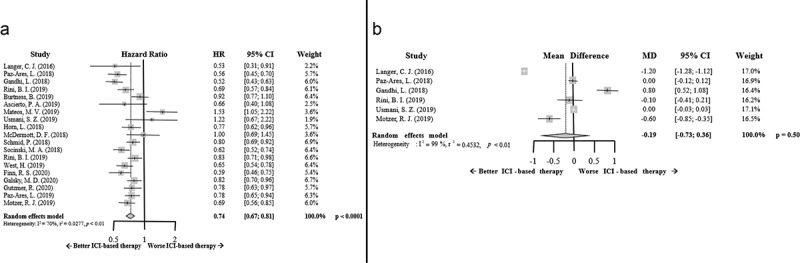
Forrest plot diagrams: Hazard ratio (HR) with 95% confidence interval (CI) for progression-free survival between arms (a); Mean difference (MD) with 95% CI for time-to-response between arms (b)
On the other hand, no differences were found between arms in relation to the TTR results (mean difference (MD) −0.19; 95% CI −0.73–0.36, p = .50). Despite a trend for a faster response with ICI-based combinatory therapy, this difference was not statistically significant (Figure 2b). Of note, TTR data were found in only six studies.
Efficacy endpoints: OS and ORR
The OS results showed a benefit supporting the ICI-based combinatory therapy concerning the standard of care (HR 0.76; 95% CI 0.69–0.84, p < .0001). Again, the results in myeloma patients concerning this analysis are more controversial (Figure 3a).
Figure 3.
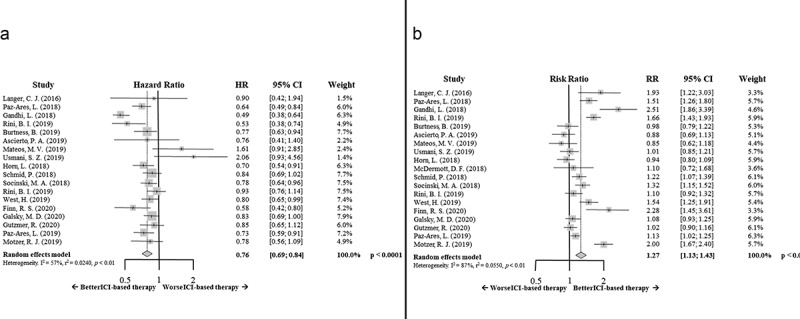
Forrest plot diagrams: Hazard ratio (HR) with 95% confidence interval (CI) for overall survival between arms (a); Risk ratio (RR) with 95% CI for overall response rate between arms (b)
ICI-based therapy increased the response rate compared to standard of care (relative risk (RR) 1.27; 95% CI 1.13–1.43, p < .0001) (Figure 3b).
Safety endpoints: ≥ G3 AEs
Concerning the safety profile of this new combined strategy, the ICI-based therapy showed a slight, but statistically significant, increase of ≥ G3 AEs compared to standard of care (RR 1.07; 95% CI 1.01–1.14, p = .03) (Figure 4).
Figure 4.
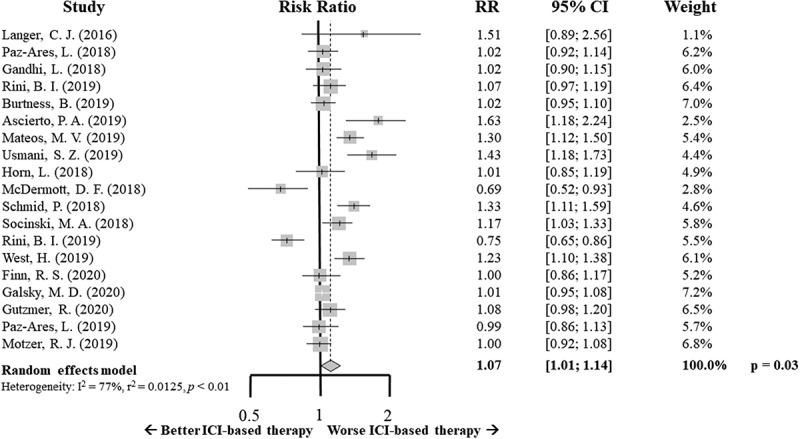
Forrest plot diagram: Risk ratio (RR) with 95% confidence interval (CI) for grade 3 or more adverse events between arms
All p-value of the Egger test was higher than 0.05, there was no evidence of small-study effects.
Subgroup analysis by backbone treatment (chemotherapy versus targeted therapy)
The type of treatment combined with ICIs did not seem to impact any selected endpoints. Both subgroups (chemotherapy or targeted therapy) presented similar results to those obtained in the global analysis without any difference between them (Table 1).
Table 1.
Subgroup analyses according to backbone treatment (chemotherapy versus targeted therapy) and primary tumor type. p-values for subgroup differences concerning progression-free survival (PFS), time-to-response (TTR), overall survival (OS), overall response rate (ORR), and grade 3 or more adverse events (≥ G3 AEs)
|
p-value for subgroup differences |
|
|
| By backbone treatment | By primary tumor type | |
| PFS | 0.8605 | < 0.0001 |
| TTR | 0.5623 | 0.3459 |
| OS | 0.6849 | 0.0151 |
| ORR | 0.6035 | < 0.0001 |
| ≥ G3 AEs | 0.097 | < 0.0001 |
Subgroup analysis by primary tumor type
Concerning TTR, no differences were found between treatment arms regarding the primary tumor type (Table 1).
Multiple myeloma patients treated with the ICI-based combinatory therapy seemed to present a potentially deleterious effect in terms of PFS and OS when compared to the control arm; this difference reached statistical significance when considering all primary tumor types (Table 1).
The ORR results according to tumor type were more heterogeneous: hepatocellular carcinoma and NSCLC patients presented the most favorable data with the ICI-based combinatory therapy while the benefits in myeloma, BRAF-mutated melanoma, and HNSCC patients in favor of ICI-based arm are no so clear (Table 1).
Concerning ≥ G3 AEs, multiple myeloma and, to a lesser extent, TNBC patients treated with ICI-based therapy experimented a higher number of these events in comparison with other tumor types (Table 1).
Discussion
The combinations of ICIs with chemotherapy/targeted therapy improve PFS and RR without harm on TTR
Our report evaluating 19 RCTs with ICIs-chemotherapy/targeted therapy combinatory strategy versus standard treatment (chemotherapy or targeted therapy without ICIs as appropriate) in advanced cancer patients shows that the combination therapy led not only to a significantly better OS but also an improvement in PFS and ORR.
Although ICIs have shown to be an active treatment in multiple tumor types, highlighting long-lasting responses, one of the concerns is the early mortality rate when using it as monotherapy. This early detrimental effect may be due to a slow onset of action of ICIs resulting in higher disease progression and death during the first months of treatment. Conversely, chemotherapy is characterized by a more rapid anti-tumor response but often followed by tumor re-growth; therefore, combining both therapies could overcome these limitations. An important finding of this study is that the combinations with ICIs did not have a detrimental effect on TTR or delay in response. As mentioned above, the percentage of patients who achieved an objective response is also superior with the combination treatment arm, supporting the benefit of this strategy even in highly symptomatic patients where ICIs alone could be insufficient. All the efficacy endpoints are suggesting an overall benefit for the combinatory strategy in the absence of predictive biomarkers for a better selection of the population.
Minimal increase of manageable toxicities
The addition of ICIs to chemotherapy/targeted therapy appears to slightly increase the frequency of ≥ G3 AEs (7%), although they seem to be manageable and consistent with the known toxicity profile of chemotherapy or ICI as monotherapy. No limiting overlapping toxicities seem to harm the administration of these kinds of combinations.
Still a long way to go: better combinations in properly selected populations
Given the bad prognosis of advanced cancer, several combination treatments have been investigated over the years. However, many of them have failed in showing a significant benefit in efficacy outcomes. For example, the combination of chemotherapy plus hormone therapy33 or poly-ADP ribose polymerase (PARP) inhibitors34 in breast or ovarian cancers, respectively, or chemotherapy and cyclin-dependent kinases (CDK) four-sixths inhibitors35 do not have shown a meaningful improvement in survival but an increased risk of significant toxicities.
However, the distinct and probably synergistic mechanisms of action of chemotherapy and immunotherapy have been investigated for years. At present, several preclinical studies have suggested an immunomodulatory role of cytotoxic agents. Chemotherapy has been shown to induce depletion of immunosuppressive cells, such as regulatory T cells, tumor-associated macrophages, and myeloid-derived suppressor cells. Additionally, the cytotoxic effect of chemotherapy may trigger an immunogenic tumor cells death, enhancing T-cell infiltration and immune response, making chemotherapy an ideal partner to combine with immunotherapy.36
Unfortunately, the benefit of ICI as well as that of the combinations with other agents differs significantly among patients. This variety of responses37 along with the higher toxicity and exponential costs should highlight the requirement of the development of predictive biomarkers associated with drug improvements. Several investigations have been carried out to identify biomarkers to select patients who will best respond to ICI. Tumor-infiltrating lymphocytes, PD-L1 expression, tumor mutational burden, or DNA mismatch repair gene deficiency have been related with clinical benefit in cancer patients. However, several other factors that could also predict response to ICI, such as gut microbial diversity and composition or certain transcriptomic and epigenetic signatures, are under investigation.38 In addition, it would be interesting to investigate not only which patients benefit the most from the combination but also determine which drugs are the best partners, along with the optimal dose (i.e., full dose or metronomic administration) or even the timing (sequential or concurrent) of administration.39,40 The optimal dose is a topic of special interest since most trials have been developed empirically.
The study has numerous boundaries. It includes a rather heterogeneous population, especially multiple tumor types (highlighting the comparison between multiple myeloma and the rest of solid tumors) or types of treatment (different compounds). The purpose was to obtain a wide effect of the profile of ICI-based therapy. Each study offers its relative results compared to its corresponding control arm; however, the comparability among the different studies is controversial. In addition, there are no shared stratification factors or useful biomarkers employed among the studies included; worryingly, any of the data showed here take into consideration the PD-L1 expression status in the treated population. Subgroup analyses by treatment and tumor type were performed to avoid bias: no differences were found regarding the backbone treatment (chemotherapy versus targeted therapy); on the other hand, the results obtained in multiple myeloma patients treated with ICI-based therapy raised doubts about the current benefit of the combinatory arm in this tumor type. The comparison between anti-PD-1 versus anti-PD-L1 antibodies was not conducted. As mentioned before, all studies but one were performed in the metastatic first-line setting. Finally, other promising approaches as maintenance strategies have not been considered despite their interesting results in terms of efficacy and toxicity.
Taking together, all these data suggest that the addition of ICI (anti-PD-1/PD-L1 antibodies) to chemotherapy/targeted therapy could benefit a greater number of patients, achieving fast and more durable responses as well as prolonging survival without the early detrimental effect seen with ICI monotherapy; toxicity profile is safe, with a mild increase of ≥ G3 manageable AEs.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed here.
References
- 1.U.S. Department of Health and Human Services, Food and Drug Administration, Oncology Center of Excellence, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) . Clinical trial endpoints for the approval of cancer drugs and biologics. Guidance for industry. [accessed 2020 Jun 16].2018. December; https://www.fda.gov/media/71195/download
- 2.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015. October 22;373(17):1627–8. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee J-L, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK. et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017. 16;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Champiat S, Ferrara R, Massard C, Besse B, Marabelle A, Soria J-C, Ferté C. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol. 2018. December;15(12):748–762. doi: 10.1038/s41571-018-0111-2. [DOI] [PubMed] [Google Scholar]
- 5.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010. August 19;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carretero-González A, Lora D, Ghanem I, Zugazagoitia J, Castellano D, Sepúlveda JM, López-Martin JA, Paz-Ares L, de Velasco G. Analysis of response rate with ANTI PD1/PD-L1 monoclonal antibodies in advanced solid tumors: a meta-analysis of randomized clinical trials. Oncotarget [Internet]. 2018. February 2 [cited 2020 April16];9(9). Available from. http://www.oncotarget.com/fulltext/24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ritchie G, Gasper H, Man J, Lord S, Marschner I, Friedlander M, Lee CK. Defining the most appropriate primary end point in phase 2 trials of immune checkpoint inhibitors for advanced solid cancers: a systematic review and meta-analysis. JAMA Oncol. 2018. April 1;4(4):522. doi: 10.1001/jamaoncol.2017.5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franceschi E, Finocchiaro G, Zagonel V, Reni M, Fabi A, Caserta C, Clavarezza M, Maiello E, Carteni G, Rosti G, et al. Time to response (TTR) and early tumor shrinkage (ETS) in recurrent glioblastoma patients treated with bevacizumab: an exploratory analysis of the prospective randomized AVAREG (ML25739) phase II study. J Clin Oncol. 2015. May 20;33(15_suppl):2047–2047. doi: 10.1200/jco.2015.33.15_suppl.2047. [DOI] [Google Scholar]
- 9.Takeda M, Okamoto I, Nakagawa K. Survival outcome assessed according to tumor response and shrinkage pattern in patients with EGFR mutation–positive non–small-cell lung cancer treated with gefitinib or erlotinib. J Thorac Oncol. 2014. February;9(2):200–204. doi: 10.1097/JTO.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, Psyrri A, Basté N, Neupane P, Bratland Å, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019. November;394(10212):1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 11.Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018. December 6;379(23):2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 12.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018. 31;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 13.Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH, et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019. November;394(10212):1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009. July 21;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Green S, Cochrane Collaboration . Cochrane handbook for systematic reviews of interventions [Internet]. 2011. [accessed 2020 Jun 21] http://handbook.cochrane.org
- 16.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997. September 13;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarzer G, Carpenter JR, Rücker G. Meta-analysis with R. Springer Int Publ; 2015. https://www.springer.com/gp/book/9783319214153 [Google Scholar]
- 18.Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016. November;17(11):1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med. 2018. November 22;379(21):2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 20.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019 Mar 21;380(12):1116-1127. [DOI] [PubMed] [Google Scholar]
- 21.Ascierto PA, Ferrucci PF, Fisher R, Del Vecchio M, Atkinson V, Schmidt H, Schachter J, Queirolo P, Long GV, Di Giacomo AM, et al. Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat Med. 2019. June;25(6):941–946. doi: 10.1038/s41591-019-0448-9. [DOI] [PubMed] [Google Scholar]
- 22.Mateos M-V, Blacklock H, Schjesvold F, Oriol A, Simpson D, George A, Goldschmidt H, Larocca A, Chanan-Khan A, Sherbenou D, et al. Pembrolizumab plus pomalidomide and dexamethasone for patients with relapsed or refractory multiple myeloma (KEYNOTE-183): a randomised, open-label, phase 3 trial. Lancet Haematol. 2019. September;6(9):e459–69. doi: 10.1016/S2352-3026(19)30110-3. [DOI] [PubMed] [Google Scholar]
- 23.Usmani SZ, Schjesvold F, Oriol A, Karlin L, Cavo M, Rifkin RM, Yimer HA, LeBlanc R, Takezako N, McCroskey RD, et al. Pembrolizumab plus lenalidomide and dexamethasone for patients with treatment-naive multiple myeloma (KEYNOTE-185): a randomised, open-label, phase 3 trial. Lancet Haematol. 2019. September;6(9):e448–58. doi: 10.1016/S2352-3026(19)30109-7. [DOI] [PubMed] [Google Scholar]
- 24.McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, Fong L, Joseph RW, Pal SK, Reeves JA, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24(6):749–757. doi: 10.1038/s41591-018-0053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Hegg R, Im S-A, Shaw Wright G, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018. November 29;379(22):2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 26.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018. 14;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 27.Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, Bracarda S, Stadler WM, Donskov F, Lee JL, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019 Jun 15;393(10189):2404-2415. [DOI] [PubMed] [Google Scholar]
- 28.West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp H-G, Daniel D, McCune S, Mekhail T, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019. July;20(7):924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 29.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, Kudo M, Breder V, Merle P, Kaseb AO, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020. May 14;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 30.Galsky MD, Arija JÁA, Bamias A, Davis ID, De Santis M, Kikuchi E, Garcia-del-Muro X, De Giorgi U, Mencinger M, Izumi K, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020. May;395(10236):1547–1557. doi: 10.1016/S0140-6736(20)30230-0. [DOI] [PubMed] [Google Scholar]
- 31.Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, Pereira RP, Eigentler T, Rutkowski P, Demidov L, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020. June;395(10240):1835–1844. doi: 10.1016/S0140-6736(20)30934-X. [DOI] [PubMed] [Google Scholar]
- 32.Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019 Mar 21;380(12):1103-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sledge GW, Hu P, Falkson G, Tormey D, Abeloff M. Comparison of chemotherapy with chemohormonal therapy as first-line therapy for metastatic, hormone-sensitive breast cancer: an Eastern cooperative oncology group study. J Clin Oncol Off J Am Soc Clin Oncol. 2000. January;18(2):262–266. doi: 10.1200/JCO.2000.18.2.262. [DOI] [PubMed] [Google Scholar]
- 34.Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, Okamoto A, Moore KN, Efrat Ben-Baruch N, Werner TL, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019. December 19;381(25):2403–2415. doi: 10.1056/NEJMoa1909707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts PJ, Kumarasamy V, Witkiewicz AK, Knudsen ES. Chemotherapy and CDK4/6 inhibitors: unexpected bedfellows. Mol Cancer Ther. 2020. August;19(8):1575–1588. doi: 10.1158/1535-7163.MCT-18-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Apetoh L, Ladoire S, Coukos G, Ghiringhelli F. Combining immunotherapy and anticancer agents: the right path to achieve cancer cure? Ann Oncol Off J Eur Soc Med Oncol. 2015. September;26(9):1813–1823. doi: 10.1093/annonc/mdv209. [DOI] [PubMed] [Google Scholar]
- 37.de Velasco G, Krajewski KM, Albiges L, Awad MM, Bellmunt J, Hodi FS, Choueiri TK. Radiologic heterogeneity in responses to anti-PD-1/PD-L1 therapy in metastatic renal cell carcinoma. Cancer Immunol Res. 2016. January 1;4(1):12–17. doi: 10.1158/2326-6066.CIR-15-0197. [DOI] [PubMed] [Google Scholar]
- 38.Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019. March;19(3):133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens JHM. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol Off J Eur Soc Med Oncol. 2019. 01;30(2):219–235. doi: 10.1093/annonc/mdy551. [DOI] [PubMed] [Google Scholar]
- 40.Murciano-Goroff YR, Warner AB, Wolchok JD. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res. 2020. June;30(6):507–519. doi: 10.1038/s41422-020-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


