Abstract
Background
SEGA is a rare, slow-growing CNS neoplasm that has historically been treated by surgical resection. However, the advent of a mammalian target of rapamycin complex-1 inhibitor, everolimus, has shown promising results in recent clinical trials. We sought to provide an analysis of epidemiological and survival risk factors in this rare tumor entity, while comparing trends in surgical management before and after introduction of everolimus in SEGAs.
Methods
Patients with SEGA were queried from the National Cancer Database between 2004 and 2015. Standard statistical analysis was conducted to assess variables associated with the odds of performing surgery and survival, while controlling for confounding variables.
Results
A total of 460 patients were diagnosed with SEGA. Multivariable analysis of survival demonstrated that increased age was associated with decreased survival (HR, 1.05; P < .0001). Multivariable analysis of surgery showed increased age (odds ratio [OR], 1.02, P = .04) and tumor size 20 mm or larger (OR, 9.52-16.75, P < .0001 for all) to be associated with higher odds of performing surgery. The use of radiotherapy (OR, 0.12, P = .008) or chemotherapy (OR, 0.21, P = .008) was associated with lower odds of surgery. A comparison of surgical rates between 2004 and 2010 and 2011 and 2015 was found to be significantly different, with a lower rate of surgery seen after 2011 (60.63% vs 48.06%, P = .007).
Conclusion
Our analysis of SEGAs demonstrated that age was the only variable affecting overall survival. Surgical resection was performed in older patients with larger tumors (> 20 mm) as a primary mode of treatment, without chemoradiotherapy. Expectedly, rates of surgical resection were found to have decreased since 2011, after FDA approval of everolimus for SEGA treatment.
Keywords: everolimus, NCDB, SEGA, surgical trends, survival
SEGA has been characterized as a rare, benign neoplasm (World Health Organization grade I) that is almost exclusively associated with TSC.1–4 These astrocytic tumors arise within the ventricular system often in close proximity with the foramen of Monro, thereby disrupting the flow and absorption of cerebrospinal fluid (CSF). This disruption presents clinically as obstructive hydrocephalus, which can have a gradual or sudden course; in rare cases acute hydrocephalus or acute worsening in the backdrop of gradually progressive obstructive hydrocephalus can lead to sudden death.1,2,4–8 Additional modes of clinical presentation in patients with SEGAs include seizures and intratumoral hemorrhage.9–11 SEGAs are commonly seen in the younger population, particularly within the first 2 decades of life.2,5,8,12 Currently, gross total resection of the tumor is the standard of treatment because it addresses hydrocephalus, seizures, as well as potential tumor recurrence.7,8,13,14 Given the eloquent structures at risk with intraventricular tumor resection in a young patient population, efforts have been focused on alternative modes of management. Recent study of molecular targeted therapy, via mammalian target of rapamycin (mTOR) inhibitors, has been shown to be beneficial in treating SEGAs by reducing tumor volume, alleviating the source of hydrocephalus, and reducing seizures in patients with TSC.15–19 These promising results provide evidence for an alternative to patients who may not be candidates for surgical resection.16–18,20
Owing to the rare nature of SEGAs, the majority of studies have been limited to case series or small cohort studies, with the largest having 228 patients.1 We used the National Cancer Database to analyze the largest number of SEGA patients to date to better characterize this population as it pertains to demographics, complications, treatment modalities, and outcomes. It is crucial to examine whether management decision tendencies in SEGA, across the national landscape, has changed in recent practice with the US FDA approval of everolimus for SEGA in late 2010. This recent development could potentially result in molecular-targeted therapies gaining traction as a nonsurgical or adjunctive option to the historical standard of radical resection.
Methods
The National Cancer Database (NCDB) is a nationwide, hospital-based registry that captures approximately 70% of all patients newly diagnosed with cancer, who received care at cancer centers that are accredited by the American College of Surgeons Commission on Cancer (CoC). The NCDB receives more than 1 million cancer case reports annually from more than 1430 hospitals and portrays the majority of cancer cases nationwide.21 All patients with a histologically confirmed diagnosis of SEGAs between 2004 and 2015 were identified, as classified by the third edition of the International Classification of Diseases for Oncology: 938.4/1. Variables collected included age at diagnosis, sex, race, insurance status, tumor size, treatment modality, type of surgery performed, treatment with radiation, radiation-surgery sequence, and survival from time of diagnosis. NCDB data are publicly available and deidentified, and thus did not require review from our institutional review board.
Statistical Analysis
The outcome variable used in this study was survival status, defined as living or deceased at the time of evaluation (death from any cause, ie, all-cause mortality). All the aforementioned variables were analyzed with respect to survival status. The t test was used for the comparison of continuous variables. For categorical variables, the χ 2 test was used. Survival time was determined as the interval, described in months, between diagnosis and death or last follow-up reported in NCDB. Kaplan-Meier curves were generated for descriptive visualization of survival by different variables such as age, sex, race, treatment regimen, and tumor size. A Cox proportional hazards model was used to evaluate the effect of demographics, tumor size, treatment, and year of diagnosis on survival. A multiple logistics regression model was used to evaluate the effect of such variables on the odds of performing surgery. Both models were adjusted for potentially interacting and confounding covariates. The significance level was set at α = .05, and odds ratios (OR), hazard ratios (HR), 95% CIs, and P values were calculated. Statistical analysis was performed using R statistical software (version 3.4.0, 2017; R Foundation for Statistical Computing). This study complies with Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Results
Baseline Characteristics
A total of 460 patients were identified in the NCDB as having histologically identified SEGAs during 2004 to 2015 (Table 1). The study population had a mean age of 18.38 ± 14.90 years (range, 0-71 years) and a comparable distribution of males (n = 247, 53.7%) and females (n = 213, 46.3%). The racial spread in order of descending frequency consisted of: White (n = 332, 72.2%), Black (n = 92, 20.0%), other (n = 28, 6.1%), and unknown race (n = 8, 1.7%). The majority of tumors were smaller than 40 mm (n = 301, 65.4%), 39 (8.48%) were greater than or equal to 4 cm, and 120 (26.09%) had no size recorded. Half the patients were treated with surgery (n = 253, 55.0%), whereas radiation (n = 11, 2.39%) and chemotherapy were rarely used (n = 26, 5.65%). Chemotherapy use increased from 2011 to 2015 compared to 2004 to 2010 (n = 19 vs n = 7, respectively). A total of 424 patients were alive, and 36 patients had died at the time of data collection. Age at diagnosis (P < .0001) and insurance status (P < .001) were found to be different between patients who were alive and dead.
Table 1.
Baseline Characteristics
| Parameter | Total (n = 460) | Alive (n = 424) | Dead (n = 36) | P |
|---|---|---|---|---|
| Age at diagnosis, mean (SD), y | 18.38 (14.90) | 17.41 (14.23) | 29.92 (17.75) | < .001 |
| Sex, n (%) | .25 | |||
| Male | 247 (53.7) | 231 (54.48) | 16 (44.44) | |
| Female | 213 (46.3) | 193 (45.52) | 20 (55.56) | |
| Race, n (%) | .93 | |||
| White | 332 (72.17) | 307 (72.41) | 25 (69.44) | |
| Black | 92 (20.0) | 84 (19.81) | 8 (22.22) | |
| Other | 28 (6.09) | 26 (6.13) | 2 (5.56) | |
| Unknown | 8 (1.74) | 7 (1.65) | 1 (2.78) | |
| Insurance status, n (%) | < .001 | |||
| Private insurance | 218 (47.39) | 205 (48.35) | 13 (36.11) | |
| Medicaid | 159 (34.56) | 150 (35.38) | 9 (25.0) | |
| Medicare | 41 (8.91) | 31 (7.31) | 10 (27.78) | |
| Other government insurance | 6 (1.30) | 6 (1.42) | 0 (0.0) | |
| Not insured | 22 (4.78) | 20 (4.72) | 2 (5.56) | |
| Unknown | 14 (3.04) | 12 (2.83) | 2 (5.56) | |
| Tumor size, n (%), mm | .81 | |||
| < 40 | 301 (65.43) | 281 (66.27) | 20 (55.56) | |
| ≥ 40 | 39 (8.48) | 36 (8.49) | 3 (8.33) | |
| Unknown | 120 (26.09) | 107 (25.24) | 13 (36.11) | |
| Surgery, n (%) | .18 | |||
| Performed | 253 (55.0) | 237 (55.9) | 16 (44.44) | |
| Not performed | 207 (45.0) | 187 (44.1) | 20 (55.56) | |
| Radiation, n (%) | .20 | |||
| No | 444 (96.52) | 410 (96.70) | 34 (94.44) | |
| Yes | 11 (2.39) | 9 (2.12) | 2 (5.56) | |
| Unknown | 5 (1.09) | 5 (1.18) | 0 (0.0) | |
| Chemotherapy, n (%) | .41 | |||
| No | 418 (90.87) | 383 (90.33) | 35 (97.22) | |
| Yes | 26 (5.65) | 25 (5.90) | 1 (2.78) | |
| Unknown | 16 (3.48) | 16 (3.77) | 0 (0.0) | |
| Chemotherapy, 2004-2010 | .69 | |||
| No | 237 (97.13) | 214 (97.27) | 23 (95.83) | |
| Yes | 7 (2.87) | 6 (2.73) | 1 (4.17) | |
| Chemotherapy, 2011-2015 | .25 | |||
| No | 181 (90.50) | 169 (89.89) | 12 (100.0) | |
| Yes | 19 (9.50) | 19 (10.11) | 0 (0.0) | |
| Survival, mo, mean (SD) | 63.36 (42.81) | 66.28 (42.22) | 28.99 (34.27) | < .001 |
Survival Analysis
Kaplan-Meier log-rank testing revealed no association between race, sex, surgery, tumor size, chemotherapy or radiotherapy, and overall survival (see Supplementary Material). Multivariable analysis (Table 2) revealed increased age at diagnosis to be associated with an increased hazard risk (HR 1.05; 95% CI, 1.02-1.07; P < .0001). All other factors, including the use of chemotherapy (HR 0.83; 95% CI, 0.11-6.33; P = .86) or radiation (OR 1.39; 95% CI, 0.29-6.59; P = .68), were not shown to be associated with increased survival.
Table 2.
Multivariable Analysis of Survival and Tumor Characteristics
| Parameter | HR | 95% CI | P |
|---|---|---|---|
| Age at diagnosis, y | 1.05 | 1.02-1.07 | < .001 |
| Sex | |||
| Male | (Reference) | ||
| Female | 1.18 | 0.59-2.37 | .65 |
| Race | |||
| White | (Reference) | ||
| Black | 1.41 | 0.62-3.19 | .41 |
| Other | 1.43 | 0.33-6.17 | .64 |
| Tumor size, mm | |||
| < 20 | (Reference) | ||
| 20-29 | 1.44 | 0.51-4.05 | .49 |
| 30-39 | 1.49 | 0.42-5.21 | .54 |
| ≥ 40 | 1.30 | 0.34-4.99 | .70 |
| Unknown | 1.71 | 0.69-4.23 | .24 |
| Chemotherapy | |||
| No | (Reference) | ||
| Yes | 0.83 | 0.11-6.33 | .86 |
| Radiation | |||
| No | (Reference) | ||
| Yes | 1.39 | 0.29-6.59 | .68 |
| Year of diagnosis | |||
| 2011-2015 | (Reference) | ||
| 2004-2010 | 0.93 | 0.42-2.05 | .86 |
Abbreviation: HR, hazard ratio.
Analysis of Surgery Risk
Multivariable analysis identified several characteristics that affected the odds of performing surgery (Table 3). Increased age at diagnosis (OR 1.02; 95% CI, 1.00-1.03, P = .04) and larger tumor sizes of 20 to 29 mm (OR 9.52; 95% CI, 5.04-17.99, P < .0001), 30 to 39 mm (OR 12.73; 95% CI, 5.16-31.40, P < .0001), and 40 mm or larger (OR 16.57; 95% CI, 6.10-45.98, P < .0001) were correlated with higher odds of surgery. Surgery was rarely performed when either chemotherapy (OR 0.21; 95% CI, 0.06-0.66, P = .008) or radiation (OR 0.12; 95% CI, 0.02-0.57, P = .008) was involved in the treatment plan.
Table 3.
Multivariable Analysis of Surgery and Tumor Characteristics
| Parameter | OR | 95% CI | P |
|---|---|---|---|
| Age at diagnosis, y | 1.02 | 1.00-1.03 | .04 |
| Sex | |||
| Male | (Reference) | ||
| Female | 0.70 | 0.44-1.09 | .12 |
| Race | |||
| White | (Reference) | ||
| Black | 1.00 | 0.57-1.74 | .99 |
| Other | 1.38 | 0.53-3.56 | .51 |
| Tumor size, mm | |||
| < 20 | (Reference) | ||
| 20-29 | 9.52 | 5.04-17.99 | < .001 |
| 30-39 | 12.73 | 5.16-31.40 | < .001 |
| ≥ 40 | 16.75 | 6.10-45.98 | < .001 |
| Unknown | 3.32 | 1.92-5.74 | < .001 |
| Chemotherapy | |||
| No | (Reference) | ||
| Yes | 0.21 | 0.06-0.66 | .008 |
| Unknown | 2.04 | 0.53-7.89 | .30 |
| Radiation | |||
| No | (Reference) | ||
| Yes | 0.12 | 0.02-0.57 | .008 |
| Year of diagnosis | |||
| 2011-2015 | (Reference) | ||
| 2004-2010 | 1.40 | 0.88-2.20 | .15 |
Abbreviation: OR, odds ratio.
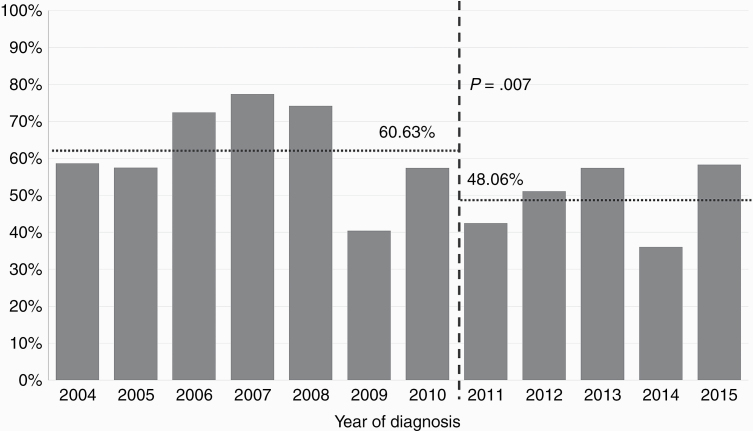
The annual rates of SEGAs that were treated surgically during 2004 to 2015 are described in Figure 1. A comparison of rates of surgery between 2004 to 2010 and 2011 to 2015 showed that rates decreased after 2011 (χ 2 = 7.26, P = .007). This observation was not seen to have interactions with other variables, such as size of the tumor, which suggests that the effects of other variables on odds of surgery were the same before and after 2011.
Fig. 1.
Rates of Surgical Intervention of SEGAs, 2004 to 2015.
Discussion
The present study set out to analyze the largest-to-date sample of patients with SEGAs, a tumor entity that had previously been restricted to case series and smaller study cohorts.1,2,22–25 We found that SEGA patients had a mean age of 18 years, exhibited a slight male predominance, and occurred mostly in the White population. The study sample discussed here is comparable to those reported in other studies in terms of distribution of age, sex, and race.1 A univariate comparison between patient groups who were alive vs dead showed mean age to be different between the 2 groups. This was echoed on multivariable analysis that showed increased age to be associated with a higher hazard risk. This finding is consistent with previous studies that found younger age to be associated with better overall survival and lower rates of long-term complications after surgery.1,22 Despite improved survival outcomes at younger age, serial neuroradiological imaging of pediatrics vs adult SEGAs revealed that pediatric SEGAs have significantly higher growth rates and thus benefit from more frequent, routine surveillance.26 The closer surveillance of pediatric SEGA patients may in turn be a factor that leads to early progression detection and contributes to improved survival rates seen in this population. Regardless, mortality was generally low in patients with SEGA; however, a study of 355 patients with TSC revealed SEGA patients to have decreased survival compared with the general population.27 Deaths in these patients are largely due to TSC-related comorbidities such as renal complications, cardiac rhabdomyomas, lung lymphangiomyomatosis, aortic aneurysm, bronchopneumonia, and neurological complications that are commonly attributed to intracranial abnormalities encompassing tumors, seizures, and severe mental handicaps.27
The main treatment for SEGAs has historically been surgical resection because of the lack of responsiveness from other modalities such as chemotherapy and radiotherapy.28 Furthermore, surgery carries the advantage of addressing the issue of acute hydrocephalus and tumor resection, as well as potentially eliminating an imminent cause of seizures. Surgical treatment is provided for SEGAs that present with obstructive hydrocephalus or demonstrate growth on serial imaging.6 There is limited and variable evidence on the timing and efficacy of surgery, especially regarding which specific subpopulations may benefit most from surgical tumor resection.13,29,30 We attempted to describe characteristics that were related to the odds of surgery through a multivariable analysis to observe trends in surgical decision making.
Our analysis showed that increased age was associated with higher odds of receiving surgery. Many SEGAs may remain indolent and thus may remain undetected before becoming symptomatic secondary to growth, which may explain our observation of targeted surgery in the higher age spectrum. In addition, a previous study demonstrated that more surgery-related complications were seen in patients younger than 3 years compared to older patients because of the higher likelihood of bilateral SEGAs, rapidly growing tumors, more severe TSC presentation, and innate complication profiles associated with high-risk pediatric surgery, such as blood loss.31 Although rapid tumor growth strongly prompts surgery, severe TSC comorbidities may complicate the decision of optimal surgical candidacy.
Odds of surgery demonstrated a positive correlation with increased tumor size. Larger SEGAs have a higher chance of obstructing the foramen of Monro and present with neurological symptoms pertaining to hydrocephalus and its sequelae, thus requiring surgical resection of the mass. Once a mass has reached a critical volume to present with obstructive hydrocephalus, options for treatment aside from alleviating tumor burden and mechanical obstruction become limited. Simultaneously, at such critical tumor mass, the risks of surgery such as motor deficit, hydrocephalus, hemorrhage, cognitive and memory decline, as well as incomplete resection increase.31 Accordingly, it has been shown that earlier intervention for smaller tumors is associated with improved outcomes. One such study of 57 SEGA patients demonstrated that surgical treatment of SEGAs larger than 3 cm resulted in 67% of adverse events, whereas patients undergoing surgery for SEGAs smaller than 2 cm were not affected by any complications.31 Several smaller studies have also shown similar results, in which treatment of larger, symptomatic tumors are associated with a higher risk of postoperative complications.7,32 Select clinicians recommend that SEGAs as small as 5 mm and located near the foramen of Monro should be removed as soon as growth is confirmed through serial imaging.6
Everolimus, an mTOR complex 1 inhibitor, has been approved for use in the treatment of SEGAs not amenable to surgery since late 2010. This was following positive results from an open-label phase 2 trial,19 making everolimus, along with other mTOR inhibitors such as rapamycin, the only systemic agents approved to date. In the aforementioned trial, 75% of patients treated with everolimus had a volumetric response of 30% or more during the 6-month treatment period and a clinically relevant reduction in the overall frequency of seizures.19 The efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1) trial, a double-blind, placebo-controlled phase 3 trial conducted shortly after the previous study, reproduced the efficacy of everolimus treatment, in which 57.7% of treated patients achieved a volumetric response of 50% or greater.15 These results suggest that everolimus may provide an effective means of reducing the clinical complications of SEGA through volume reduction and avoidance of invasive surgery, as well as management of other manifestations of TSC, such as seizures, cardiac rhabdomyomas, angiomyolipomas, and skin lesions.33,34
Development of an effective systemic therapy has important implications for the surgical management of SEGAs against the backdrop of TSC. Everolimus can be a valid option for high-risk patients because of systemic disease burden, bilaterally located SEGAs, and tumors in very young patients as demonstrated by previous case studies.35,36 In these cases, the patients either presented with multiple, recurrent lesions or significant TSC-related comorbidities that excluded them from being surgical candidates. Furthermore, the reduction in tumor volume seen with everolimus, even in patients treated for recurrent tumors after initial resection, suggested that it may be useful as an adjuvant therapy to improve outcomes with surgery.8,37 Most exciting, however, is the notion that everolimus may play a larger role in select small tumors that show progression on serial imaging, where surgery would pose a higher risk of complication. Inevitably, everolimus will not be an optimal choice for patients who suffer from very large tumors with obstructive hydrocephalus in the acute setting, maintaining the role of surgery in select cases. In addition, because TSC afflicts multiple organ systems, systemic therapy such as everolimus provides the benefit of addressing several manifestations of TSC simultaneously, including SEGAs. The development of this systemic therapy allows for more careful decision making in a pathology, which has previously been restricted to solely surgical interventions. Indeed, our study revealed that the collective rates of surgical resection decreased after 2010, when everolimus was approved by the FDA for SEGA treatment. The temporal association of the reduction of surgery rates with the introduction of everolimus lends credence to the idea that everolimus may have played a role in modifying treatment approach to reducing surgery. Collectively, everolimus’s role in treatment and optimal situations for its use remain to be elucidated.6
Our study has several limitations that must be taken into account during the interpretation of our presented results. First, the present study used the NCDB; as such, it is suspect to many limitations that arise from using registry-based data. For example, there may be confounding factors such as primary indications for surgical treatment, the specific chemotherapeutic agent and length of therapy, treatment sequence, the presence of specific comorbidities related to TSC, the severity of other TSC-related symptoms, and the specific cause of patient death, which were not available from this database but may have an impact on the assessment of survival or surgical candidacy. The NCDB may not be entirely representative of all cancer care across the United States; therefore, potential selection biases that result from differences between reporting institutions, which are accredited by the CoC, and non-CoC accredited hospitals may exist. CoC-accredited hospitals are more likely to be in urban locations and exhibit a higher degree of oncology-related specialization.38 Although there may be potential differences between the 2 in terms of patient demographics, the data set was deemed relevant for the purposes of our study, given that treatment of SEGAs and tuberous sclerosis complex is multidisciplinary in nature and would most likely be referred to a center with higher levels of cancer specialization. The low mortality rate of SEGA patients did not allow us to identify risk factors that affect survival in a meaningful way. The presence and status of TSC mutations were unavailable, which may have important effects both on survival and surgical decision making. Although histologically confirmed SEGAs are considered pathognomonic for TSC, there have been reports of SEGAs without other manifestations of TSC, which have been shown to be histologically different from those that arise in association with TSC.5,12,39,40 It has been shown that patients harboring a TSC2 mutation, one of the tumor-suppressor genes that are mutated in this condition, develop more aggressive SEGAs at a younger age compared to those with a TSC1 mutation.26,31 A quarter of our sample did not have recorded tumor sizes, which may have a large influence on our observed association between tumor size and odds of performing surgery. Most important, limitations inherent to using registry-based data such as the NCDB do not allow us to draw strong, causal relationships between the FDA approval of everolimus and rates of surgical resection in SEGA patients. Our analysis was limited to elucidating temporal associations with the rates of surgery in this large, national database. Therefore, substantial confounders that explain this decrease in surgery rates may exist. Despite these drawbacks, our study provides a description of the largest sample of SEGA patients to date. Furthermore, the NCDB collects data from multiple centers across the United States, which allows for a more representative view of this condition compared to smaller, single-institution reports.
Conclusion
In an analysis of 460 patients with SEGAs, factors that contributed to improved survival and increased odds of surgical intervention were assessed, while comparing rates of surgery before and after the approval of everolimus as a treatment option. Increased age was associated with an increased hazard risk, while no other variables significantly affected survival. Surgery was performed commonly in older patients with larger tumors 20 mm or larger as a primary treatment, without multimodal chemotherapy and radiotherapy. Rates of performing surgery were found to have decreased since 2011, after FDA approval of everolimus for SEGA treatment.
Supplementary Material
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement. None declared.
References
- 1. Nguyen HS, Doan NB, Gelsomino M, et al. Subependymal giant cell astrocytoma: a surveillance, epidemiology, and end results program–based analysis from 2004 to 2013. World Neurosurg. 2018;118:e263–e268. [DOI] [PubMed] [Google Scholar]
- 2. Jung TY, Kim YH, Jung S, Baek HJ.. The clinical characteristics of subependymal giant cell astrocytoma: five cases. Brain Tumor Res Treat. 2015;3(1):44–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jóźwiak S, Mandera M, Młynarski W. Natural history and current treatment options for subependymal giant cell astrocytoma in tuberous sclerosis complex. Semin Pediatr Neurol. 2015;22(4): 274–281. [DOI] [PubMed] [Google Scholar]
- 4. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tahiri Elousrouti L, Lamchahab M, Bougtoub N, et al. Subependymal giant cell astrocytoma (SEGA): a case report and review of the literature. J Med Case Rep. 2016;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wheless JW, Klimo P Jr. Subependymal giant cell astrocytomas in patients with tuberous sclerosis complex: considerations for surgical or pharmacotherapeutic intervention. J Child Neurol. 2014;29(11):1562–1571. [DOI] [PubMed] [Google Scholar]
- 7. Cuccia V, Zuccaro G, Sosa F, Monges J, Lubienieky F, Taratuto AL.. Subependymal giant cell astrocytoma in children with tuberous sclerosis. Childs Nerv Syst. 2003;19(4):232–243. [DOI] [PubMed] [Google Scholar]
- 8. Beaumont TL, Limbrick DD, Smyth MD. Advances in the management of subependymal giant cell astrocytoma. Childs Nerv Syst. 2012;28(7):963–968. [DOI] [PubMed] [Google Scholar]
- 9. Kim JY, Jung TY, Lee KH, Kim SK.. Subependymal giant cell astrocytoma presenting with tumoral bleeding: a case report. Brain Tumor Res Treat. 2017;5(1):37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ogiwara H, Morota N. Subependymal giant cell astrocytoma with intratumoral hemorrhage. J Neurosurg Pediatr. 2013;11(4): 469–472. [DOI] [PubMed] [Google Scholar]
- 11. De Waele L, Lagae L, Mekahli D. Tuberous sclerosis complex: the past and the future. Pediatr Nephrol. 2015;30(10):1771–1780. [DOI] [PubMed] [Google Scholar]
- 12. Sinson G, Sutton LN, Yachnis AT, Duhaime AC, Schut L.. Subependymal giant cell astrocytomas in children. Pediatr Neurosurg. 1994;20(4):233–239. [DOI] [PubMed] [Google Scholar]
- 13. de Ribaupierre S, Dorfmüller G, Bulteau C, et al. Subependymal giant-cell astrocytomasin pediatric tuberous sclerosis disease: when should we operate? Neurosurgery. 2007;60(1):83–90. [DOI] [PubMed] [Google Scholar]
- 14. Berhouma M. Management of subependymal giant cell tumors in tuberous sclerosis complex: the neurosurgeon’s perspective. World J Pediatr. 2010;6(2):103–110. [DOI] [PubMed] [Google Scholar]
- 15. Franz DN, Belousova E, Sparagana S, et al. Long-term use of everolimus in patients with tuberous sclerosis complex: final results from the EXIST-1 study. PLoS One. 2016;11(6):e0158476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krueger DA, Care MM, Agricola K, Tudor C, Mays M, Franz DN.. Everolimus long-term safety and efficacy in subependymal giant cell astrocytoma. Neurology. 2013;80(6):574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arroyo MS, Krueger DA, Broomall E, Stevenson CB, Franz DN.. Acute management of symptomatic subependymal giant cell astrocytoma with everolimus. Pediatr Neurol. 2017;72:81–85. [DOI] [PubMed] [Google Scholar]
- 18. Franz DN, Belousova E, Sparagana S, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381(9861):125–132. [DOI] [PubMed] [Google Scholar]
- 19. Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363(19):1801–1811. [DOI] [PubMed] [Google Scholar]
- 20. French JA, Lawson JA, Yapici Z, et al. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet. 2016;388(10056):2153–2163. [DOI] [PubMed] [Google Scholar]
- 21. Bilimoria KY, Stewart AK, Winchester DP, Ko CY.. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goh S, Butler W, Thiele EA. Subependymal giant cell tumors in tuberous sclerosis complex. Neurology. 2004;63(8):1457–1461. [DOI] [PubMed] [Google Scholar]
- 23. Kumar R, Singh V. Subependymal giant cell astrocytoma: a report of five cases. Neurosurg Rev. 2004;27(4):274–280. [DOI] [PubMed] [Google Scholar]
- 24. Adriaensen MEAPM, Schaefer-Prokop CM, Stijnen T, Duyndam DAC, Zonnenberg BA, Prokop M.. Prevalence of subependymal giant cell tumors in patients with tuberous sclerosis and a review of the literature. Eur J Neurol. 2009;16(6):691–696. [DOI] [PubMed] [Google Scholar]
- 25. Guo A, Suresh V, Liu X, Guo F.. Clinicopathological features and microsurgical outcomes for giant pediatric intracranial tumor in 60 consecutive cases. Childs Nerv Syst. 2017;33(3):447–455. [DOI] [PubMed] [Google Scholar]
- 26. Tsai JD, Wei CC, Tsao TF, et al. Association between the growth rate of subependymal giant cell astrocytoma and age in patients with tuberous sclerosis complex. Childs Nerv Syst. 2016;32(1):89–95. [DOI] [PubMed] [Google Scholar]
- 27. Shepherd CW, Gomez MR, Lie JT, Crowson CS.. Causes of death in patients with tuberous sclerosis. Mayo Clin Proc. 1991;66(8):792–796. [DOI] [PubMed] [Google Scholar]
- 28. Roth J, Roach ES, Bartels U, et al. Subependymal giant cell astrocytoma: diagnosis, screening, and treatment. recommendations from the International Tuberous Sclerosis Complex Consensus Conference 2012. Pediatr Neurol. 2013;49(6):439–444. [DOI] [PubMed] [Google Scholar]
- 29. Sun P, Krueger D, Liu J, Guo A, Rogerio J, Kohrman MH.. Surgical resection of subependymal giant cell astrocytomas (SEGAs) and changes in SEGA-related conditions: a US national claims database study. Curr Med Res Opin. 2012;28(4):651–656. [DOI] [PubMed] [Google Scholar]
- 30. Amin S, Carter M, Edwards RJ, et al. The outcome of surgical management of subependymal giant cell astrocytoma in tuberous sclerosis complex. Eur J Paediatr Neurol. 2013;17(1):36–44. [DOI] [PubMed] [Google Scholar]
- 31. Kotulska K, Borkowska J, Roszkowski M, et al. Surgical treatment of subependymal giant cell astrocytoma in tuberous sclerosis complex patients. Pediatr Neurol. 2014;50(4):307–312. [DOI] [PubMed] [Google Scholar]
- 32. Torres OA, Roach ES, Delgado MR, et al. Early diagnosis of subependymal giant cell astrocytoma in patients with tuberous sclerosis. J Child Neurol. 1998;13(4):173–177. [DOI] [PubMed] [Google Scholar]
- 33. Moavero R, Coniglio A, Garaci F, Curatolo P.. Is mTOR inhibition a systemic treatment for tuberous sclerosis? Ital J Pediatr. 2013;39:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krueger DA, Wilfong AA, Mays M, et al. Long-term treatment of epilepsy with everolimus in tuberous sclerosis. Neurology. 2016;87(23):2408–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perek-Polnik M, Jóźwiak S, Jurkiewicz E, Perek D, Kotulska K.. Effective everolimus treatment of inoperable, life-threatening subependymal giant cell astrocytoma and intractable epilepsy in a patient with tuberous sclerosis complex. Eur J Paediatr Neurol. 2012;16(1): 83–85. [DOI] [PubMed] [Google Scholar]
- 36. Yalon M, Ben-Sira L, Constantini S, Toren A. Regression of subependymal giant cell astrocytomas with RAD001 (everolimus) in tuberous sclerosis complex. Childs Nerv Syst. 2011;27(1):179–181. [DOI] [PubMed] [Google Scholar]
- 37. Franz DN, Agricola KD, Tudor CA, Krueger DA.. Everolimus for tumor recurrence after surgical resection for subependymal giant cell astrocytoma associated with tuberous sclerosis complex. J Child Neurol. 2013;28(5):602–607. [DOI] [PubMed] [Google Scholar]
- 38. Bilimoria KY, Bentrem DJ, Stewart AK, Winchester DP, Ko CY.. Comparison of Commission on Cancer–approved and –nonapproved hospitals in the United States: implications for studies that use the National Cancer Data Base. J Clin Oncol. 2009;27(25):4177–4181. [DOI] [PubMed] [Google Scholar]
- 39. Raju GP, Urion DK, Sahin M. Neonatal subependymal giant cell astrocytoma: new case and review of literature. Pediatr Neurol. 2007;36(2):128–131. [DOI] [PubMed] [Google Scholar]
- 40. Watanabe Y, Oki S, Migita K, Isobe N, Okazaki T, Nabika S.. A case of subependymal giant cell astrocytoma not associated with tuberous sclerosis [article in Japanese]. No Shinkei Geka. 2003;31(5): 543–548. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.