Abstract
Background
Amino acid PET imaging of brain tumors has been shown to play an important role in predicting tumor grade, delineation of tumor margins, and differentiating tumor recurrence from the background of postradiation changes, but is not commonly used in clinical practice because of high cost. We propose that PET/MRI imaging of patients grouped to the day of tracer radiosynthesis will significantly decrease the cost of PET imaging, which will improve patient access to PET.
Methods
Seventeen patients with either primary brain tumors or metastatic brain tumors were recruited for imaging on 3T PET/MRI and were scanned on 4 separate days in groups of 3 to 5 patients. The first group of consecutively imaged patients contained 3 patients, followed by 2 groups of 5 patients, and a last group of 4 patients.
Results
For each of the patients, standard of care gadolinium-enhanced MRI and dynamic PET imaging with 18F-FDOPA amino acid tracer was obtained. The total cost savings of scanning 17 patients in batches of 4 as opposed to individual radiosynthesis was 48.5% ($28 321). Semiquantitative analysis of tracer uptake in normal brain were performed with appropriate accumulation and expected subsequent washout.
Conclusion
Amino acid PET tracers have been shown to play a critical role in the characterization of brain tumors but their adaptation to clinical practice has been limited because of the high cost of PET. Scheduling patient imaging to maximally use the radiosynthesis of imaging tracer significantly reduces the cost of PET and results in increased availability of PET tracer use in neuro-oncology.
Keywords: cost, FDOPA, PET/MRI, radioisotope, radiotracer, synthesis
Amino acid PET imaging of brain tumors has been shown to play an important role in the prediction of tumor grade, delineation of tumor margins, and differentiating tumor recurrence from the background of postradiation changes.1–8 The most common amino acid PET tracers that are used in imaging of brain tumors include 11C-methionine, 18F-FDOPA, and 18F-FET.1–8 Thus far, combined PET/CT has been used for PET imaging of brain tumors with subsequent coregistration of PET images onto separately acquired MRI. Introduction of simultaneous PET/MRI opened up the possibility of simultaneous imaging with MRI and PET.5,9–11 In body applications, simultaneous PET/MRI imaging of neuroendocrine tumors with 68Ga-DOTA-TATE and 68Ga-DOTA-TOC provides excellent evaluation of tumor metastatic burden with PET and additional detailed evaluation of liver lesions with dedicated MRI of the liver.12 PET/MRI has also been helpful in the evaluation of prostate cancer with 68Ga-PSMA-11, which allows simultaneous acquisition of dedicated pelvis MRI with the PET scan, thus providing detailed MRI evaluation of the pelvis for tumor extent in the prostate, seminal vesicles, and pelvic lymph nodes.12–14
Applications of PET/MRI in neurologic diseases include imaging of brain tumors,3,5,6,8,15–17 epilepsy,18,19 and neurodegenerative disorders.20 In brain tumors, imaging with 18F-FDOPA evaluates amino acid uptake by cells via L-type amino acid transporter 1 (LAT1) transporter that is present both on epithelial cells of the blood vessels and on the glioblastoma cells, allowing the tracer to bypass the blood-brain barrier and be transported into the glioblastoma cells. LAT1 transporter is involved in transporting a variety of amino acids, including the 11C-methionine and 18F-fluoro-ethyl-tyrosine (FET) tracers, and is expressed in up to 90% of glioblastoma cells.3–5,8,15–17,21–23
Even though amino acid PET has shown to be effective in evaluating brain tumors, the high cost of PET imaging and tracer synthesis has mostly kept this type of imaging in research and out of clinical practice.10 Our study evaluated the feasibility of performing simultaneous 18F-FDOPA PET/MRI on patients with either primary or metastatic brain tumors. Our goal was to identify the most efficient method of patient imaging and focused on maximizing the use of each batch of 18F-FDOPA production prepared for imaging of our patients. We started with imaging of 3 patients per day and increased to up to 5 patients per day from one batch of 18F-FDOPA production, resulting in efficient use of our radiochemistry facility and significant cost savings that may help expedite translation of 18F-FDOPA PET/MRI imaging to the clinic.
Methods
Seventeen patients with either primary brain tumors or metastatic brain tumors from lung and breast cancers were recruited for imaging on a 3T PET/MRI (GE SIGNA PET/MR). Patients were scanned in groups of 3, 4, or 5 per day. Patient groups were scheduled and a batch of tracer was synthesized the morning of the scan. This study was performed in compliance with HIPAA regulations and approved by institutional IRB. All patients signed IRB-approved consent forms prior to imaging.
The MRI portion of the exam included localizer sequence, axial brain volume imaging (BRAVO) T1 (a GE 3-dimensional [3D] inversion recovery spoiled gradient echo T1 weighted), axial BRAVO T1 postgadolinium, axial diffusion weighted imaging (DWI) (2-mm slice thickness), axial 3D susceptibility weighted angiography (SWAN), 3D CUBE (3D fast spin echo) fluid-attenuated inversion recovery (FLAIR), axial T2-weighted image (3-mm slice thickness) acquired after intravenous gadolinium administration, arterial spin labeling (ASL).
PET imaging was performed immediately after injection of 18F-FDOPA (185-222 MBq) and for the duration of the exam providing 35 to 45 minutes of PET data for each of the patients. PET data were obtained in list mode and dynamic multiframe data were created for each minute for the first 10 minutes after injection and for every 5 minutes for the time points at 10, 15, 20, 25, 30, 35, 40, and 45 minutes. The dynamic multiframe data were reconstructed using the vendor-provided time-of-flight–ordered subsets expectation maximization algorithm with 28 subsets, 5 iterations, and spatial Gaussian postfilter with 2 mm full-width at half maximum cutoff frequency. Regions of interest (ROI) were manually drawn along the borders of the tumor by a neuroradiologist (M.S.A.). The size of the ROI depended on the size of the tumor. Mean, maximum, and minimum standardized uptake values were generated for each time point. Cost of imaging scans were obtained from billing records.
Results
Seventeen patients with primary brain gliomas and patients with brain metastases from breast or lung cancer were imaged (Table 1). There were 10 patients with glioblastoma, isocitrate dehydrogenase (IDH) wild-type treated with radiation therapy and subsequent chemotherapy. There were 2 patients with anaplastic astrocytoma (patient 1: 1p19q intact, IDH1 R132H mutant, O6-methylguanine methyltransferase (MGMT) unmethylated; patient 2: IDH1 R132H wild-type, MGMT methylated, PTEN deleted, EGFR not amplified); and one patient with diffuse astrocytoma (molecular markers unknown) status postchemoradiotherapy. There were 4 patients with metastatic cancer—breast (2) or lung (2)—that demonstrated increased enhancement after radiation therapy. All of the patients were imaged within 1 year of radiation therapy.
Table 1.
Patient Characteristics. Seventeen PET/MRI Imaging Exams Were Performed on a GE PET/MRI 3 Tesla Scanner
| Characteristics | |
|---|---|
| Age, y | 57.1 ± 11.8 |
| Sex | 10 male, 7 female |
| Tumor types | Glioblastoma (10) Anaplastic astrocytoma (2) Astrocytoma (1) Metastatic breast cancer (2) Metastatic lung cancer (2) |
| PET imaging time, min | 35-45 |
| MRI sequences | DWI, ASL, T1, T2, 3D FLAIR, SPGR PG, T1 PG, SWAN |
Abbreviations: 3D FLAIR, 3-dimensional fluid-attenuated inversion recovery; ASL, arterial spin labeling; SPGR PG, spoiled gradient-recalled sequence post-gadolinium; SWAN, susceptibility-weighted angiography.
Groups of 3 to 5 patients were imaged on a single day on a GE PET/MRI 3T scanner. The first group of consecutively imaged patients contained 3 patients; second group 5 patients; third group 5 patients; and the last group 4 patients. The cost of the batch production of tracer for each of the groups is described in Table 2. Although the exact pricing shown in Table 2 is specific to our institution, a similar pricing practice is common at other radiopharmaceutical facilities across the country. For the 3-patient batch, the cost of tracer synthesis was $2605 per batch. If each of these patients were imaged individually, the cost of the tracer batch would be $7200. This results in a $4595 cost savings for a batch of 3 patients. For 2 batches of 5 patients, the cost savings for tracer synthesis is $9062 and $8532, respectively. For a batch of 4 patients, the cost savings for tracer synthesis is $6132. This totals to a cost savings of $28 321 for imaging of 17 patients with 18F-FDOPA PET. Therefore, the cost per patient in batch group is $1784.1 ± 138 vs $3715.8 ± 571 if patients were imaged on separate days.
Table 2.
Imaging of Patients the Same day as the Batch of 18F-DOPA Is Produced Results in Significant Cost Savings
| Batch scan | Cost of individual scans | |
|---|---|---|
| Batch 1 tracer | $2605 | $2400 × 3=$7200 |
| Pt 1 MRI | $1030 | $1030 |
| Pt 2 MRI | $1030 | $1030 |
| Pt 3 MRI | $1030 | $1030 |
| Batch 2 tracer | $2938 | $2400 × 5 = $12 000 |
| Pt 4 MRI | $1030 | $1030 |
| Pt 5 MRI | $1030 | $1030 |
| Pt 6 MRI | $1030 | $1030 |
| Pt 7 MRI | $1030 | $1030 |
| Pt 8 MRI | $1030 | $1030 |
| Batch 3 tracer | $3468 | $2400 × 5 = $12 000 |
| Pt 9 MRI | $1030 | $1030 |
| Pt 10 MRI | $1030 | $1030 |
| Pt 11 MRI | $1030 | $1030 |
| Pt 12 MRI | $1030 | $1030 |
| Pt 13 MRI | $1030 | $1030 |
| Batch 3 tracer | $3468 | $2400 × 4 = $9600 |
| Pt 14 MRI | $1030 | $1030 |
| Pt 15 MRI | $1030 | $1030 |
| Pt 16 MRI | $1030 | $1030 |
| Pt 17 MRI | $1030 | $1030 |
| Total | $29 989 | $58 310 |
Abbreviation: Pt, patient.
Production of a batch of tracer includes costs of materials and personnel. In our study, multiple patients were scanned the same day a batch of tracer was produced. In our first group, 3 patients were imaged with a batch of tracer costing $2605. If these 3 patients were imaged individually, the separate production costs of tracer would have been $7200. Cost of the MRI portion of the exam was the same for batch-imaged patients and if patients were imaged separately.
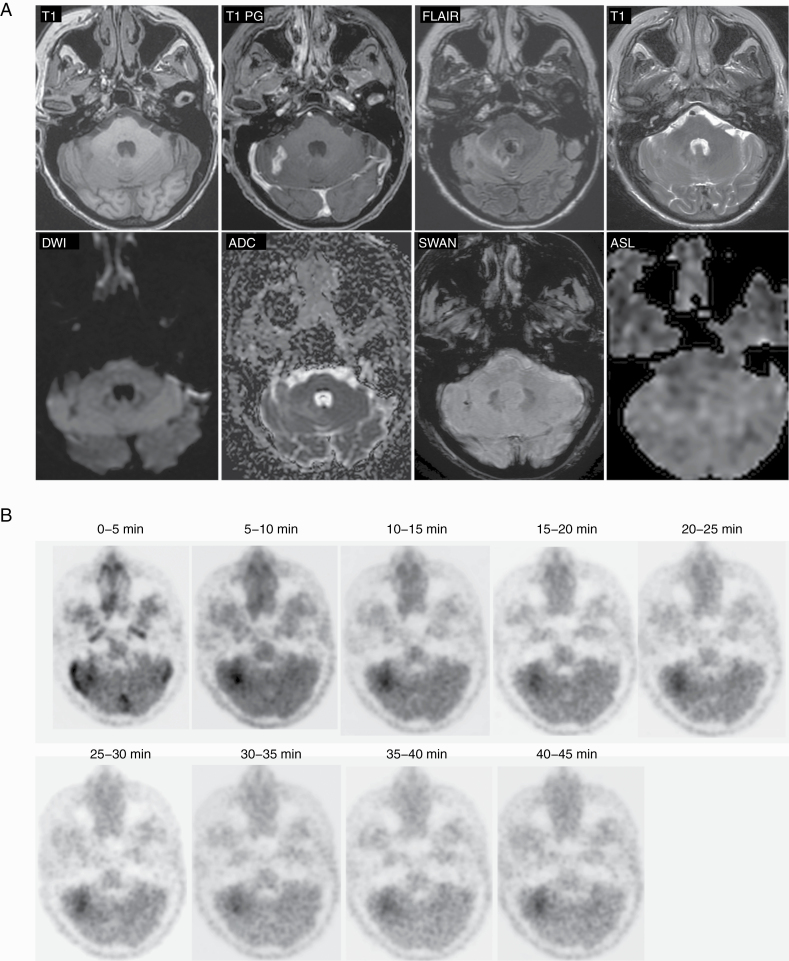
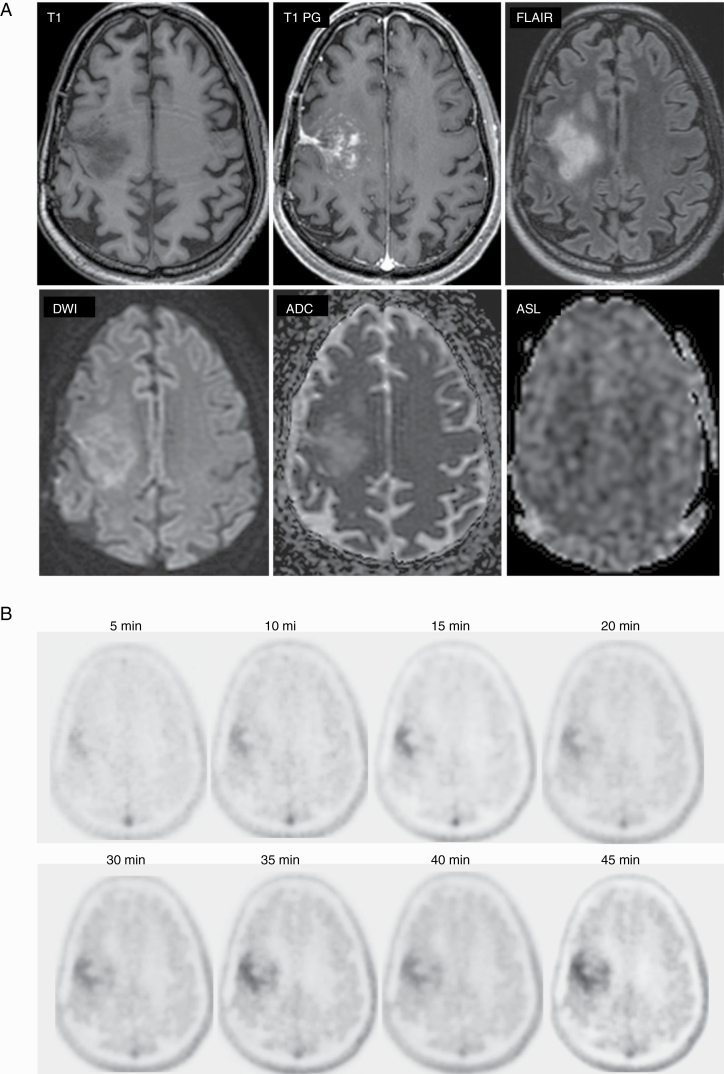
For each of the patients, clinical-grade MRI was obtained that included T1 pregadolinium and postgadolinium imaging, 3D FLAIR, T2, DWI, susceptibility weighted imaging with SWAN, and perfusion imaging with ASL (Figures 1 and 3). Clinical-quality images were obtained for all 17 of the imaged patients, and clinical interpretation was performed on all of the MRI portions of the scan. MRI and PET images were diagnostic both for brain metastases (see Figure 1) and high-grade gliomas (see Figure 3).
Figure 1.
18F-DOPA PET/MRI imaging of patient with metastatic breast cancer to the right cerebellar hemisphere status post–Gamma Knife radiosurgery 7 months prior to imaging. Diagnostic quality MRI of the brain was obtained at the same time as PET imaging. PET imaging was performed for 45 minutes.
Figure 3.
18F-DOPA PET/MRI imaging of patient with glioblastoma, isocitrate dehydrogenase 1 wild-type and MGMT (O6-methylguanine methyltransferase) methylated and undergoing pembrolizumab treatment. Diagnostic quality MRI of the brain was obtained at the same time as PET imaging demonstrating heterogeneously enhancing tumor with no evidence of increased perfusion. PET imaging was performed for 45 minutes and demonstrated focal tracer uptake within the region of the tumor.
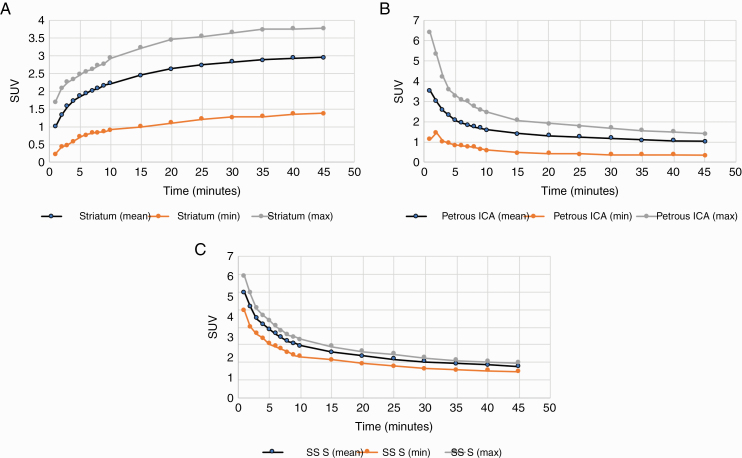
Dynamic PET acquisition was obtained for each of the 17 patients for 35 to 45 minutes after injection of the tracer (see Figure 2). Dynamic PET analysis was successful in all of the patients. Semiquantitative analysis of tracer uptake within the striatum demonstrated an appropriate gradual increase in tracer uptake within the striatum over a period of 35 minutes, with reaching of plateau by 40 minutes after tracer injection. Semiquantitative analysis of tracer washout from the internal carotid artery demonstrated a rapid decrease in blood pool tracer within the first 10 to 15 minutes, with reaching of plateau by 20 minutes after injection. Semiquantitative analysis of tracer washout from the superior sagittal sinus demonstrates a rapid decrease in tracer uptake from the venous blood pool within the first 10 to15 minutes and reaching a plateau by 20 minutes after injection.
Figure 2.
Analysis of 18F-DOPA uptake within A, striatum, and B, petrous internal carotid artery and superior sagittal sinus (SSS). Mean, maximum, and minimum standardized uptake values were measured within a region of interest placed within either caudate nucleus, petrous portion of the internal carotid artery, or within the superior sagittal sinus. The values were measured on images acquired over time to determine kinetic curves of tracer uptake.
Discussion
Our study shows that amino acid hybrid PET/MRI imaging of brain tumors is feasible and significant cost savings could be obtained through patient batching. The availability of PET tracers and significant cost of tracer synthesis and delivery have limited the use of PET in clinical practice and research. An average PET/CT with novel tracer can cost up to $5000, depending on the institution. Therefore, in addition to the discovery of novel tracers and their applications to clinical practice, it is critical to consider factors that contribute to the high cost of PET imaging. Our study demonstrates that clustering patients to a single clinical day with single-batch synthesis of tracer leads to up to 48.5% in direct cost savings. These costs do not account for factors of clinical charges to the patient and insurance reimbursement, because 18F-FDOPA is not currently FDA approved for neuro-oncology use. Depending on regional and local payers, imaging can be considered appropriate and therefore its cost absorbed by insurance with an investigational new drug application (IND) in place. By performing cost-recovery IND, the cost of the radiopharmaceutical would decrease as well.
18F-FDOPA PET tracer is an analog of phenylalanine amino acid normally transported into glioblastoma cells via the LAT1 transporter. It is used to determine glioblastoma tumor margins after resection and to differentiate tumor recurrence from background postradiation changes. In our study, we show that simultaneous acquisition of dynamic 18F-FDOPA PET and clinical-quality MRI of the brain is possible in patients with primary brain tumors such as glioblastoma and astrocytoma and in patients with metastatic brain tumors from lung and breast primaries. All of our patients were treated for their primary disease with either resection and/or radiation therapy and were recruited during the time of suspected recurrence. In our recruited patients, we performed 17 PET/MRI hybrid scans while preparing only 4 batches of 18F-FDOPA. This resulted in more efficient use of our radiochemistry laboratory and significantly reduced the price of radiotracer preparation. In all of our patients, we were able to obtain dynamic continuous PET imaging ranging from 35 to 45 minutes and all patients with diagnostic MRI received a clinical read and were evaluated by the neuro-oncology tumor board. The limitations of our study include small sample size and a heterogeneous collection of patients with high-grade gliomas, astrocytomas, and metastatic breast and lung cancer. In addition, there is an inherent limitation of the PET scan, which is the exposure to radiation from the 18F-labeled tracer that is not present in MRI scans. The radiation dose from imaging of the brain was similar between 18F-FDG and 18F-FDOPA.24 Therefore, use of amino acid PET in clinical practice should follow the guidance of clinical trials that define the intervention window where PET should be used and where follow-up MRI is sufficient. To establish these guidelines and methods for translating advanced molecular imaging methods into clinical practice, extensive research is needed and our method will provide a way to significantly decrease the cost of implementing research PET scans.
The major challenge of batching PET/MRI scans was timely recruitment of patients. We recruited patients by direct communication with neuro-oncologists and by participating in a tumor board, which allowed us to identify 3 to 5 patients per scanning day. All of the recruited patients presented for imaging but on one of the last imaging days, only 4 patients were able to be recruited. Adequate recruitment to imaging may present as a challenge in using this method, although patients were motivated to undergo this imaging scan and none turned down imaging. Another challenge of this method is communication of batch request to the radiochemist for synthesis the morning of the scan. The synthesis would start early in the morning around 4:30 am and would complete by 10:30 am. Therefore, if a patient cancels the morning of the scan, there is no time to recruit another patient in his or her place, which highlights the need to discuss the obstacles of imaging with patients prior to the day of PET/MRI.
To our knowledge, this is the first study describing the use of single-batch PET amino acid tracer preparation for scanning of multiple patients. This method allows imaging of patients with PET tracers in a more cost-efficient manner and can result in the increased availability of PET tracer use in neuro-oncology practice.
Funding
This work was supported by National Institutes of Health [T32 grant 5T32EB001631-12 to M.S.A.]; and National Center for Advancing Translational Science (NCATS) [CTSA grant KL2 TR001862 to M.S.A.].
Conflict of interest statement. None declared.
References
- 1. Zaragori T, Ginet M, Marie PY, et al. Use of static and dynamic [18F]-F-DOPA PET parameters for detecting patients with glioma recurrence or progression. EJNMMI Res. 2020;10(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tinkle CL, Duncan EC, Doubrovin M, et al. Evaluation of 11C-methionine PET and anatomic MRI associations in diffuse intrinsic pontine glioma. J Nucl Med. 2019;60(3):312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiaravalloti A, Fiorentini A, Villani V, et al. Factors affecting 18F FDOPA standardized uptake value in patients with primary brain tumors after treatment. Nucl Med Biol. 2015;42(4):355–359. [DOI] [PubMed] [Google Scholar]
- 4. Law I, Albert NL, Arbizu J, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46(3):540–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marner L, Henriksen OM, Lundemann M, et al. Clinical PET/MRI in neurooncology: opportunities and challenges from a single-institution perspective. Clin Transl Imaging. 2017;5(2):135–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Misch M, Guggemos A, Driever PH, et al. F-18-FET-PET guided surgical biopsy and resection in children and adolescence with brain tumors. Child Nerv Syst 2015;31(2): 261–26 7. [DOI] [PubMed] [Google Scholar]
- 7. Verger A, Stegmayr C, Galldiks N, et al. Evaluation of factors influencing 18F-FET uptake in the brain. Neuroimage Clin. 2018;17:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu J, Zheng J, Xu W, et al. Accuracy of 18F-FDOPA positron emission tomography and 18F-FET positron emission tomography for differentiating radiation necrosis from brain tumor recurrence. World Neurosurg. 2018;114:e1211–e1224. [DOI] [PubMed] [Google Scholar]
- 9. Fraum TJ, Fowler KJ, McConathy J. PET/MRI: emerging clinical applications in oncology. Acad Radiol. 2016;23(2):220–236. [DOI] [PubMed] [Google Scholar]
- 10. Mayerhoefer ME, Prosch H, Beer L, et al. PET/MRI versus PET/CT in oncology: a prospective single-center study of 330 examinations focusing on implications for patient management and cost considerations. Eur J Nucl Med Mol Imaging. 2020;47(1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Riola-Parada C, García-Cañamaque L, Pérez-Dueñas V, et al. Simultaneous PET/MRI vs PET/CT in oncology. A systematic review. Rev Esp Med Nucl Imagen Mol. 2016;35(5):306–312. [DOI] [PubMed] [Google Scholar]
- 12. Wibmer AG, Hricak H, Ulaner GA, et al. Trends in oncologic hybrid imaging. Eur J Hybrid Imaging. 2018;2(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burger IA, Müller J, Donati OF, et al. 68Ga-PSMA-11 PET/MR detects local recurrence occult on mpMRI in prostate cancer patients after HIFU. J Nucl Med. 2019;60(8):1118–1123. [DOI] [PubMed] [Google Scholar]
- 14. Grubmüller B, Baltzer P, Hartenbach S, et al. PSMA ligand PET/MRI for primary prostate cancer: staging performance and clinical impact. Clin Cancer Res. 2018;24(24):6300–6307. [DOI] [PubMed] [Google Scholar]
- 15. Bhunia S, Vangala V, Bhattacharya D, et al. Large amino acid transporter 1 selective liposomes of l-DOPA functionalized amphiphile for combating glioblastoma. Mol Pharm. 2017;14(11):3834–3847. [DOI] [PubMed] [Google Scholar]
- 16. Gauvain K, Ponisio MR, Barone A, et al. 18F-FDOPA PET/MRI for monitoring early response to bevacizumab in children with recurrent brain tumors. Neurooncol Pract. 2018;5(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lohmann P, Werner JM, Shah NJ, et al. Combined amino acid positron emission tomography and advanced magnetic resonance imaging in glioma patients. Cancers (Basel) 2019;11(2):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oldan JD, Shin HW, Khandani AH, et al. Subsequent experience in hybrid PET-MRI for evaluation of refractory focal onset epilepsy. Seizure. 2018;61:128–134. [DOI] [PubMed] [Google Scholar]
- 19. Strohm T, Steriade C, Wu G, et al. FDG-PET and MRI in the evolution of new-onset refractory status epilepticus. AJNR Am J Neuroradiol. 2019;40(2):238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mainta IC, Perani D, Delattre BM, et al. FDG PET/MR imaging in major neurocognitive disorders. Curr Alzheimer Res. 2017;14(2):186–197. [DOI] [PubMed] [Google Scholar]
- 21. Vander Borght T, Asenbaum S, Bartenstein P, et al. ; European Association of Nuclear Medicine (EANM) . EANM procedure guidelines for brain tumour imaging using labelled amino acid analogues. Eur J Nucl Med Mol Imaging. 2006;33(11):1374–1380. [DOI] [PubMed] [Google Scholar]
- 22. Tsuyuguchi N, Terakawa Y, Uda T, et al. Diagnosis of brain tumors using amino acid transport PET imaging with 18F-fluciclovine: a comparative study with L-methyl-11C-methionine PET imaging. Asia Ocean J Nucl Med Biol. 2017;5(2):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Youland RS, Kitange GJ, Peterson TE, et al. The role of LAT1 in F-18-DOPA uptake in malignant gliomas. J Neuro-Oncol 2013;111(1):11–1 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martí-Climent JM, Prieto E, Morán V, et al. Effective dose estimation for oncological and neurological PET/CT procedures. EJNMMI Res. 2017;7(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]