Abstract
Over the past 4 years, advances in molecular pathology have enhanced our understanding of CNS tumors, providing new elements to refine their classification and improve the 2016 World Health Organization (WHO) Classification of CNS tumors. The Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy—Not Official WHO (cIMPACT-NOW) was formed in late 2016 by a group of neuropathology and neuro-oncology experts to provide practical recommendations (published as cIMPACT-NOW updates) to improve the diagnosis and classification of CNS tumors, in advance of the publication of a new WHO Classification of CNS tumors. Here we review the content of all the available cIMPACT-NOW updates and discuss the implications of each update for the diagnosis and management of patients with CNS tumors.
Keywords: brain tumor classification, cIMPACT-NOW, glioblastoma, IDH-mutant gliomas, molecular pathology
The 2016 World Health Organization (WHO) Classification of CNS tumors formally incorporated molecular characteristics into the definition of many of these tumors. This not only reflects a paradigm shift in their classification, but also affects the clinical management of patients with these tumors.1–3 Since 2016, ongoing discoveries in molecular pathology have advanced our understanding of many of the entities organized under the WHO 2016 classification. Given that some of these more recent discoveries carry important implications for clinical practice and for the design and interpretation of clinical trials, an international group of leading neuropathologists—all of whom were directly involved in establishing the WHO 2016 classification—formed the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy—Not Official WHO (cIMPACT-NOW) to communicate them in advance of the release of a new WHO CNS tumor classification.4 The importance of the cIMPACT-NOW updates to neuro-oncology practice was foreseen from the beginning, and the group involved a clinical advisory panel.4 Meanwhile, the fact that these updates have been published in neuropathology journals4–11 may have limited the propagation of this information to the wider clinical neuro-oncology community. The present article reviews the highlights of the published cIMPACT-NOW updates 1 through 7 and discusses their implications for the management of patients with CNS tumors in current neuro-oncology practice.
cIMPACT-NOW Update 1: Not Otherwise Specified and Not Elsewhere Classified
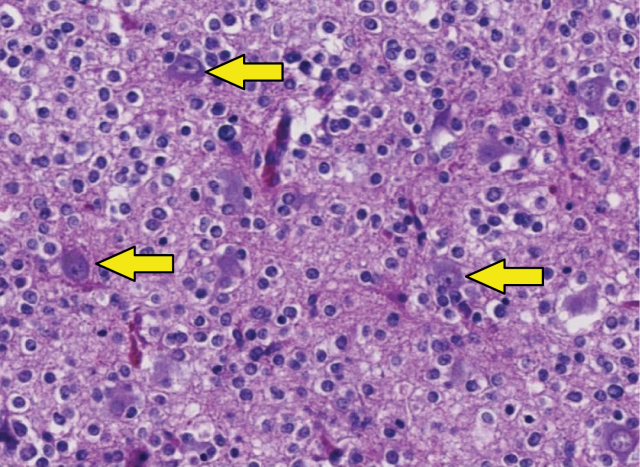
This first update refines the meaning of the “not otherwise specified” (NOS) designation in diagnoses (eg, “glioblastoma, NOS”) and limits it to diagnoses in which molecular testing is not available (eg, in low-resource settings) or was performed but did not yield adequate results (assay failure), or was deliberately not performed (eg, not testing isocitrate dehydrogenase [IDH] status in an elderly patient with glioblastoma because of lack of implications for therapeutic management).5 When confronting an NOS diagnosis, the clinician may want to discuss the need and/or possibility for additional molecular testing with the pathologist (eg, a diagnosis of “glioblastoma, NOS” in a relatively young [< 55 years] patient). This update also introduces the designation “not elsewhere classified” (NEC), which is to be used when molecular testing has been performed and yielded adequate results, but the results do not lead to a precise categorization of the tumor within the framework of the WHO 2016 classification.5 The NEC designation would apply, for example, to a diffuse glioma in an adult patient that histologically has prototypical oligodendroglial histology, but that is IDH-wildtype and in which differential diagnostic alternatives are not a good fit either. In such a case, the integrated diagnosis could be reported as “oligodendroglioma, NEC” (Figure 1).
Figure 1.
Example of oligodendroglioma, not elsewhere classified (NEC). In the hematoxylin-eosin–stained section, the histology of this tumor in the temporal lobe of a 40-year-old woman is fully compatible with a diffuse glioma (arrows indicate preexistent neurons that are overrun by diffusely infiltrating tumor cells). The tumor cells have a prototype oligodendroglial phenotype: “fried-egg” appearance with round nuclei and a clear halo. However, unlike “canonical” oligodendrogliomas as defined in the World Health Organization 2016 classification, this tumor was isocitrate dehydrogenase (IDH) wild-type and did not show codeletion of chromosome arms 1p and 19q. After ruling out other tumors that may have an oligodendroglial phenotype (especially dysembryoplastic neuroepithelial tumor, [extraventricular] neurocytoma, clear cell ependymoma, and pilocytic astrocytoma) and demonstrating that this tumor did not have molecular features of IDH–wild-type glioblastoma (see Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy—Not Official WHO update 3), the tumor was signed out as “oligodendroglioma, NEC.”
cIMPACT-NOW Update 2: Clarifying the Diagnosis of Diffuse Midline Glioma, Histone 3 K27M-Mutant, and of Diffuse Astrocytoma/Anaplastic Astrocytoma, Isocitrate Dehydrogenase Mutant6
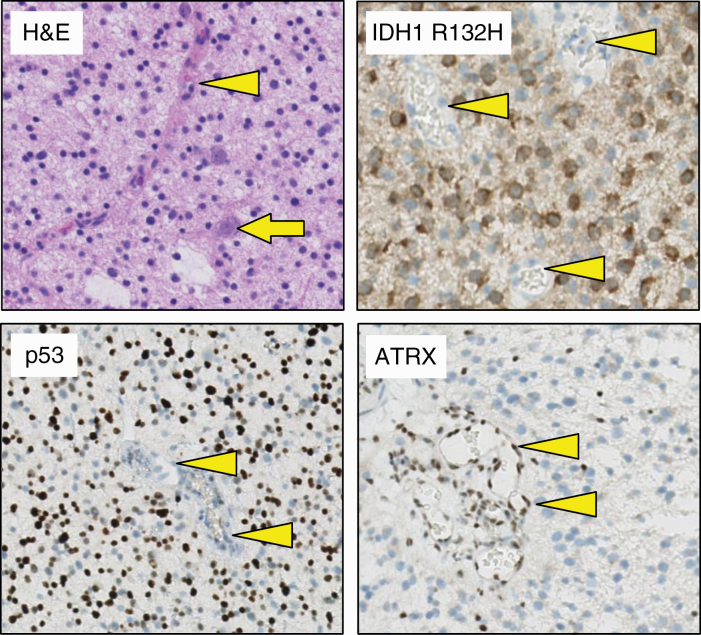
This second update addresses 2 separate issues. First, it clarifies that for a tumor to be signed out as diffuse midline glioma, histone 3 (H3) K27M-mutant, all the attributes listed in the diagnosis must be satisfied. In other words, the tumor must show diffuse infiltrative growth in the CNS parenchyma, affect “midline” structures (brainstem, thalamus, spinal cord), have glioma histology, and demonstrate the presence of an H3 K27M mutation.6 This clarification is important because there are other tumors with H3 K27M mutations that are not diffuse midline gliomas (eg, pilocytic astrocytomas and ependymomas) and have different prognoses.12 The second issue addresses an alternative route for obtaining a bona fide diagnosis of diffuse astrocytoma, IDH mutant, or anaplastic astrocytoma, IDH mutant. Per the 2016 WHO classification, these diagnoses require the presence of an IDH1 or IDH2 mutation AND the absence of a 1p/19q codeletion. The update suggests surrogate markers for this latter criterion: The diagnosis diffuse astrocytoma, IDH mutant, and anaplastic astrocytoma, IDH mutant can also be made in the absence of 1p/19q testing as long as the tumors immunohistochemically show clear loss of ATP-dependent X-linked helicase (ATRX) nuclear expression and/or strong and diffuse nuclear staining for p536 (Figure 2).
Figure 2.
Immunohistochemical surrogate markers enabling discrimination of astrocytoma, isocitrate dehydrogenase (IDH)-mutant from oligodendroglioma, IDH mutant. Hematoxylin-eosin–stained section of a tumor in a 52-year-old man reveals a diffuse, histologically low-grade glioma (preexistent neuron indicated by arrow). Using immunohistochemistry, this tumor can readily be characterized as IDH-mutant (tumor cells positive for IDH1 R132H mutant protein). In addition, because the tumor cell nuclei are strongly and extensively positive for p53 but negative for ATRX, immunohistochemistry allows for designating this tumor as astrocytoma, IDH-mutant (and discarding the diagnosis oligodendroglioma, IDH-mutant and 1p/19q-codeleted). The tumor microvasculature (indicated by arrowheads) serves as an internal control for the immunohistochemical stains (negative for IDH R132H–mutant protein and p53, positive for ATRX).
cIMPACT-NOW Update 3: Molecular Clues for Recognizing Histologically Lower-Grade, Diffuse Astrocytic Gliomas, Isocitrate Dehydrogenase (IDH)–Wild-Type (WT) as Glioblastoma, IDH-WT (WHO Grade IV)
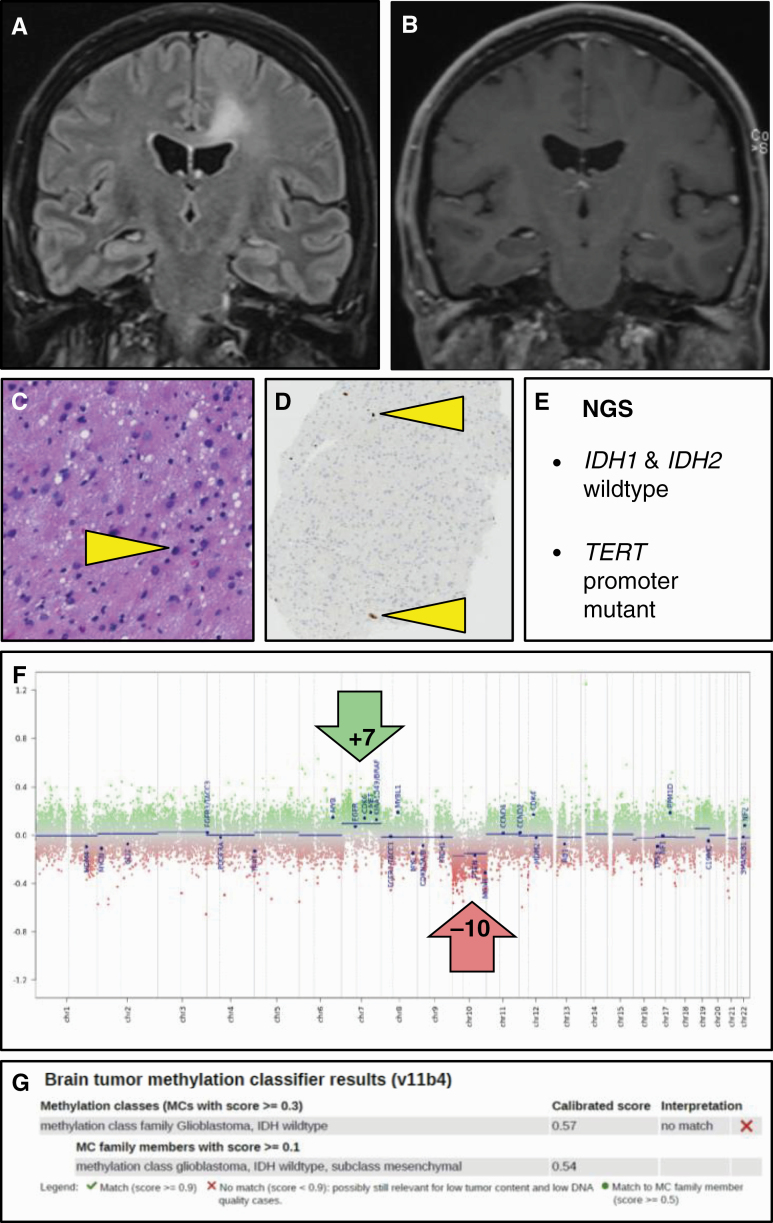
The presence of necrosis and/or florid microvascular proliferation is a cornerstone for the histopathological diagnosis of a diffuse astrocytic glioma, IDH–wild-type as glioblastoma (WHO grade IV). However, even if these histological features are lacking, for example, in biopsies of lesions in deep or eloquent areas, most histologically lower-grade (WHO grade II or III) diffuse astrocytic gliomas, IDH–wild-type in adult patients behave as a WHO grade IV lesion. The third cIMPACT-NOW update presents molecular criteria that can be used for upgrading the diagnosis of such histologically lower-grade, IDH–wild-type, astrocytomas to glioblastoma, IDH–wild-type (WHO grade IV). This concerns the following 3 criteria or any combination thereof: concurrent gain of whole chromosome 7 and loss of whole chromosome 10 (+7/–10); TERT promoter mutation; epidermal growth factor receptor (EGFR) amplification7 (Figure 3). A recent study fully supports this recommendation: For 71 adult patients with histologically lower-grade, IDH–wild-type diffuse astrocytic gliomas and MRI findings consistent with WHO grade II or III, the presence of 1 or more of the 3 aforementioned molecular markers indeed implied a prognosis consistent with that of glioblastoma.13 Consequently, molecular analysis of WHO grade II or III diffuse astrocytic, IDH–wild-type gliomas in adult patients is strongly recommended, as demonstration of +7/–10, EGFR amplification and/or TERT promoter mutation allows for upgrading the tumor to WHO grade IV with important therapeutic and prognostic implications. Of note, because the formal diagnosis proposed in this update (“Diffuse astrocytic glioma, IDH–wild-type, with molecular features of glioblastoma, WHO grade IV”) is somewhat cumbersome, cIMPACT-NOW update 6 suggests just calling these tumors glioblastomas.
Figure 3.
Histologically low-grade isocitrate dehydrogenase (IDH)–wild-type astrocytoma with molecular features of glioblastoma. A, T2–fluid-attenuated inversion recovery MRI of this 54-year-old male patient reveals a lesion that is compatible with diffuse low-grade glioma; in B, T1-weighed postgadolinium scans, the lesion is not enhancing. C and D, Hematoxylin-eosin staining of the needle biopsy material reveals only a slight increase in cellularity with mild nuclear pleomorphism and variably increased size of the (eosinophilic) cytoplasm, while Ki-67 staining reveals only very few proliferating cells (some positive nuclei indicated by arrowheads); these histopathological findings are compatible with the diagnosis of diffuse low-grade glioma. E, Next-generation sequencing of this lesion, however, reveals the absence of IDH1 and IDH2 mutation, but the presence of a TERT promoter mutation, meaning that the lesion according to cIMPACT-NOW update 3 qualifies as glioblastoma, IDH–wild-type, F, The fact that the copy number profile (obtained by performing methylome analysis) reveals gain of complete chromosome 7 combined with loss of complete chromosome 10 fully supports this diagnosis. G, Analysis of the methylome profile of this lesion using the Heidelberg Brain Tumor Classifier14,15 also suggests the diagnosis glioblastoma, IDH–wild-type as best match; the low tumor cell content in this biopsy material may well explain the suboptimal score for this diagnosis (as well as the relatively subtle levels of chromosome 7 gain and chromosome 10 loss in F). cIMPACT-NOW, Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy—Not Official WHO.
cIMPACT-NOW Update 4: Classification of “Pediatric-Type” Diffuse Gliomas, Isocitrate Dehydrogenase–Wild-Type, Histone 3–Wild-Type on the Basis of Presence of Myb Proto-Oncogene, Myb Proto-Oncogene Like 1, FGFR1 Alteration, B-Raf Proto-Oncogene, Serine/Threonine Kinase V600E Mutation, or Other Mitogen-Activated Protein Kinase Pathway Alterations
This update highlights the diagnostic relevance of particular molecular alterations that are frequently found in histologically low-grade, diffuse gliomas in children. If such a tumor does not have an IDH or H3 K27M mutation, and does not have molecular alterations associated with glioblastoma (see update 3), based on its molecular features, the tumor can be diagnosed as diffuse glioma, Myb proto-oncogene (MYB)-altered, or Myb proto-oncogene like 1 (MYBL1)-altered, fibroblast growth factor receptor 1 (FGFR1)-mutant, FGFR1 tyrosine-kinase duplicated, B-Raf proto-oncogene, serine/threonine kinase (BRAF) V600E–mutant (but not if it has a concurrent deletion in CDKN2A/B), or as “Mitogen-Activated Protein Kinase (MAPK) pathway-altered.” 8 This refined categorization of pediatric-type diffuse gliomas allows for greater precision when discussing prognosis with the children and/or their parents and has potential implications for targeted treatment (eg, targeting BRAF V600E mutations with BRAF inhibitors like dabrafenib16) as well as the design of clinical trials. When confronted with a diagnosis of an IDH- and H3–wild-type diffuse glioma in a child, it is thus important to further characterize the tumor at the molecular level to find out if it concerns a tumor with a specific alteration that signifies a generally favorable prognosis and could potentially be targeted.
cIMPACT-NOW Update 5: Improved Grading of Isocitrate Dehydrogenase–Mutant Astrocytomas on the Basis of Cyclin-Dependent Kinase Inhibitor 2A/B Homozygous Deletion Status17
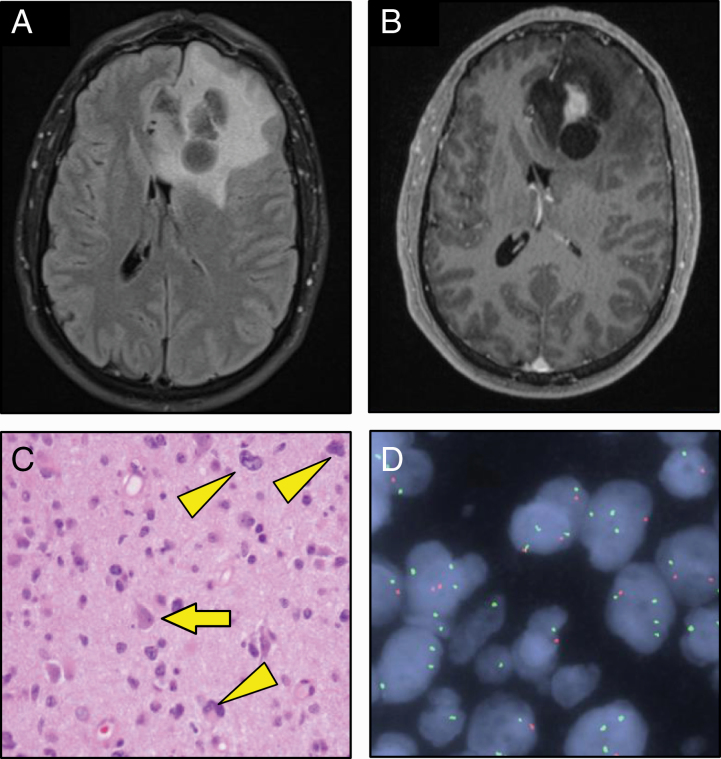
Although IDH-mutant diffuse astrocytic tumors are known to have a more favorable prognosis than their IDH–wild-type counterparts,18,19 the presence of a homozygous deletion of cyclin-dependent kinase inhibitor 2A/B (CDKN2A/B) is now recognized as an important negative prognostic factor within the former group.20 In fact, IDH-mutant astrocytomas that harbor homozygous CDKN2A/B deletion behave clinically as high-grade malignant tumors.20 The cIMPACT-NOW update 5 recommends grading IDH-mutant astrocytomas as WHO grade II in the absence of anaplastic features, significant mitotic activity, and homozygous CDKN2A/B deletion; as WHO grade III if anaplastic features and significant mitotic activity are present but there is no homozygous deletion of CDKN2A/B; and as WHO grade IV not only if the tumor histologically shows microvascular proliferation and/or necrosis, but also if molecular analysis reveals the presence of homozygous CDKN2A/B deletion17 (Figure 4). Testing for CDKN2A/B deletions is important for optimal counseling of patients with an IDH-mutant diffuse astrocytoma because the presence of homozygous CDKN2A/B deletion in these tumors signifies high-grade malignant behavior.
Figure 4.
Homozygous cyclin-dependent kinase inhibitor 2A/B (CDKN2A/B) loss in isocitrate dehydrogenase (IDH)-mutant astrocytoma: grade 4. A and B, MRI images of a left frontal lobe lesion in a 45-year-old man demonstrating increased A, T2–fluid-attenuated inversion recovery signal and contrast enhancement on B, T1 postgadolinium sequences. C, Histology with infiltrating pleomorphic astrocytic cells with high nuclear-to-cytoplasmic ratios (arrowheads) and only a few scattered mitoses; because necrosis and microvascular proliferation were absent, the histopathological diagnosis was anaplastic astrocytoma, IDH-mutant, World Health Organization grade III (arrow indicates preexistent neuron). D, Fluorescent in situ hybridization assay to analyze CDKN2A copy number status. A CDKN2A locus-specific probe (red) and a chromosome 9 centromeric probe (CEP9; green) are hybridized and used to assess the CDKN2A copy number status scored in approximately 50 nuclei.21 A signal pattern of more than 10% of nuclei with no CDKN2A signal and at least one CEP9 signal is considered diagnostic of homozygous deletion of CDKN2A.21 The present sample demonstrated loss of CDKN2A signal and preservation of CEP9 signal in 24 of 50 cells counted, consistent with homozygous deletion of CDKN2A and thus reason to upgrade the tumor grade to IV.
cIMPACT-NOW Update 6: Recommendations on Emerging New Entities and Diagnostic Principles for Future CNS Tumor Classification and Grading (cIMPACT-Utrecht meeting report)11
The cIMPACT-6 update summarizes the consensus from a working group meeting in September 2019 in Utrecht, the Netherlands, preparing the next WHO classification of CNS tumors. The update makes a number of recommendations regarding diagnostic principles and tumor nomenclature and acknowledges the utility of methylome profiling for CNS tumor classification and diagnosis. In line with cIMPACT-NOW update 3, it is indeed now recommended to designate diffuse, histologically lower-grade, IDH–wild-type astrocytomas that have molecular features of glioblastoma directly as “glioblastoma, IDH–wild-type” because this would help to eliminate confusion and facilitate inclusion of these patients into clinical trials. Furthermore, the cIMPACT-Utrecht meeting not only approves the suggestion of cIMPACT-NOW update 5 to use the presence of homozygous CDKN2A/B deletion as a marker for the highest malignancy grade in the group of diffuse, IDH-mutant astrocytomas, but proposes the term “Astrocytoma, IDH-mutant, grade 4” (rather than glioblastoma, IDH-mutant) for this subgroup. This latter term makes it easier to discriminate the IDH-mutant astrocytic tumors of the highest malignancy grade from the (much more frequent and even more aggressive) IDH–wild-type glioblastomas. Lastly, update 6 proposes definitions and characteristic features for multiple emerging new entities, many of which can be accurately characterized by specific molecular features (eg, diffuse glioma, H3.3 G34-mutant, and spinal ependymoma, MYCN proto-oncogene [MYCN]-amplified).
cIMPACT-NOW Update 7: Refining the Classification of Ependymomas Using Molecular Features22
This most recent cIMPACT-NOW update harnesses findings from methylome profiling to upgrade the classification of ependymomas. Most notably, the update recommends that ependymomas be classified by both anatomic site and molecular features to allow for improved assessment of prognosis and therapeutic management. Supratentorial (ST) ependymomas, when possible, should be classified according to the presence of chromosome 11 open reading frame 95 (c11orf95) or yes-associated protein 1 (YAP1) gene fusions. At this time, there are no sufficient data to assign a WHO grade to these. For example, by far the most frequent ST ependymoma, ependymoma with a c11orf95 fusion (previously called ependymoma, RELA fusion-positive), can be graded as 2 or 3. Based on the degree of histone H3 K27-trimethylation, posterior fossa ependymomas should be classified as type A or type B (PFA or PFB), characterized by respectively the absence or presence of H3 K27me3 staining of tumor cell nuclei. PFA ependymomas (particularly those with gain of chromosome 1q) are known to have a worse prognosis, but more survival data need to be collected before a definitive grade can be assigned to these posterior fossa tumors. The update also recognizes ependymoma of the spinal cord with MYCN amplification as a new entity associated with poor clinical outcome and recommends considering myxopapillary ependymomas of the spinal cord as grade 2 (rather than grade 1) tumors. Lastly, the update recommends not awarding the papillary, clear cell, and tanycytic variant of classic ependymoma with a separate status in the CNS tumor classification anymore because of a lack of specific clinical utility.
Concluding Remarks
Since the publication of the revised fourth edition of the WHO Classification of CNS Tumors in 2016, cIMPACT-NOW has published several guidelines for improved pathological diagnosis of these neoplasms. Most of these translate to recent advances in our understanding of the molecular underpinnings of CNS tumors. Salient recommendations include those that provide guidance for how even in the absence of histopathological characteristics of the highest malignancy grade, molecular markers can be used to reach a diagnosis of glioblastoma, IDH–wild-type or astrocytoma, IDH-mutant, grade IV. The cIMPACT-NOW guidelines (Table 1) have important implications for clinical practice and for the design and interpretation of clinical trials. Additionally, cIMPACT-NOW has proposed several changes regarding diagnostic principles and nomenclature, as well as made suggestions on which tumor types have now emerged as mature enough to deserve a separate status in a next WHO classification.
Table 1.
Implications of Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy—Not Official World Health Organization Updates 1 to 7 for Clinical Practice
| cIMPACT-NOW update | Implications | Ref. |
|---|---|---|
| 1 | • NOS added to pathological diagnosis: results of molecular testing required for a more precise diagnosis as listed in WHO classification not available; (re-)consider the need and/or possibility for additional molecular testing | 5 |
| • NEC added to pathological diagnosis: indicates a diagnosis that, after adequate (molecular) testing, is not listed as such in most recent WHO classification | ||
| 2 | • Not every H3 K27M–mutant tumor is a diffuse midline glioma and carries the same dismal prognosis | 6 |
| • Diffuse (anaplastic) astrocytoma, IDH-mutant can be diagnosed using ATRX and/or p53 expression as immunohistochemical surrogate markers for absence of 1p/19q codeletion | ||
| 3 | • Demonstration of +7/–10, EGFR amplification, and/or TERT promoter mutation in WHO grade II or III, IDH–wildtype diffuse gliomas in adult patients allows for the diagnosis glioblastoma, IDH–wild-type | 7 |
| 4 | • IDH– and H3–wild-type diffuse glioma in children that are driven by MYB or MYBL1 gene rearrangement, FGFR1 mutation, or FGFR1 tyrosine kinase domain duplication, BRAF V600E mutation, or by another MAPK pathway alteration generally have favorable prognosis | 8 |
| • Molecular defect in these tumors represents a potential therapeutic target | ||
| 5 | • Homozygous CDKN2A/B deletion in IDH-mutant diffuse astrocytoma signifies grade IV malignant behavior | 15 |
| 6 | • Presentation of | 11 |
| -New general principles for classification of CNS tumors | ||
| -Suggested revisions of nomenclature for some of these tumors | ||
| -More recently recognized (sub)types of CNS tumors that appear ready for inclusion in next WHO classification of CNS tumors | ||
| 7 | • Improved classification of ependymomas based on anatomic region and molecular characteristics | 19 |
| -Supratentorial: classified based on presence of c11orf95 vs YAP1 gene fusions (c11orf95 fusion–positive ependymomas largely overlapping with RELA fusion-positive ependymomas as described in WHO 2016 classification) | ||
| -PF: PF ependymomas can be classified as type A or type B (PFA or PFB), characterized by respectively absence or presence of H3 K27me3 staining of tumor cell nuclei; gain of Chr. 1q signifies worse prognosis in PFA ependymomas | ||
| -Ependymoma of the spinal cord with MYCN amplification is a newly recognized entity associated with poor clinical outcome | ||
| -Myxopapillary ependymomas of the spinal cord should be considered grade 2 (rather than grade 1) tumors |
Abbreviations: ATRX, ATP-dependent X-linked helicase; BRAF, B-Raf proto-oncogene, serine/threonine kinase; CDKN2A/B, cyclin-dependent kinase inhibitor 2A/B; Chr., chromosome; c11orf95, chromosome 11 open reading frame 95; cIMPACT-NOW, Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy—Not Official World Health Organization; EGFR, epidermal growth factor receptor; FGFR1, fibroblast growth factor receptor 1; H3, histone H3; IDH, isocitrate dehydrogenase; MAPK, mitogen-activated protein kinase; MYB, Myb proto-oncogene; MYBL1, Myb proto-oncogene like 1; MYCN, MYCN proto-oncogene; NEC, not elsewhere classified; NOS, not otherwise specified; PF, posterior fossa; YAP1, yes-associated protein 1.
Although most of the cIMPACT-NOW recommendations summarized in this table can indeed be expected to be incorporated in the next (fifth) edition of the WHO Classification of CNS Tumors (publication foreseen in the first half of 2021), because of even more recent insights some modifications may be introduced as well.
Preparations for the next, fifth edition of the WHO Classification of CNS Tumors are now in full swing (publication scheduled in less than a year from now). While most of the cIMPACT-NOW recommendations summarized previously are likely to be incorporated in the new WHO classification, because of even more recent insights some modifications may be introduced as well. Meanwhile, because it can be expected that our understanding of the biology of CNS tumors will continue to expand at a rapid pace, continuation of the efforts of cIMPACT-NOW–like consortia may be very helpful for optimal (evidence-based, balanced, rapid) translation of novel insights into clinical diagnostics, with the ultimate goal of providing the best possible care to our CNS tumor patients.
Funding
The authors have no conflicts of interest to declare or funding sources to report.
Conflict of interest statement. None declared.
References
- 1. Louis DN, Ohgaki H, Wiestler OD, et al. World Health Organization Classification of Tumours of the Central Nervous System. 4th ed. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, eds. Lyon: International Agency for Research on Cancer; 2016. [Google Scholar]
- 2. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 3. Kristensen BW, Priesterbach-Ackley LP, Petersen JK, Wesseling P.. Molecular pathology of tumors of the central nervous system. Ann Oncol. 2019;30(8):1265–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Louis DN, Aldape K, Brat DJ, et al. Announcing cIMPACT-NOW: the Consortium to inform molecular and practical approaches to CNS tumor taxonomy. Acta Neuropathol. 2017;133(1):1–3. [DOI] [PubMed] [Google Scholar]
- 5. Louis DN, Wesseling P, Paulus W, et al. cIMPACT-NOW update 1: not otherwise specified (NOS) and not elsewhere classified (NEC). Acta Neuropathol. 2018;135(3):481–484. [DOI] [PubMed] [Google Scholar]
- 6. Louis DN, Giannini C, Capper D, et al. cIMPACT-NOW update 2: diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant. Acta Neuropathol. 2018;135(4):639–642. [DOI] [PubMed] [Google Scholar]
- 7. Brat DJ, Aldape K, Colman H, et al. cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV.” Acta Neuropathol. 2018;136(5):805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ellison DW, Hawkins C, Jones DTW, et al. cIMPACT-NOW update 4: diffuse gliomas characterized by MYB, MYBL1, or FGFR1 alterations or BRAFV600E mutation. Acta Neuropathol. 2019;137(4):683–687. [DOI] [PubMed] [Google Scholar]
- 9. Louis DN, Ellison DW, Brat DJ, et al. cIMPACT-NOW: a practical summary of diagnostic points from Round 1 updates. Brain Pathol. 2019;29(4):469–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yeaney GA, Brat DJ. What every neuropathologist needs to know: update on cIMPACT-NOW. J Neuropathol Exp Neurol. 2019;78(4): 294–296. [DOI] [PubMed] [Google Scholar]
- 11. Louis DN, Wesseling P, Aldape K, et al. cIMPACT-NOW update 6 : new entity and diagnostic principle recommendations of the cIMPACT-Utrecht meeting on future CNS tumor classification and grading. Brain Pathol. 2020;30(4):844–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pratt D, Natarajan SK, Banda A, et al. Circumscribed/non-diffuse histology confers a better prognosis in H3K27M-mutant gliomas. Acta Neuropathol. 2018;135(2):299–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tesileanu CMS, Dirven L, Wijnenga MMJ, et al. Survival of diffuse astrocytic glioma, IDH1/2 wildtype, with molecular features of glioblastoma, WHO grade IV: a confirmation of the cIMPACT-NOW criteria. Neuro Oncol. 2020;22(4):515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Capper D, Stichel D, Sahm F, et al. Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: the Heidelberg experience. Acta Neuropathol. 2018;136(2):181–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown NF, Carter T, Kitchen N, Mulholland P.. Dabrafenib and trametinib in BRAFV600E mutated glioma. CNS Oncol. 2017;6(4):291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brat DJ, Aldape K, Colman H, et al. cIMPACT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020;139(3):603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cancer Genome Atlas Research Network, Brat DJ, Verhaak RGW, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weller M, Weber RG, Willscher E, et al. Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol. 2015;129(5):679–693. [DOI] [PubMed] [Google Scholar]
- 20. Shirahata M, Ono T, Stichel D, et al. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018;136(1):153–166. [DOI] [PubMed] [Google Scholar]
- 21. Chung CTS, Santos GDC, Hwang DM, et al. FISH assay development for the detection of p16/CDKN2A deletion in malignant pleural mesothelioma. J Clin Pathol. 2010;63(7):630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ellison DW, Aldape KD, Capper D, et al. cIMPACT-NOW update 7: advancing the molecular classification of ependymal tumors. [Published online ahead of print June 5, 2020.] Brain Pathol. doi: 10.1111/bpa.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vera-Bolanos E, Aldape K, Yuan Y, et al. ; CERN Foundation . Clinical course and progression-free survival of adult intracranial and spinal ependymoma patients. Neuro Oncol. 2015;17(3):440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]