Abstract
Background
The main goal of this functional MRI (fMRI) study was to examine whether cognitive deficits in glioma patients prior to treatment are associated with abnormal brain activity in either the central executive network (CEN) or default mode network (DMN).
Methods
Forty-six glioma patients, and 23 group-matched healthy controls (HCs) participated in this fMRI experiment, performing an N-back task. Additionally, cognitive profiles of patients were evaluated outside the scanner. A region of interest–based analysis was used to compare brain activity in CEN and DMN between groups. Post hoc analyses were performed to evaluate differences between low-grade glioma (LGG) and high-grade glioma (HGG) patients.
Results
In-scanner performance was lower in glioma patients compared to HCs. Neuropsychological testing indicated cognitive impairment in LGG as well as HGG patients. fMRI results revealed normal CEN activation in glioma patients, whereas patients showed reduced DMN deactivation compared to HCs. Brain activity levels did not differ between LGG and HGG patients.
Conclusions
Our study suggests that cognitive deficits in glioma patients prior to treatment are associated with reduced responsiveness of the DMN, but not with abnormal CEN activation. These results suggest that cognitive deficits in glioma patients reflect a reduced capacity to achieve a brain state necessary for normal cognitive performance, rather than abnormal functioning of executive brain regions. Solely focusing on increases in brain activity may well be insufficient if we want to understand the underlying brain mechanism of cognitive impairments in patients, as our results indicate the importance of assessing deactivation.
Keywords: central executive network, cognitive deficits, default mode network, functional MRI, glioma
In his landmark paper from 1990, Mesulam proposed that brain functions are subserved by large-scale networks, and that the relationship between function and structure is dynamic, as well as both localized and distributed.1 This network approach offered an alternative for the traditional clinical view of a modular and fixed organization of the brain. It paved the way toward more efficient and safer brain tumor surgery. Our improved understanding of the complex architecture of the motor and language network, combined with the use of intraoperative mapping techniques, has increased resectability and reduced the amount of late severe neurological deficits.2,3
In contrast, brain regions or networks that underlie cognitive deficits in glioma patients remain largely elusive. That is unfortunate, because results from neuropsychological assessments in brain tumor patients have indicated that cognitive deficits occur frequently4–7 and may have a large negative impact on normal socio-professional functioning and quality of life.8,9 These deficits occur across different cognitive domains and across different types, grading, and location of the brain tumor.4–7 Therefore, it is more likely that an overarching mechanism is responsible for these deficits than dysfunction of a specific peritumoral region, in line with the large-scale network view. A better understanding of the neural correlates of cognitive deficits in glioma patients will be a necessary first step toward preventing cognitive deficits after (surgical) treatment, and developing specific rehabilitation methods.
A number of large-scale networks have been associated with cognitive performance, whereby involved brain areas either increase or decrease their activity during goal-oriented behavior.10–14 The central executive network (CEN) is engaged during a range of cognitive tasks and shows a typical pattern of increased brain activity as compared to rest.10–12 The default mode network (DMN) also shows changes in activity during a variety of cognitive tasks, but brain activity within this network decreases compared to rest.13,14 Deactivation of DMN regions is therefore often interpreted as a necessary inhibition of brain processes that may interfere with cognitive task performance,15 whereas CEN activation is typically associated with execution of the task itself.10–12 Conversely, abnormal functioning of either of these 2 networks may underlie cognitive impairments in brain tumor patients.
To test this hypothesis, we performed a functional MRI (fMRI) experiment and studied glioma patients (prior to treatment) as well as healthy controls (HCs) during cognitive task performance. Brain activity levels within the CEN and DMN network in glioma patients were compared to brain activity levels in HCs. Because cognitive functions are more often impaired in high-grade glioma (HGG) patients than in low-grade glioma (LGG) patients16, we performed post hoc analyses to investigate possible differences in cognitive performance or brain activity levels between these subgroups.
Methods
Study Population
All newly diagnosed patients with a presumed diffuse glioma undergoing surgical tumor resection at the Elisabeth-TweeSteden Hospital in Tilburg (the Netherlands) between July 2016 and February 2019 were invited to participate in this prospective, 3T fMRI study. Exclusion criteria included 1) age younger than 18 years and older than 75 years, 2) history of intracranial surgery, 3) history of cranial radiotherapy, 4) history of neurological or psychiatric disorders, 5) lack of basic proficiency in Dutch, 6) inability to undergo fMRI scan session because of severe visual, motor, or cognitive problems or poor general health, and 7) contraindications for the MRI scan (such as magnetic elements in the body or claustrophobia).
To be able to investigate possible differences between LGG and HGG patients in post hoc analyses, patients were divided into 2 groups based on a combination of histopathological and molecular tumor characteristics. Patients with a World Health Organization (WHO) grade II tumor with an isocitrate dehydrogenase (IDH)1 mutation (IDH1+, astrocytoma as well as oligodendroglioma [with 1p19q codeletion]) were classified as LGG. Patients with a WHO grade II tumor with IDH1 wild-type (IDH1–), WHO grade III tumor (IDH1+ or IDH1–), or a WHO grade IV tumor were classified as HGG.17 One patient had a WHO grade I dysembryoplastic neuroepithelial tumor and was excluded from further analyses to increase the study homogeneity in terms of participants, considering the less-infiltrative nature of this tumor.
Because of technical problems, 3 LGG and 2 HGG patients had incomplete fMRI task data and were therefore excluded from our analyses. Ultimately, 46 glioma patients (21 LGG and 25 HGG patients) were included in this study. Patients were scanned 1 to 5 days prior to surgery. Because of postponement of the surgery, 1 LGG patient and 1 HGG patient were scanned 20 days and 37 days prior to surgery, respectively.
Furthermore, 37 healthy volunteers were recruited through online advertisement as a control group. Exclusion criteria included 1) age younger than 18 years and older than 75 years, 2) previously diagnosed neurological or psychiatric disorders, 3) severe concussion of the brain with loss of consciousness in the past, or 4) contraindications for the MRI scan (such as pregnancy, magnetic elements in the body, and claustrophobia).
To minimize the effect of age on task performance as well as brain activity, HCs were matched on a group level with the glioma group so the mean age and range in age did not significantly differ between the glioma patient group and the HC group. From the initial 37 HCs, ultimately 23 group-matched HCs were included for further analyses.
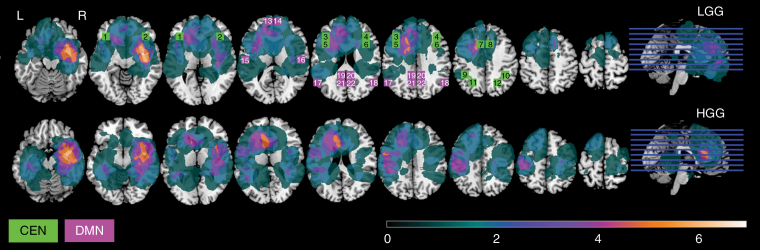
Detailed sociodemographic characteristics of the HC and glioma group and clinical patient characteristics of the glioma group can be found in Table 1. Additionally, sociodemographic and clinical characteristics are presented for LGG and HGG patients separately to further specify both groups that are used in post hoc analyses. The distribution of tumor localization can be found in Figure 1 for LGG and HGG patients separately.
Table 1.
Sociodemographic and Clinical Characteristics
| Variable | HCs (N = 23) | Glioma (N = 46) | LGG (N = 21) | HGG (N = 25) |
|---|---|---|---|---|
| Age, y, mean ± SD (range) | 43.5 ± 16.2 (18-69) | 46.7 ± 14.1 (18-71) | 39.9 ± 12.5 (19-67) | 52.3 ± 13.0 (18-71) |
| Sex, male/female | 8 (35)/15 (65) | 29 (63)/17 (37) | 14 (67)/7 (33) | 15 (60)/10 (40) |
| Handedness, left/right | 2 (9)/21 (91) | 6 (13)/40 (87) | 2 (10)/19 (90) | 4 (16)/21 (84) |
| Histopathological diagnosis | ||||
| WHO grade II | ||||
| IDH1+, astrocytoma | 13 (28) | 13 (59) | – | |
| IDH1+, oligodendroglioma | 8 (17) | 8 (36) | – | |
| IDH1– | 2 (4) | – | 2 (8) | |
| WHO grade III | ||||
| IDH1+ | 4 (9) | – | 4 (16) | |
| IDH1– | – | – | – | |
| WHO grade IV | 19 (41) | – | 19 (76) | |
| Tumor hemisphere, left/right/both | 23 (50)/22 (48)/1 (2) | 10 (48)/10 (48)/1 (4) | 13 (52)/12 (48)/– | |
| Tumor volume, cm3, mean (range) | 64.79 (5.13-233.99) | 61.09 (5.13-233.99) | 67.90 (5.24-189.64) | |
| Antiepileptic medication | 27 (59) | 14 (67) | 13 (52) |
Abbreviations: HCs, healthy controls; HGG, high-grade glioma; IDH, isocitrate dehydrogenase; LGG, low-grade glioma; WHO, World Health Organization.
Sociodemographic characteristics are presented for HCs. For glioma patients, sociodemographic and clinical patient characteristics are presented. Additionally, sociodemographic and clinical characteristics are presented for LGG and HGG patients separately, as these groups are compared in post hoc analyses. Values are indicated as number of participants (%) unless indicated otherwise.
Figure 1.
The distribution of tumor localization is presented here for low-grade glioma (LGG, upper panel, n = 21) and high-grade glioma (HGG, lower panel, n = 25) patients separately. The color scale shows minimal overlap (dark green) to maximal overlap (white). Additionally, selected regions of interest (ROIs) are illustrated. ROIs belonging to the central executive network (CEN, in purple) and the default mode network (DMN, in green) are superimposed on the tumor distribution to indicate the ROI location with respect to tumor location. The numbers correspond to the numbers indicated in Table 2, where further characteristics of the individual ROIs can be found.
All participants gave written informed consent prior to the scan session. This study was approved by the independent medical ethical committee (protocol number: NL51147.028.14).
Neuropsychological Assessment
As part of clinical care, patients were neuropsychologically evaluated prior to surgery. Cognitive performance was examined using the formal Dutch translation of the computerized neuropsychological battery Central Nervous System Vital Signs (CNS VS).18 To obtain a cognitive profile of both patient groups, test results of the 7 neuropsychological tests that are examined in this battery were used: verbal memory, visual memory, symbol digit coding, finger-tapping, Stroop III, shifting attention, and a continuous performance test19.
In-Scanner Task Design
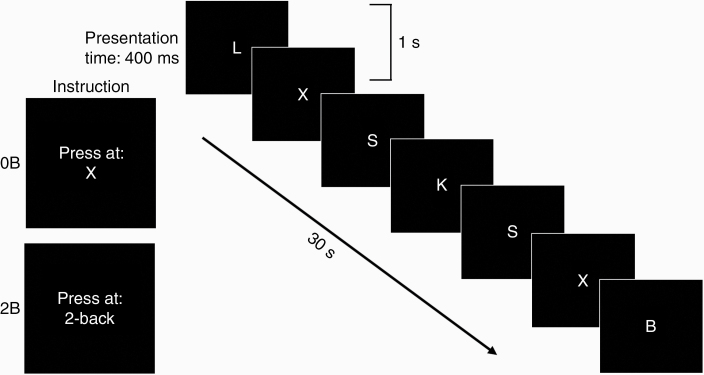
To examine brain activity during cognitive performance, HCs as well as glioma patients performed a 2-back working memory (WM) task (2B) inside the scanner.20 As baseline, we included a 0-back task (0B) to exclude activation associated with motor and visual processes. Stimuli were identical for both conditions as participants paid attention to a sequence of consonants that was presented in the center of the screen. Because of different instructions, task difficulty varied between conditions. For 0B, participants needed to respond to the target consonant “X.” For 2B, participants needed to respond if a stimulus was equal to a stimulus that was presented 2 trials before (Figure 2).
Figure 2.
Schematic outline of the N-back task. The task consisted of 2 conditions with increasing task difficulty, due to different instructions (presented for 4 seconds prior to the relevant task block). Stimuli were identical for both conditions and consisted of a fast sequence of consonants. For 0-back (0B), participants needed to respond to the target consonant “X.” For 2-back (2B), participants needed to respond if a stimulus was equal to a stimulus that was presented 2 trials before.
The task was presented in blocks of 30 seconds and rest blocks of 15 seconds. The experiment also comprised conditions that were unrelated to this article. 2B as well as 0B consisted of 2 blocks and the number of targets was equal for both conditions (12 targets per block). Stimuli were presented for 400 ms with an interstimulus interval of 1 second (Figure 2). Instructions for each condition were presented for 4 seconds prior to the relevant task block. The participants responded to a target by pushing a button on a button box with their right hand.
Prior to the fMRI scanning session, the N-back WM task was practiced on a laptop outside the scanner to make participants familiar with the task and to reduce possible practice effects during the fMRI scanning session.
Image Acquisition
Scans were performed on a 3T Philips Achieva scanner (Philips Medical Systems) using a 32-channel head coil. A 3-dimensional (3D) T1-weighted structural image was acquired for anatomical registration purposes (scan parameters: repetition time [TR]/echo time [TE]: 8.4/3.8 ms, field of view [FOV]: 254 × 254 × 158 mm3, flip angle: 8°, voxel size 1 mm isotropic, 158 slices [sagittal orientation]). fMRI images were obtained using an echo-planar imaging pulse sequence (scan parameters: TR/TE: 2000/28 ms, FOV: 240 × 240 × 111 mm, voxel size: 3 × 3 × 3 mm, 219 volumes). Each run also included other conditions and tasks unrelated to this paper.
fMRI Preprocessing
fMRI data were preprocessed and analyzed using SPM12. All scans were registered to the first scan to correct for participant movement during the experiment and slice-timing correction was applied. Subsequently, the images were coregistered to the anatomical image and spatially normalized to standard Montreal Neurological Institute (MNI) space using parameters derived from the spatial normalization of the anatomical image. Individual scans were spatially smoothed with a 3D Gaussian filter (full-width at half-maximum: 12 mm) to minimize effects of functional anatomical differences between participants.
fMRI Analysis
For the evaluation of the fMRI data, we performed a blocked generalized linear model regression analysis with separate regressors for the 0B and 2B condition. The generalized linear model was designed such that the beta value represented a percentage signal change. Signal changes were calculated compared to rest for 0B and 2B separately.
A region of interest (ROI) analysis was performed for the evaluation of signal changes, using a systematic system of equal-sized ROIs.21,22 Furthermore, this ROI-based analysis provides high statistical power thanks to the larger regions and strongly reduced need for correction for multiple tests.23 Additionally, the use of systematically designed ROIs allows for comparison of signal changes between networks and regions, as well as quantitative replication of the findings of this study23,24.
Region of Interest Selection
For our fMRI analysis, we focused on the brain activity within the CEN and the DMN, because these networks have been shown to be consistently involved in cognitive processes.10–14 We based our ROI selection on brain activity clusters that have been previously reported10,25–27 to avoid circular analysis.28 We selected cube-shaped ROIs with dimensions of 15 × 15 × 15 mm from a predefined grid in MNI space21,22 so they overlapped with the previously reported peak coordinates. By using a predefined shape and size for the ROIs, we further minimized the effect of circularity because the borders were not influenced by noise.28 All ROIs were placed symmetrically for both hemispheres. The location and other characteristics of the selected ROIs are presented in Figure 1 and Table 2. Ultimately, the CEN consisted of regions within the dorsal- and ventral lateral prefrontal cortex, the premotor cortex, the anterior cingulate, and regions along the intraparietal sulcus.10 The DMN consisted of brain regions within the medial prefrontal cortex,25 posterior cingulate,26 angular gyrus,26 precuneus,27 and medial temporal cortex. Brain activity was averaged over all ROIs within the CEN and DMN respectively for further analyses.
Table 2.
Description of Selected Regions of Interest
| ROI | ROI full name | BA | MNIx | MNIy | MNIz |
|---|---|---|---|---|---|
| Central executive network | |||||
| 1 | Left ventrolateral prefrontal cortex | 47 | –39 | 30 | 0 |
| 2 | Right ventrolateral prefrontal cortex | 47 | 39 | 30 | 0 |
| 3 | Left dorsolateral prefrontal cortex | 48 | –39 | 30 | 30 |
| 4 | Right dorsolateral prefrontal cortex | 48 | 39 | 30 | 30 |
| 5 | Left premotor cortex | 44 | –39 | 15 | 30 |
| 6 | Right premotor cortex | 44 | 39 | 15 | 30 |
| 7 | Left anterior cingulate | 32 | –9 | 15 | 45 |
| 8 | Right anterior cingulate | 32 | 9 | 15 | 45 |
| 9 | Left intraparietal sulcus lateral anterior part | 40 | –39 | –45 | 45 |
| 10 | Right intraparietal sulcus lateral anterior part | 40 | 39 | –45 | 45 |
| 11 | Left intraparietal sulcus medial posterior part | 7 | –24 | –60 | 45 |
| 12 | Right intraparietal sulcus medial posterior part | 7 | 24 | –60 | 45 |
| Default mode network | |||||
| 13 | Left medial prefrontal cortex | 10 | –9 | 60 | 15 |
| 14 | Right medial prefrontal cortex | 10 | 9 | 60 | 15 |
| 15 | Left medial temporal cortex | 48 | –54 | –15 | 15 |
| 16 | Right medial temporal cortex | 48 | 54 | –15 | 15 |
| 17 | Left angular gyrus | 39 | –54 | –60 | 30 |
| 18 | Right angular gyrus | 39 | 54 | –60 | 30 |
| 19 | Left posterior cingulate | 23 | –9 | –45 | 30 |
| 20 | Right posterior cingulate | 23 | 9 | –45 | 30 |
| 21 | Left precuneus | 0 | –9 | –60 | 30 |
| 22 | Right precuneus | 0 | 9 | –60 | 30 |
Abbreviations: BA, Brodmann area; MNIxyz, Montreal Neurological Institute coordinates; ROI, region of interest.
The numbers of individual ROIs correspond to the ROIs presented in Figure 1. MNI coordinates represent the center point of the 15 × 15 × 15 mm cube-shaped ROIs.
Statistical Analyses
To obtain a better cognitive profile of the glioma patient group, raw neuropsychological test scores of patients were converted into sociodemographically adjusted z-scores based on a Dutch normative sample.19 Individual patient test scores were considered impaired if the z-score was less than or equal to 1.5.29 Subsequently, we calculated the percentage of patients with an impaired score for each test separately and determined the percentage of patients with an impaired score for one or more tests in the glioma group, as well as in LGG and HGG patients separately.
To evaluate the in-scanner task performance of HCs as well as glioma patients, we determined the number of missed targets and the number of false alarms separately for 0B and 2B in each group. Subsequently, we calculated the increase in percentage of incorrect responses between 2B and 0B (combining false alarms and missed targets), analogous to the fMRI analyses. Finally, we used these results to conduct separate analyses of covariance (ANCOVAs) to test for group differences between glioma patients and HCs. Age (in years) was included as a covariate to minimize the effect of this factor on task performance in each group. Post hoc analyses included separate ANCOVAs with age as a covariate to test for possible differences between LGG and HGG patients.
To identify brain activity specifically associated with cognitive performance, brain activity during 0B was subtracted from brain activity during 2B in both networks. Subsequently, we conducted separate ANCOVAs for activity averaged over all ROIs included in the CEN and all ROIs included in the DMN, comparing activity in these 2 networks between glioma patients and HCs. Age was included as a covariate to control for effects of this factor on activity in each group. Again, post hoc analyses included separate ANCOVAs with age as a covariate to test for possible differences between LGG and HGG patients. All statistical analyses were performed using SPSS 24.
Results
Neuropsychological Assessment
Forty-four out of 46 glioma patients (21 LGG patients and 23 HGG patients) underwent neuropsychological assessment prior to surgery. Percentages of patients with impaired scores ranged over tests from 11% to 30%, whereas in a normative sample 7% of impaired scores is expected for each test. Post hoc analyses showed that percentages of patients with impaired scores ranged over tests from 5% to 24% in the LGG group and from 9% to 45% in the HGG group. In total, 57% of the LGG patients had an impaired score for 1 test, and 19% had an impaired score for 2 or more tests. In the HGG group, 17% had an impaired score for 1 test, whereas 61% of the patients had an impaired score for 2 or more tests.
In-Scanner Task Performance
As expected, the percentage of incorrect responses (mean ± SEM) increased during the most difficult condition in HCs (0B: 0.04 ± 0.32; 2B: 11.04 ± 1.49) as well as glioma patients (0B: 0.74 ± 0.23; 2B: 16.41 ± 1.05) (Figure 3A).
Figure 3.
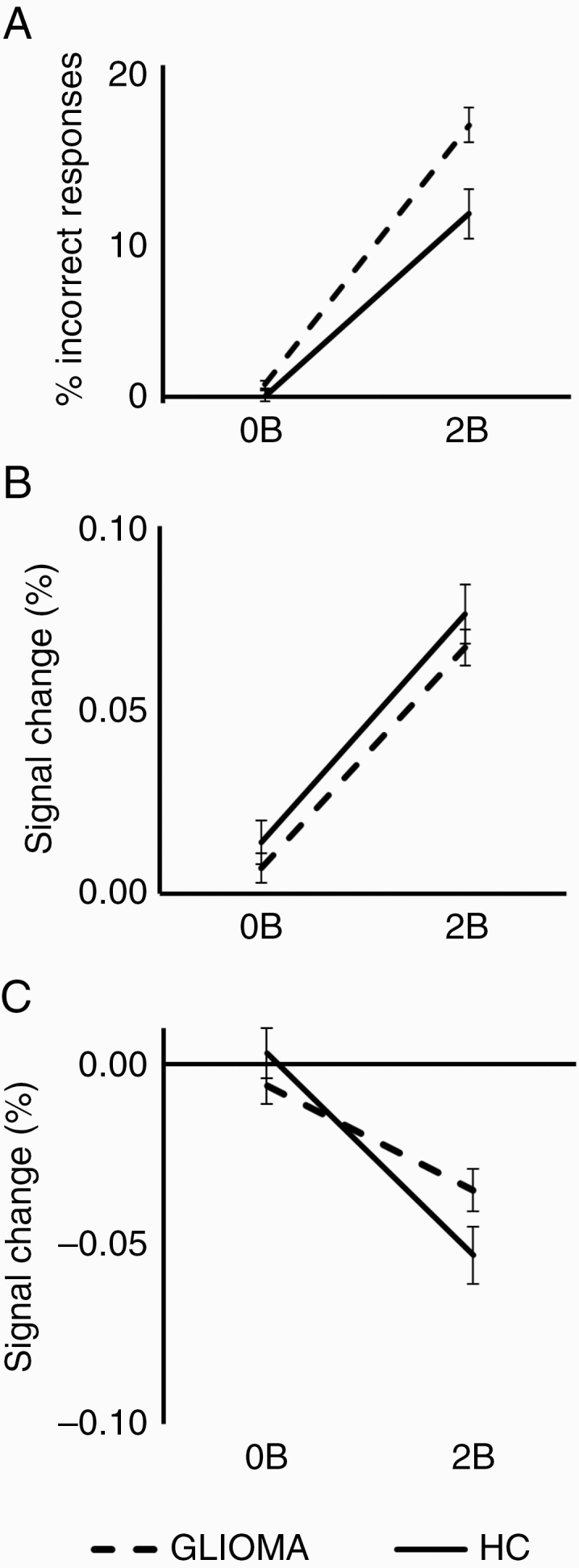
Task performance and brain activity measures of healthy controls (HC, solid line) and glioma patients (GLIOMA, dashed line) are presented here for the 0-back (0B) and the 2-back (2B) condition. Age-corrected results are presented by showing results for the mean age of all participants (mean age = 45.6 years). A, The percentage of incorrect responses (false alarms and missed targets combined) are shown (± SEM) for each group. Taking age into account, glioma patients showed reduced task performance compared to HCs. C and D, Signal change within the central executive network (CEN, C), and the default mode network (DMN, D) are shown (± SEM). Note the similar increase in activity within the CEN for HCs and glioma patients with increasing task difficulty. For the DMN, patients show reduced deactivation during 2B.
Taking age into account, performance differences were found between HCs and glioma patients, as glioma patients had an increased number of incorrect responses compared to HCs (F = 6.04, P = .02). The presented task performance results are corrected for age by showing results for the mean age of all participants (mean age = 45.6 years) (Figure 3A). Post hoc analyses showed no task performance differences between LGG and HGG patients (F = 1.63, P = .21).
Region of Interest Analysis
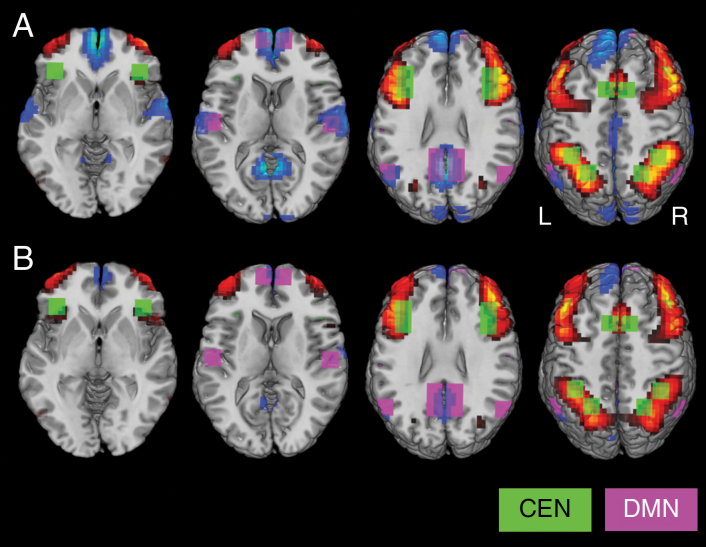
Task-induced brain activity patterns in CEN and DMN (difference between 2B and 0B) are presented in Figure 4 for HCs and glioma patients, respectively. The brain activity patterns presented in Figure 4 are not corrected for age because we prefer to show the actual measured signal changes (which is in line with most fMRI studies). We included age as a covariate in our statistical design to evaluate whether differences in measured brain activity levels between HCs and glioma patients are statistically significant. Detailed results of these ROI analyses are presented in Figure 3B and 3C. The results presented in Figure 3 are corrected for age by showing results for the mean age of all participants (mean age = 45.6 years). Taking age into account, ROI analyses revealed no abnormal brain activity levels within the CEN in glioma patients compared to HCs (F = 0.01, P = .91). Furthermore, brain activity levels within the CEN did not differ between both patient groups (F = 0.79, P = .38). However, glioma patients did show reduced deactivation of the DMN compared to HCs (F = 5.56, P = .02), indicating reduced responsiveness of the DMN during cognitive performance. The level of deactivation did not differ between both patient groups (F = 0.26, P = .61).
Figure 4.
Illustration of the brain activity patterns induced by the N-back task for A, healthy control group (HC), and B, glioma patients (GLIOMA). The contrast between the 2-back and 0-back task condition is presented here. Voxels in which the brain activity exceeded the threshold value of b = 0.05 are indicated in red, whereas voxels in which the brain activity was below the threshold value of b = –0.05 are indicated in blue. Regions of interest (ROIs) are superimposed on the brain activity patterns (CEN, in green; DMN, in purple). Note that the positive brain activity patterns are quite similar between groups within CEN, whereas glioma patients show less negative brain activity compared to the HC group within the DMN.
Discussion
The main goal of this fMRI study was to examine whether cognitive deficits in glioma patients prior to surgical or other treatment can be associated with abnormal brain activity in either CEN or DMN during cognitive performance.
Cognitive evaluation of the glioma patient group using a battery of neuropsychological tests outside the scanner indicated impaired cognitive functioning in glioma patients (LGG and HGG), as well as a more severely affected cognitive functioning in HGG compared to LGG patients, matching observations in the literature of these patients.4,16 CEN activity did not show a difference between any of the groups. In contrast, glioma patients did show significantly reduced deactivation in DMN compared to HCs. This effect did not differ significantly between the 2 patient groups. Therefore, our study suggests that preoperative cognitive impairments in glioma patients are associated with reduced responsiveness of the DMN, whereas we did not find evidence for involvement of the CEN.
As expected, our fMRI results showed increased activity in the CEN as well as reduced activation in the DMN compared to rest. Increased brain activity in CEN is typically understood to represent the execution of a cognitive task.10–12 Similar to the etiology of sensorimotor or language (ie, classical neurological) deficits, cognitive deficits may occur when these eloquent areas or pathways for executive processes are damaged during surgery.30 Some brain-mapping studies have therefore imported preoperatively acquired WM-related brain activity into cranial neuronavigation to evaluate and preserve WM function31.
Although this is important, it may only be part of the story. Deactivation of the DMN is well described in healthy individuals13,14 and occurs in a variety of cognitive tasks. Deactivation of the DMN is therefore generally understood to be a necessary prerequisite for goal-oriented behavior, such as for a cognitive task.13,15 The DMN has previously been associated with processes that are performed during rest, but interfere with goal-oriented behavior, such as “mind wandering.” 32 DMN brain activity should therefore be suppressed during goal-oriented cognitive tasks. Other studies have suggested an association of DMN with mental effort33 or regulation of bodily homeostasis.34
We found significantly reduced deactivation of the DMN during task performance in glioma patients. These results suggest that the various cognitive deficits in glioma patients that are already present prior to surgery may be associated with a reduced capacity to inhibit this DMN. Since deactivation of the DMN is generally understood to be a prerequisite for goal-oriented behavior, one may speculate that it could potentially indicate cognitive impairment and therefore serve as a cognitive biomarker in glioma patients. For example, it could be used to monitor effects of neurofeedback training.35 From a surgical perspective, sparing this network could be important in preventing further loss of cognitive functions. At least, our findings imply that solely examining increases in brain activity in fMRI is likely insufficient to characterize the neuroanatomy of cognitive functions in glioma patients.
In summary, cognitive deficits that are found in glioma patients prior to surgery appear not to be related to deficits in brain regions associated with task execution, but rather to a reduced capacity to achieve a brain state that is optimal for general cognitive functioning. Our results thus support our assumption that various cognitive deficits in glioma patients may be associated with abnormal functioning of one overarching brain function, rather than dysfunction of several task-specific brain regions or networks.
Task-based fMRI studies that examined cognitive functions in glioma patients prior to surgery are unfortunately rather scarce and often do not include healthy individuals as a reference.31 Therefore, it is difficult to evaluate our results in regard to similar studies in glioma patients. Comparable studies in patients with other neurological or psychiatric disorders have shown altered cognitive brain activity patterns compared to HCs.36–46 In regard to CEN activity, previous results are rather inconsistent, as both increased36–39 as well as decreased40,41 executive activity compared to HCs have often been reported. For instance, in a study of aneurysmal subarachnoid hemorrhage patients, cognitive impairment was associated with increased activity in executive brain regions.36 This suggests a lack of efficiency in CEN, which could result in reduced performance, especially during difficult tasks. In contrast, studies of patients with schizophrenia43 and mild traumatic brain injury44 have suggested that cognitive impairments in these patient groups are associated with a reduced capacity to modulate brain activity in the CEN when tasks vary in difficulty. Whereas increased executive activity may be explained by a lack of efficiency, a decrease in executive activity can be caused by poorer performance.41 This seemed not to be the case in our study because CEN activity was normal for glioma patients. This is a strong argument that the patients kept performing the task despite a somewhat poorer performance, since brain activity within the CEN is a marker for task execution.10–12
In line with our findings in glioma patients, several previous task-based fMRI studies in patients with neurological or psychiatric disorders have also reported reduced DMN deactivation during cognitive performance.38–42 The combination of normal CEN activity and diminished DMN deactivation was previously also reported in studies of chronic pain patients45 and patients with remitted major depression.46 Because patient in-scanner task performance was normal in these studies, Čeko et al45 suggested that a responsive executive network may be sufficient for successful cognitive performance, regardless of DMN deactivation. In our study, reduced deactivation in the DMN was accompanied by decreased task performance in glioma patients, despite a responsive CEN. This suggests that in contrast to the study by Čeko and colleagues,45 a normally responsive executive network alone may not be sufficient for normal cognitive task performance. Cognitive impairments have previously also been reported in chronic pain patients.47 An alternative interpretation of the results presented by Čeko et al45 may be that despite normal performance on the relatively short fMRI task, subtle cognitive impairments did exist in their patient group, explaining the reduced DMN deactivation that they found.
Some limitations have to be taken into account when interpreting the results of this study. Because of our selection criteria that patients should be able to undergo and complete the fMRI scan session, patients with severe visual, motor, or cognitive problems or a poor health condition were excluded from this study. Therefore, the cognitive abilities of the participating glioma patients may be an overestimation of the actual cognitive abilities in this population. Hence, our results may be an underestimation of the problems that occur in glioma patients. Furthermore, age may be a factor that influences task performance as well as the level of brain activity. Therefore, we have minimized the effect of age by matching HCs on a group level with the glioma group so that the mean age and range in age did not significantly differ between the glioma patient group and the HC group. Additionally, age was included as a covariate in all statistical analyses to further control for the possible effect of age in each group. Therefore, the influence of a possible age effect on our results regarding the comparison between patients and HC was minimal. For our post hoc analyses, the average age of HGG patients was considerably older than the average age of the LGG patient group. Whereas LGG occurs most commonly in the second through fourth decades of life, the average age of HGG patients is considerably higher at diagnosis, as the incidence of HGG increases with age.48,49 Therefore, the relatively large age difference between LGG and HGG patients is inherent to glioma subtype. By including age as a covariate in our post hoc analyses, the effect of age was minimized in our comparison between LGG and HGG patients. Finally, the infiltrative character of glioma tumors can induce tissue distortion, which complicates the comparison of brain activity levels between glioma patients and HCs. To minimize the effect of deformation, images were spatially normalized into standard MNI space during preprocessing using deformation fields that quantify the amount of displacement for each location in 3D space. Combining all patients, 11.6% of all ROIs within the CEN showed some tumor overlap, whereas in the DMN this was 7.6%. Considering the relatively low tumor overlap, and the fact that we did not find differences in brain activity levels within the CEN between glioma patients and HCs, while tumor overlap was larger in CEN than in DMN, we believe the influence of deformation and tumor overlap on our results regarding reduced DMN deactivation was minimal.
In conclusion, our study suggests that cognitive deficits in glioma patients prior to treatment are associated with reduced capacity to deactivate the DMN, whereas we found no evidence of abnormal CEN function. Thus, it appears that cognitive deficits in glioma patients do not reflect abnormal functioning of executive brain regions, but rather a reduced capacity to achieve a brain state necessary for normal cognitive task performance. Solely focusing on increases in brain activity may be insufficient to study and understand cognitive impairment in patients, as our results indicate the importance of assessing deactivation. Our results constitute an important step toward a better understanding of the underlying mechanism of cognitive decline in glioma patients and provide a lead to development of a biomarker to guide the effects of new surgical treatment or rehabilitation methods.
Acknowledgments
We would like to thank the participating patients, the whole team of the neurosurgery department of the Elisabeth-TweeSteden Hospital that contributed to the inclusion of patients, and the radiology technicians for their contribution to this study.
Funding
This work was supported by ZonMw, the Dutch Organization of Health Research and Development [project number 842003004].
Conflict of interest statement. None declared.
References
- 1. Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28(5):597–613. [DOI] [PubMed] [Google Scholar]
- 2. Southwell DG, Birk HS, Han SJ, Li J, Sall JW, Berger MS.. Resection of gliomas deemed inoperable by neurosurgeons based on preoperative imaging studies. J Neurosurg. 2018;129(3):567–575. [DOI] [PubMed] [Google Scholar]
- 3. De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS.. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012;30(20):2559–2565. [DOI] [PubMed] [Google Scholar]
- 4. Miotto EC, Junior AS, Silva CC, et al. Cognitive impairments in patients with low grade gliomas and high grade gliomas. Arq Neuropsiquiatr. 2011;69(4):596–601. [DOI] [PubMed] [Google Scholar]
- 5. van Loenen IS, Rijnen SJM, Bruijn J, Rutten GJM, Gehring K, Sitskoorn MM.. Group changes in cognitive performance after surgery mask changes in individual patients with glioblastoma. World Neurosurg. 2018;117:e172–e179. [DOI] [PubMed] [Google Scholar]
- 6. Rijnen SJM, Meskal I, Bakker M, et al. Cognitive outcomes in meningioma patients undergoing surgery: individual changes over time and predictors of late cognitive functioning. Neuro Oncol. 2019;21(7):911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159–168. [DOI] [PubMed] [Google Scholar]
- 8. Aaronson NK, Taphoorn MJ, Heimans JJ, et al. Compromised health-related quality of life in patients with low-grade glioma. J Clin Oncol. 2011;29(33):4430–4435. [DOI] [PubMed] [Google Scholar]
- 9. Allen DH, Loughan AR. Impact of cognitive impairment in patients with gliomas. Semin Oncol Nurs. 2018;34(5):528–546. [DOI] [PubMed] [Google Scholar]
- 10. Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3(4):255–274. [DOI] [PubMed] [Google Scholar]
- 11. D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M.. The neural basis of the central executive system of working memory. Nature. 1995;378(6554):279–281. [DOI] [PubMed] [Google Scholar]
- 12. Smith EE, Jonides J, Marshuetz C, Koeppe RA.. Components of verbal working memory: evidence from neuroimaging. Proc Natl Acad Sci U S A. 1998;95(3):876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL.. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shulman GL, Corbetta M, Fiez JA, et al. Searching for activations that generalize over tasks. Hum Brain Mapp. 1997;5(4):317–322. [DOI] [PubMed] [Google Scholar]
- 15. Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–447. [DOI] [PubMed] [Google Scholar]
- 16. van Kessel E, Baumfalk AE, van Zandvoort MJE, Robe PA, Snijders TJ.. Tumor-related neurocognitive dysfunction in patients with diffuse glioma: a systematic review of neurocognitive functioning prior to anti-tumor treatment. J Neurooncol. 2017;134(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wesseling P, Capper D. WHO 2016 Classification of gliomas. Neuropathol Appl Neurobiol. 2018;44(2):139–150. [DOI] [PubMed] [Google Scholar]
- 18. Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS vital signs. Arch Clin Neuropsychol. 2006;21(7):623–643. [DOI] [PubMed] [Google Scholar]
- 19. Rijnen SJM, Meskal I, Emons WHM, et al. Evaluation of normative data of a widely used computerized neuropsychological battery: applicability and effects of sociodemographic variables in a Dutch sample. Assessment. 2020;27(2):373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cohen JD, Perlstein WM, Braver TS, Braver TS. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386(6625):604–608. [DOI] [PubMed] [Google Scholar]
- 21. Jansma JM, Rutten GM. A proposal for a system that facilitates quantitative replication of fMRI studies. Poster presented at: 23rd annual meeting of the Organization for Human Brain Mapping; June 25–29, 2017; Vancouver, British Columbia, Canada. https://archive.aievolution.com/2017/hbm1701/index.cfm?do=abs.viewAbs&abs=4047.
- 22. Schouwenaars IT, de Dreu MJ, Rutten GM, Ramsey NF, Jansma JM.. The effect of response frequency on cognitive brain activity during an alertness task. Neuroreport. 2019;30(17):1166–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poldrack RA. Region of interest analysis for fMRI. Soc Cogn Affect Neurosci. 2007;2(1):67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zandbelt BB, Gladwin TE, Raemaekers M, et al. Within-subject variation in BOLD-fMRI signal changes across repeated measurements: quantification and implications for sample size. Neuroimage. 2008;42(1):196–206. [DOI] [PubMed] [Google Scholar]
- 25. Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT.. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci. 2009;29(46):14496–14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb Cortex. 2008;18(11):2553–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh KD, Fawcett IP. Transient and linearly graded deactivation of the human default-mode network by a visual detection task. Neuroimage. 2008;41(1):100–112. [DOI] [PubMed] [Google Scholar]
- 28. Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI.. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12(5):535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lezak MD, Howieson DB, Bigler ED, Tranel D.. Neuropsychological Assessment. 5th ed. New York, NY: Oxford University Press, 2012. [Google Scholar]
- 30. Mandonnet E, Cerliani L, Siuda-Krzywicka K, et al. A network-level approach of cognitive flexibility impairment after surgery of a right temporo-parietal glioma. Neurochirurgie. 2017;63(4):308–313. [DOI] [PubMed] [Google Scholar]
- 31. Braun V, Albrecht A, Kretschmer T, Richter H-P, Wunderlich A.. Brain tumour surgery in the vicinity of short-term memory representation—results of neuronavigation using fMRI images. Acta Neurochir (Wien). 2006;148(7):733–739. [DOI] [PubMed] [Google Scholar]
- 32. Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN.. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jansma JM, Ramsey NF, de Zwart JA, van Gelderen P, Duyn JH.. fMRI study of effort and information processing in a working memory task. Hum Brain Mapp. 2007;28(5):431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Teves D, Videen TO, Cryer PE, Powers WJ.. Activation of human medial prefrontal cortex during autonomic responses to hypoglycemia. Proc Natl Acad Sci U S A. 2004;101(16):6217–6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mayeli A, Misaki M, Zotev V, et al. Self-regulation of ventromedial prefrontal cortex activation using real-time fMRI neurofeedback-Influence of default mode network. Hum Brain Mapp. 2020;41(2):342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ellmore TM, Rohlffs F, Khursheed F. FMRI of working memory impairment after recovery from subarachnoid hemorrhage. Front Neurol. 2013;4:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dettwiler A, Murugavel M, Putukian M, Cubon V, Furtado J, Osherson D.. Persistent differences in patterns of brain activation after sports-related concussion: a longitudinal functional magnetic resonance imaging study. J Neurotrauma. 2014;31(2):180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fryer SL, Woods SW, Kiehl KA, et al. Deficient suppression of default mode regions during working memory in individuals with early psychosis and at clinical high-risk for psychosis. Front Psychiatry. 2013;4:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106(4):1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gosselin N, Bottari C, Chen JK, et al. Electrophysiology and functional MRI in post-acute mild traumatic brain injury. J Neurotrauma. 2011;28(3):329–341. [DOI] [PubMed] [Google Scholar]
- 41. Pomarol-Clotet E, Salvador R, Sarró S, et al. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol Med. 2008;38(8):1185–1193. [DOI] [PubMed] [Google Scholar]
- 42. Fassbender C, Zhang H, Buzy WM, et al. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jansma JM, Ramsey NF, van der Wee NJ, Kahn RS.. Working memory capacity in schizophrenia: a parametric fMRI study. Schizophr Res. 2004;68(2-3):159–171. [DOI] [PubMed] [Google Scholar]
- 44. McAllister TW, Sparling MB, Flashman LA, Guerin SJ, Mamourian AC, Saykin AJ. Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14(5):1004–1012. [DOI] [PubMed] [Google Scholar]
- 45. Čeko M, Gracely JL, Fitzcharles MA, Seminowicz DA, Schweinhardt P, Bushnell MC. Is a responsive default mode network required for successful working memory task performance? J Neurosci. 2015;35(33):11595–11605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bartova L, Meyer BM, Diers K, et al. Reduced default mode network suppression during a working memory task in remitted major depression. J Psychiatr Res. 2015;64:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hart RP, Martelli MF, Zasler ND. Chronic pain and neuropsychological functioning. Neuropsychol Rev. 2000;10(3):131–149. [DOI] [PubMed] [Google Scholar]
- 48. Diwanji TP, Engelman A, Snider JW, Mohindra P.. Epidemiology, diagnosis, and optimal management of glioma in adolescents and young adults. Adolesc Health Med Ther. 2017;8:99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS.. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20(suppl 4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]