Abstract
Late adverse effects of cancer treatments represent a significant source of morbidity and also financial hardship among brain tumor patients. These effects can be produced by direct neurologic damage of the tumor and its removal, and/or by complementary treatments such as chemotherapy and radiotherapy, either alone or combined. Notably, young adults are the critical population that faces major consequences because the early onset of the disease may affect their development and socioeconomic status.
The spectrum of these late adverse effects is large and involves multiple domains. In this review we classify the main long-term adverse effects into 4 sections: CNS complications, peripheral nervous system complications, secondary neoplasms, and Economic impact. In addition, CNS main complications are divided into nonfocal and focal symptoms.
Owing to all the secondary effects mentioned, it is essential for physicians to have a high level of clinical suspicion to prevent and provide early intervention to minimize their impact.
Keywords: adverse events, brain tumors, glioma, metastases, long-term survivors
Unfortunately, survival rates in brain cancer patients are not very hopeful despite current advances in treatment. The most prevalent malignant brain tumors (BTs), glioblastoma (GBM) and brain metastases (BMs), present with expected median global survival rates of less than 2 years in most clinical situations. It therefore follows that most major research efforts in clinical neuro-oncology have focused on increasing overall survival and that less attention has been paid to the long-lasting and late adverse events induced by the treatments. However, despite this pessimistic scenario and the slow improvement in treatment over past and recent years, some patients do achieve long-term overall survival.
Patients with low-grade gliomas (LGGs) can reach median survival rates between 8 to 13 years depending on the treatment administered,1 and 15% to 20% of this population can achieve overall survival rates of almost 20 years from diagnosis.2 Even in the case of GBMs, patients with isocitrate dehydrogenase–mutated tumors or with methylation of the MGMT promoter gene, having good performance status at diagnosis, can expect survival rates greater than 20% to 30% at 4 years under the combined standard treatment.3,4 It is also worth mentioning that 0.71% of the global GBM population remain alive 10 years after diagnosis.5 In the BM setting, independently of selected patients treated with radical approaches like surgery or radiosurgery, a survival rate of more than 4 years can be found in up to 40% of the patients with favorable clinical and molecular prognostic factors.6 For this not preponderant but existing population of long-term survivors, the treating neuro-oncologists must focus either on early identification of these patients and the treatment side effects or on providing early treatment for adverse events when possible.
Current treatments, either alone or administered in combined schedules, can induce side effects. It is well known that radiotherapy (RT) and chemotherapy (ChT) cause short-term and even medium-term complications. This is in part due to the focus numerous clinical trials place on the efficacy of treatment. However, there is far less information on the long-term consequences of these treatments. This is due in part to the lower number of long-term survivors, and because frequently therapeutic schedules combine RT and ChT regimens, which hinders the identification of the side effect responsible, or even differentiation of the effect from tumor progression.
The aim of this report is to provide a narrative review of the main long-term adverse events related to BT treatments, classified by clinical manifestations, to increase physicians’ awareness of this problem. The adverse events and clinical consequences considered for this purpose are any triggered by or related to the oncologic treatments, independently of when they appear, and those that the patient will have to face for the rest of their lives. Because of this wide range of scope, this review is mainly focused on the adulthood population, although age frontiers progressively lose significance when we address the chronic treatment consequences in long-term cancer survivor patients. Likewise, we will leave beyond the scope of this clinical practice review the pathophysiology behind these adverse events; these mechanisms are quite different among the adverse events, and are only partially known in the best of cases. Deeper knowledge about these mechanisms can be obtained from the monographic reviews cited in the text.
Nonfocal CNS Adverse Events
Cognition Impairment
Cognitive impairment is a frequent and under evaluated neurological symptom in the BT population. In fact, up to 50% to 90% of long-term survivors will exhibit some cognitive dysfunction during the course of the disease with a consequent impairment of their quality of life.7
Cognitive impairment occurs in the majority of treatment-naive glioma (up to 62%), being more common in high-grade glioma (HGG) than LGG and also in BM patients (nearly 50%), suggesting that the tumor by itself is highly responsible for neurocognitive dysfunction. However, the literature about pretreatment cognitive toxicity in this population is characterized by small-scale studies and strong heterogeneity.8 Likewise, the surgery impact on cognition in LGG is controversial.
Several studies have shown that surgery in eloquent areas is related to cognitive deficits that tend to resolve in the following 3 months.9 However, another study comparing the effect of surgery on cognition in LGG in a group of patients with radiological suspicion of LGG as a control group showed that the group that underwent surgery exhibited worse cognitive functioning and quality of life than those who did not.10 How these factors may affect the long-term development of cognitive deficits is not yet known.
ChT-induced cognitive impairment or “chemobrain” has mostly been described in non-CNS tumor patients, although the literature for the BM population is scarce. In the primary BT population, ChT may have greater access to the brain because of the disease burden that diminishes the integrity of the blood-brain barrier (BBB) or because of the formulation of agents that more readily cross the BBB.11 Unfortunately, data directly supporting chemobrain in BT population are limited.12 In glioma population, 2 studies of LGG and anaplastic gliomas13,14 showed that the addition of PCV (procarbazine, lomustine, and vincristine) to RT did not result in significantly higher rates of Mini-Mental State Exam score decline. Unfortunately, no more extensive appropriate cognitive assessment was performed.
Conversely to chemobrain, cognitive toxicity induced by RT in the BT population is well established. Particularly, in the BM population, 2 randomized studies comparing oligometastatic patients treated with focal RT alone vs focal RT plus whole-brain radiotherapy (WBRT) showed a detrimental effect of the WBRT at 3 to 4 months (24% vs 52%)15 and 1 year (63.5% vs 91.7%)16 of follow-up. Likewise, a randomized clinical trial in oligometastatic patients who underwent surgery compared focal RT on the surgical cavity vs WBRT showing a significant difference in cognitive impairment at 6 months in the WBRT arm (52% vs 85%).17
Concerning cognitive deficits in LGG following RT, a longitudinal study showed cognitive function decline (especially in attention) after a median of 12 years from diagnosis.18 Moreover, a recent study that included oligodendroglioma (grades II and III) treated with RT alone (20%) or combined with ChT (80%) has shown cognitive impairment as early as 2 years after combined treatment. After 5 and 10 years, survivors of any or both treatment modalities reported a 38% and 70% rate of cognitive deterioration, respectively, accompanied by global cortical atrophy and white-matter changes on MRI.19 Regarding World Health Organization grade IV, no data on long-term cognitive impairment are available.
Classically, the cognitive toxicity induced by RT has been classified as acute (and reversible), early delayed, and late delayed toxicity. Late cognitive deterioration usually appears 6 months after RT. It appears as a progressive and irreversible subcortical cognitive decline.20 Risk factors associated with RT-induced cognitive toxicity described so far are age (< 7 or > 60 years), dose greater than 2 Gy per fraction, total cumulative radiation dose, irradiated brain volume, concomitant ChT, and cardiovascular risk factors.21 RT-related neuroimaging anomalies include abnormalities of the periventricular white matter (Figure 1A), ventricular dilation, and cortical atrophy. Additionally, long-term RT has been associated with loss of hippocampal volume and cortical thickness decline in temporal and limbic regions in patients with primary BT7.22 In line with these results, prophylactic cranial irradiated patients showed a bilateral gray-matter reduction in basal ganglia and white-matter microstructural changes.23
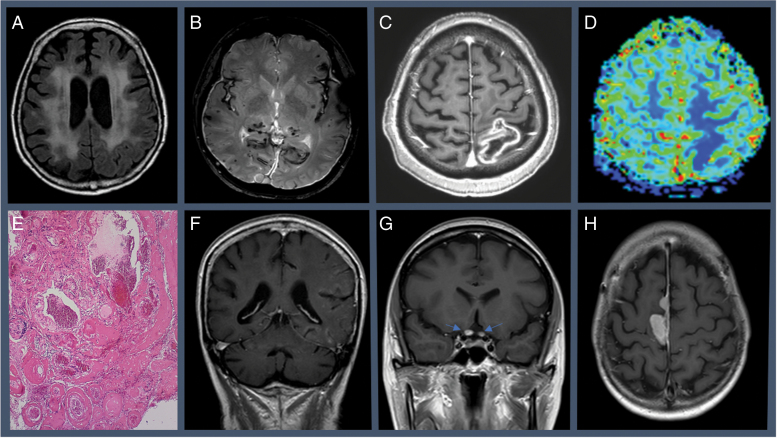
Fig. 1.
A, Leukoencephalopathy induced by whole-brain radiotherapy. T2/fluid-attenuated inversion recovery MRI showing hyperintensities affecting bilateral subcortical white matter. B, Radio-induced cavernomas and meningeal siderosis in an adult patient with a medical history of glioma World Health Organization grade I treated with radiotherapy in his childhood. T2-weighted gradient-echo MRI sequence. C, Cerebral radiation necrosis (focal area of contrast-enhancement on T1-weighted MRI) with lower relative cerebral blood volume levels, D, in a patient treated with radiosurgery for brain metastasis; and the histopathological findings after surgical resection, showing astrogliosis, infiltration by macrophages, necrosis, hyalinosis, and extensive vasculopathic changes that include E, ectasia, fibrinoid necrosis, and obliterative mural fibroplasia. F, SMART (stroke-like migraine attacks after radiation therapy) syndrome from a patient showing diffuse unilateral cortical T1-weighted gadolinium enhancement of cerebral gyri during the acute neurological event. G, Radiation-induced optic neuropathy of a patient with pituitary metastasis treated with stereotactic radiosurgery. Contrast-enhancement on T1-weighted MRI showing focal enhancement of both intracranial optic nerves. H, Radiation-induced meningioma.
With respect to potential therapeutic approaches, i) nonpharmacological measures such as cognitive rehabilitation in glioma patients24 and hippocampal sparing25 and ii) pharmacological therapies such as memantine in BM patients26 have been successfully tested. Recent results in BM patients treated with hippocampal sparing and memantine showed a lower incidence of cognitive deterioration at 6 months compared to a group treated with WBRT plus memantine (59.5% vs 68.2%).27
Other drugs studied in this population failed to improve cognitive deficits, including donepezil, methylphenidate, modafinil, and motexafin gadolinium.
Fatigue
Most cancer patients are at risk of suffering from fatigue. Focusing on BT, fatigue has been reported in about 40% to 70% of patients during active systemic treatment, and in about 80% during cranial RT.28 Despite the strong connection between active treatment and fatigue, the latter might not disappear with the finalization of treatment. Studies of general cancer survivors have shown that approximately 20% to 30% of patients might suffer from persistent fatigue.29 Regarding the BT population, most of these data come from patients with BM (especially from breast and lung cancer). However, the evidence is more limited in patients with primary BT, in whom fatigue with HGG tends to be more common than LGG (including the pediatric population),30 whereas in the long-term (> 2 years), fatigue tends to be higher in LGG because of a longer life expectancy.31
Before starting any treatment for fatigue, it is important to exclude conditions that may contribute to worsening fatigue, such as oncological status (excluding recurrence or progression), emotional distress, anemia, sleep disturbances, nutritional deficiencies, metabolic disorders, endocrine dysfunction (particularly hypothyroidism or adrenal insufficiency), other medical comorbidities, and poor physical condition. It is also important to reduce the dosage or eliminate unnecessary supportive medication (if possible). In addition, it is essential to be aware of any recent medication changes and their possible drug interactions.
Finally, after treatable contributing factors have been identified, it is important to plan measures to reduce fatigue. Several clinical trials have been conducted to evaluate the efficacy of diverse therapeutic approaches: i) nonpharmacological intervention: Aerobic exercise is considered as the strongest evidence (level 1) to improve fatigue. However, in BT patients the evidence is limited, although it may also potentially be effective. Psychosocial intervention such as cognitive-behavioral therapy, educational therapies, and meditation may be also useful (level 1); and ii) pharmacological intervention: methylphenidate and modafinil32 have been proposed but the results are controversial, especially in primary BT, for which most of the studies were uncontrolled and enrolled only a small number of patients.
Endocrinopathies
Endocrinopathies are among the most common permanent sequelae resulting from BT therapy. They are highly striking in children, in whom the growing process, puberty, and gonadal development can be seriously impaired; they are much underdiagnosed in adults.33,34 In addition to RT,35 surgical resection, or rarely ChT, causes toxic damage to the hypothalamus and pituitary gland resulting in several hormonal deficiencies.36 Endocrine dysfunction may also appear as a consequence of the tumor itself, or patients may develop peripheral endocrinopathies as a result of multimodality cancer treatment.34 The incidence rate reported is highly variable (43%-93%)37 due to differences in BT populations, depth of assessments, and timing of evaluation. The largest series including adult-onset BT patients showed a rate of 88.8% of hypopituitarism.33
Endocrinopathies are a time-dependent phenomenon. Progressive loss of hypothalamic function occurs in the first decade with subsequent decline in pituitary function between 10 and 20 years after cranial RT. The latter is due to a loss of pituitary cells responsible for secreting growth hormone (GH), thyrotropin, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and adrenocorticotropic hormone (ACTH),36 which can involve multiple hormones in up to 40%34,36 and a panhypopituitarism in some cases.38 Importantly, the spectrum of endocrine disorders in BT survivors may vary from a subtle laboratory abnormality with limited clinical significance to potentially lethal syndromes.37
Many studies have demonstrated that higher doses of RT are associated with an increased risk of endocrine dysfunction.35,36 Remarkable differences observed in the incidence of anterior pituitary hormone deficiencies suggest different radiosensitivities of the various hypothalamus-pituitary cell lines. One retrospective study including 58 adult-onset patients with glioma distant from the hypothalamus-pituitary axis without known hormone deficit prior to RT followed for a median period of 8.2 years described dose thresholds as follows: greater than 10 Gy, 30 Gy, 32 Gy, and 40.8 Gy for the GH, luteinizing hormone/follicle-stimulating hormone, ACTH, and thyrotropin axis, respectively, which is in line with those reported in childhood cancer survivors.34
A high rate of endocrine deficiency was observed in an adult series, in which almost 85% of patients developed pituitary hormone deficits over a follow-up of 8 years, even after excluding high-risk (parasellar) BTs.34 Despite the expected higher risk in suprasellar tumors, many studies show that more than 30% of patients with posterior fossa and supratentorial tumors also developed endocrinopathies after cranial RT without showing differences in the course and the severity of hormone deficiencies.39 In some types of RT, to date, proton beam radiation has not been associated with a significant reduction in this latter complication.40
Among endocrine dysfunctions, GH deficiency (GHD) is the earliest and most frequent, with an increasing risk in doses greater than 18 Gy.36 In suprasellar or posterior fossa tumor at doses greater than 30 Gy, risk for GHD may be more than 80% by 10 years after RT.34,36 ChT has also been associated with GHD in BT survivors. Although an important part of the adult population is going to suffer from GHD, the replacement benefit is not clear, and the potential effect of extraneous GH on tumor growth has to be considered before take any treatment decision.
With respect to central and primary hypothyroidism, 53% and 21% of 254 BT survivors presented with thyroid dysfunction 2 years after therapy.39 Primary hypothyroidism occurred most commonly in patients with posterior fossa tumors such as medulloblastoma, likely reflecting direct thyroid injury following neck irradiation.41 Conversely, there is conflicting evidence regarding a higher incidence of hypothyroidism in BT survivors receiving RT and ChT compared with those treated with RT alone.37
Hyperprolactinemia may develop in 6% to 10% of BT survivors,39 typically in female patients, being transitory in most cases.34 Contradictory results have been reported between RT dose and hyperprolactinemia,36,39 and a potential synergistic effect of ChT has been suggested.42
Hypogonadism due to gonadotropin deficiency has also been frequently reported, with cumulative incidences between 20% and 50% of patients at long-term follow-up and related to ChT and RT.43 However, transient or permanent testicular or ovarian damage most frequently occurs secondary to adjuvant ChT. Gonadotoxicity is dose dependent and can be induced by alkylating agents such as temozolomide, procarbazine, cisplatin, and vinblastine, or spinal RT. Given the risk of treatment-induced infertility, adult patients should be counseled regarding fertility preservation before initiating therapy. Furthermore, sexual dysfunction, which is not limited to organ dysfunction, is a common issue in BT patients (up to 53%) despite rarely being addressed in daily clinical practice. However, other factors such as concomitant medications and brain-side tumor resection can influence sexual dysfunction.
ACTH deficiency was found in 14% of BT survivors at a median of 2.2 years after therapy,39 being lower (< 5%) when RT was not involving the pituitary gland.44 However, the most frequent cause of adrenal insufficiency in patients with BT is prolonged glucocorticoid treatment. Conversely, diabetes insipidus, unlike anterior pituitary dysfunction, is an uncommon complication and usually associated with BT infiltration or surgical manipulation at suprasellar tumors,42 affecting between 30% and 77% of craniopharyngioma survivors.36
In summary, endocrine dysfunction can be subclinical and associated with unspecific symptoms. As a treatable condition, a high level of clinical suspicion is critical, in addition to routine surveillance and laboratory screening not only in children but also in adult-onset BT long-term survivors. Supplementary treatment according to follow-up recommendations and guidelines for enhancing the quality of life of BT survivorship should be considered.
Focal CNS Adverse Events
Cerebrovascular Disorders
Therapy-related vascular disorders including ischemic and hemorrhagic stroke and cerebral venous thrombosis occur in BT patients. Recently, a population-based study among 8 million cancer patients identified a rate of stroke of 21.64/100 000/year and a standardized mortality ratio of stroke of 2.17, making the risk of stroke for cancer patients more than twice that of the general population. BT patients with a stroke have the highest mortality.45 In addition, another study showed that the risk of ischemic and hemorrhagic strokes was higher in BT patients in the first 6 months after diagnosis. However, the risk of both ischemic and hemorrhagic strokes decreases 1 year after diagnosis.46
A retrospective study in primary BT patients who had suffered strokes showed that up to 25% had strokes related to delayed effects of RT. The median latency from RT to stroke diagnosis was 3.2 years (range, 0.5-30 years). The most common BTs associated with strokes were gliomas (60%), meningiomas (25%), and primary CNS lymphoma (6%).47 However, ChT has also been associated with CNS vasculopathy, although the risk is rather low (incidence less than 1%). However, studies may have included patients who had also been treated with RT, which would increase this risk. Among ChT or other agents, the risk of stroke is higher for some specific regimens such as methotrexate, 5‑fluorouracil, platinum compounds, L‑asparaginase, and bevacizumab.48,49 However, most events occur during treatment or during a relatively short period of time after the end of treatment.
The vascular complications of cranial RT include stroke (both hemorrhagic and ischemic), lacunar lesions, vascular occlusive disease including moyamoya syndrome, and vascular malformations. Postradiation vasculopathy usually occurs in medium- and large‑sized vessels, with subsequent stenosis or occlusion typically occurring more extensively within the radiation portal.50 Radiation-induced vascular injury is a complex process and involves both arterial and capillary damage, because veins are less sensitive. Vascular malformations including cavernoma (see Figure 1B), telangiectasia, and aneurysm are late vascular complications of cranial RT. The estimated cumulative incidence at 15 years of cavernoma is 5%, and the risk of hemorrhage is 1% per year, which is similar to non-RT–induced cavernomas.51 Moyamoya syndrome incidence increases over time (the median period for development is more than 3 years). An increase in the incidence of moyamoya has been associated with neurofibromatosis type 1 (NF1), young age, and tumor location.52
The management of strokes in this patient population has been extrapolated from stroke therapy in adult noncancer patients. There are limited data describing interventional techniques specifically for radiation-induced cranial vasculopathies. However, regarding angioplasty and stenting for radiation-induced carotid stenosis, similar rates of efficacy and stroke risk have been demonstrated in comparison to patients with nonradiation-induced stenosis, with the exception of insistent restenosis (25.7% vs 4.2%).53
Cerebral Radiation Necrosis
Cerebral radiation necrosis (RN) is still a diagnosis challenge. It can occur as early as 3 months after RT and may appear even later than 10 years after treatment.54 The incidence of RN in HGG ranges between 5% and 7%.55 In BMs treated with stereotactic radiosurgery, which carries a higher risk for RN, reported incidence ranges between 14% and 24%.56 Risk factors include tumor volume, prescribed dose, fraction size, volume of normal brain irradiated, previous use of radiation, and the use of concurrent systemic therapy.
Clinical signs and symptoms associated with RN are variable based on location and degree of tissue injury and associated edema. However, it can also be present in asymptomatic patients. MRI features of RN and tumor progression overlap, typically appearing as focal areas of contrast enhancement on T1-weighted images and T2/fluid-attenuated inversion recovery hyperintensities reflecting perilesional edema57 (see Figure 1, C-E). Certain enhancement patterns such as “Swiss cheese,” “soap bubble,” or “cut green pepper” have 25% positive predictive value in support of RN.58 Owing to the difficulties of obtaining diagnosis certainty, the use of various advanced imaging modalities, either combined or in isolation, could be useful. MRI perfusion studies, using dynamic susceptibility contrast-enhanced perfusion MRI, might show the tissue necrosis as lower relative cerebral blood volume levels.57 However, the use of this technique is hampered by the current lack of standardization. MR spectroscopy has proven to be sensitive and specific in reliably differentiating between progressive tumor (higher choline (Cho)/creatine (Cr) and Cho/N-acetyl-aspartate ratios) and RN (higher lactate/Cr and lower Cho/Cr ratios),59 although we must remember that tumor necrosis can generate false-negative results. Among nuclear medicine studies, amino acid tracers such as 18F-dihydroxyphenylalanine (18F-DOPA) and 11C-methionine (11C-MET) seem more reliable for diagnosis than glucose metabolites.60,61 A recent imaging meta-analysis showed that the best diagnostic accuracy methods for RN in glioma are 18F-fluoroethyl-L-tyrosine (18F-FET) PET and 99mTc-sestamibi (MIBI) single-photon emission CT (SPECT) and the combination of DWI and MR spectroscopy and perfusion imaging. However, for BMs, no difference in diagnostic accuracy between methods was noted.62
The management of RN primarily depends on the presence of symptoms. For clinically asymptomatic patients, close clinical and radiographic monitoring may be sufficient because focal lesions may stabilize and spontaneously resolve over time without treatment.56 For symptomatic patients, oral corticosteroids are the preferred first-line treatment. There are no studies guiding the dose of steroids, but usually dexamethasone (4-8 mg/day) is used.
In a small randomized clinical trial, treatment with the VEGF-targeting antibody bevacizumab resulted in clinical and radiographic improvement in patients with biopsy-proven RN refractory to corticosteroids.63 A pooled analysis showed that the use of bevacizumab had a radiographic response rate of 97% and a clinical improvement rate of 79% with a mean decrease in dexamethasone of 6 mg.64 As such, bevacizumab appears to be a promising agent; however, the durability of response and toxicities such as hemorrhage, thrombosis, and impaired wound healing must be taken into account. For patients who remain symptomatic despite conservative management, or in whom there is uncertainty as to the diagnosis, surgical resection can be considered. Histopathology from surgically resected lesions commonly shows a mix of residual tumor cells and RN.65 There were other therapies reported whose efficacy was unclear: antiplatelet therapy, anticoagulation, and hyperbaric oxygen.
It is interesting to highlight a recent positive phase 2 clinical trial that tested nerve growth factor as a therapy for reverse temporal RN. Comparing groups treated with corticoids vs corticoids plus nerve growth factor (n = 14 in both arms), 4 vs 13 patients responded (P = .001) at 9 months.66 Other phase 2 clinical trials are currently ongoing including pentoxifylline combined with vitamin E (NCT01508221) and laser interstitial thermal therapy (NCT01651078).
Delayed Relapsing-Remitting Radiotherapy-Induced Neurologic Syndromes
Brain radiation has been related to delayed and sudden focal and nonfocal neurological disorders in long-term survivors. Currently, these disorders are divided into 3 not always well-differentiated distinct entities: stroke-like migraine attacks after radiation therapy (SMART) syndrome,67 peri-ictal pseudoprogression (PIPG),68 and acute late-onset encephalopathy after radiation therapy (ALERT) syndrome69,70 (Table 1). These syndromes share common features, including long-interval from brain irradiation, acute onset, and the association with transient enhancing MRI abnormalities, reversibility, and recurrence.
Table 1.
Clinical and Radiological Features of Delayed Relapsing-Remitting Radiotherapy-Induced Neurologic Syndromes (Adapted From 68 and 70)
| SMART syndrome | PIPG | ALERT syndrome | |
|---|---|---|---|
| Clinical symptoms |
|||
| Seizures | Frequently, mostly generalized seizures | Very frequently, mostly partial seizures, including subclinical epileptic discharges | Rare |
| Headache | Constant, migraine-like attacks | No | No |
| Others | Stroke-like deficits | Postictal deficits | Encephalopathy |
| MRI findings T1-weighted imaging with gadolinium |
Diffuse unilateral cortical (parieto-occipital) gadolinium enhancement of cerebral gyri | Focal cortical and/or leptomeningeal enhancing lesions | Bilateral cortico-subcortical multiple patchy enhancement ± leptomeningeal enhancement |
| Treatment | Symptomatic (headache treatment) | Symptomatic (antiepileptic drugs) | High doses of corticosteroids |
| Clinical course | Full recovery within d to few wk; relapses possible | Full recovery within wk to few mo; relapses possible | Full recovery in wk, sometimes need intensive care unit, relapses possible |
Abbreviations: ALERT, acute late-onset encephalopathy after radiotherapy; PIPG, peri-ictal pseudoprogression; SMART, stroke-like migraine attacks after radiation therapy.
SMART syndrome is a recognized syndrome that consists of prolonged reversible paroxysmal episodes of neurological dysfunction associated with headache. MRI at the time of symptoms demonstrates a transient and cortical gadolinium enhancement of the affected cerebral hemisphere (see Figure 1F). These symptoms are sometimes accompanied by generalized seizures and ipsilateral slowing electroencephalogram activity. Functional imaging studies provide additional insight into the pathophysiology of SMART. 18F-fluorodeoxyglucose PET during symptomatic episodes has demonstrated a hypermetabolism merging with gyral thickening and gadolinium enhancement on MRI in the affected areas.71 SPECT findings of focal cortical hyperperfusion in the symptomatic regions can also be useful in the diagnosis.72 Both imaging techniques’ findings have suggested that radiation-induced vascular dysfunction may cause these hyperperfusion states. However, most patients (82%) exhibited interictal epileptiform activity, electrographic seizures, or clinical seizure activity. Whether seizures are the origin, or a consequence of SMART and image test results, is still unclear.
The interval between radiation and SMART diagnosis is on average 20 years.71 Neurological symptoms can last for weeks, and although half of patients (55%) will fully recover over an average of 2 months, the remaining 45% will have permanent neurological deficits. SMART revised criteria were published in 2006.73
MRI changes are normally transient, developing within 2 to 7 days, typically resolve in 2 to 5 weeks in line with symptoms, and may not be found in every attack. However, permanent MRI changes such as cortical laminar necrosis have also been described in up to 27% of patients, developing as early as 17 days after symptom onset. MRI findings do not respect vascular borders and occur in regions included in the radiation field. However, there is prominent involvement of the temporoparietal and occipital lobes with relative sparing of the frontal lobes in all cases reported67 (see Figure 1, C and D). Clinical course may be complicated by a superimposed cerebral infarction.
Interestingly, a series on long-term survivors after brain RT was published last year, including patients with delayed relapsing-remitting RT-induced neurologic syndromes (n = 26) together with an identification of 87 additional cases from the literature. Moreover, a small proportion of patients with small-vessel infarcts was also included (n = 6).74
Patients with stroke-like syndromes commonly present with a mosaic of symptoms, including focal deficits (77%), encephalopathy (50%), seizures (35%), and headache (35%). Most of them (73%) have acute consistent MRI alterations.
Regarding SMART treatment, in case of a seizure, rapid control is essential but no other therapeutic option has demonstrated a clear benefit. Treatment includes high-dose steroids in 65% of cases, but their efficacy is uncertain because of the transient nature of this disorder. Concerning the outcome, 85% of patients recover completely but 62% experience relapses (median follow-up, 3.5 years).
Reports of what has been subsequently termed PIPG demonstrate MRI findings similar to SMART but highlight the absence of headache, less significant neurologic impairment, and more rapid clinical recovery as differentiating aspects.68 Although PIPG probably represents the same spectrum of phenomena as SMART, reported cases tend to show more meningeal enhancement than the cortical enhancement seen in SMART.
Later in 2013, Di Stefano and colleagues described ALERT syndrome. It is a distinctive acute, transient but long-lasting (4-24 days) neurologic syndrome characterized by impaired consciousness progressing to coma and signs of diffuse or multifocal brain dysfunction with clinical improvement after high-dose steroids. Clinical severity was such that all patients required intermediate or intensive care. Several consecutive electroencephalogram recordings performed during the acute phase showed bilateral/diffuse slow abnormalities. MRI showed multiple bilateral areas of subcortical patchy enhancement and/or leptomeningeal enhancement. Most of the patients (60%) recovered completely. Patients with permanent residual neurologic deficits recurred when chronic steroid therapy was tapered down.69
Peripheral Nervous System Adverse Events
Cranial Neuropathies
Among cranial nerve treatment-induced damage, hearing loss is another frequent late side effect,43 although frequently underreported or minimized by patients. It is important to note that this deafness may sometimes be present before treatment, due to the location of the primary tumor, and worsen with administered therapies. For instance, posterior and middle cranial fossa RT, platinum-based ChT, and surgery may contribute to sensorineural ototoxicity and hearing loss, although current RT conformal techniques have reduced radiation-induced cochlea damage. Toxicity to cochlear hair cells is the main cause of treatment-related hearing loss and results in deficits in hearing high-frequency sounds. The damage is typically irreversible and bilateral and can be asymmetrical. In a study based on an internet-based survey in BT survivors, hearing loss, tinnitus, or vertigo was reported by 57.4% of patients, in a median time since diagnosis of only 1 year.43 Based on time from diagnosis, survivors who had been diagnosed at an early age reported an increase of vestibular and hearing changes in all cases.43 The platinum risk toxicity, including carboplatin schedules, is dose dependent.
Visual loss is an extremely severe problem also associated with BT therapy. However, the rates are variable depending on whether the evaluation of sequelae includes subjective or objective assessments. Noteworthy, one single-center study included 42 surviving medulloblastoma pediatric patients treated with craniospinal radiation with a median follow-up of 15.6 years. In this study the most frequently reported impairment was vision (58.8%) with ophthalmological objective sequelae identified in only 17%.
BT located in the skull base or parasellar region (pituitary adenomas), meningiomas, and craniopharyngiomas receiving RT are highly at risk for cranial nerve deficits, especially radiation-induced optic neuropathy (RION) (see Figure 1G). The typical clinical presentation is a rapid, painless, and irreversible monocular visual loss, although the second optic nerve may also be rapidly involved (days to months).75 RION occurs typically between 10 and 20 months after treatment, but the onset may range from 3 months to 9 years.75 The 2 most important treatment-related risk factors for optic nerve/chiasm injury are total dose and fraction size.76 RION requires cumulative doses of radiation that usually exceed 54 Gy (1.8-2 Gy/fraction). For single-fraction stereotactic radiosurgery, the incidence of RION is rare in doses lower than 8 Gy, but increases at doses between 8 to 12 Gy, and becomes greater (> 10%) at 12 to 15 Gy.77 An inverse relationship between the radiation dose and the latency to the onset of symptoms has been suggested.75 In addition, an increased risk of RION has been reported with increasing age, preexisting compression of the optic nerve, and chiasm by tumor.75 Therefore, one limitation of most of the retrospective studies is knowing whether the late-sequelae were present before treatment. This makes it difficult to ascertain whether the late effects were a result of the treatment or the initial disease. In addition, there are conflicting results regarding a possible higher risk of optic neuropathy in BT patients receiving RT in the optic nerve fields after initiating bevacizumab as salvage therapy.78
Vagal, recurrent laryngeal, hypoglossal as well as sympathetic nerve neuropathies can also be observed as late-delayed effects of RT for skull-base BT. Radiation-induced hypoglossal neuropathy can present as paroxysmal dysarthria due to tongue myokymia.79 Likewise, vincristine has also been associated with recurrent unilateral or bilateral laryngeal nerve paralysis, but usually with good prognosis and resolution.80
Finally, bilateral ptosis associated with cranial neuropathy has also been observed in a few patients treated with vincristine and carboplatin for LGG.81
Chemotherapy-Induced Peripheral Neuropathies
Chemotherapy-induced peripheral neuropathies (CIPNs) are also frequent but mostly underreported complications in BT survivors.81 This treatment adverse event has been extensively analyzed in patients with systemic tumors82 but less in BT patients. Microtubule inhibitors like vinca alkaloids or the platinum compounds are used to treat patients with LGG, anaplastic gliomas, or medulloblastomas, sometimes in combination. Vincristine frequently causes peripheral neuropathy that can involve sensory, motor, and autonomic nerve fibers.82 Other chemotherapeutic agents, like cisplatin or carboplatin, involve sensory neurons of the dorsal root ganglion and are manifested as sensory polyneuropathy.
Rates of reported neuropathy vary according studies, probably related to the design and kind of assessments performed.
In adults, the standard ChT treatment for diffuse LGG and anaplastic oligodendrogliomas includes vincristine, 2 doses per cycle (1.4 mg/m2) during 6 cycles. In the NOA-04 trial, comparing temozolomide to PCV in anaplastic glioma, ChT was discontinued for neuropathy in 7.4% of patients in the PCV monotherapy arm.83 Similarly, the rate of 2% to 8% of grades 3 to 4 peripheral neuropathy was identified in anaplastic glioma patients receiving PCV in Radiation Therapy Oncology Group84 and European Organisation for Research and Treatment of Cancer85 trials. Grade 3 or 4 of autonomic neuropathy was reported in 2% of treated patients.84 To our knowledge, no data regarding rates of lower grades of peripheral neuropathy are available.
Most medulloblastoma protocols worldwide include vincristine during RT and ChT. Peripheral neuropathy is known as a significant dose‐limiting toxicity in these patients, and approximately 50% of patients require dose reduction; in fact, it is the exception to complete a full course of maintenance vincristine. In adults, this figure seems higher, even when vincristine during radiation induction is not tolerated, which is the case for many patients. In this vein, adults with medulloblastoma treated on the Packer protocol experienced considerably greater CIPNs than children treated on an identical protocol (50% vs 29%, respectively).86
There are scarce data available on the prognosis of neuropathy in BT survival. However, information from other systemic cancers shows that vincristine has a fairly good prognosis after long follow-up, but complete recovery is observed in only 25% of cases.87 In addition, platinum-induced neuropathy remains in around 20% to 30% of patients 20 years after treatment ends.88 Other regimens, such as weekly vinblastine, appear to offer similar tumor control without significant neurotoxicity.
Finally, it is important to remember that neurotoxic agents like vincristine are not recommended in patients with hereditary neuropathies, in whom undiagnosed hereditary peripheral neuropathy could be unmasked. These patients are asymptomatic prior to the diagnosis of BT and rapidly experience quadriparesis following a relatively low cumulative dose of vincristine.89 Owing to this, a careful family history and examination can be very helpful before vinca-alkaloid use, especially in BT children.
Secondary Neoplasms
The development of secondary neoplasms (SNs) is one of the most serious delayed consequences of oncological therapy that may affect survival, causing up to 10% of deaths in BT survivors.90,91 The interval to SN development may differ depending on histology because it is shorter in glioma than meningioma.
The Childhood Cancer Survivor Study, which assessed the long-term survivorship experience of 1887 adult survivors of childhood CNS tumors treated between 1970 and 1986, identified a cumulative incidence for all SNs of 10.7% at 25 years from diagnosis.76 Another study of overall cancer population survivors found a lower incidence of this: 20% at 30 years.92
There is strong evidence that suggests a link between cranial RT and SNs. Children and young adults are at an increased risk of developing SNs, especially those who received RT at younger than age 5 years. Age, large volume, and dose have been shown to be associated with SNs, despite being observed with low doses of RT (as an example, tinea capitis treated with 100 200 cGy WBRT).80 In the Childhood Cancer Survivor Study, 25 years after diagnosis, patients receiving cranial RT of 50 Gy or more had a higher cumulative incidence (7.1%) than those receiving less than 50 Gy (5.2%), and it was 1.0% in those without RT exposure.90
SNs are reported to be mostly malignant. The most common types of SNs are quite similar across studies. They include solid tumors like meningiomas (see Figure 1H), gliomas, malignant schwannomas, and sarcomas, or hematological diseases.91 A large retrospective, registered-based study that used the Surveillance, Epidemiology, and End Results database and included 3627 patients found the cumulative incidence of subsequent malignant neoplasms (SMNs) was of 6.4% at 30 years for pediatric BT survivors. These SMNs were BTs (40.2%), thyroid cancers (11.1%), leukemias (9.4%), sarcomas (8.5%), and other malignancies (30.8%).91 In another large study, among 2779 patients with a primary diagnosis of a CNS tumor, 81 survivors had 97 SNs, 64 (66.0%) of which were SMNs.93 More specifically, among a cohort of 155 adult GBM long-term survivors, 11% of patients during a median time of 7.8 years after diagnosis suffered an SN.94
It is important to note that the development of SMN in BT survivors is linked not only to exposure to RT but also to ChT. One of the cornerstones of treatment of glioma is based in alkylating agents, such as temozolomide, carmustine, or lomustine, which are known to be leukemogenic agents. Secondary primary hematological malignancies such as myelodysplasia, acute myeloid leukemia, or non-Hodgkin lymphoma have developed in BT patients in association with exposure to alkylating agents.95 No conclusive data have been found to indicate whether temozolomide is more likely to induce secondary hematological malignancies than nitrosoureas or to enhance the leukemogenic activity of other alkylators.95 One of the largest studies exploring this issue included 359 consecutive all-ages glioma patients who had received alkylating agents alone or combined in Japan from 1990 to 2009. In this retrospective study, the incidences of hematological malignancies were 1.33% and 1.41% in the nitrosourea and temozolomide groups, respectively, whereas in the group receiving temozolomide and nitrosoureas, incidences were 3.17%.95
Among potential factors that increase the risk of development of SNs in BT survivors, in addition to RT and ChT there is also genetic predisposition. In one study, children with a known cancer predisposition syndrome accounted for 22% of the BT population who developed SNs. NF1 is a characteristic example of this condition.93 NF1-affected children with a primary BT present a 2.4-fold and 3.5-fold higher risk of developing SNs and SMNs, respectively, when compared with non–NF1-affected childhood cancer survivors.96 Another study explored whether among treated NF1-affected children, the risk of SN was higher for those exposed to radiation and/or alkylating agents and identified that RT was associated with an increased risk (2.8-fold higher), but alkylating ChT was not.96 Although these SMNs can be curable, there is no clinical practice consensus regarding screening and surveillance between different centers. Some centers have decided to perform a surveillance brain MRI every 2 years for all patients who received cranial RT during childhood, extending this need up to 6 decades.97
Economic Impact on Brain Tumor Patients
BT patients are not exempt from long-term adverse treatment effects and its economic cost. Notably, it is estimated that a quarter of cancer survivors experience high financial hardship as a result, mainly of an increased out-of-pocket health care costs and reduced income due to employment disability.98
However, despite the magnitude of the problem, only a few studies have been conducted to investigate the economic costs for cancer survivors, especially in BT patients.
In 2018 the All-Party Parliamentary Group on Brain Tumours carried out a study addressing patients and their families, but also physicians, researchers, academics, charities, and drug companies, analyzing the economic and social impact of BT diagnosis. This study reveals that all these economic and social costs have to be sustained by the patients, families, and society at large.
The main costs included loss of income due to additional expenses of the cancer treatment care and costly home modifications. It is estimated that average household expenses have reached $18 774 per year $8687 for all other types of cancer. As a result, BT costs for working-age people have increased to an average of $757 per year, becoming the third highest behind lung ($1.524 billion) and breast ($806 million). The consequences are less severe among young adults because they are financially dependent on their parents, but in terms of social impact the consequences may be worse because the early onset of the disease will be a burden for the rest of their lives.99 Along the same line, another study reveals that about 40% of cancer survivors may warrant medical psychological intervention during the survivorship period.100
Another cost for BT patients is the productivity loss sustained by the inability to work because of cancer disease or treatment side effects, but also to premature cancer-related mortality. Related to mortality, an Irish study found that BT patients have the fourth premature mortality cost ($49.18 million: 8.3%), behind lung, colorectum, and breast cancer.101
Considering all the above, governments should aim to develop national health care plans that cover cancer survivors’ specific needs. Recently, the European Commission and European and American oncology societies have provided a set of recommendations and guidelines for patients, physicians, and national survivorship care plan development.
Conclusions
The range of late adverse events in long-term BT survivors is wide, depending on tumor location, treatment administered, and time from last therapeutic intervention. These adverse events usually have an important impact not only on patients’ neurological function or performance status, but also their financial sustainability, and this can sometimes prove life-threatening (see summary Table 2). For these reasons, in an attempt to maintain patients’ quality of life, a high level of clinical suspicion is critical to minimize its impact, especially in young long-term BT survivors. Although efforts in neuro-oncology should still focus on increasing the current suboptimal patient survival expectancy, physicians must not forget the subpopulation of already existing long-term survivors and their potential problems induced by the administered treatments.
Table 2.
Summary of Main Treatment Adverse Events in Long-Term Brain Tumor Survivors
| Category | Complication | Timing of onset | Frequency at long term, % | Clinical symptoms | Risk factors | MRI findings | Treatment |
|---|---|---|---|---|---|---|---|
| Nonfocal CNS | Cognition impairment | 3-4 mo to ≥ 2 y after initiated treatment | 50%-90% | Cognitive functions decline, especially in attention | Surgery in eloquent areas ChT RT (WBRT and SRS) Supportive treatment (AEDs) |
Changes in periventricular white matter, ventricular dilation, cortical atrophy, loss of hippocampal volume | Cognitive rehabilitation Hippocampal sparing Memantine |
| Fatigue | Usually appears during active treatment (40%-80%) | 20%-30% | Physical, emotional, and mental exhaustion | Progression of tumor, anemia, endocrine dysfunction, emotional distress, comorbidities | Not applicable | Aerobic exercise Psychosocial intervention Methylphenidate and modafinil (uncertain) |
|
| Endocrinopathies | 10-20 y | 43%-93% | GH deficiency > hypothyroidism > hypogonadism > ACTH deficiency > hyperprolactinemia | RT Surgical resection of hypothalamus and pituitary gland ChT (rarely) |
Not applicable | Supplementary treatment according to guidelines, except for GHD replacement (uncertain) | |
| Focal CNS | Cerebrovascular disorders | 3-4 y (RT) During active treatment (ChT) |
25% related to RT 1% related to ChT |
Focal neurological deficits | RT ChT (MTX, 5-fluorouracil, platinum compounds, L-asparaginase, BVZ) |
Stroke, lacunar lesions, vascular occlusive disease (Moyamoya syndrome), and vascular malformations, carotid stenosis | Adapted from stroke guidelines therapy in noncancer adult patients |
| Cerebral radiation necrosis | 3 mo to 10 y after RT | 5-%7% (HGG) 14%-24% (BMs) |
Asymptomatic or focal neurological deficits | RT | Focal area of contrast-enhancement on T1-weighted, lower rCBV levels, higher Lact/Cr and lower Cho/Cr ratios, nuclear medicine studies (amino acid tracers) | Clinical and radiographic monitoring (if asymptomatic) Oral corticosteroids or BVZ (if neurological symptoms) |
|
| SMART syndrome | ≥ 20 y | Rare | Headache (migraine-like attacks), seizures (mostly generalized seizures), transient stroke-like deficits | RT | Diffuse unilateral cortical (parieto-occipital) gadolinium enhancement of cerebral gyri | Symptomatic headache treatment | |
| Peripheral nervous system | Cranial neuropathies | 3 mo to 9 y | 57%-60% (cochlea damage and RION) | Cochlea damage > RION > vagal, recurrent laryngeal, hypoglossal, sympathetic nerve neuropathies | RT (skull base, parasellar region, posterior cranial fossa) ChT (platinum, vincristine) |
RION (focal enhancement of intracranial optic nerve) | Dose reduction Symptomatic treatment |
| CIPNs | During active treatment, right after last cycle | 20%-30% (platinum compounds) | Sensory, motor, autonomic polyneuropathy | ChT (platinum compounds, vincristine) Hereditary neuropathies |
Not applicable | Dose reduction Symptomatic treatment | |
| Secondary neoplasms | Secondary neoplasms | 25-30 y | 11%-20% | Asymptomatic, focal neurological deficits, seizures, hematological malignancies | RT ChT (leukemogenic agents: temozolomide, carmustine or lomustine) Genetic predisposition (NF1) |
Meningiomas, gliomas, malignant schwannomas, sarcomas | Oncological treatment |
Abbreviations: ACTH, adrenocorticotropic hormone; AEDs, antiepileptic drugs; BMs, brain metastases; BVZ, bevacizumab; Cho, choline; ChT, chemotherapy; CIPNs, chemotherapy-induced peripheral neuropathies; Cr, creatine; GH, growth hormone; GHD, growth hormone deficiency; HGG, high-grade gliomas; Lact, lactate; NF1, neurofibromatosis type 1; MTX, methotrexate; RION, radiation-induced optic neuropathy; rCBV, relative cerebral blood volume; RT, radiotherapy; SMART, stroke-like migraine attacks after radiation therapy; SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy.
SMART syndrome (not including peri-ictal pseudoprogression and acute late-onset encephalopathy after radiotherapy syndrome as presented in detail in Table 1).
Funding
This work was supported by the Department of Health of the Government of Catalonia CERCA Program (grant number SLT008/18/00028 to J.B.).
Conflict of interest statement. None declared.
References
- 1. Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Youland RS, Schomas DA, Brown PD, et al. Changes in presentation, treatment, and outcomes of adult low-grade gliomas over the past fifty years. Neuro Oncol. 2013;15(8):1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 4. Leibetseder A, Ackerl M, Flechl B, et al. Outcome and molecular characteristics of adolescent and young adult patients with newly diagnosed primary glioblastoma: a study of the Society of Austrian Neurooncology (SANO). Neuro Oncol. 2013;15(1):112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tykocki T, Eltayeb M. Ten-year survival in glioblastoma. A systematic review. J Clin Neurosci. 2018;54:7–13. [DOI] [PubMed] [Google Scholar]
- 6. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Makale MT, McDonald CR, Hattangadi-Gluth JA, Kesari S.. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol. 2017;13(1):52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schimmel WCM, Gehring K, Hanssens PEJ, Sitskoorn MM.. Cognitive functioning and predictors thereof in patients with 1-10 brain metastases selected for stereotactic radiosurgery. J Neurooncol. 2019;145(2):265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klein M. Neurocognitive functioning in adult WHO grade II gliomas: impact of old and new treatment modalities. Neuro Oncol. 2012;14(suppl 4):iv17–iv24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reijneveld JC, Sitskoorn MM, Klein M, Nuyen J, Taphoorn MJ.. Cognitive status and quality of life in patients with suspected versus proven low-grade gliomas. Neurology. 2001;56(5):618–623. [DOI] [PubMed] [Google Scholar]
- 11. Schagen SB, Wefel JS. Chemotherapy-related changes in cognitive functioning. EJC Suppl. 2013;11(2):225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levin VA, Yung WK, Bruner J, et al. Phase II study of accelerated fractionation radiation therapy with carboplatin followed by PCV chemotherapy for the treatment of anaplastic gliomas. Int J Radiat Oncol Biol Phys. 2002;53(1):58–66. [DOI] [PubMed] [Google Scholar]
- 13. Wang M, Cairncross G, Shaw E, et al. ; Radiation Therapy Oncology Group (RTOG); North Central Cancer Treatment Group (NCCTG); Southwest Oncology Group (SWOG); National Cancer Institute of Canada Clinical Trials Group (NCIC CTG); Eastern Cooperative Oncology Group (ECOG) . Cognition and quality of life after chemotherapy plus radiotherapy (RT) vs. RT for pure and mixed anaplastic oligodendrogliomas: Radiation Therapy Oncology Group trial 9402. Int J Radiat Oncol Biol Phys. 2010;77(3):662–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prabhu RS, Won M, Shaw EG, et al. Effect of the addition of chemotherapy to radiotherapy on cognitive function in patients with low-grade glioma: secondary analysis of RTOG 98-02. J Clin Oncol. 2014;32(6):535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. [DOI] [PubMed] [Google Scholar]
- 16. Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–818. [DOI] [PubMed] [Google Scholar]
- 19. Cayuela N, Jaramillo-Jiménez E, Càmara E, et al. Cognitive and brain structural changes in long-term oligodendroglial tumor survivors. Neuro Oncol. 2019;21(11):1470–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurologist. 2003;9(4):180–188. [DOI] [PubMed] [Google Scholar]
- 21. Dietrich J, Monje M, Wefel J, Meyers C.. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008;13:1285–1295. [DOI] [PubMed] [Google Scholar]
- 22. Cayuela N, Simó M. Neurotoxicidad cognitiva inducida por la radioterapia cerebral en adultos. Rev Neurol. 2019;68:160–168. [PubMed] [Google Scholar]
- 23. Simó M, Vaquero L, Ripollés P, et al. Brain damage following prophylactic cranial irradiation in lung cancer survivors. Brain Imaging Behav. 2016;10(1):283–295. [DOI] [PubMed] [Google Scholar]
- 24. Gehring K, Sitskoorn MM, Gundy CM, et al. Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol. 2009;27(22):3712–3722. [DOI] [PubMed] [Google Scholar]
- 25. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brown PD, Pugh S, Laack NN, et al. ; Radiation Therapy Oncology Group (RTOG) . Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown PD, Gondi V, Pugh S, et al. ; for NRG Oncology . Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG Oncology CC001. J Clin Oncol. 2020;38(10):1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schiff D, Lee EQ, Nayak L, et al. Medical management of brain tumors and the sequelae of treatment. Neuro Oncol 2015;17(4):488–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bower JE. Cancer-related fatigue: Mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pace A, Dirven L, Koekkoek JAF, et al. ; European Association of Neuro-Oncology palliative care task force . European Association for Neuro-Oncology (EANO) guidelines for palliative care in adults with glioma. Lancet Oncol. 2017;18(6):e330–e340. [DOI] [PubMed] [Google Scholar]
- 31. Drappatz J, Wen PY. Principles of supportive care. In: Primary Central Nervous System Tumors: Pathogenesis and Therapy. Norden AD, Reardon DA, Wen PY, eds. New York: Springer; 2011:72–73. [Google Scholar]
- 32. Gehring K, Patwardhan SY, Collins R, et al. A randomized trial on the efficacy of methylphenidate and modafinil for improving cognitive functioning and symptoms in patients with a primary brain tumor. J Neurooncol. 2012;107(1):165–174. [DOI] [PubMed] [Google Scholar]
- 33. Kyriakakis N, Lynch J, Orme SM, et al. Pituitary dysfunction following cranial radiotherapy for adult-onset nonpituitary brain tumours. Clin Endocrinol (Oxf). 2016;84(3):372–379. [DOI] [PubMed] [Google Scholar]
- 34. Kyriakakis N, Lynch J, Orme SM, et al. Hypothalamic-pituitary axis irradiation dose thresholds for the development of hypopituitarism in adult-onset gliomas. Clin Endocrinol (Oxf). 2019;91(1):131–140. [DOI] [PubMed] [Google Scholar]
- 35. Liu APY, Hastings C, Wu S, et al. Treatment burden and long-term health deficits of patients with low-grade gliomas or glioneuronal tumors diagnosed during the first year of life. Cancer. 2019;125(7):1163–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Howell JC, Rose SR. Pituitary disease in pediatric brain tumor survivors. Expert Rev Endocrinol Metab. 2019;14(4):283–291. [DOI] [PubMed] [Google Scholar]
- 37. Gurney JG, Kadan-Lottick NS, Packer RJ, et al. ; Childhood Cancer Survivor Study . Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer. 2003;97(3):663–673. [DOI] [PubMed] [Google Scholar]
- 38. Huynh-Le MP, Walker AJ, Burger PC, et al. Management of pediatric intracranial low-grade gliomas: long-term follow-up after radiation therapy. Childs Nerv Syst. 2016;32(8):1425–1430. [DOI] [PubMed] [Google Scholar]
- 39. Lawson SA, Horne VE, Golekoh MC, et al. Hypothalamic-pituitary function following childhood brain tumors: analysis of prospective annual endocrine screening. Pediatr Blood Cancer. 2019;66(5):e27631. [DOI] [PubMed] [Google Scholar]
- 40. Eaton BR, Esiashvili N, Kim S, et al. Endocrine outcomes with proton and photon radiotherapy for standard risk medulloblastoma. Neuro Oncol. 2016;18(6):881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Paulino AC. Hypothyroidism in children with medulloblastoma: a comparison of 3600 and 2340 cGy craniospinal radiotherapy. Int J Radiat Oncol Biol Phys. 2002;53(3):543–547. [DOI] [PubMed] [Google Scholar]
- 42. Stava CJ, Jimenez C, Vassilopoulou-Sellin R. Endocrine sequelae of cancer and cancer treatments. J Cancer Surviv. 2007;1(4):261–274. [DOI] [PubMed] [Google Scholar]
- 43. Sloane K, Vachani C, Hampshire MK, Metz JM, Hill-Kayser CE.. Late effects in survivors of central nervous system tumors: reports by patients and proxies. J Cancer Surviv. 2016;10(2):234–240. [DOI] [PubMed] [Google Scholar]
- 44. Constine LS, Woolf PD, Cann D, et al. Hypothalamic-pituitary dysfunction after radiation for brain tumors. N Engl J Med. 1993;328(2):87–94. [DOI] [PubMed] [Google Scholar]
- 45. Zaorsky NG, Zhang Y, Tchelebi LT, Mackley HB, Chinchilli VM, Zacharia BE.. Stroke among cancer patients. Nat Commun. 2019;10(1):5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zöller B, Ji J, Sundquist J, Sundquist K.. Risk of haemorrhagic and ischaemic stroke in patients with cancer: a nationwide follow-up study from Sweden. Eur J Cancer. 2012;48(12):1875–1883. [DOI] [PubMed] [Google Scholar]
- 47. Kreisl TN, Toothaker T, Karimi S, DeAngelis LM.. Ischemic stroke in patients with primary brain tumors. Neurology. 2008;70(24):2314–2320. [DOI] [PubMed] [Google Scholar]
- 48. Saynak M, Cosar-Alas R, Yurut-Caloglu V, Caloglu M, Kocak Z, Uzal C.. Chemotherapy and cerebrovascular disease. J BUON. 2008;13(1):31–36. [PubMed] [Google Scholar]
- 49. Auer TA, Renovanz M, Marini F, Brockmann MA, Tanyildizi Y.. Ischemic stroke and intracranial hemorrhage in patients with recurrent glioblastoma multiforme, treated with bevacizumab. J Neurooncol. 2017;133(3):571–579. [DOI] [PubMed] [Google Scholar]
- 50. Rogers LR. Cerebrovascular complications in cancer patients. Neurol Clin. 2003;21(1):167–192. [DOI] [PubMed] [Google Scholar]
- 51. Nimjee SM, Powers CJ, Bulsara KR. Review of the literature on de novo formation of cavernous malformations of the central nervous system after radiation therapy. Neurosurg Focus. 2006;21(1):e4. [DOI] [PubMed] [Google Scholar]
- 52. Desai SS, Paulino AC, Mai WY, Teh BS.. Radiation-induced moyamoya syndrome. Int J Radiat Oncol Biol Phys. 2006;65(4):1222–1227. [DOI] [PubMed] [Google Scholar]
- 53. Yu SC, Zou WX, Soo YO, et al. Evaluation of carotid angioplasty and stenting for radiation-induced carotid stenosis. Stroke. 2014;45(5):1402–1407. [DOI] [PubMed] [Google Scholar]
- 54. Parvez K, Parvez A, Zadeh G. The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int J Mol Sci. 2014;15(7):11832–11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ruben JD, Dally M, Bailey M, Smith R, McLean CA, Fedele P.. Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65(2):499–508. [DOI] [PubMed] [Google Scholar]
- 56. Minniti G, Clarke E, Lanzetta G, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Barajas RF Jr, Chang JS, Segal MR, et al. Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2009;253(2):486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dequesada IM, Quisling RG, Yachnis A, Friedman WA.. Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic-pathological study. Neurosurgery. 2008;63(5):898–903; discussion 904. [DOI] [PubMed] [Google Scholar]
- 59. Smith EA, Carlos RC, Junck LR, Tsien CI, Elias A, Sundgren PC.. Developing a clinical decision model: MR spectroscopy to differentiate between recurrent tumor and radiation change in patients with new contrast-enhancing lesions. AJR Am J Roentgenol. 2009;192(2):W45–W52. [DOI] [PubMed] [Google Scholar]
- 60. Kim YH, Oh SW, Lim YJ, et al. Differentiating radiation necrosis from tumor recurrence in high-grade gliomas: assessing the efficacy of 18F-FDG PET, 11C-methionine PET and perfusion MRI. Clin Neurol Neurosurg. 2010;112(9):758–765. [DOI] [PubMed] [Google Scholar]
- 61. Lizarraga KJ, Allen-Auerbach M, Czernin J, et al. (18)F-FDOPA PET for differentiating recurrent or progressive brain metastatic tumors from late or delayed radiation injury after radiation treatment. J Nucl Med. 2014;55(1):30–36. [DOI] [PubMed] [Google Scholar]
- 62. Furuse M, Nonoguchi N, Yamada K, et al. Radiological diagnosis of brain radiation necrosis after cranial irradiation for brain tumor: a systematic review. Radiat Oncol. 2019;14(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tye K, Engelhard HH, Slavin KV, et al. An analysis of radiation necrosis of the central nervous system treated with bevacizumab. J Neurooncol. 2014;117(2):321–327. [DOI] [PubMed] [Google Scholar]
- 65. Telera S, Fabi A, Pace A, et al. Radionecrosis induced by stereotactic radiosurgery of brain metastases: results of surgery and outcome of disease. J Neurooncol. 2013;113(2):313–325. [DOI] [PubMed] [Google Scholar]
- 66. Wang XS, Ying HM, He XY, Zhou ZR, Wu YR, Hu CS.. Treatment of cerebral radiation necrosis with nerve growth factor: a prospective, randomized, controlled phase II study. Radiother Oncol. 2016;120(1):69–75. [DOI] [PubMed] [Google Scholar]
- 67. Black DF, Morris JM, Lindell EP, et al. Stroke-like migraine attacks after radiation therapy (SMART) syndrome is not always completely reversible: a case series. AJNR Am J Neuroradiol. 2013;34(12):2298–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rheims S, Ricard D, van den Bent M, et al. Peri-ictal pseudoprogression in patients with brain tumor. Neuro Oncol. 2011;13(7):775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Di Stefano AL, Berzero G, Vitali P, et al. Acute late-onset encephalopathy after radiotherapy: an unusual life-threatening complication. Neurology. 2013;81(11):1014–1017. [DOI] [PubMed] [Google Scholar]
- 70. Regal PJ, Di Stefano AL, Berzero G, Marchioni E.. Acute late-onset encephalopathy after radiotherapy: an unusual life-threatening complication. Neurology. 2014;82(12):1102. [DOI] [PubMed] [Google Scholar]
- 71. Pruitt A, Dalmau J, Detre J, Alavi A, Rosenfeld MR.. Episodic neurologic dysfunction with migraine and reversible imaging findings after radiation. Neurology. 2006;67(4):676–678. [DOI] [PubMed] [Google Scholar]
- 72. Cordato DJ, Brimage P, Masters LT, et al. Post-cranial irradiation syndrome with migraine-like headaches, prolonged and reversible neurological deficits and seizures. J Clin Neurosci. 2006;13(5):586–590. [DOI] [PubMed] [Google Scholar]
- 73. Bartleson JD, Krecke KN, O’Neill BP, Brown PD.. Reversible, strokelike migraine attacks in patients with previous radiation therapy. Neuro Oncol. 2003;5(2):121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Di Stefano AL, Berzero G, Ducray F, et al. Stroke-like events after brain radiotherapy: a large series with long-term follow-up. Eur J Neurol. 2019;26(4):639–650. [DOI] [PubMed] [Google Scholar]
- 75. Danesh-Meyer HV. Radiation-induced optic neuropathy. J Clin Neurosci. 2008;15(2):95–100. [DOI] [PubMed] [Google Scholar]
- 76. Farzin M, Molls M, Kampfer S, et al. Optic toxicity in radiation treatment of meningioma: a retrospective study in 213 patients. J Neurooncol. 2016;127(3):597–606. [DOI] [PubMed] [Google Scholar]
- 77. Pollock BE, Link MJ, Leavitt JA, Stafford SL.. Dose-volume analysis of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Neurosurgery. 2014;75(4):456–460; discussion 460. [DOI] [PubMed] [Google Scholar]
- 78. Chamberlain MC, Raizer J, Schiff D, Sherman JH.. Optic neuropathy in patients with glioblastoma receiving bevacizumab. Neurology. 2010;75(3):289–290; author reply 290. [DOI] [PubMed] [Google Scholar]
- 79. Geraldes T, Alemany M, Lozano A, Velasco R.. Hypoglossal myokymia presenting as paroxysmal dysarthria following head and neck radiotherapy. J Neurol Neurosurg Psychiatry. 2017;88(8):709. [DOI] [PubMed] [Google Scholar]
- 80. Stone JB, DeAngelis LM. Cancer-treatment-induced neurotoxicity—focus on newer treatments. Nat Rev Clin Oncol. 2016;13(2):92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rosca L, Robert-Boire V, Delisle JF, Samson Y, Perreault S.. Carboplatin and vincristine neurotoxicity in the treatment of pediatric low-grade gliomas. Pediatr Blood Cancer. 2018;65(11):e27351. [DOI] [PubMed] [Google Scholar]
- 82. Velasco R, Bruna J. Chemotherapy-induced peripheral neuropathy: an unresolved issue [article in Spanish]. Neurologia. 2010;25(2):116–131. [PubMed] [Google Scholar]
- 83. Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27(35):5874–5880. [DOI] [PubMed] [Google Scholar]
- 84. Intergroup Radiation Therapy Oncology Group Trial 9402; Cairncross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24(18):2707–2714. [DOI] [PubMed] [Google Scholar]
- 85. van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24(18):2715–2722. [DOI] [PubMed] [Google Scholar]
- 86. Greenberg HS, Chamberlain MC, Glantz MJ, Wang S.. Adult medulloblastoma: multiagent chemotherapy. Neuro Oncol. 2001;3(1):29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Haim N, Epelbaum R, Ben-Shahar M, Yarnitsky D, Simri W, Robinson E.. Full dose vincristine (without 2-mg dose limit) in the treatment of lymphomas. Cancer. 1994;73(10):2515–2519. [DOI] [PubMed] [Google Scholar]
- 88. Glendenning JL, Barbachano Y, Norman AR, Dearnaley DP, Horwich A, Huddart RA.. Long-term neurologic and peripheral vascular toxicity after chemotherapy treatment of testicular cancer. Cancer. 2010;116(10):2322–2331. [DOI] [PubMed] [Google Scholar]
- 89. Kissoon T, Gururangan S, Sladky J. Acute neurotoxicity following vincristine due to Charcot-Marie-Tooth disease in a young child with medulloblastoma. Neurooncol Pract. 2019;6(3):179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Armstrong GT, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101(13):946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Perkins SM, Fei W, Mitra N, Shinohara ET.. Late causes of death in children treated for CNS malignancies. J Neurooncol. 2013;115(1):79–85. [DOI] [PubMed] [Google Scholar]
- 92. Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102(14):1083–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tsui K, Gajjar A, Li C, et al. Subsequent neoplasms in survivors of childhood central nervous system tumors: risk after modern multimodal therapy. Neuro Oncol. 2015;17(3):448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kim JY, Jackman JG, Woodring S, et al. Second primary cancers in long-term survivors of glioblastoma. Neurooncol Pract. 2019;6(5):386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Momota H, Narita Y, Miyakita Y, Shibui S.. Secondary hematological malignancies associated with temozolomide in patients with glioma. Neuro Oncol. 2013;15(10):1445–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bhatia S, Chen Y, Wong FL, et al. Subsequent neoplasms after a primary tumor in individuals with neurofibromatosis type 1. J Clin Oncol. 2019;37(32):3050–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Turcotte LM, Neglia JP, Reulen RC, et al. Risk, risk factors, and surveillance of subsequent malignant neoplasms in survivors of childhood cancer: a review. J Clin Oncol. 2018;36(21):2145–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ekwueme DU, Zhao J, Rim SH, et al. Annual out-of-pocket expenditures and financial hardship among cancer survivors aged 18-64 years—United States, 2011-2016. MMWR Morb Mortal Wkly Rep. 2019;68(22):494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Brain Tumour Research. Brain Tumours: a cost too much to bear? Report of the All-Party Parliamentary Group (APPG) on Brain Tumours. Inquiry into the economic and social impacts of brain tumours. https://www.braintumourresearch.org/media/news/news-item/2018/10/15/exposing-the-financial-impact-of-a-brain-tumour. Accessed March 2, 2020.
- 100. Lagergren P, Schandl A, Aaronson NK, et al. ; European Academy of Cancer Sciences . Cancer survivorship: an integral part of Europe’s research agenda. Mol Oncol. 2019;13(3):624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hanly PA, Sharp L. The cost of lost productivity due to premature cancer-related mortality: an economic measure of the cancer burden. BMC Cancer. 2014;14:224. [DOI] [PMC free article] [PubMed] [Google Scholar]