Abstract
Background
The aim of our study is to determine the incidence, timing, and risk factors for cerebral vasculopathy after cranial proton and photon radiation for pediatric brain tumors.
Methods
We performed a single-institution retrospective review of a cohort of children treated with proton radiation for brain tumors. MRA and/or MRI were reviewed for evidence of cerebral vascular stenosis and infarcts. Twenty-one similar studies (17 photon, 4 proton) were identified by systematic literature review.
Results
For 81 patients with median follow-up of 3 years, the rates of overall and severe vasculopathy were 9.9% and 6.2% respectively, occurring a median of 2 years post radiation. Dose to optic chiasm greater than 45 Gy and suprasellar location were significant risk factors. Results were consistent with 4 prior proton studies (752 patients) that reported incidence of 5% to 6.7%, 1.5 to 3 years post radiation. With significantly longer follow-up (3.7-19 years), 9 studies (1108 patients) with traditional photon radiation reported a higher rate (6.3%-20%) and longer time to vasculopathy (2-28 years). Significant risk factors were neurofibromatosis type 1 (NF-1; rate 7.6%-60%) and suprasellar tumors (9%-20%). In 10 studies with photon radiation (1708 patients), the stroke rate was 2% to 18.8% (2.3-24 years post radiation).
Conclusions
Childhood brain tumor survivors need screening for vasculopathy after cranial radiation, especially with higher dose to optic chiasm, NF-1, and suprasellar tumors. Prospective studies are needed to identify risk groups, and ideal modality and timing, for screening of this toxicity.
Keywords: cerebral vasculopathy, childhood brain tumors, photon radiation, proton radiation, stroke
The use of multimodality treatment regimens that include radiation has significantly improved survival outcomes for children with brain tumors.1–5 However, these gains come at a cost, with significant radiation-related late effects in long-term survivors of childhood brain tumors.6–9 One of these well-described late effects is vasculopathy, with cerebral vessel stenosis, moyamoya disease, and consequently ischemic stroke.10–12 In the Childhood Cancer Survivor Study (CCSS), for 1871 childhood brain tumor survivors more than 5 years off therapy, the relative risk for a late-occurring stroke was 29 compared to a sibling comparison group.10 This risk was particularly increased for children who received cranial irradiation at a dose higher than 30 Gy, and even higher with a dose greater than 50 Gy. The risk of stroke after cranial radiation persists into adulthood, with the incidence continuing to rise with longer follow-up. In the CCSS cohort, for children who received greater than 50 Gy cranial radiation, the cumulative incidence of stroke rose from 1.1% at 10 years post diagnosis to 12% after 30 years.13 In an analysis of the Surveillance, Epidemiology, and End Results Program database, children with brain tumors remained at a very high risk of death due to stroke for the remainder of their life, with a standardized mortality ratio of fatal stroke greater than 100.14 The long-term follow-up guidelines from the Children’s Oncology Group recommend annual screening by history and neurological exam for cerebrovascular complications for survivors of childhood cancers who received radiation to the brain, and suggest potential consideration for MRI and MRA as clinically indicated. The role and timing of these imaging modalities in screening for radiation-related vasculopathy is uncertain.
Much of the literature on vasculopathy after radiation for brain tumors pertains to conventional photon radiation. Increasingly, however, proton radiation is now preferred at many centers with access to this modality to treat children with brain tumors.15–17 By minimizing the exit dose and radiation to normal brain tissue surrounding the target field, protons can potentially reduce the risk and incidence of late effects, compared to traditional photon radiation.18 It remains unclear how the risk and timing of cerebral vasculopathy after protons compares with photon radiation. In this retrospective study we describe the incidence of cerebral vasculopathy for a cohort of patients at a single institution who received proton radiation for childhood brain tumors. In addition, we performed a systematic review, summarizing the existing literature on cerebral vasculopathy and stroke after photon and proton radiation for pediatric CNS tumors.
Methods
Systematic Literature Search
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) method was used for the systematic literature search.19,20 Databases searched were Embase, Classic+Embase (OvidSP); MEDLINE and Epub Ahead of Print, in-Process & Other Non-Indexed Citations (OvidSP); PubMed; Scopus; and Web of Science Core Collection. A librarian (S.C.) customized search strategies for each database by incorporating controlled vocabulary terms and/or keywords designed to retrieve literature addressing all of these concepts: childhood/pediatric, intracranial/brain neoplasms/tumors/cancer, radiation/radiotherapy, photons, protons, vasculopathy, and stroke. After internal and external de-duplication, 7792 unique titles from the literature search were identified.
Eligibility Criteria and Data Extraction
In our study, vasculopathy referred to cerebral vessel stenosis or moyamoya vasculopathy or stroke. For studies to be eligible for this systematic review, they needed to report data regarding the incidence of vasculopathy after radiation for a childhood brain tumor, for a cohort of at least 5 patients, with a median follow-up of at least 1 year. The studies could be prospective or retrospective. Two authors (A.S. and A.B.) independently reviewed all 7792 titles and identified relevant studies (Figure 1). In case of disagreement, the 2 reviewers reached consensus by discussion. First, eligibility was determined for all studies by review of title and abstract. The full text was assessed for selected publications that appeared to be on topic, to ascertain data availability, completeness, and relevance based on the predetermined inclusion criteria. Papers with missing or duplicated data were further excluded. From eligible studies, data were extracted as available, including the number of patients in the cohort, patient and tumor characteristics, demographics, details of surgery and chemotherapy, dose of radiation, radiation modality, median follow-up, and the incidence, timing, and risk factors for vasculopathy and stroke after cranial radiation.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Flow Diagram.
Retrospective Study at a Single Institution
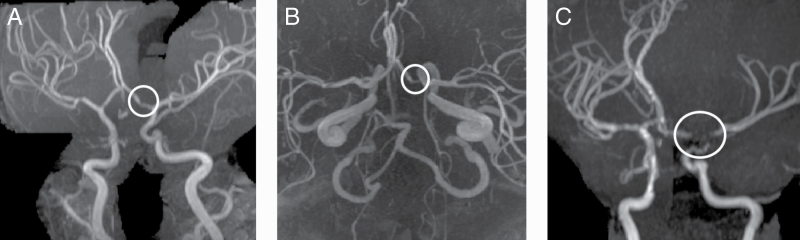
Data were added to the systematic review from a previously unpublished single-institution retrospective review at University of Oklahoma Health Sciences Center for a cohort of 81 patients treated with proton radiation for childhood brain tumors. All these patients had MRIs of the brain before radiation (baseline) as well as after radiation for surveillance for tumor recurrence and late effects. The study radiologist reviewed the last brain MRI and MRA for every patient. Vasculopathy was defined as narrowing of the proximal segment of a cerebral blood vessel compared to the distal segment. Grading for vasculopathy was defined as mild if the narrowing was less than 25%, moderate if narrowing was 25% to 75%, and severe if there was more than 75% narrowing of the proximal vessel segment (Figure 2). For patients identified as having vasculopathy, prior MRIs and MRAs were reviewed to determine the date when this complication was first apparent on imaging. The study radiation oncologist recorded proton radiation data for all patients, including radiation volume, total radiation dose, and the dose to the optic chiasm.
Figure 2.
Magnetic Resonance Angiography Images of Grades of Vasculopathy Based on Degree of Cerebral Vascular Narrowing Compared to Normal Proximal Segment. A, Mild vasculopathy with less than 25% narrowing; B, Moderate vasculopathy with 25% to 75% narrowing; C, Severe vasculopathy with greater than 75% narrowing; area of vasculopathy is encircled in white.
For the single-institution cohort, the significance of categorical variables as predictors of vasculopathy was analyzed using the Fisher exact test (age, sex, surgery, chemotherapy and radiation volume, suprasellar tumor location). Radiation dose to the optic chiasm was analyzed as a continuous variable using the Wilcoxon Mann-Whitney test and also as a categorical variable (dose greater or less than the median dose for the cohort: 45 Gy RBE).
Results
Single-Institution Retrospective Review
The cohort included 81 patients with a median age of 8 years at the time of radiation, and median follow-up of 3 years post radiation (Table 1). A majority of the patients had supratentorial tumors (63%). The incidence of vasculopathy was found to be 9.9% (8/81 patients), detected at a median interval of 2 years (1.4-3.7 years), after proton radiation was completed. The severity of vasculopathy was mild in 3 patients, moderate for 1, and severe for 4 patients. The incidence of moderate/severe vasculopathy was 6.2% (5/81 patients), occurring only in patients with suprasellar tumors, at a median interval of 2 years (1.4-2.3 years) post radiation. The incidence was significantly higher for patients with suprasellar tumors (22.7%) and those whose radiation dose to the optic chiasm was greater than the median dose for the cohort, 45 Gy (RBE) (16.1%). Of the 5 patients found to have moderate/severe vasculopathy, only 2 presented with symptoms or signs of stroke or TIA, whereas the rest were diagnosed on surveillance imaging. For both severe vasculopathy and vasculopathy overall, suprasellar tumor location (P = .001 and P = .004, respectively) and radiation dose to the optic chiasm greater than 45 Gy (P = .026 and P = .029, respectively) were found to be significant risk factors
Table 1.
Demographic and Clinic Characteristics of Single-Institution Cohort
| Characteristic | No. |
|---|---|
| No. of patients | 81 (No NF-1 patients) |
| Male/Female | 47/34 |
| Median age at start of radiation, y | 8 (range, 0.8-20.6) |
| Median duration of follow-up, y | 3 (range, 0.1-8.2) |
| Tumor location | • 22 suprasellar |
| • 29 supratentorial | |
| • 30 infratentorial | |
| Median maximum dose to optic chiasm, Gy | 45 RBE (range, 0-63) |
Abbreviation: NF-1, neurofibromatosis type 1.
Systematic Literature Review
Of the 7792 studies identified by systematic literature review, a majority (7699) were excluded by abstract review alone. Of the remaining 93 studies assessed with full manuscript review, 21 met the inclusion criteria for final analysis (Figure 1). The findings of these included studies are summarized in Table 2.
Table 2.
Summary of Literature on Vasculopathy and Stroke After Cranial Photon and Proton Radiation
| Vasculopathy rate (all severities) (No. of studies) | Moyamoya/severe vasculopathy rate (No. of studies) | Stroke rate (No. of studies) | Median time to event, y (No. of studies) |
|---|---|---|---|
| Photon radiation (9 studies) | |||
| • All diagnoses | • All diagnoses | • All diagnoses | • Vasculopathy |
| 2%-8.7% (3) | 2%-4.3% (3) | 2%-18.8% (6) | 2-28 (8) |
| • NF-1: 7.6%-60% (5) | • Suprasellar | • NF-1: 26%-60% (2) | • Stroke: 2.3-24 (8) |
| • Suprasellar | 5%-17.8% (3) | • Suprasellar | |
| 9%-20% (5) | 16.6%-26% (3) | ||
| Proton radiation (5 studies) | |||
| • 5%-9.9% (5 studies) | • 2.6%-6.2% (2) | • 1.1%-8.3% (4 studies) | • Vasculopathy: 1.5-3 (3) |
| • Stroke: 2.25-2.4 (2) |
Abbreviation: NF-1, neurofibromatosis type 1.
Vasculopathy After Cranial Radiation for Childhood Brain Tumors
Nine studies were identified evaluating the incidence of vasculopathy after photon radiation for childhood brain tumors, with a median follow-up ranging from 3.7 to 19 years and a total of 1108 patients (Table 3).11,21–28 The rate of vasculopathy reported ranged from 6.3 to 20% for nonneurofibromatosis type 1 (non–NF-1) patients, with the median time to detection ranging from 2 to 28 years post radiation. These rates were higher for patients with NF-1 (7.6%-60%)11,22–25 and suprasellar tumors (9%-20%).24–28 Six of these studies further reported the incidence of severe vasculopathy or moyamoya disease, with rates ranging from 2% to 4.3% for all diagnoses overall in 3 studies.11,21,22 The rate of severe vasculopathy was significantly higher for patients with suprasellar tumors (5%-17.8%; 3 studies).25–27 The highest incidence of moyamoya disease was reported for patients with NF-1 and optic pathway glioma (60%).25
Table 3.
Vasculopathy and Stroke After Cranial Photon Radiation
| Study | No. | Diagnoses | Median follow-up, y (range) | Late-effect rate, % | Median time to event, y (range) | Significant predictor of late-effect |
|---|---|---|---|---|---|---|
| Vasculopathy after photon radiation (9 studies) | ||||||
| Wu et al21 | 391 | All | 7.3 (NR) | • Moyamoya: 2 | 3 (2-20) | • Not identified |
| • 6/8 cases in suprasellar tumors with suprasellar RT dose > 4000 cGy | ||||||
| Ullrich et al11 | 345 | All | 4.5 (0.9-8.1) | • All severities: 6.3 | Moyamoya: | • NF-1 (3-fold risk) |
| • Moyamoya: 3.5 | • Non–NF-1: 4.6 | • Dose to optic chiasm (7% risk/1 Gy) | ||||
| • NF-1: 21 | • NF1: 3.2 | |||||
| Nordstrom et al22 | 115 | All (4 protons) (4 NF-1) | 4.6 (2.9-8. 6) (until last MRA) | • All severities: 8.7 | 4.8 (1.4-12) | • Not identified |
| ➢5-y CI: 5.4 | ||||||
| ➢10-y CI: 16 | ||||||
| • NF-1: 25 | ||||||
| • Moyamoya: 4.3 | ||||||
| Merchant et al23 | 78 | Low-grade glioma | 7.4 (NR) | • All severities: 6.4 | 2.6 (1-8.3) | • Age < 5 y at RT |
| ➢NF-1: 7.6 | ||||||
| ➢Non–NF-1: 6.2 | ||||||
| • 6-y CI: 4.79 | ||||||
| Grill et al24 | 69 | Optic pathway glioma | 7 (NR) | • All severities: 19 | 3 (0.5-12) | • NF-1 |
| ➢NF-1: 29.7 | ||||||
| ➢Non–NF-1: 6.2 | ||||||
| • Symptomatic: 15.9 | ||||||
| Kestle et al25 | 28 | Optic pathway glioma | NR | • Moyamoya: 17.8 | • NF-1: 3.1 | • NF-1 |
| ➢NF-1: 60 | • Non–NF-1: 4.5 | |||||
| ➢Non–NF-1: 8.7 | ||||||
| Winkfield et al26 | 43 | Craniopharyngioma | NR | • Moyamoya: 9 | NR | • Not identified |
| Liu et al27 | 20 | Craniopharyngioma | 3.7 (0.3-7.1) | • All severities: 20 | Moyamoya: 2 | • Intracystic bleomycin |
| • Moyamoya: 5 | ||||||
| Lo et al28 | 19 | Craniopharyngioma | 19.2 (8-41) | • All severities: 16 | 28 (25-34) | • Not identified |
| Stroke after photon radiation (10 studies) | ||||||
| El-Fayech et al51 | 447 | All | NR | 5.8 | NR | • Radiation dose to circle of Willis |
| Bowers et al52 | 385 | All | 3 (0.1-9.6) | 2.8 | 2.3 (0.3-18.6) | • Cranial radiation |
| • Optic pathway glioma | ||||||
| Mueller et al53 | 325 | All | 7.3 (2.4-15) | • 5-y CI: 2 | 12 (1-24) | • Radiation dose (5% hazard per 1 Gy) |
| • 10-y CI: 4 | ||||||
| Campen et al54 | 265 | All | 6.8 | 4.9 | 4.9 (0.09-8.3) | • Radiation to circle of Willis (12/13 patients) |
| Passos et al55 | 100 | All | 16.7 (6-29) | 2 | NR | • Not identified |
| Omura et al56 | 32 | All | 5.2 (1.3-14) | 18.8 | 5.8 (2-12) | • Radiation dose to circle of Willis |
| Cappelli et al57 | 54 | Optic pathway glioma | NR | • Overall: 16.6 | 2.5 (0.5-6) | • All received radiation to optic pathway (median dose 54 Gy) |
| ➢NF-1: 26 | ||||||
| ➢Non–NF-1: 4.5 | ||||||
| Kestle et al25 | 28 | Optic pathway glioma | NR | • Overall: 17.8 | • NF-1: 3.1 | • NF1 |
| ➢NF-1: 60 | • Non–NF1: 4.5 | |||||
| ➢Non–NF1: 8.7 | ||||||
| Christopherson et al58 | 53 | Medulloblastoma | 15.4 (0.4-44.4) | 1.8 | 24 | • Not identified |
| Lo et al28 | 19 | Craniopharyngioma | 19.2 (8-41) | 26 | 5 (2-35) | • Not identified |
Abbreviations: cGy, centigray; NF-1, neurofibromatosis type 1; NR, not reported; RT, radiation therapy.
In the 9 retrospective studies and 19 case reports/series29–47 identified in our review, patients reported to have developed vasculopathy after photon radiation included 74 patients with suprasellar tumors (52 optic pathway glioma, 21 craniopharyngioma, and 1 meningioma), 6 patients with low-grade glioma without a specified location, and 16 patients with nonsuprasellar tumors (7 medulloblastoma, 3 supratentorial low-grade glioma, 1 supratentorial PNET, 1 high-grade glioma, 1 supratentorial ependymoma, 1 pineal germ cell tumor, 1 retinoblastoma, and 1 clival chordoma). Significant risk factors for postradiation vasculopathy identified in these studies included dose to the optic chiasm in 1 study,11 NF-1 in 3 studies,11,24,25 and age younger than 5 years at start of radiation, in 1 study.23
Five studies (including the present study) were identified that evaluated the incidence of vasculopathy after proton radiation, with 833 patients and a median follow-up of 2.7 to 5.6 years (Table 4).12,48–50 The incidence of vasculopathy was found to be similar to that with photons, ranging from 5% to 9.9%. However, the time to severe vasculopathy described was earlier at a median duration ranging from 1.5 to 3 years post radiation (detected earliest at 1 year and latest at 7.5 years post radiation).48,50 Similar to studies with photon radiation, a majority of patients who developed vasculopathy after protons had suprasellar tumors (40/62 patients: 56%), including 32 patients with craniopharyngioma, 3 with optic pathway glioma, and 1 with a germ cell tumor. The most common nonsuprasellar diagnoses for patients who developed vasculopathy after proton therapy (28/62 patients: 16%) included medulloblastoma/embryonal tumor (9 patients) and ependymoma (8 patients). Dose to the optic chiasm of greater than 54 Gy often for suprasellar tumors was identified as a significant risk factor for this complication after protons, in 2 studies,12 and age at radiation younger than 5 years was found to increase risk in 1 study.12
Table 4.
Vasculopathy and Stroke After Cranial Proton Radiation
| Study | No. | Diagnoses | Median follow-up, y (range) | Late-effect rate, % | Median time to event, y (range) | Significant predictors of late-effect |
|---|---|---|---|---|---|---|
| Vasculopathy after proton radiation | ||||||
| Kralik et al48 | 75 | All | 4.3 (0.6-9.6) | All severities: 6.7 | 1.5 (1-7.5) | • Not identified |
| Hall et al12 | 644 | All | 3 (0.1-9.6) | 3-y CI | NR | • Mild vasculopathy: |
| • All severities: 6.4 | ➢Age < 5 y | |||||
| ➢Dose to optic chiasm ≥ 54 Gy RBE | ||||||
| • Severe vasculopathy | ||||||
| •Severe: 2.6 | ➢Dose to optic chiasm ≥ 54 Gy RBE | |||||
| Lee et al49 | 13 | All | 2.7 (0.4-7.8) | 7.6 | NR | • Not identified |
| Greenfield et al50 | 20 | Germ cell tumor | 5.6 (0.3-8.2) | 5 | 3 (1 patient) | • Not identified |
| Bavle et al, present study | 81 | All | 3 (0.1-8.2) | • All severities: 9.9 | • All severities: 2 (1.4-3.7) | • Suprasellar tumor |
| • Severe: 6.2 | • Severe: 2 (1.4-2.3) | • Dose to optic chiasm > 45 Gy RBE | ||||
| Stroke/TIA after proton radiation | ||||||
| Kralik et al48 | 75 | All | 4.3 (0.6-9.6) | 5.3 | 2.4 (1-7.5) | • None identified |
| Hall et al12 | 644 | All | 3 (0.1-9.6) | 1.1 | NR | • Dose to optic chiasm ≥ 54 Gy RBE |
| Lee et al49 | 12 | All | 2.1 (0.4-7.8) | 8.3 | NR | •Not identified |
| Bavle et al, present study | 81 | All | 3 (0.1-8.2) | 2.4 | 2.25 (2.2-2.3) | •Not identified |
Abbreviation: NR, not reported.
Stroke After Cranial Radiation for Childhood Brain Tumors
The incidence of stroke or TIA after photon radiation was reported in 10 retrospective studies, with median follow-up ranging from 3 to 19 years, and a total of 1708 patients (Table 3).10,25,28,51–58 The reported incidence ranged from 2% to 18.8%, occurring 2.3 to 24 years post radiation. A total of 138 patients were reported to have had a stroke or TIA after photon radiation in 10 retrospective studies and 21 case reports/ series.29,30,32,33,35–37,39,40,42–44,59–67 A majority of cases had received treatment for suprasellar tumors (82/138 cases: ~59%), most commonly optic pathway glioma (63 patients) and craniopharyngioma (15 patients). Of the 42 cases with nonsuprasellar tumors, the most common diagnoses were medulloblastoma (20 patients) and high-grade glioma (12 patients). Significant risk factors for postradiation stroke identified in these studies included dose to the optic chiasm or circle of Willis in 4 studies,51,54,56,57 NF-1 in 1,25 and cranial radiation in 2 studies.10,52
After proton radiation, 4 studies reported an incidence of stroke ranging from 1.1% to 8.3% after a median follow-up of 2.1 to 4.3 years (Table 4).12,48,49 The median duration from proton treatment to stroke was reported in 2 studies to be 2.4 years48 and 2.25 years (present study), respectively. Dose to the optic chiasm of 54 Gy or greater RBE was found to be a significant risk factor for stroke in one of these studies.12
Discussion
The cohort size and incidence of vasculopathy in our analysis were very similar to those reported by Kralik et al in the first retrospective study of cerebral vasculopathy after proton radiation for childhood CNS tumors, with a rate of 6.7% for moderate to severe vasculopathy.48 In the largest study of vasculopathy after proton radiation for childhood CNS and skull base tumors, reported by Hall et al, among 644 patients with a median follow-up of 3 years, the 3-year cumulative rate was 6.4% for vasculopathy and 2.6% for severe vasculopathy.12 Radiation dose to the optic chiasm greater than 54 Gy RBE, and age younger than 5 years at time of radiation were found to be significant risk factors for vasculopathy. The former risk factor, but not the latter, was found to be significant in our study as well. Our study had the limitations inherent to a retrospective review. All patients were treated at a single institution, with a sample size that was not large, limiting the generalizability of our findings. Similar to other studies with protons, the duration of follow-up was not very long. Hence the incidence of this complication more than 5 years off therapy could not be determined, and importantly the functional implications and evolution of asymptomatic image-defined vasculopathy could not be studied.
To better define the incidence, risk factors, and median time to the development of vasculopathy and stroke after photon and proton radiation, we performed a systematic review of the literature using the PRISMA method. However, a meta-analysis could not be performed because of the significant heterogeneity in studies for definitions of vasculopathy, grading of severity and symptoms, and methods of detection. Whereas some studies reported the rate of vasculopathy overall, without grading severity, others studied the incidence of more severe vasculopathy like moyamoya disease. In many studies, the term vasculopathy was not defined and hence rates could not be compared with other studies, and data from these studies could not be combined for analysis. The symptoms of vasculopathy reported also varied, ranging from stroke or TIA alone to including headaches, seizures, and other neurological deficits. There was also a wide variation in the diagnoses included in these studies, with some cohorts comprising only suprasellar tumors, while a few analyzed cohorts that included any brain tumor diagnosis. A few papers analyzed NF-1 patients as a subset, whereas many did not have or mention NF-1 patients.
As would be expected, the follow-up was significantly longer for studies with photon radiation compared to protons, which is a relatively newer radiation modality. For both types of radiation, the highest incidence of image-defined vasculopathy and stroke was in patients with suprasellar tumors, who received a higher radiation dose to the optic chiasm or circle of Willis. The most common nonsuprasellar tumor with this late effect was medulloblastoma. The median time to vasculopathy and stroke after photon radiation varied widely, between 2 to 5 years for the former, and as long as 12 to 24 years for the latter. Image-defined vasculopathies could have been detected earlier by the use of imaging surveillance, in contrast to older studies that reported symptomatic vasculopathy, with stroke or TIA, based on retrospective patient reports. This variation in the method of detection of both image-defined and symptomatic vasculopathy also makes it challenging to objectively compare the time to vasculopathy after protons compared to photon radiation. Studies with proton radiation also had a shorter follow-up compared to those with photons, and hence the true incidence of this complication with the 2 modalities could not be compared.
The literature indicates that the survivors of childhood brain tumors, especially with certain risk factors, are at risk for vasculopathy and stroke, both early and late off therapy, after either proton or photon cranial radiation. All patients who have received cranial radiation should have an annual neurological examination and must be educated regarding the risk of vasculopathy and stroke, and the symptoms and signs of these complications. A potential screening strategy for the early detection of vasculopathy, prior to symptomatic presentation and the onset of neurological deficits, especially in high-risk patients (suprasellar tumors, high radiation dose to the optic chiasm, young age at radiation), is to perform an MRA of the brain before radiation, and then annually or biennially for at least the first 5 years after radiation. The feasibility and efficacy of such a screening strategy needs evaluation as part of prospective, multi-institutional pediatric neuro-oncology therapeutic trials, with continued systematic long-term follow-up.
Funding
The authors did not receive any financial support for the research, authorship and/or publication of this paper.
Conflict of interest statement. None declared.
References
- 1. Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. [DOI] [PubMed] [Google Scholar]
- 2. Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25): 4202–4208. [DOI] [PubMed] [Google Scholar]
- 3. Merchant TE, Li C, Xiong X, Kun LE, Boop FA, Sanford RA.. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10(3):258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jensen AW, Laack NNI, Buckner JC, Schomberg PJ, Wetmore CJ, Brown PD.. Long-term follow-up of dose-adapted and reduced-field radiotherapy with or without chemotherapy for central nervous system germinoma. Int J Radiat Oncol Biol Phys. 2010;77(5):1449–1456. [DOI] [PubMed] [Google Scholar]
- 5. Kiehna EN, Merchant TE. Radiation therapy for pediatric craniopharyngioma. Neurosurg Focus. 2010;28(4):E10. [DOI] [PubMed] [Google Scholar]
- 6. Bavle A, Tewari S, Sisson A, Chintagumpala M, Anderson M, Paulino AC.. Meta-analysis of the incidence and patterns of second neoplasms after photon craniospinal irradiation in children with medulloblastoma. Pediatr Blood Cancer. 2018;65(8):e27095.29693784 [Google Scholar]
- 7. Kahalley LS, Ris MD, Mahajan A, et al. Prospective, longitudinal comparison of neurocognitive change in pediatric brain tumor patients treated with proton radiotherapy versus surgery only. Neuro Oncol. 2019;21(6):809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eaton BR, Esiashvili N, Kim S, et al. Endocrine outcomes with proton and photon radiotherapy for standard risk medulloblastoma. Neuro Oncol. 2016;18(6):881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whelan KF, Stratton K, Kawashima T, et al. Ocular late effects in childhood and adolescent cancer survivors: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2010;54(1):103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bowers DC, Liu Y, Leisenring W, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24(33):5277–5282. [DOI] [PubMed] [Google Scholar]
- 11. Ullrich NJ, Robertson R, Kinnamon DD, et al. Moyamoya following cranial irradiation for primary brain tumors in children. Neurology. 2007;68(12):932–938. [DOI] [PubMed] [Google Scholar]
- 12. Hall MD, Bradley JA, Rotondo RL, et al. Risk of radiation vasculopathy and stroke in pediatric patients treated with proton therapy for brain and skull base tumors. Int J Radiat Oncol Biol Phys. 2018;101(4):854–859. [DOI] [PubMed] [Google Scholar]
- 13. Mueller S, Fullerton HJ, Stratton K, et al. Radiation, atherosclerotic risk factors, and stroke risk in survivors of pediatric cancer: a report from the Childhood Cancer Survivor Study. Int J Radiat Oncol Biol Phys. 2013;86(4):649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zaorsky NG, Zhang Y, Tchelebi LT, Mackley HB, Chinchilli VM, Zacharia BE.. Stroke among cancer patients. Nat Commun. 2019;10(1):5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Indelicato DJ, Rotondo RL, Uezono H, et al. Outcomes following proton therapy for pediatric low-grade glioma. Int J Radiat Oncol Biol Phys. 2019;104(1):149–156. [DOI] [PubMed] [Google Scholar]
- 16. Indelicato DJ, Bradley JA, Rotondo RL, et al. Outcomes following proton therapy for pediatric ependymoma. Acta Oncol. 2018;57(5):644–648. [DOI] [PubMed] [Google Scholar]
- 17. Macdonald SM, Sethi R, Lavally B, et al. Proton radiotherapy for pediatric central nervous system ependymoma: clinical outcomes for 70 patients. Neuro Oncol. 2013;15(11):1552–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gondi V, Yock TI, Mehta MP. Proton therapy for paediatric CNS tumours—improving treatment-related outcomes. Nat Rev Neurol. 2016;12(6):334–345. [DOI] [PubMed] [Google Scholar]
- 19. Shamseer L, Moher D, Clarke M, et al. ; PRISMA-P Group . Preferred reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu YH, Chang FC, Liang ML, et al. Incidence and long-term outcome of postradiotherapy moyamoya syndrome in pediatric patients with primary brain tumors: a single institute experience in Taiwan. Cancer Med. 2016;5(8):2155–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nordstrom M, Felton E, Sear K, et al. Large vessel arteriopathy after cranial radiation therapy in pediatric brain tumor survivors. J Child Neurol. 2018;33(5):359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Merchant TE, Kun LE, Wu S, Xiong X, Sanford RA, Boop FA.. Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol. 2009;27(22):3598–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grill J, Couanet D, Cappelli C, et al. Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol. 1999;45(3):393–396. [DOI] [PubMed] [Google Scholar]
- 25. Kestle JR, Hoffman HJ, Mock AR. Moyamoya phenomenon after radiation for optic glioma. J Neurosurg. 1993;79(1):32–35. [DOI] [PubMed] [Google Scholar]
- 26. Winkfield KM, Tsai HK, Yao X, et al. Long-term clinical outcomes following treatment of childhood craniopharyngioma. Pediatr Blood Cancer. 2011;56(7):1120–1126. [DOI] [PubMed] [Google Scholar]
- 27. Liu AK, Bagrosky B, Fenton LZ, et al. Vascular abnormalities in pediatric craniopharyngioma patients treated with radiation therapy. Pediatr Blood Cancer. 2009;52(2):227–230. [DOI] [PubMed] [Google Scholar]
- 28. Lo AC, Howard AF, Nichol A, et al. A cross-sectional cohort study of cerebrovascular disease and late effects after radiation therapy for craniopharyngioma. Pediatr Blood Cancer. 2016;63(5):786–793. [DOI] [PubMed] [Google Scholar]
- 29. Beyer RA, Paden P, Sobel DF, Flynn FG.. Moyamoya pattern of vascular occlusion after radiotherapy for glioma of the optic chiasm. Neurology. 1986;36(9):1173–1178. [DOI] [PubMed] [Google Scholar]
- 30. Bitzer M, Topka H. Progressive cerebral occlusive disease after radiation therapy. Stroke. 1995;26(1):131–136. [DOI] [PubMed] [Google Scholar]
- 31. Brandicourt P, Bonnet L, Béjot Y, Drouet C, Moulin T, Thines L.. Moya-Moya syndrome after cranial radiation for optic glioma with NF1. Case report and literature review of syndromic cases. Neurochirurgie. 2018;64(1):63–67. [DOI] [PubMed] [Google Scholar]
- 32. Chiewvit P, Janyavanich V, Soonthonpong N, Churoj A, Chawalparit O, Suthipongchai S.. Moyamoya syndrome: post cranial irradiation of pineal gland tumor. Interv Neuroradiol. 2001;7(2):167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Debrun G, Sauvegrain J, Aicardi J, Goutieres F.. Moyamoya, a nonspecific radiological syndrome. Neuroradiology. 1975;8(4):241–244. [Google Scholar]
- 34. Han JY, Choi JW, Wang KC, et al. Coexistence of radiation-induced meningioma and moyamoya syndrome 10 years after irradiation against medulloblastoma: a case report. J Korean Med Sci. 2017;32(11):1896–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hirata Y, Matsukado Y, Mihara Y, Kochi M, Sonoda H, Fukumura A.. Occlusion of the internal carotid artery after radiation therapy for the chiasmal lesion. Acta Neurochir (Wien). 1985;74(3-4): 141–147. [DOI] [PubMed] [Google Scholar]
- 36. Ikeyama Y, Abiko S, Kurokawa Y, et al. Radiation-induced cerebrovasculopathy: a case report and review of the literature [article in Japanese]. No Shinkei Geka [Neurol Surg]. 1993;21(8):751–757. [PubMed] [Google Scholar]
- 37. Ishiyama K, Tomura N, Kato K, et al. A patient with Moyamoya-like vessels after radiation therapy for a tumor in the basal ganglia [article in Japanese]. No To Shinkei [Brain Nerve]. 2001;53(10): 969–973. [PubMed] [Google Scholar]
- 38. Jamjoom AB, Malabarey T, Jamjoom ZAB, Al-Sohaibani M, Hulailah A, Kolawole T.. Cerebro-vasculopathy and malignancy: catastrophic complications of radiotherapy for optic nerve glioma in a von Recklinghausen neurofibromatosis patient. Neurosurg Rev. 1996;19(1):47–51. [DOI] [PubMed] [Google Scholar]
- 39. Manion B, Sung WS. Radiation-induced moyamoya disease after childhood astrocytoma. J Clin Neurosci. 2011;18(10):1403–1405. [DOI] [PubMed] [Google Scholar]
- 40. Maruyama K, Mishima K, Saito N, Fujimaki T, Sasaki T, Kirino T.. Radiation-induced aneurysm and moyamoya vessels presenting with subarachnoid haemorrhage. Acta Neurochir (Wien). 2000;142(2):139–143. [DOI] [PubMed] [Google Scholar]
- 41. Mori K, Takeuchi J, Ishikawa M, Handa H, Toyama M, Yamaki T.. Occlusive arteriopathy and brain tumor. J Neurosurg. 1978;49(1):22–35. [DOI] [PubMed] [Google Scholar]
- 42. Okuno T, Prensky AL, Gado M. The moyamoya syndrome associated with irradiation of an optic glioma in children: report of two cases and review of the literature. Pediatr Neurol. 1985;1(5):311–316. [DOI] [PubMed] [Google Scholar]
- 43. Painter MJ, Chutorian AM, Hilal SK. Cerebrovasculopathy following irradiation in childhood. Neurology. 1975;25(2):189–194. [DOI] [PubMed] [Google Scholar]
- 44. Reynolds MR, Haydon DH, Caird J, et al. Radiation-induced moyamoya syndrome after proton beam therapy in the pediatric patient: a case series. Pediatr Neurosurg. 2016;51(6):297–301. [DOI] [PubMed] [Google Scholar]
- 45. Scala M, Vennarini S, Garrè ML, et al. Radiation-induced moyamoya syndrome after proton therapy in child with clival chordoma: natural history and surgical treatment. World Neurosurg. 2019;123:306–309. [DOI] [PubMed] [Google Scholar]
- 46. Serdaroğlu A, Simşek F, Gücüyener K, Oguz A, Karadeniz C, Balibey M.. Moyamoya syndrome after radiation therapy for optic pathway glioma: case report. J Child Neurol. 2000;15(11):765–767. [DOI] [PubMed] [Google Scholar]
- 47. Servo A, Puranen M. Moyamoya syndrome as a complication of radiation therapy. Case report. J Neurosurg. 1978;48(6):1026–1029. [DOI] [PubMed] [Google Scholar]
- 48. Kralik SF, Watson GA, Shih CS, Ho CY, Finke W, Buchsbaum J.. Radiation-induced large vessel cerebral vasculopathy in pediatric patients with brain tumors treated with proton radiation therapy. Int J Radiat Oncol Biol Phys. 2017;99(4):817–824. [DOI] [PubMed] [Google Scholar]
- 49. Lee KA, O’Sullivan C, Daly P, et al. Proton therapy in paediatric oncology: an Irish perspective. Ir J Med Sci. 2017;186(3):577–582. [DOI] [PubMed] [Google Scholar]
- 50. Greenfield BJ, Jaramillo S, Abboud M, et al. Outcomes for pediatric patients with central nervous system germ cell tumors treated with proton therapy. Clin Transl Radiat Oncol. 2016;1:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. El-Fayech C, Haddy N, Allodji RS, et al. Cerebrovascular diseases in childhood cancer survivors: role of the radiation dose to Willis circle arteries. Int J Radiat Oncol Biol Phys. 2017;97(2):278–286. [DOI] [PubMed] [Google Scholar]
- 52. Bowers DC, Mulne AF, Reisch JS, et al. Nonperioperative strokes in children with central nervous system tumors. Cancer. 2002;94:1094–1101. [PubMed] [Google Scholar]
- 53. Mueller S, Sear K, Hills NK, et al. Risk of first and recurrent stroke in childhood cancer survivors treated with cranial and cervical radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86(4):643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Campen CJ, Kranick SM, Kasner SE, et al. Cranial irradiation increases risk of stroke in pediatric brain tumor survivors. Stroke. 2012;43(11):3035–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Passos J, Nzwalo H, Marques J, et al. Late cerebrovascular complications after radiotherapy for childhood primary central nervous system tumors. Pediatr Neurol. 2015;53(3):211–215. [DOI] [PubMed] [Google Scholar]
- 56. Omura M, Aida N, Sekido K, Kakehi M, Matsubara S.. Large intracranial vessel occlusive vasculopathy after radiation therapy in children: clinical features and usefulness of magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 1997;38(2):241–249. [DOI] [PubMed] [Google Scholar]
- 57. Cappelli C, Grill J, Raquin M, et al. Long-term follow up of 69 patients treated for optic pathway tumours before the chemotherapy era. Arch Dis Child. 1998;79(4):334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Christopherson KM, Rotondo RL, Bradley JA, et al. Late toxicity following craniospinal radiation for early-stage medulloblastoma. Acta Oncol. 2014;53(4):471–480. [DOI] [PubMed] [Google Scholar]
- 59. Bansal LR, Belair J, Cummings D, Zuccoli G.. Late-onset radiation-induced vasculopathy and stroke in a child with medulloblastoma. J Child Neurol. 2015;30(6):800–802. [DOI] [PubMed] [Google Scholar]
- 60. Chau LQ, Levy ML, Crawford JR. Delayed radiation-induced stroke mimics recurrent tumor in an adolescent with remote history of low-grade brainstem glioma. Pediatr Neurol. 2019;98:87–88. [DOI] [PubMed] [Google Scholar]
- 61. Elwood K, Laack N, Warad DM, Keating G, Eckel L, Nageswara Rao AA.. Injury to insult: infarction after radiotherapy in the treatment of pediatric brain tumor. Pediatr Neurol. 2015;52(5):552–553. [DOI] [PubMed] [Google Scholar]
- 62. Grenier Y, Tomita T, Marymont MH, Byrd S, Burrowes DM.. Late postirradiation occlusive vasculopathy in childhood medulloblastoma. Report of two cases. J Neurosurg. 1998;89(3):460–464. [DOI] [PubMed] [Google Scholar]
- 63. Ishikawa N, Tajima G, Yofune N, Nishimura S, Kobayashi M.. Moyamoya syndrome after cranial irradiation for bone marrow transplantation in a patient with acute leukemia. Neuropediatrics. 2006;37(6): 364–366. [DOI] [PubMed] [Google Scholar]
- 64. Kunitaka M, Akai T, Akioka N, Tomita T, Nagai S, Kuroda S.. Lacunar stroke, cavernous angioma, and fusiform aneurysm due to irradiation for pilocytic astrocytoma—a case report. J Stroke Cerebrovasc Dis. 2018;27(8):e165–e167. [DOI] [PubMed] [Google Scholar]
- 65. Mineura K, Sasajima T, Kowada M, Saitoh H, Shishido F.. Radiation-induced vasculopathy implicated by depressed blood flow and metabolism in a pineal glioma. Br J Radiol. 1993;66(788):727–733. [DOI] [PubMed] [Google Scholar]
- 66. Mitchell WG, Fishman LS, Miller JH, et al. Stroke as a late sequela of cranial irradiation for childhood brain tumors. J Child Neurol. 1991;6(2):128–133. [DOI] [PubMed] [Google Scholar]
- 67. Nakazaki K, Titoku S, Ota S, et al. Lacunar infarction in brain tumor patients: chronic stage complication after radiation therapy [article in Japanese]. No Shinkei Geka [Neurol Surg]. 2007;35(10):1019–1023. [PubMed] [Google Scholar]