Abstract
Background
Diffuse intrinsic pontine gliomas (DIPGs) are a leading cause of brain tumor deaths in children. Current standard of care includes focal radiation therapy (RT). Despite clinical improvement in most patients, the effect is temporary and median survival is less than 1 year. The use and benefit of reirradiation have been reported in progressive DIPG, yet standardized approaches are lacking. We conducted a survey to assess reirradiation practices for DIPG in North America.
Methods
A 14-question REDCap survey was disseminated to 396 North American physicians who care for children with CNS tumors.
Results
The response rate was 35%. Participants included radiation-oncologists (63%; 85/135) and pediatric oncologists/neuro-oncologists (37%; 50/135). Most physicians (62%) treated 1 to 5 DIPG patients per year, with 10% treating more than 10 patients per year. Reirradiation was considered a treatment option by 88% of respondents. Progressive disease and worsening clinical status were the most common reasons to consider reirradiation. The majority (84%) surveyed considered reirradiation a minimum of 6 months following initial RT. Doses varied, with median total dose of 2400 cGy (range, 1200-6000 cGy) and fraction size of 200 cGy (range, 100-900 cGy). Concurrent use of systemic agents with reirradiation was considered in 46%, including targeted agents (37%), biologics (36%), or immunotherapy (25%). One-time reirradiation was the most common practice (71%).
Conclusion
Although the vast majority of physicians consider reirradiation as a treatment for DIPG, total doses and fractionation varied. Further clinical trials are needed to determine the optimal radiation dose and fractionation for reirradiation in children with progressive DIPG.
Keywords: diffuse intrinsic pontine glioma, reirradiation
A leading cause of death from CNS malignancies in children is diffuse intrinsic pontine gliomas (DIPGs). These are aggressive tumors that represent 75% to 80% of pediatric brainstem tumors and 10% of all childhood CNS tumors.1–3 The prognosis for children with DIPGs is significantly worse than other brainstem tumors and other malignant gliomas given their location because the pons contains vital structures critical for life-sustaining functions such as breathing, blood pressure, and heart rate.3 Despite numerous clinical trials of chemotherapy and biological response modifiers, median survival remains less than 1 year from diagnosis.1–6 No efficacious treatment exists for recurrent/progressive disease following radiotherapy, and time to death after recurrence is approximately 3 months.7 Various treatment approaches, including reirradiation and systemic agents, are used with no standard of care for these patients in the refractory and recurrent setting.
Radiotherapy serves as the only treatment modality that has been shown to lengthen survival after diagnosis with DIPG.8,9 With increasing evidence of its safety in pediatric CNS tumors, reirradiation is more frequently being used for children with recurrent/progressive DIPG.10–12 Because standard reirradiation approaches are lacking, we conducted a survey to characterize physician practice patterns regarding reirradiation at time of DIPG recurrence or progression.
Methods
A 14-question REDCap13 survey (Supplemental Table 1) regarding reirradiation practices was developed by pediatric neuro-oncologists and radiation oncologists and emailed to 396 North American physicians identified through an International Pediatric Neuro-Oncology and Radiation-Oncology database. Pediatric oncologists/pediatric neuro-oncologists were included because they often serve as patients’ primary physicians and follow patients with DIPG, prescribe systemic adjuvant therapy, and refer patients to radiation oncologists at the time of diagnosis for initial radiation along with at time of recurrence/progression for consideration of reirradiation. Survey responses were collected and analyzed with descriptive statistics. Data were analyzed using t tests and chi-square tests, as appropriate.
Results
The overall response rate was 35%. Two participants were excluded because they did not treat DIPG patients. Participants included radiation-oncologists (63%; 85/135) and pediatric oncologist/pediatric neuro-oncologists (37%; 50/135).
Most physicians (62%) surveyed treated 1 to 5 DIPG patients per year, 24% treated 6 to 10 DIPG patients per year, and 10% treated more than 10 patients per year. Most respondents (55%) practice at institutions that treat greater than 50 new pediatric neuro-oncology patients annually (Table 1). Four radiation oncologists and one pediatric neuro-oncologist/pediatric oncologist did not respond to this question.
Table 1.
Number of New Pediatric Neuro-Oncology Patients Seen in Consultation per Year
| 0-25 new patients, % (n) | 26-50 new patients, % (n) | 51-75 new patients, % (n) | 76-100 new patients, % (n) | > 100 new patients, % (n) | |
|---|---|---|---|---|---|
| All respondents | 30 (41) | 14%(19) | 15 (20) | 11 (15) | 29 (39) |
| Radiation oncologist | 41 (35) | 19 (16) | 14 (12) | 7 (6) | 18 (15) |
| Pediatric neuro-oncologist/pediatric oncologist | 12 (6) | 6 (3) | 16 (8) | 18 (9) | 48 (24) |
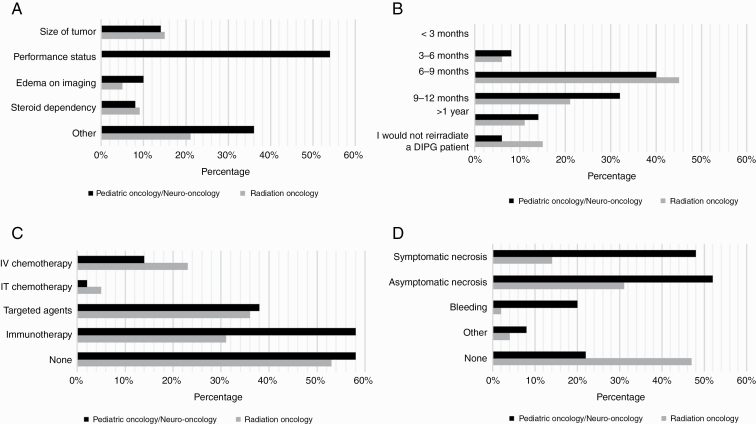
Reirradiation was considered a potential treatment option by 88% of respondents, 85% (69/81) of radiation oncologists, and 94% (46/49) of pediatric neuro-oncologist/oncologists. Worsening clinical status and progressive disease were the most common reasons to consider reirradiation. Responses were relatively similar among the physician specialties, with 67% of radiation oncologists and 78% pediatric neuro-oncologists/oncologists considering worsening clinical status as an indication and 65% and 66% considering progressive disease or tumor growth, respectively. Other considerations for reirradiation included patients’ initial response to radiotherapy. Contraindications to reirradiation included poor performance status (70%), size of the tumor (18%), edema on imaging (8%), and steroid dependency (11%) (Figure 1A). Additional reasons for not considering reirradiation included short interval to progression, evidence of radiation necrosis, sedation requirements of the child, poor/no clinical response to initial radiotherapy, and intratumoral hemorrhage.
Figure 1.
Reirradiation practices. A, Contraindications to reirradiation: Contraindications included size of tumor (18%), poor performance status (70%), edema on imaging (8%), and steroid dependency (11%). Other reasons included short interval to progression, evidence of radiation therapy necrosis, sedation requirements in the child, poor/no clinical response to initial radiotherapy, and intratumoral hemorrhage. B, Time from initial radiation to reirradiation: C, Concurrent therapy used with reirradiation: Concurrent use of systemic agents with reirradiation was considered in 46% of respondents, with targeted agents (37%), biologics (34%), immunotherapy (25%), intravenous chemotherapy (19%), and intrathecal chemotherapy (4%). D, Complications with reirradiation: Complications seen with reirradiation included asymptomatic necrosis (43%), symptomatic necrosis (30%), and bleeding (9%). Other reasons included edema and steroid dependency.
The majority (84%) surveyed considered reirradiation a minimum of 6 months following initial radiotherapy, with none suggesting reirradiation less than 3 months from initial radiotherapy (Figure 1B). A small number of participants (12%) would not consider reirradiation, and 3 radiation oncologists did not answer this question.
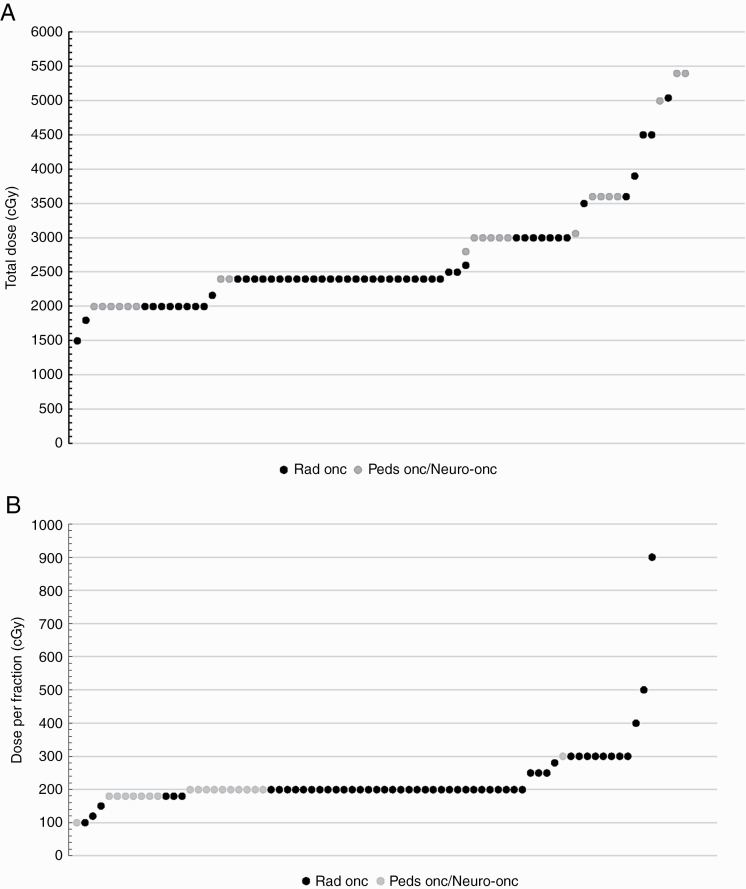
Photon radiotherapy for reirradiation in DIPG was considered by 98% of respondents, whereas 18% would consider treatment with proton reirradiation. Notably, all radiation oncologists answered this question, whereas 28% (14/50) of medical oncologists did not provide an answer. Although all physicians, regardless of their specialty, were asked about radiation doses administered, of those physicians that considered reirradiation, only 44% (22/50) of the pediatric oncologists/pediatric neuro-oncologists specified doses used; all others indicated radiation dosing was determined by their radiation oncologists. All radiation oncologists who considered reirradiation responded to this question with a specified dose. Although radiation therapy (RT) doses varied with a median total dose of 2400 cGy (range, 1200-6000 cGy) (Figure 2A) and median dose per fraction of 200 cGy (range, 100-900 cGy) (Figure 2B), most respondents (87%) recommended a total dose between 2000 and 3600 cGy, regardless of specialty, and 91% considered a fractionation regimen with 180 to 300 cGy/fraction. One radiation oncologist considered focal stereotactic radiosurgery/radiotherapy with 3000 to 4500 cGy in 1 to 5 fractions, and another would consider 5040 cGy in 120-cGy twice-daily fractions. One-time reirradiation was the most common practice (71%) (66% among radiation oncologists and 70% among pediatric oncologists), whereas 18% overall (15% of radiation oncologists, 18% of medical oncologists) would consider reirradiation twice and 5% (4% of radiation oncologists and 6% of medical oncologists) more than 2 times. Interestingly, 12% of all respondents would not consider reirradiation, specifically 15% of radiation oncologists and 6% of pediatric oncologists/neuro-oncologists would not reirradiate a DIPG patient.
Figure 2.
Reirradiation dosing. A, Total reirradiation dose for diffuse intrinsic pontine glioma (DIPG) patients based on physician responses: Radiation therapy doses varied, with median total dose of 2400 cGy (range, 1200-6000 cGy). B, Dose per fraction of reirradiation in DIPG based on physician responses: Median dose per fraction was 200 cGy (range, 100-900 cGy).
Concurrent use of systemic agents with reirradiation was considered in 46% of respondents (47% of radiation oncologists and 42% of pediatric oncologists/pediatric neuro-oncologists), mainly with targeted agents (37%), or immunotherapy (25%). Less commonly, intravenous cytotoxic chemotherapy (19%) and intrathecal chemotherapy (4%) were also considered. Eleven participants, all radiation oncologists, chose not to respond to this question, whereas all pediatric oncologists/pediatric neuro-oncologists responded. Of those that responded, the use of adjuvant therapy did not differ between radiation oncologists or pediatric oncologists/pediatric neuro-oncologists, with the exception of immunotherapy being more frequently used by pediatric oncologists/pediatric neuro-oncologists. Interestingly, 23% (17/74) of radiation oncologists and 14% (7/50) of pediatric oncologists/pediatric neuro-oncologists considered intravenous chemotherapy, whereas intrathecal chemotherapy was considered in 5% (4/74) and 2% (1/50), targeted agents or biologics in 36% (27/74) and 38% (19/50), and immunotherapy in 31% (23/74) and 58% (29/50) (P = .003), of radiation oncologists and pediatric oncologists/pediatric neuro-oncologists, respectively (Figure 1C).
Of the respondents who have performed reirradiation, 42% reported reirradiation was well tolerated without any complications. Of the complications experienced with reirradiation, 43% of respondents noted asymptomatic necrosis and 30% reported symptomatic necrosis. Less common side effects included bleeding and edema and steroid dependency (Figure 1D).
Discussion
RT remains an essential component of treatment for many pediatric CNS tumors, including DIPG. Reirradiation has been safely used in the treatment of recurrent ependymomas and medulloblastomas with survival benefit and has been shown to be a safe approach in progressive DIPG that may prolong survival, although no randomized phase 2 study has been conducted.7,10,12 Lack of consistency exists with reirradiation practices. Despite most radiation oncologists and pediatric neuro-oncologists/pediatric oncologists considering 2000 to 3600 cGy reirradiation as a treatment option, the number of times one would reirradiate, the use of systemic agents along with reirradiation, and the indications or contraindications to such a treatment differed. This survey illustrates the discrepancy among CNS tumor providers in the management of recurrent or progressive DIPG and further supports the need for standardized approaches.
A few groups have retrospectively investigated outcomes of a relatively small cohort of recurrent/progressive DIPG patients treated with reirradiation (Table 2). The University of Texas MD Anderson Cancer Center treated 5 patients with reirradiation using 1800 cGy in 10 fractions (1 patient) or 2000 cGy in 10 fractions (4 patients) along with concurrent chemotherapy in second or subsequent progressive DIPG. The patients in this cohort tolerated reirradiation well, with minimal adverse events, none of which were greater than grade 2 toxicities. The median time to progression was 5 months.14 An Italian group used reirradiation along with nimotuzumab and vinorelbine in a phase 2 trial for newly diagnosed DIPG patients; reirradiation was employed at progression. Twenty patients were noted to have progressive disease, of whom 16 had local progression. Eleven patients were treated with focal reirradiation (1980 cGy in 180-cGy daily fractions). Of the 5 patients with disseminated disease, 4 underwent focal reirradiation to the primary as well as metastatic sites of disease. This approach was well tolerated without any unexpected adverse effects or decline in neurological status. The median survival was 6 months (range, 6 weeks-14 months) following reirradiation.15,16 A retrospective European review of DIPG cases included 31 patients who were reirradiated at first progression with doses ranging from 1800 cGy to 3000 cGy, and some of the patients received concurrent systemic therapy. Clinical improvement was noted in 77% of the patients and no life-threatening or fatal toxicities reported. The median survival in this larger cohort was 6.4 months following reirradiation compared to 3 months in a historical cohort not treated with reirradiation at time of progression.17 Another retrospective review was conducted that included 16 DIPG patients in Canada treated with reirradiation at progression. Focal reirradiation was administered in 14 patients at doses of 2160 to 3600 cGy. Two patients received whole-brain reirradiation (3060 cGy) because of disseminated disease. The median time from diagnosis to progression was 10.5 months (range, 4-37 months). One patient received a third course of RT 6 months after reirradiation at a dose of 2160 cGy. One patient received concurrent systemic therapy with bevacizumab; all others were treated with radiotherapy alone. Seven patients received chemotherapy following reirradiation with various agents including temozolomide, valproic acid, nimotuzumab, and bevacizumab. Reirradiation was well tolerated in all patients, with the exception of one patient who experienced pontine necrosis progressing to cerebellar dysfunction and quadriparesis after 3000 cGy in 10 fractions. Steroids were avoided in 6 patients and discontinued in 4 patients by the end of reirradiation in this cohort. Median survival post reirradiation was 6.48 months (range, 3.8-13.3 months) compared to 3 months (range, 3.8-13.9 months) in historical cohorts of 46 patients with progressive DIPG not treated with reirradiation.7
Table 2.
Summary of Studies With 5 or More Patients Evaluating Reirradiation in DIPG
| Authors/y of publication | Population | No. | Median time from initial RT to re-RT, mo | Reirradiation doses | Concurrent systemic therapy | Median survival post re-RT | Conclusion |
|---|---|---|---|---|---|---|---|
| Fontanilla et al (2012)14 | Progressive DIPG | 5 | 12.5 | 18 Gy or 20 Gy (focal) | NA | 5 mo | Reirradiation was well tolerated with minimal adverse events |
| Massimino et al (2014)15,16 | Newly diagnosed DIPG with reirradiation at progression | 25 at diagnosis, 11 re-RT | NR | 19.8 Gy (focal) | Nimotuzmab and vinorelabine | 6 mo (range, 6 wks-14 mo) | Combination of Nimotuzmab/vinorelabine was well tolerated |
| Vanan and Eisenstat (2015)5 | Progressive DIPG | 10 | NR | 21.6-36 Gy (focal) or 30.6 Gy (WB) | Valproic acid (1), bevacizumab (1), temozolomide (1) | 9 mo (range, 5-13 mo) | Neurological improvement noted in all but one re-RT patient |
| Janssens et al (2017)17 | Progressive DIPG | 31 | NR | 18-30 Gy (focal) | Re-RT alone (16), re-RT combined with systemic agents (15) including nimotuzumab/vinorelbine (9), etoposide (1), valproic acid + celecoxib (1), sirolimus (2), valproic acid, temsirolimus + irinotecan (1), bevacizumab (1) | 6.4 mo | Patients who respond to upfront RT benefit from re-RT |
| Lassaletta et al (2017)7 | Progressive DIPG | 16 | 13 | 21.6-36 Gy (focal) or 30.6 Gy (WB) | Bevacizumab in one patient, all other RT alone. Seven patients received chemotherapy following reirradiation with various agents including temozolomide, valproic acid, nimotuzumab and bevacizumab | 6.48 mo (range, 3.8-13.3 mo) | Re-RT was safe and feasible in patients with progressive DIPG |
| Kline et al (2018)6 | Progressive DIPG | 31, re-RT in 12 | 11.8 | 24 Gy (focal) | Nivolumab and re-RT (8) | 6.8 mo (re-RT with nivolumab); 6.0 mo (re-RT alone) | Re-RT with concurrent PD-1 inhibition was tolerated and may offer survival benefit in recurrent DIPG |
| Amsbaugh et al (2019)18 | Progressive DIPG | 12 | 12.3 | 24-30.8 Gy (focal) | NA | 5.8 mo | Re-RT is safe and demonstrated clinical improvement |
Abbreviations: DIPG, diffuse intrinsic pontine glioma; NA, not applicable; NR, not reported; PD-1, programmed death-1; re-RT, reirradiation; RT, radiation; WB, whole brain.
Recently a prospective phase 1/2 trial investigated DIPG reirradiation safe dosing using 3 efficacy domains: imaging assessment, clinical symptoms, and patient- or family-reported quality of life.18 Patients had a median of 12.3 months from initial radiotherapy to reirradiation, suggesting that these may be a more favorable cohort of patients because most patients recur earlier. Six patients received 2400 cGy in 12 fractions with improvement in at least 2 of 3 efficacy domains. Of the 4 patients receiving 2640 cGy in 12 fractions, 2 patients demonstrated improvement in at least 2 of 3 efficacy domains. Two patients received 3080 cGy in 14 fractions, and one patient experienced grade 3 toxicity. Treatment was well tolerated, with no other reported toxicity (grade ≥ 3). From start of reirradiation, median progression-free survival and overall survival were 4.5 and 5.8 months, respectively. These findings suggest that 2400 cGy in 12 fractions is safe and provides clinical benefit and improvement in quality of life.18
Toxicities from reirradiation appear to be minimal, although existent, in the published literature.14,18 Commonly, respondents noted necrosis (asymptomatic or symptomatic), edema, and steroid dependency. Despite these side effects, 88% of those surveyed advocated reirradiation, illustrating its possible beneficial role in palliative therapy.
In keeping with the results of previous studies, our survey demonstrated that reirradiation is considered well tolerated, with the majority of physicians treating with photon irradiation at doses of 2000 to 3600 cGy. Further prospective studies are needed to determine the optimal dose and dose per fraction in the setting of reirradiation. Worsening clinical status and progressive disease were the most common reasons for considering reirradiation, which also aligns with prior studies. In our cohort, a larger portion of the pediatric oncologists/pediatric neuro-oncologists felt that worsening clinical status alone warranted consideration of reirradiation compared to radiation oncologists. This may be attributed to the medical oncology team serving as primary providers for these patients, monitoring their clinical status, and being more familiar with DIPG disease progression manifesting clinically without radiographic progression.
The perceived benefit of reirradiation in children with DIPG is difficult to assess. Most DIPG reirradiation studies are retrospective or encompass a small cohort of patients, and therefore indications for and contraindications to reirradiation are difficult to assess. In previous phase 1 and 2 studies, timing from initial radiation, life expectancy of at least 2 months, performance status (Lansky or Karnofsky 40 or higher), and no prior grade 3 or greater CNS toxicity were considerations prior to reirradiating patients.15,18 These findings align with our survey results, with poor performance status serving as the most common reason for avoiding reirradiation. We identified additional contraindications including size of tumor, tumor-associated edema on imaging, steroid dependency, evidence of radiation necrosis, sedation requirements of the child, poor/no clinical response to initial radiotherapy, and intratumoral hemorrhage. Although the survey did not capture more detailed information surrounding these contraindications, the majority of respondents focused on aspects of safety of the child, most encompassing potential complications or side effects of further radiation. Steroid dependency was seen as a contraindication in our study; further details were not collected, but this may be due in part to worsening clinical status and/or poorer perceived life expectancy, with the potential for further complications with reirradiation. Studies are needed to identify more standardized approaches to reirradiation, including contraindications to treatment. The timing from initial radiotherapy to reirradiation also varies, with some patients treated as early as 3 months after completion of RT in the European experience. In those surveyed, 84% considered reirradiation a minimum of 6 months following initial RT, with none considering reirradiation less than 3 months from initial radiotherapy. Further investigations are also needed to determine the timing of reirradiation and whether there is an increased risk to reirradiation within 6 months.
Several limitations of this study are acknowledged. As an emailed survey, this study is restricted by those respondents that completed the questionnaire. Given that an overall response rate of 35% was obtained, although consistent with other survey response rates, the study is limited by selection bias and sample size. As producers of a survey-based study, we are limited by the options and answers, thus further details surrounding the modality of radiation (proton vs photon), contraindications, or indications to reirradiation could not be provided. All responses were anonymous; thus, we are unable to comment on geographical practices or confirm/validate responses based on institutional size. However, we acknowledge the anonymous responses may show differing responses among providers at the same institution, which may be of interest in future studies to better understand how management decisions are made when there are conflicting opinions about the course of action at the time of DIPG recurrence/progression.
Conclusion
DIPG, which accounts for 10% to 20% of all childhood brain tumors, continues to have a grim outcome, leading to devastating and debilitating neurological symptoms and an overall survival of approximately 1 year from diagnosis. Only RT has been shown to improve overall survival. As research continues to investigate novel treatment options, reirradiation may also be considered. Prior studies demonstrated a median survival of 5 to 7 months following reirradiation, although prospective randomized phase 2 studies are lacking and no standard exists. Although considered a safe and feasible therapeutic option by practitioners caring for these patients, the doses of reirradiation, indications and contraindications for reirradiation, and use of adjuvant systemic therapy considered by oncologists in this North American survey varied. Future clinical trials of reirradiation to assess optimal dose, fractionation, interval between radiotherapy, and the concurrent use of systemic agents are necessary.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Supplementary Material
Acknowledgments
All authors contributed to this work.
Conflict of interest statement. None declared.
References
- 1. Jansen MH, Veldhuijzen van Zanten SE, Sanchez Aliaga E, et al. . Survival prediction model of children with diffuse intrinsic pontine glioma based on clinical and radiological criteria. Neuro Oncol. 2015;17(1):160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pierre-Aurélien B, Alexandru S, Federico DR, Justyna K, Carmine M, Didier F. Diffuse intrinsic pontine glioma in children: document or treat? World Neurosurg. 2016;93:485.e11–485.e14. [DOI] [PubMed] [Google Scholar]
- 3. Warren KE. Diffuse intrinsic pontine glioma: poised for progress. Front Oncol. 2012;2:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sison J, Tran H, Margol A, et al. . Palliative care options for a young adult patient with a diffuse intrinsic pontine glioma. Cureus. 2017;9(8):e1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vanan MI, Eisenstat DD. DIPG in children—what can we learn from the past? Front Oncol. 2015;5:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kline C, Liu SJ, Duriseti S, et al. . Reirradiation and PD-1 inhibition with nivolumab for the treatment of recurrent diffuse intrinsic pontine glioma: a single-institution experience. J Neurooncol. 2018;140(3):629–638. [DOI] [PubMed] [Google Scholar]
- 7. Lassaletta A, Strother D, Laperriere N, et al. . Reirradiation in patients with diffuse intrinsic pontine gliomas: the Canadian experience. Pediatr Blood Cancer. 2018;65(6):e26988. [DOI] [PubMed] [Google Scholar]
- 8. Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7(3):241–248. [DOI] [PubMed] [Google Scholar]
- 9. Jalali R, Raut N, Arora B, et al. . Prospective evaluation of radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. Int J Radiat Oncol Biol Phys. 2010;77(1):113–118. [DOI] [PubMed] [Google Scholar]
- 10. Bouffet E, Hawkins CE, Ballourah W, et al. . Survival benefit for pediatric patients with recurrent ependymoma treated with reirradiation. Int J Radiat Oncol Biol Phys. 2012;83(5):1541–1548. [DOI] [PubMed] [Google Scholar]
- 11. Tsang DS, Murray L, Ramaswamy V, et al. . Craniospinal irradiation as part of re-irradiation for children with recurrent intracranial ependymoma. Neuro Oncol. 2019;21(4):547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bakst RL, Dunkel IJ, Gilheeney S, et al. . Reirradiation for recurrent medulloblastoma. Cancer. 2011;117(21):4977–4982. [DOI] [PubMed] [Google Scholar]
- 13. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fontanilla HP, Pinnix CC, Ketonen LM, et al. . Palliative reirradiation for progressive diffuse intrinsic pontine glioma. Am J Clin Oncol. 2012;35(1):51–57. [DOI] [PubMed] [Google Scholar]
- 15. Massimino M, Biassoni V, Miceli R, et al. . Results of nimotuzumab and vinorelbine, radiation and re-irradiation for diffuse pontine glioma in childhood. J Neurooncol. 2014;118(2):305–312. [DOI] [PubMed] [Google Scholar]
- 16. Massimino M, Biassoni V, Miceli R, et al. . Correction to: Results of nimotuzumab and vinorelbine, radiation and re-irradiation for diffuse pontine glioma in childhood. J Neurooncol. 2018;138(3):679–680. [DOI] [PubMed] [Google Scholar]
- 17. Janssens GO, Gandola L, Bolle S, et al. . Survival benefit for patients with diffuse intrinsic pontine glioma (DIPG) undergoing re-irradiation at first progression: a matched-cohort analysis on behalf of the SIOP-E-HGG/DIPG working group. Eur J Cancer. 2017;73:38–47. [DOI] [PubMed] [Google Scholar]
- 18. Amsbaugh MJ, Mahajan A, Thall PF, et al. . A phase 1/2 trial of reirradiation for diffuse intrinsic pontine glioma. Int J Radiat Oncol Biol Phys. 2019;104(1):144–148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.