Abstract
The health and economic burden imposed by skin cancer is substantial, creating an urgent need for the development of improved molecular strategies for its prevention and treatment. Cutaneous exposure to solar ultraviolet (UV) radiation is a causative factor in skin carcinogenesis, and TLR4-dependent inflammatory dysregulation is an emerging key mechanism underlying detrimental effects of acute and chronic UV exposure. Direct and indirect TLR4 activation, upstream of inflammatory signaling, is elicited by a variety of stimuli, including pathogen-associated molecular patterns (such as lipopolysaccharide) and damage-associated molecular patterns (such as HMGB1) that are formed upon exposure to environmental stressors, such as solar UV. TLR4 involvement has now been implicated in major types of skin malignancies, including nonmelanoma skin cancer, melanoma and Merkel cell carcinoma. Targeted molecular interventions that positively or negatively modulate TLR4 signaling have shown promise in translational, preclinical, and clinical investigations that may benefit skin cancer patients in the near future.
Keywords: molecular intervention, skin cancer, TLR4
1 |. INTRODUCTION: THE ROLE OF TLR4 SIGNALING IN SKIN
Toll-like receptors (TLRs) have long been studied for their role in the response to microbial insult and skin damage. TLRs are transmembrane receptors that form homo- or heterodimers to stimulate an acute inflammatory response as a result of binding ligands derived from either microbial sources (ie, pathogen-associated molecular patterns [PAMPs]) or self-derived stress signals (ie, damage-associated molecular patterns [DAMPs]). TLRs reside in either the extracellular membrane or endosomal membranes, amenable to sensing microbial or stress signals. TLR stimulation is linked to activation of cytokines, interleukins, and other mediators of the innate immune response.
In general, TLR activation leads to rapid secretion of interferons (IFN-α and IFN-β), proinflammatry cytokines (TNF-α and interleukin-6 [IL-6]), and inflammasome activation.1 Inflammasomes are multi-meric protein complexes that assemble upon sensing of a variety of stress factors. Their formation results in caspase-1-mediated activation that processes the biologically inactive IL-1β and IL-18 precursors into active cytokines to induce an inflammatory response.
The majority of literature on TLRs has focused on their activity in macrophages, but cells throughout the body express these receptors, including epithelial cells. In the skin, keratinocytes are often the first line of defense against microbial insults or stress due to wounding, and, therefore, coordinate with macrophages to elicit an immune response. Recent work has implicated TLRs as key players in the instigation and coordination of inflammatory responses in healthy skin, as well as in many epithelial conditions linked to chronic or hyperactive inflammation.2–5
Among the eleven different members of the TLR family expressed in humans, TLR4 has been shown to play a specific role in both skin inflammation and cancer.6–10 TLR4 activity can be stimulated by multiple different PAMPs and DAMPs, including bacterially-derived lipopolysaccharide (LPS) and endogenously produced high-mobility group box 1 (HMGB1). Activation of TLR4 is a complex process involving specific ligands acting in conjunction with accessory proteins, such as myeloid differentiation protein 2 (MD2), lipopolysaccharide binding protein, and a cluster of differentiation antigen 14 (CD14). Stimulation of TLR4/MD2 dimerization and internal signaling through either the TIRAP/MyD88 or TRAM/TRIF-regulated intracellular pathways leads to the activation of transcription factors, such as NF-κB, AP-1, and IRF-3, all of which regulate inflammatory signaling, as well as apoptosis, survival and differentiation6,11,12 (Figure 1). In addition, TLR4 signaling also causes inflammasome activation elicited in primary human keratinocytes exposed to viral infection or UV radiation.13 The NLRP3 inflammasome is the most well-characterized member of this family and functions by sensing intracellular PAMPs and DAMPs and activating caspase-1/IL-1 converting enzyme, causing secretion of mature inflammatory cytokines and, possibly, stimulation of pyroptosis.14
FIGURE 1.
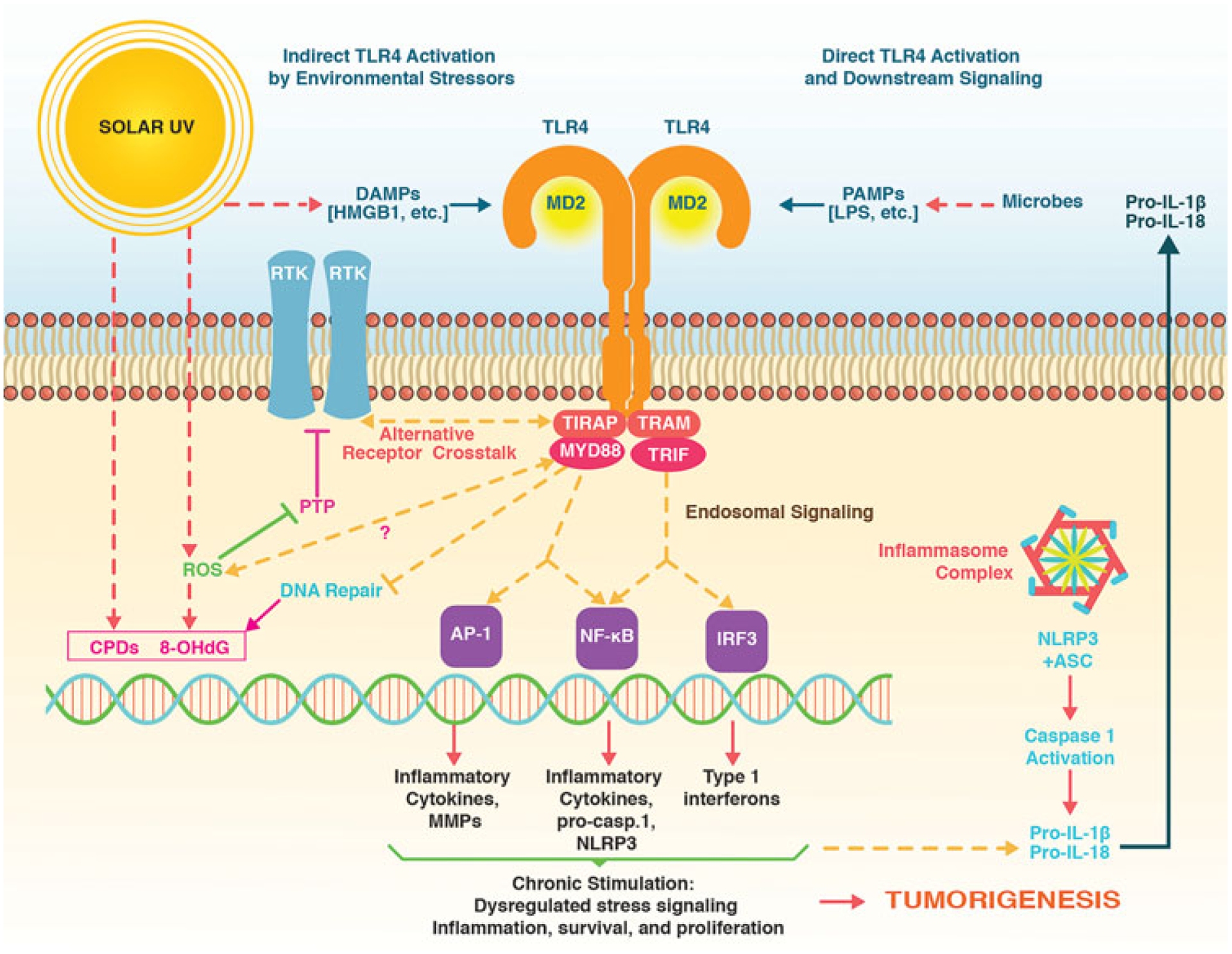
TLR4 as a key modulator of inflammatory skin carcinogenesis. Direct and indirect TLR4 activation upstream of inflammatory signaling is elicited by a variety of stimuli, including pathogen-associated molecular patterns (PAMPs, such as LPS) and damage-associated molecular patterns (DAMPs, such as HMGB1) that are formed upon exposure to environmental stressors such as solar UV. TLR4-dependent signaling activates inflammatory transcription factors (AP-1, NF-kB, IRF-3) that orchestrate gene expression with inclusion of inflammasome components. In addition, TLR4 antagonizes repair of DNA lesions that form in response to solar UV exposure including cyclobutane-pyrimidine dimers (CPDs) and reactive oxygen species (ROS)-derived 8-oxo-deoxyGuanosine (8-OHdG) and enhances receptor tyrosine kinase (RTK) signaling through molecular crosstalk that remains poorly defined. TLR4 signaling may also cause redox alterations and ROS formation, further enhancing RTK signaling through oxidative inhibition of protein tyrosine phosphatases (PTPs). Chronic activation of TLR4-controlled signaling has now been identified as a novel molecular target in solar UV-induced skin tumorigenesis amenable to pharmacological intervention. HMGB1, high-mobility group box 1; LPS, lipopolysaccharide; TLR4, toll-like receptor 4; UV, ultraviolet light.
TLR4 expression in cutaneous antigen-presenting cells and keratinocytes is upregulated and activated in response to UV.15–17 TLR4 also responds to endogenous ligands (eg, HMGB1) released in an autocrine fashion from keratinocytes as a consequence of cytotoxic environmental stress including solar UV.18 Blocking cutaneous HMGB1 reduces inflammatory cytokine production and inflammatory cell recruitment.18 MyD88 is upregulated in human epidermis in response to acute UVB exposure, and overexpression of dominant-negative MyD88 prevents UV-induced NF-κB and AP-1 activation.19 Likewise, MyD88 is overexpressed in photoaged skin compared with sun-protected skin from the same donor.19 Photoimmunosuppression is an established key mechanism of solar UV-driven skin carcinogenesis.20–24 TLR4/MyD88-dependent inhibition of DNA repair and enhancement of UV-induced apoptosis in cutaneous APCs is thought to underlie systemic photoimmunosuppression. This effect is attenuated in TLR4 KO or MYD88-deficient mice.2,25,26 Intriguingly, TLR4 signaling controls interleukin-10 (IL-10) expression, and IL-10 KO mice are resistant to photocarcinogenesis, attributed to the key role of IL-10 in UV-induced activation of regulatory T-cells and photoimmunosuppression.27
TLR4 has now been recognized as an important determinant of skin barrier function with specific roles in wound healing, tissue remodeling, and innate immunity. Consistent with these physiological roles, it has also been shown that signaling through keratinocyte-derived HMGB1 and TLR4 represents an important factor underlying skin inflammation.6,18 TLR4 involvement has now been implicated in numerous skin pathologies, including atopic dermatitis and psoriasis.4,5 Strikingly, environmental stressors including UV radiation, pollutants, and particulate matter have been identified as activators of cutaneous TLR4 signaling17,25,26,28–31 (Figure 1). Recent evidence also suggests that TLR4 serves as a novel molecular target in skin carcinogenesis.2,7,32
2 |. TLR4 IN NONMELANOMA SKIN CANCER
Nonmelanoma skin cancer (NMSC) is the most common malignancy worldwide and is rapidly increasing in incidence, representing an expanding public health burden of considerable magnitude.33–35 The average annual number of adults treated for skin cancer in the United States (NMSC or melanoma) increased from 3.4 million in 2002–2006 to 4.9 million in 2007–2011. During this period, the average annual total cost for skin cancer, the largest proportion representing keratinocytic tumors, increased from $3.6 billion to $8.1 billion.36 NMSCs also represent a major cause of morbidity after organ transplantation. Squamous cell carcinomas (SCCs) are the most common cutaneous malignancies seen in this population, with a 65 to 100 fold greater incidence in organ transplant recipients compared to the general population.37 Therefore, an urgent need exists for the development of improved molecular strategies for prevention and treatment of cutaneous SCCs. Cutaneous exposure to solar UV radiation is a causative factor in skin carcinogenesis, and inflammatory dysregulation is a key mechanism underlying detrimental effects of acute and chronic UV exposure.2,38–41
TLR4 is overexpressed in many tumor types, with the possible exception of lung tumors.42,43 Our own research has shown that TLR4 expression is significantly increased in cutaneous SCCs compared with normal skin controls as also reported by others.16,31 We have also demonstrated that TLR4 staining is confined to the basal layer of normal human epidermis with progressively increased thickness detectable in sun-damaged skin and AKs from matched patient samples. Tissue microarrays and proteomic analysis also indicated that TLR4 increased in expression in cutaneous SCC compared with normal controls. In addition, our analysis indicates that downstream intermediates of TLR4, such as MyD88 and NF-kB also increase in protein expression during the progression to NMSC.31 However, a recent report noted a different pattern of epidermal staining, in which the TLR4 expression increased as cells differentiated away from the basal layers of the skin with no differential staining detectable between normal skin and cutaneous SCCs.44 These opposing immunohistochemical data may be attributable to different antibodies recognizing TLR4 isoforms, and highlight the need for further expression analysis of TLR4 in the skin. Strikingly, the same report examined the role of TLR4 in cutaneous SCC (without the inclusion of UV exposure), providing evidence that genetic TLR4 antagonism in HaCaT keratinocytes results in increased proliferation, and that overexpression of TLR4 in cutaneous SCC-derived cells reduced xenograft growth.44 This highlights the need for further mechanistic studies and improved analytical tools assessing the activity of TLR4 in cutaneous SCC.44
In addition to its role as a key regulator of inflammatory cellular signaling, the importance of TLR4 in the development of NMSC has been attributed to a variety of other molecular mechanisms. TLR4 is integral for UV-induced photoimmunosuppression, as well as DNA repair of dendritic and epidermal cells after UV exposure. Indeed, mice lacking TLR4 or its effector protein MyD88 show enhanced clearance of UV-induced DNA damage, which has been linked to regulation of nucleotide excision repair.25,26 Multiple reports have also correlated TLR4 with wound healing of skin and other tissues.3,45,46 The key study linking TLR4 to NSMC, however, compared the skin tumor responses of mice treated with DMBA for tumor initiation and croton oil (a potent inflammatory agent) for progression. In this study, mice deficient in TLR4, but not TLR2 or TLR9, showed significant inhibition of tumorigenesis compared with wild-type controls.7 This result is in agreement with other studies showing that TLR4 knockout mice had reduced incidence of colitis-associated intestinal tumors.47 Moreover, a skin tumorigenesis experiment employing bone marrow transplants on irradiated mice to produce chimeras indicates that both bone marrow-derived cells and radioresistant (non-bone marrow-derived) cells are required for skin tumor development under these circumstances.7 Notably, another report has utilized TLR4 deficient mice to examine chemically-induced skin carcinogenesis and found that DMBA treatment caused significantly more tumors in the knockout strain compared with wild-type mice.48 However, this experiment utilized only DMBA for both initiation and promotion of the tumors. DMBA is not an inflammatory mediator of promotion and has additionally been shown to cause contact hypersensitivity, which can actually protect against the carcinogenic effects of this compound.49,50 Thus, while the two TLR4 knockout mouse experiments reported to date produced seemingly opposing results, it may be hypothesized that overactivity of TLR4 contributes to inflammation-related skin tumorigenesis and that TLR4 inhibition may reduce tumor initiation and progression in the skin.
Our own recent work suggests that pharmacological inhibition of TLR4 may be a promising strategy for the prevention and/or treatment of UV-induced NMSC. Initially, we confirmed the role of TLR4 in UV-induced stress signaling by utilizing siRNA in human and mouse keratinocytes. Knockdown of TLR4 in this manner significantly reduced both NF-κB and AP-1 signaling after UV exposure.31 Next, using the small molecule TLR4 inhibitor resatorvid (TAK-242), we showed that pharmacological inhibition of this receptor in vitro blocked both LPS and UV-induced signaling in cultured keratinocytes. When resatorvid was applied topically after an acute dose of solar-simulated UV in SKH-1 hairless mice, epidermal stimulation of NF-κB and p38 MAP kinase were dramatically reduced and UV-induced apoptosis was increased.31 This promising result led us to perform a long-term photocarcinogenesis trial in SKH-1 mice, which showed that topical resatorvid significantly inhibited both the size and the number of tumors in this model.32 Tumors from this experiment showed reduced p38 phosphorylation and increased apoptosis, as in the acute model.32 Thus, it may be concluded that topical pharmacological inhibition of TLR4 using resatorvid may be amenable to clinical development as a prevention/treatment strategy against UV-induced NMSC.
Others have shown similar positive results when natural product-based agents were utilized to block TLR4 signaling. Indeed, the phytochemical flavone baicalin protects against UVA-induced skin inflammation and DNA damage through inhibition of TLR4.17,51 However, the specific molecular mechanism underlying TLR4 inhibition by this compound remains to be elucidated.
3 |. TLR4 IN HEAD AND NECK SQUAMOUS CELL CARCINOMA
TLR4 overexpression and activation have been substantiated in other types of squamous cell carcinoma, including esophageal SCC (ESCC) and head and neck SCC (HNSCC). Specifically, TLR4 protein expression was increased in ESCC tumor tissues compared with adjacent normal tissues, and TLR4 overexpression was significantly correlated with tumor differentiation grade and lymph node metastasis.52 In addition, LPS-induced TLR4 signaling in ESCC cells promoted tumor proliferation and regulated inflammatory cytokine expression. Together, these data suggest that TLR4 signaling contributes to the progression of ESCC.52 Other studies have found that baseline TLR4 expression is increased in esophageal adenocarcinoma cells compared with normal esophageal cells, and that stimulation with LPS results in intracellular MyD88/TRAF6/NF-κB signaling and increased proliferation in all three cell lines tested. This proliferation was significantly reduced in the presence of the NF-κB inhibitor Bay11–7082.53 Likewise, in HNSCC there is evidence that TLR4 intensity correlates with tumor grade, and that TLR4 activation promotes tumor development and protects the tumor from immune attack, suggesting that TLR4 engagement on tumor cells supports HNSCC progression.54
4 |. TLR4 IN MELANOMA
The role of TLR receptors in cutaneous melanoma has been addressed by recent research focusing on both molecular etiology and therapeutic development.55 Expression of TLR4 has been documented in human melanoma cells cultured from tumor specimens, and multiple studies have reported TLR4 overexpression in human melanoma tumors as well as a negative association between the TLR4 expression and relapse-free survival.7–9 Tissue microarray analysis has determined pronounced TLR4 and MyD88 overexpression in radial and vertical growth phase melanoma specimens.7 It was also suggested that TLR4 expression may be a new prognostic factor of unfavorable disease progression in cutaneous malignant melanoma.8 In addition, clinical outcomes in vaccinated melanoma patients have been associated with TLR4 gene polymorphisms that impact dendritic cell function.56 Strikingly, overexpression of the TLR4 agonist HMGB1 in melanoma predicts patient survival.57
Moreover, paracrine factors secreted by keratinocytes in response to stress such as solar UV, HMGB1, and calprotectin (S100A8/A9 heterodimer) cause melanocyte and melanoma cell activation through TLR4 agonism.58 TLR4 signaling has also been shown to promote the migration of human melanoma cells.59 Consistent with this finding, independent research has shown that TLR4 was a key factor in solar UV-driven progression and metastasis of melanoma, attributed to inflammatory induction of angiotropism with perivascular invasion.60 Strikingly, epidermal keratinocytes played a key role in TLR4-dependent melanoma progression through release of the TLR4 agonist HMGB1 in response to UV exposure.60 Confirming the key role of TLR4 signaling in murine melanoma progression, metastasis of the primary tumor to the lung was blocked by genetic knockout of TLR4 as well as by antibody-based inhibition. Clinical data support this hypothesis, substantiating a positive correlation between increased angiotropism and an elevated risk of metastasis.60 Similarly, it has been shown that TPA-induced, neutrophil-dependent inflammatory responses in mouse models selectively promote metastatic spread of melanoma in a TLR4-dependent manner.61 Further evidence for a role of TLR4 in melanomagenesis is supported by the finding that miR-145–5p, an antagonist of TLR4 expression, is downregulated in tumor tissue of human melanoma patients, and inhibits tumor occurrence and metastasis through the NF-κB signaling pathway by targeting TLR4 in malignant melanoma.62
Notably, while inhibition of TLR4 may block inflammatory melanoma metastasis, early research indicates that TLR4 is an important factor in adjuvant-mediated immune-dependent tumor regression.63 Early research indicates that immunotherapeutic intervention using Mycobacterium bovis bacillus Calmette-Guerin cell wall skeleton (BCG-CWS) for TLR4 activation shows activity in B16 murine melanoma models, a therapeutic effect not observed in MyD88-deficient mice.63 Importantly, activation of TLR4 on B16 murine melanoma cells in vitro inhibits subsequent tumor growth in vivo, an effect not observed in athymic mice.64 Others have reported that inoculation with the putative TLR4 agonist Brucella species lumazine synthase (BLS, a highly immunogenic decameric protein) may reduce B16 murine melanoma tumor growth,65 an effect attributed to BLS signaling via TLR4 causing innate and adaptive immune responses, with induction of dendritic cell maturation and CD8+ T-cell cytotoxicity.65 Consequently, current clinical investigations are focusing on immunostimulatory/adjuvant effects of TLR4 agonism. Specialized clinical trials in melanoma patients are ongoing using the glucopyranosyl lipid A-based TLR4 agonist GLA-SE with the MART-1 antigen (melanoma antigen recognized by T-cells-1), with frequency and IFNγ production of vaccine-peptide specific CTLs serving as an outcome measure of immune activation (Table 1; NCT02320305). Moreover, an autologous dendritic cell vaccine generated by single-step antigen loading and TLR4 activation is being examined in melanoma patients (Table 1; NCT01530698).
TABLE 1.
Clinical trials targeting TLR4 in skin cancer
| ClinicalTrials.gov Identifier | Title | Agent | Type | Status |
|---|---|---|---|---|
| NCT02035657 | A proof-of-concept trial of GLA-SE in patients with Merkel cell carcinoma | GLA-SE (TLR4 agonist) | Phase 1 | Completed, no results posted |
| NCT01530698 | Single-step antigen loading and TLR activation of dendritic cells in melanoma patients | Autologous dendritic cell vaccine | Interventional, open label | Completed, no results posted |
| NCT02320305 | MART-1 antigen with or without TLR4 agonist GLA-SE in treating patients with stage II-IV melanoma that has been removed by surgery | MART-1 antigen and GLA-SE (TLR4 agonist) | Interventional, open label | Active, not recruiting |
| NCT03122366 | TLR4 polymorphisms and risk of skin cancer | None | Observational | Active, not recruiting |
Abbreviations: MART-1, melanoma antigen recognized by T-cells-1; TLR, toll-like receptors.
Numerous pharmacological studies have demonstrated feasibility of targeting TLR4 in melanoma using small molecule modulators of natural and synthetic origin. The natural product andrographolide has been shown to block melanoma tumor growth in a murine model by inactivating the TLR4-NF-κB signaling axis.66,67 Tea polyphenols inhibit the proliferation, migration, and invasion of melanoma cells through the downregulation of TLR4.68 Inhibition of p38 and ERK1/2 pathways by the TLR2 and TLR4-antagonistic natural product and xanthone derivative sparstolonin B has been shown to suppress LPS-driven inflammation-induced melanoma metastasis in a B16 mouse model.69 Recently, the small molecule TLR4 antagonist resatorvid (TAK-242) has been successfully used to block murine melanoma progression.60 It has also been shown that the established chemotherapeutic paclitaxel exerts TLR4-directed antitumor activity in an immune competent B16 melanoma mouse model, attributed to TLR4 control of M1/M2 macrophage polarization, reprogramming tumor-associated macrophages to an M1 profile.70
5 |. TLR4 IN MERKEL CELL CARCINOMA
Immune evasion is an established hallmark of cancer.71 Importantly, immune evasion mechanisms have become the focus of current investigations aiming at therapeutic targeting of Merkel cell carcinoma (MCC), a rare but highly aggressive skin cancer with neuroendocrine features.72 A viral etiology characterized by the presence of Merkel cell polyomavirus (MCV) as well as chronic exposure to solar ultraviolet light have been identified as key factors driving the majority of MCC cases, characterized by solitary cutaneous or subcutaneous nodules. Photoimmunosuppression and photomutagenesis, associated with solar UV exposure, have been mechanistically implicated in both viral and nonviral mediated MCC.72
With relevance to MCV-positive and negative tumors, immunomodulatory interventions have attracted much attention in the context of therapeutic development, including investigations on the PD1/PD-L1 axis and likewise, TLR4-dependent stimulation of antitumor immunity, the focus of a recent proof-of-concept clinical trial (Table 1). Recently, TLR expressions (TLRs 2, 4, 5, 7, 9) have been implicated in MCC, and a strong positive correlation was established between TLR4 and MCV expression.10 Remarkably, G100, a clinical LPS mimetic TLR4 agonist, induces antitumor immune responses and tumor regression in patients with MCC upon intratumoral administration, an effect attributed to APC activation and increased infiltration of CD8+ and CD4+ lymphocytes, facilitating an inflamed immunologically active microenvironment and systemic immune responses.73
5.1 |. TLR4-directed molecular interventions and clinical translation for skin cancer
Based on the emerging role of TLR4 in skin tumorigenesis, the availability of validated pharmacological modulators targeting TLR4 signaling has facilitated a considerable research effort aiming at experimental and investigational therapeutic intervention.74 Given the complexity underlying TLR4-dependent signaling, a number of TLR4-directed modulators have now been identified and are in various stages of preclinical and clinical development as recently reviewed expertly.75,76
A large number of pharmacologically validated TLR4 modulators of natural and synthetic origin is now available, promising to allow for clinical oncologically-relevant translation in the near future. Numerous specialized reviews have recently provided comprehensive coverage of TLR4 pharmacology.75–77 Much emphasis has centered on natural products with TLR4-antagonistic activity including promiscuous multitarget agents, such as baicalin, curcumin, sulforaphane, andrographolide, and glycyrrhizin, often derived from phytochemical dietary sources that seem amenable to chemopreventive repurposing.11,17,66,67 Moreover, a number of potent and selective TLR4 antagonists of synthetic origin have been identified and have now entered preclinical and clinical assessment.31,32,77,78 Of note, TLR4-directed antagonism can also be achieved targeting direct downstream effectors, such as the MyD88-directed small molecule ST-2825.79 Remarkably, most of the pharmacologically active TLR4 agonists are derived from the lipid A moiety of LPS (including eritoran and GLA-SE), some of which have now entered oncological clinical trials (Table 1). Contemporary drug discovery is adding an additional number of MD2-directed TLR4 agonistic small molecule candidates for future clinical use, including novel carboxylate-based glycolipids.80
Ongoing preclinical and clinical research will define the potential of TLR4-directed approaches for skin cancer prevention and intervention and will also identify the patient population that most benefits from these specific strategies.81 Clinically, TLR4 agonists are currently being tested for their ability to act as immune-stimulating adjuvants targeting melanoma and MCC (Table 1). In addition, TLR4 modulation is now being tested in other clinical fields. For example, the TLR4 agonist, GLA-SE, is used in conjunction with radiation therapy targeting adult sarcomas (Clinicaltrials.gov, NCT02180698), and an antibody-based approach to TLR4 inhibition (NI-0101) has recently completed clinical trials for safety assessments with positive results in rheumatoid arthritis (clincialtrials.gov, NCT01808469, NCT03241108).
6 |. CONCLUSIONS
Modulation of TLR signaling for therapeutic gain has attracted much attention, and TLR7-directed intervention using imiquimod and derivatives thereof has shown efficacy in the treatment of cutaneous premalignant and malignant conditions including actinic keratosis and basal cell carcinoma, respectively. Cumulative evidence as summarized in this review suggests that modulation of TLR4 signaling might represent an equally valuable molecular target for the prevention and treatment of nonmelanoma and melanoma skin cancers. The therapeutic requirement for TLR4 agonism versus antagonism may be highly dependent upon the type of cancer and the progression status of each patient.6,42,81 Thus, the mechanistic dichotomy between TLR4 agonistic and antagonistic approaches, both of which have shown clinical promise, needs further elucidation and will benefit from the increasing availability of TLR4 modulatory therapeutics.
Funding information
National Cancer Institute, Grant/Award Numbers: R03 CA230949, P01 CA027502, R01 CA229418
REFERENCES
- 1.Kawamura T, Ogawa Y, Aoki R, Shimada S. Innate and intrinsic antiviral immunity in skin. J Dermatol Sci. 2014;75(3):159–166. [DOI] [PubMed] [Google Scholar]
- 2.Lewis W, Simanyi E, Li H, et al. Regulation of ultraviolet radiation induced cutaneous photoimmunosuppression by toll-like receptor-4. Arch Biochem Biophys. 2011;508(2):171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L, Guo S, Ranzer MJ, DiPietro LA. Toll-like receptor 4 has an essential role in early skin wound healing. J Invest Dermatol. 2013;133(1):258–67.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Weng H, Song JF, Deng YH, Li S, Liu HB. Activation of the HMGB1TLR4NFkappaB pathway may occur in patients with atopic eczema. Mol Med Rep. 2017;16(3):2714–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith RL, Hebert HL, Massey J, et al. Association of Toll-like receptor 4 (TLR4) with chronic plaque type psoriasis and psoriatic arthritis. Arch Dermatol Res. 2016;308(3):201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickinson SE, Wondrak GT. TLR4-directed molecular strategies targeting skin photodamage and carcinogenesis. Curr Med Chem. 2018;25(40):5487–5502. [DOI] [PubMed] [Google Scholar]
- 7.Mittal D, Saccheri F, Venereau E, Pusterla T, Bianchi ME, Rescigno M. TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. EMBO J. 2010;29(13):2242–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eiro N, Ovies C, Fernandez-Garcia B, et al. Expression of TLR3, 4, 7 and 9 in cutaneous malignant melanoma: relationship with clinico-pathological characteristics and prognosis. Arch Dermatol Res. 2013;305(1):59–67. [DOI] [PubMed] [Google Scholar]
- 9.Goto Y, Arigami T, Kitago M, et al. Activation of Toll-like receptors 2, 3, and 4 on human melanoma cells induces inflammatory factors. Mol Cancer Ther. 2008;7(11):3642–3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jouhi L, Koljonen V, Bohling T, Haglund C, Hagstrom J. The expression of Toll-like receptors 2, 4, 5, 7 and 9 in Merkel cell carcinoma. Anticancer Res. 2015;35(4):1843–1849. [PubMed] [Google Scholar]
- 11.Molteni M, Gemma S, Rossetti C. The role of Toll-Like receptor 4 in infectious and noninfectious inflammation. Mediators Inflamm. 2016;2016:6978936–6978939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. [DOI] [PubMed] [Google Scholar]
- 13.Sand J, Haertel E, Biedermann T, et al. Expression of inflammasome proteins and inflammasome activation occurs in human, but not in murine keratinocytes. Cell Death Dis. 2018;9(2):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vince JE, Silke J. The intersection of cell death and inflammasome activation. Cell Mol Life Sci. 2016;73(11–12):2349–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Bi Z, Wang Y. Wang Y. Increased MAPK and NF-kappaB expression of Langerhans cells is dependent on TLR2 and TLR4, and increased IRF-3 expression is partially dependent on TLR4 following UV exposure. Mol Med Rep. 2011;4(3):541–546. [DOI] [PubMed] [Google Scholar]
- 16.Weng H, Deng Y, Xie Y, Liu H, Gong F. Expression and significance of HMGB1, TLR4 and NF-kappaB p65 in human epidermal tumors. BMC Cancer. 2013;13(311):311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Min W, Ahmad I, Chang ME, Burns EM, Qian Q, Yusuf N. Baicalin protects keratinocytes from Toll-like receptor-4 mediated DNA damage and inflammation following ultraviolet irradiation. Photochem Photobiol. 2015;91:1435–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamore SD, Cabello CM, Wondrak GT. HMGB1-directed drug discovery targeting cutaneous inflammatory dysregulation. Curr Drug Metab. 2010;11(3):250–265. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y, Kim H, Kim S, et al. Myeloid differentiation factor 88 regulates basal and UV-induced expressions of IL-6 and MMP-1 in human epidermal keratinocytes. J Invest Dermatol. 2009;129(2):460–467. [DOI] [PubMed] [Google Scholar]
- 20.Welsh MM, Karagas MR, Applebaum KM, Spencer SK, Perry AE, Nelson HH. A role for ultraviolet radiation immunosuppression in nonmelanoma skin cancer as evidenced by gene-environment interactions. Carcinogenesis. 2008;29(10):1950–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halliday GM. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat Res. 2005;571(1–2):107–120. [DOI] [PubMed] [Google Scholar]
- 22.Yu SH, Bordeaux JS, Baron ED. The immune system and skin cancer. Adv Exp Med Biol. 2014;810:182–191. [DOI] [PubMed] [Google Scholar]
- 23.Murphy GM. Ultraviolet radiation and immunosuppression. Br J Dermatol. 2009;161(Suppl 3):90–95. [DOI] [PubMed] [Google Scholar]
- 24.de Gruijl FR. UV-induced immunosuppression in the balance. Photochem Photobiol. 2008;84(1):2–9. [DOI] [PubMed] [Google Scholar]
- 25.Ahmad I, Simanyi E, Guroji P, et al. Toll-like receptor-4 deficiency enhances repair of UVR-induced cutaneous DNA damage by nucleotide excision repair mechanism. J Invest Dermatol. 2014;134(6):1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harberts E, Zhou H, Fishelevich R, Liu J, Gaspari AA. Ultraviolet radiation signaling through TLR4/MyD88 constrains DNA repair and plays a role in cutaneous immunosuppression. J Immunol. 2015;194(7):3127–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loser K, Apelt J, Voskort M, et al. IL-10 controls ultraviolet-induced carcinogenesis in mice. J Immunol. 2007;179(1):365–371. [DOI] [PubMed] [Google Scholar]
- 28.He M, Ichinose T, Yoshida Y, et al. Urban PM2.5 exacerbates allergic inflammation in the murine lung via a TLR2/TLR4/MyD88-signaling pathway. Sci Rep. 2017;7(1):11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huls A, Klumper C, MacIntyre EA, et al. Atopic dermatitis: interaction between genetic variants of GSTP1, TNF, TLR2, and TLR4 and air pollution in early life. Pediatr Allergy Immunol. 2018;29(6):596–605. [DOI] [PubMed] [Google Scholar]
- 30.Seo SW, Park SK, Oh SJ, Shin OS. TLR4-mediated activation of the ERK pathway following UVA irradiation contributes to increased cytokine and MMP expression in senescent human dermal fibroblasts. PLoS One. 2018;13(8):e0202323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janda J, Burkett NB, Blohm-Mangone K, et al. Resatorvid-based pharmacological antagonism of cutaneous TLR4 blocks UV-induced NF-kappaB and AP-1 signaling in keratinocytes and mouse skin. Photochem Photobiol. 2016;92(6):816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blohm-Mangone K, Burkett NB, Tahsin S, et al. Pharmacological TLR4 antagonism using topical resatorvid blocks solar UV-induced skin tumorigenesis in SKH-1 mice. Cancer Prev Res (Phila). 2018;11:265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu LS, Colegio OR. Molecularly targeted therapies for nonmelanoma skin cancers. Int J Dermatol. 2013;52(6):654–665. [DOI] [PubMed] [Google Scholar]
- 34.Wondrak GT. Sunscreen-based skin protection against solar insult: molecular mechanisms and opportunities. Fundamentals of Cancer Prevention. 2014;30(2):301–320. [Google Scholar]
- 35.Natarajan VT, Ganju P, Ramkumar A, Grover R, Gokhale RS. Multifaceted pathways protect human skin from UV radiation. Nat Chem Biol. 2014;10(7):542–551. [DOI] [PubMed] [Google Scholar]
- 36.Guy GP Jr., Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and costs of skin cancer treatment in the u.s., 2002–2006 and 2007–2011. Am J Prev Med. 2015;48(2):183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chockalingam R, Downing C, Tyring SK. Cutaneous squamous cell carcinomas in organ transplant recipients. J Clin Med. 2015;4(6):1229–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wondrak GT, Jacobson MK, Jacobson EL. Endogenous UVA-photosensitizers: mediators of skin photodamage and novel targets for skin photoprotection. Photochem Photobiol Sci. 2006;5(2):215–237. [DOI] [PubMed] [Google Scholar]
- 39.Cadet J, Mouret S, Ravanat JL, Douki T. Photoinduced damage to cellular DNA: direct and photosensitized reactions. Photochem Photobiol. 2012;88(5):1048–1065. [DOI] [PubMed] [Google Scholar]
- 40.Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol. 2001;79(6):547–568. [DOI] [PubMed] [Google Scholar]
- 41.Rhodes LE, Gledhill K, Masoodi M, et al. The sunburn response in human skin is characterized by sequential eicosanoid profiles that may mediate its early and late phases. FASEB J. 2009;23(11):3947–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mai CW, Kang YB, Pichika MR. Should a Toll-like receptor 4 (TLR-4) agonist or antagonist be designed to treat cancer? TLR-4: its expression and effects in the ten most common cancers. Onco Targets Ther. 2013;6:1573–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bauer AK, Upham BL, Rondini EA, Tennis MA, Velmuragan K, Wiese D. Toll-like receptor expression in human non-small cell lung carcinoma: potential prognostic indicators of disease. Oncotarget. 2017;8(54):91860–91875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iotzova-Weiss G, Freiberger SN, Johansen P, et al. TLR4 as a negative regulator of keratinocyte proliferation. PLoS One. 2017;12(10):e0185668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suga H, Sugaya M, Fujita H, et al. TLR4, rather than TLR2, regulates wound healing through TGF-beta and CCL5 expression. J Dermatol Sci. 2014;73(2):117–124. [DOI] [PubMed] [Google Scholar]
- 46.Bai S, Zhou H, Wu L. Bone marrow stromal cells improved functional recovery in spinal cord injury rats partly via the Toll-like receptor-4/nuclear factor-kappaB signaling pathway. Exp Ther Med. 2019;17(1):444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukata M, Chen A, Vamadevan AS, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133(6):1869–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yusuf N, Nasti TH, Long JA, et al. Protective role of Toll-like receptor 4 during the initiation stage of cutaneous chemical carcinogenesis. Cancer Res. 2008;68(2):615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slaga TJ. Overview of tumor promotion in animals. Environ Health Perspect. 1983;50:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klemme JC, Mukhtar H, Elmets CA. Induction of contact hypersensitivity to dimethylbenz(a)anthracene and benzo(a)pyrene in C3H/HeN mice. Cancer Res. 1987;47(22):6074–6078. [PubMed] [Google Scholar]
- 51.Sherwani MA, Yang K, Jani A, et al. Protective effect of baicalin against TLR4-mediated UVA-induced skin inflammation. Photochem Photobiol. 2018;95:605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zu Y, Ping W, Deng T, Zhang N, Fu X, Sun W. Lipopolysaccharide-induced toll-like receptor 4 signaling in esophageal squamous cell carcinoma promotes tumor proliferation and regulates inflammatory cytokines expression. Dis Esophagus. 2017;30(2):1–8. [DOI] [PubMed] [Google Scholar]
- 53.Kohtz PD, Halpern AL, Eldeiry MA, et al. Toll-like receptor-4 Is a mediator of proliferation in esophageal adenocarcinoma. Ann Thorac Surg. 2019;107(1):233–241. [DOI] [PubMed] [Google Scholar]
- 54.Szczepanski MJ, Czystowska M, Szajnik M, et al. Triggering of Toll-like receptor 4 expressed on human head and neck squamous cell carcinoma promotes tumor development and protects the tumor from immune attack. Cancer Res. 2009;69(7):3105–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coati I, Miotto S, Zanetti I, Alaibac M. Toll-like receptors and cutaneous melanoma. Oncol Lett. 2016;12(5):3655–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tittarelli A, Gonzalez FE, Pereda C, et al. Toll-like receptor 4 gene polymorphism influences dendritic cell in vitro function and clinical outcomes in vaccinated melanoma patients. Cancer Immunol Immunother. 2012;61(11):2067–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Q, Li J, Wen T, et al. Overexpression of HMGB1 in melanoma predicts patient survival and suppression of HMGB1 induces cell cycle arrest and senescence in association with p21 (Waf1/Cip1) up-regulation via a p53-independent, Sp1-dependent pathway. Oncotarget. 2014;5(15):6387–6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shirley SH, von Maltzan K, Robbins PO, Kusewitt DF. Melanocyte and melanoma cell activation by calprotectin. J Skin Cancer. 2014;2014:846249–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takazawa Y, Kiniwa Y, Ogawa E, et al. Toll-like receptor 4 signaling promotes the migration of human melanoma cells. Tohoku J Exp Med. 2014;234(1):57–65. [DOI] [PubMed] [Google Scholar]
- 60.Bald T, Quast T, Landsberg J, et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature. 2014;507(7490):109–113. [DOI] [PubMed] [Google Scholar]
- 61.Bald T, Landsberg J, Jansen P, Gaffal E, Tuting T. Phorbol ester-induced neutrophilic inflammatory responses selectively promote metastatic spread of melanoma in a TLR4-dependent manner. Oncoimmunology. 2016;5(2):e1078964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin C, Wang A, Liu L, Wang G, Li G, Han Z. miR-145–5p inhibits tumor occurrence and metastasis through the NF-kappaB signaling pathway by targeting TLR4 in malignant melanoma. J Cell Biochem. 2019. [published online ahead of print January 30, 2019]. 10.1002/jcb.28388 [DOI] [PubMed] [Google Scholar]
- 63.Akazawa T, Masuda H, Saeki Y, et al. Adjuvant-mediated tumor regression and tumor-specific cytotoxic response are impaired in MyD88-deficient mice. Cancer Res. 2004;64(2):757–764. [DOI] [PubMed] [Google Scholar]
- 64.Andreani V, Gatti G, Simonella L, Rivero V, Maccioni M. Activation of Toll-like receptor 4 on tumor cells in vitro inhibits subsequent tumor growth in vivo. Cancer Res. 2007;67(21):10519–10527. [DOI] [PubMed] [Google Scholar]
- 65.Rossi AH, Farias A, Fernandez JE, Bonomi HR, Goldbaum FA, Berguer PM. Brucella spp. lumazine synthase induces a TLR4-mediated protective response against B16 melanoma in mice. PLoS One. 2015;10(5):e0126827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang QQ, Zhou DL, Ding Y, et al. Andrographolide inhibits melanoma tumor growth by inactivating the TLR4/NF-kappaB signaling pathway. Melanoma Res. 2014;24(6):545–555. [DOI] [PubMed] [Google Scholar]
- 67.Liu G, Chu H. Andrographolide inhibits proliferation and induces cell cycle arrest and apoptosis in human melanoma cells. Oncol Lett. 2018;15(4):5301–5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen X, Chang L, Qu Y, Liang J, Jin W, Xia X. Tea polyphenols inhibit the proliferation, migration, and invasion of melanoma cells through the downregulation of TLR4. Int J Immunopathol Pharmacol. 2018;32:394632017739531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang YM, Cao QY, Guo XY, et al. Inhibition of p38 and ERK1/2 pathways by Sparstolonin B suppresses inflammation-induced melanoma metastasis. Biomed Pharmacother. 2018;98:382–389. [DOI] [PubMed] [Google Scholar]
- 70.Wanderley CW, Colon DF, Luiz JPM, et al. Paclitaxel reduces tumor growth by reprogramming tumor-associated macrophages to an M1 profile in a TLR4-dependent manner. Cancer Res. 2018;78(20):5891–5900. [DOI] [PubMed] [Google Scholar]
- 71.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 72.Becker JC, Stang A, DeCaprio JA, et al. Merkel cell carcinoma. Nat Rev Dis Primers. 2017;3:17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhatia S, Miller NJ, Lu H, et al. Intratumoral G100, a TLR4 agonist, induces antitumor immune responses and tumor regression in patients with Merkel cell carcinoma. Clin Cancer Res. 2019;25(4):1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shetab Boushehri MA, Lamprecht A. TLR4-based immunotherapeutics in cancer: a review of the achievements and shortcomings. Mol Pharm. 2018;15(11):4777–4800. [DOI] [PubMed] [Google Scholar]
- 75.Peri F, Calabrese V. Toll-like receptor 4 (TLR4) modulation by synthetic and natural compounds: an update. J Med Chem. 2014;57(9):3612–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li J, Csakai A, Jin J, Zhang F, Yin H. Therapeutic developments targeting toll-like receptor-4-mediated neuroinflammation. Chem-MedChem. 2016;11(2):154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dickinson SE, Wondrak GT. TLR4-directed molecular strategies targeting skin photodamage and carcinogenesis. Curr Med Chem. 2018;25(40):5487–5502. [DOI] [PubMed] [Google Scholar]
- 78.Rice TW, Wheeler AP, Bernard GR, et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit Care Med. 2010;38(8):1685–1694. [DOI] [PubMed] [Google Scholar]
- 79.Loiarro M, Capolunghi F, Fanto N, et al. Pivotal advance: inhibition of MyD88 dimerization and recruitment of IRAK1 and IRAK4 by a novel peptidomimetic compound. J Leukoc Biol. 2007;82(4):801–810. [DOI] [PubMed] [Google Scholar]
- 80.Cochet F, Facchini FA, Zaffaroni L, et al. Novel carboxylate-based glycolipids: TLR4 antagonism, MD-2 binding and self-assembly properties. Sci Rep. 2019;9(1):919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sfanos KS. Targeting toll-like receptors in cancer prevention. Cancer Prev Res (Phila). 2018;11:251–254. [DOI] [PubMed] [Google Scholar]


