Abstract
Multidrug-resistant (MDR) Acinetobacter baumannii presents a critical challenge to human health worldwide and polymyxins are increasingly used as a last-line therapy. Due to the rapid emergence of resistance during polymyxin monotherapy, synergistic combinations (e.g. with rifampicin) are recommended to treat A. baumannii infections. However, most combination therapies are empirical, owing to a dearth of understanding on the mechanism of synergistic antibacterial killing. In the present study, we employed metabolomics to investigate the synergy mechanism of polymyxin B-rifampicin against A. baumannii AB5075, an MDR clinical isolate. The metabolomes of A. baumannii AB5075 were compared at 1 and 4 h following treatments with polymyxin B alone (0.75 mg/L, i.e. 3×MIC), rifampicin alone (1 mg/L, i.e. 0.25×MIC) and their combination. Polymyxin B monotherapy significantly perturbed glycerophospholipid and fatty acid metabolism at 1 h, reflecting its activity on bacterial outer membrane. Rifampicin monotherapy significantly perturbed glycerophospholipid, nucleotide and amino acid metabolism, which are related to the inhibition of RNA synthesis. The combination treatment significantly perturbed the metabolism of nucleotides, amino acids, fatty acids and glycerophospholipids at 1 and 4 h. Notably, the intermediate metabolite pools from pentose phosphate pathway were exclusively enhanced by the combination, while most metabolites from the nucleotide and amino acid biosynthesis pathways were significantly decreased. Overall, the synergistic activity of the combination was initially driven by polymyxin B which impacted pathways associated with outer membrane biogenesis; and subsequent effects were mainly attributed to rifampicin via the inhibition of RNA synthesis. This study is the first to reveal the synergistic killing mechanism of the polymyxin-rifampicin combination against polymyxin-susceptible MDR A. baumannii at the network level. Our findings provide new mechanistic insights for optimizing this synergistic combination in patients.
Keywords: Acinetobacter baumannii, metabolomics, polymyxin B, rifampicin, synergy
1. Introduction
Multidrug-resistant (MDR) Acinetobacter baumannii is a serious threat to human health globally [1]. In 2017, the World Health Organization identified carbapenem-resistant A. baumannii as a ‘Critical’ pathogen that urgently requires novel treatment options [1-3]. Polymyxins are a class of cationic lipopeptide antibiotics with activity mainly against Gram-negative bacteria [4]. Polymyxins were discovered in the 1940s but abandoned in the 1970s due to nephrotoxicity. However, severe infections caused by Gram-negative ‘superbugs’, particularly MDR A. baumannii, have revived polymyxins in the clinic since the early 2000s [5-9]. The exact mode of action of polymyxins is still unclear, but involves initial electrostatic and hydrophobic interactions with the negatively charged lipid A component of lipopolysaccharide (LPS), followed by disorganization of cell membranes and eventually cell death [10-12]. A. baumannii can rapidly develop resistance to polymyxins via lipid A modifications (e.g. phosphoethanolamine [pEtN] or galactosamine [GalN]) or loss of LPS [13].
Combination therapies are strongly recommended for polymyxins to minimize the emergence of resistance [14, 15]. A number of studies have indicated favorable microbiological response to the combination of intravenous colistimethate (an inactive prodrug of colistin) and rifampicin in patients [16-19]. Rifampicin is an RNA polymerase targeting antibiotic and pre-clinical studies have demonstrated that polymyxin-rifampicin combination synergistically kills MDR Gram-negative bacteria (e.g. A. baumannii) and suppresses the emergence of polymyxin resistance [20-22]. However, the exact mechanism underlying this synergy still remains unknown. In the present study, we employed metabolomics to elucidate the mechanism of time-dependent synergistic killing against MDR A. baumannii by the polymyxin-rifampicin combination.
2. Materials and Methods
2.1. Bacterial isolate, antibiotics and reagents
A. baumannii AB5075, an isolate from a patient at the Walter Reed Army Medical Center in USA, was examined in the present study as a type strain. A. baumannii AB5075 is resistant to ampicillin/sulbactum, amikacin, aztreonam, cefepime, levofloxacin and tobramycin [23]. MICs of polymyxin B and rifampicin were 0.25 mg/L and 4 mg/L, respectively. Tryptone soy broth (TSB; Oxoid, Thebarton, Australia) with 20% glycerol was used for strain storage at − 80°C. AB5075 was streaked on nutrient agar before each experiment and sub-cultured in cation-adjusted Muller-Hinton broth (CAMHB; 20-25 mg/L Ca2+ and 10-12.5 mg/L Mg2+; Oxoid, Thebarton, Australia). Solution of polymyxin B sulfate (Sigma-Aldrich, Castle Hill, Australia) was freshly prepared in sterile Milli-Q water (Millipore, North Ryde, Australia) and filtered with 0.22 μm Millex GP filters (Millipore, Bedford, MA) before use. Rifampicin (Sigma-Aldrich, Castle Hill, Australia) was dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich, Castle Hill, Australia).
2.2. Metabolomics experiments
2.2.1. Bacterial culture preparation.
CAMHB culture of AB5075 was prepared overnight at 37°C with vigorous shaking and early logarithmic culture was prepared by 100-fold dilution in 200 mL fresh CAMHB (OD600 = 0.5). Four groups were examined: (i) the untreated control, (ii) polymyxin B alone (0.75 mg/L), (iii) rifampicin alone (1 mg/L), and (iv) the combination of polymyxin B (0.75 mg/L) and rifampicin (1 mg/L). Concentrations of both antibiotics were determined based upon their MICs and pharmacokinetics in patients [24, 25]. Five biological replicates of each group were prepared independently from different colonies of AB5075. Samples were collected at 0, 1, and 4 h, subsequently normalized to OD600 of 0.5; and 10 mL of each normalized sample was transferred to 15-mL Falcon tubes for metabolite extraction.
2.2.2. Metabolite extraction.
Metabolites were extracted as previously described [26]. Briefly, for each sample, cell pellet was collected from 10 mL culture through centrifugation at 3,220 × g at 4°C for 10 min. Cell pellet was then washed twice with cold saline (0.9%) and resuspended in 500 μL chloroform:methanol:water (1:3:0.9, v/v) containing four 1-μM generic internal standards (i.e. CHAPS, CAPS, PIPES and TRIS, Sigma-Aldrich, Castle Hill, Australia). Samples were immediately frozen in liquid nitrogen and thawed on ice to release intracellular metabolites. Subsequently, cell debris was removed by centrifugation at 14,000 × g at 4°C for 10 min and 200 μL particle-free supernatant was transferred to an injection vial for liquid chromatography-mass spectrometry (LC-MS/MS) analysis [26]. Pooled biological quality control (PBQC) samples were prepared by mixing 10 μL of each tested sample and processed as outline above.
2.2.3. LC-MS/MS analysis of metabolites.
Metabolite samples were analyzed with a Q-Exactive Orbitrap mass spectrometer (Thermo Fisher), coupled to a Dionex high-performance liquid chromatograph (U3000 RSLC HPLC, Thermo Fisher) with a ZIC-pHILIC column (5 μm, polymeric, 150 × 4.6 mm; SeQuant, Merck) maintained at 25°C. The MS system was operated in both positive and negative electron spray ionization [27] mode at 35,000 resolution with a detection range from 85 to 1,275 m/z. The LC mobile phase solutions were (A) 20 mM ammonium carbonate, and (B) acetonitrile, with a flow rate of 0.3 mL/min. Metabolite samples (10 μL) were eluted in a step-gradient from 80% solvent B to 50% solvent B in 15 min, then reduced to 5% solvent B during the next 3 min. This was followed by a washing step with 5% solvent B for 3 min, and a final 8-min re-equilibration with 80% solvent B [25, 28]. Samples were randomized before analysis in a single LC-MS/MS batch to minimize operation variation, and a mixture of >250 authentic standards was used to assist in metabolite identification.
2.3. Data processing and bioinformatic analysis
The raw LC-MS data were processed using mzMatch and IDEOM (http://mzmatch.sourceforge.net/ideom.php) [29]. Briefly, raw data were converted to mzXML using ProteoWizard and mzMatch was subsequently used to align and filter peaks based on the intensity (>100,000 counts), shape (codadw) of >0.8 and relative standard deviation (RSD) of <0.5 (reproducibility) [30]. Putative metabolites were identified based on LC retention time and m/z value. Peak intensities were normalized by the median, log transformed and scaled (by auto-scale function) using MetaboAnalyst 4.0 [31]. Principal component analysis (PCA) was applied for all four groups at each time point. One-way ANOVA was used to determine significantly changed metabolites with false discovery rate (FDR) < 0.05 and fold change (FC) ≥2 (i.e. log2FC ≥ 1 or ≤ −1). Pathway analysis was performed using KEGG (Kyoto Encyclopedia of Genes and Genomes) and BioCyc databases [32, 33].
3. Results
3.1. Metabolic profiles of A. baumannii AB5075 treated with polymyxin B, rifampicin alone and in combination
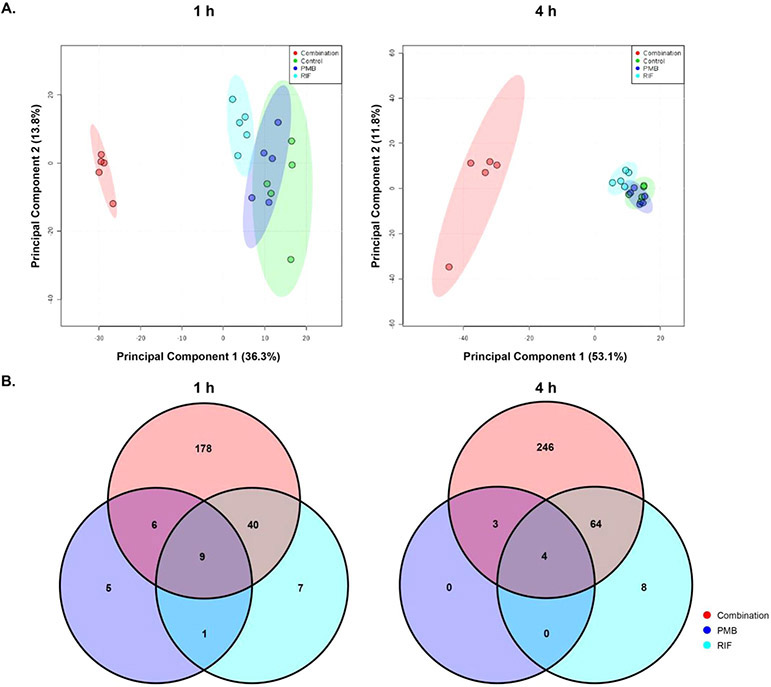
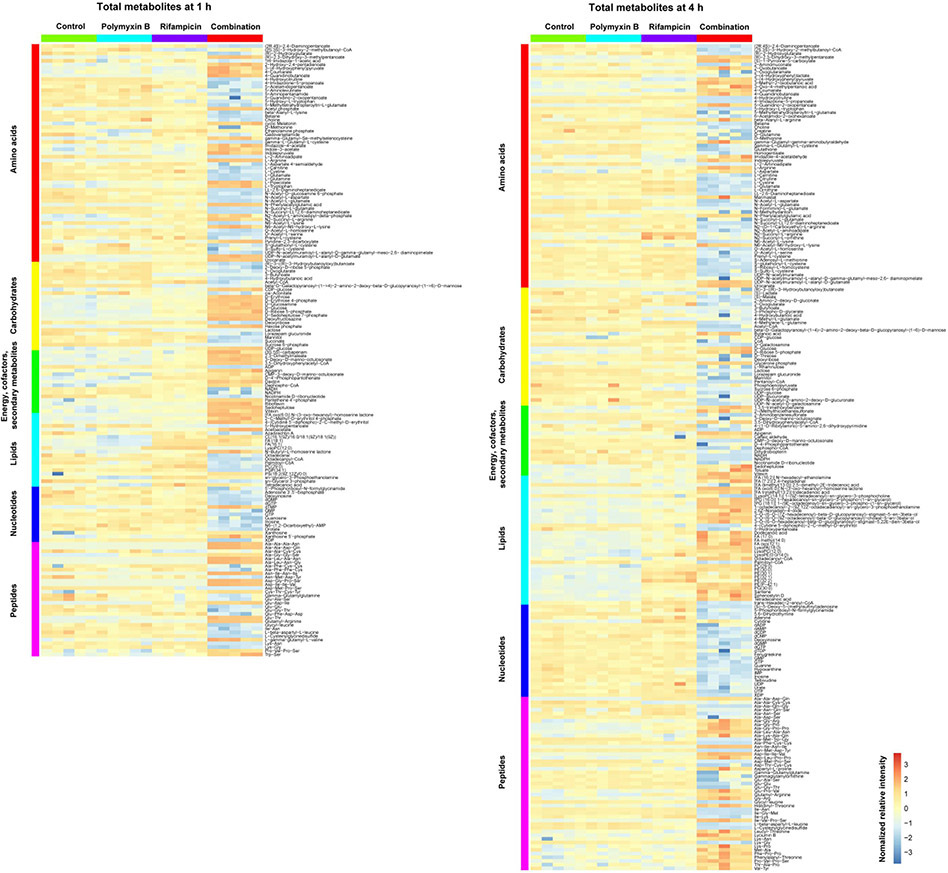
Across all conditions, a total of 858 putatively identified metabolites were obtained, including: 56 metabolites in central carbon metabolism; 153 metabolites in amino acid metabolism; 51 metabolites in nucleotide metabolism and 163 in phospholipid metabolism. Among them, 477 metabolites were mapped to the KEGG pathways, representing a 55.6% coverage of the total predicted AB5075 metabolic pathways. The median relative standard deviations (RSDs) of the samples varied in 16–32%, which were within the acceptable limits for metabolomic studies [26]. ANOVA and PCA results showed that polymyxin B and rifampicin alone induced minor metabolic changes, while the combination significant changed the abundance of 233 and 317 metabolites at 1 and 4 h, respectively (Fig. 1A and B). A large proportion of the significantly changed metabolites were from metabolism of amino acids, carbohydrates, energy, lipids, nucleotides, and peptides (Fig. 2).
Figure 1. Metabolomic analyses of MDR A. baumannii AB5075 treated with polymyxin B (PMB) alone, rifampicin (RIF) alone and in combination (PMB/RIF) at 1 and 4 h.
(A) Principal component analysis (PCA) plots of the first two components indicate the metabolic changes in AB5075 with the treatments of polymyxin B (blue), rifampicin (cyan) and combination (red) at 1 h (left) and 4 h (right). (B) Venn diagrams of the significantly perturbed metabolites in AB5075 with polymyxin B (blue), rifampicin (cyan) and combination (red) treatments at 1 h and 4 h. Significantly perturbed metabolites were selected based on log2FC ≤ −1 or ≥ 1 and FDR < 0.05.
Figure 2. Heatmap profiles of the relative abundance of metabolites in A. baumannii AB5075 treated with polymyxin B alone (PMB), rifampicin alone (RIF) and the combination at 1 (left) and 4 h (right).
Total metabolites are grouped into different classes: amino acids, carbohydrates, energy, cofactors and secondary metabolites, lipids, nucleotides and peptides.
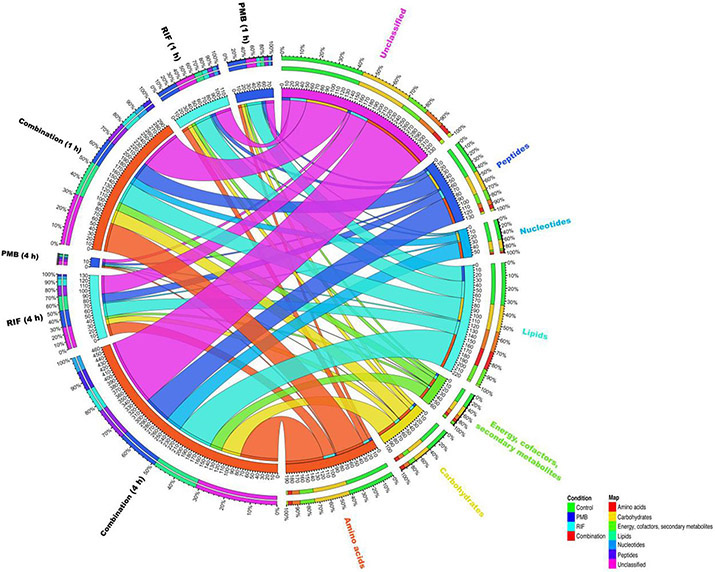
Pathway analyses revealed that multiple metabolic pathways were significantly perturbed by polymyxin B and rifampicin alone and in combination. In detail, at 1 and 4 h rifampicin alone significantly perturbed lipid and nucleotide metabolism; whereas at 1 h polymyxin B treatment alone mostly impacted phospholipid metabolism. Compared to each treatment per se, the combination significantly perturbed a greater number of metabolic pathways, including nucleotide biosynthesis, amino acid metabolism, phospholipid metabolism, fatty acid metabolism and central carbon metabolism (Fig. 3).
Figure 3. Bipartite graph connects different treatment groups with significantly altered metabolites of each major class in A. baumannii AB5075.
Total metabolites are grouped into different classes: amino acids, carbohydrates, energy, cofactors and secondary metabolites, lipids, nucleotides and peptides.
3.2. Polymyxin B, rifampicin alone and the combination significantly perturbed phospholipid and glycerophospholipid metabolism
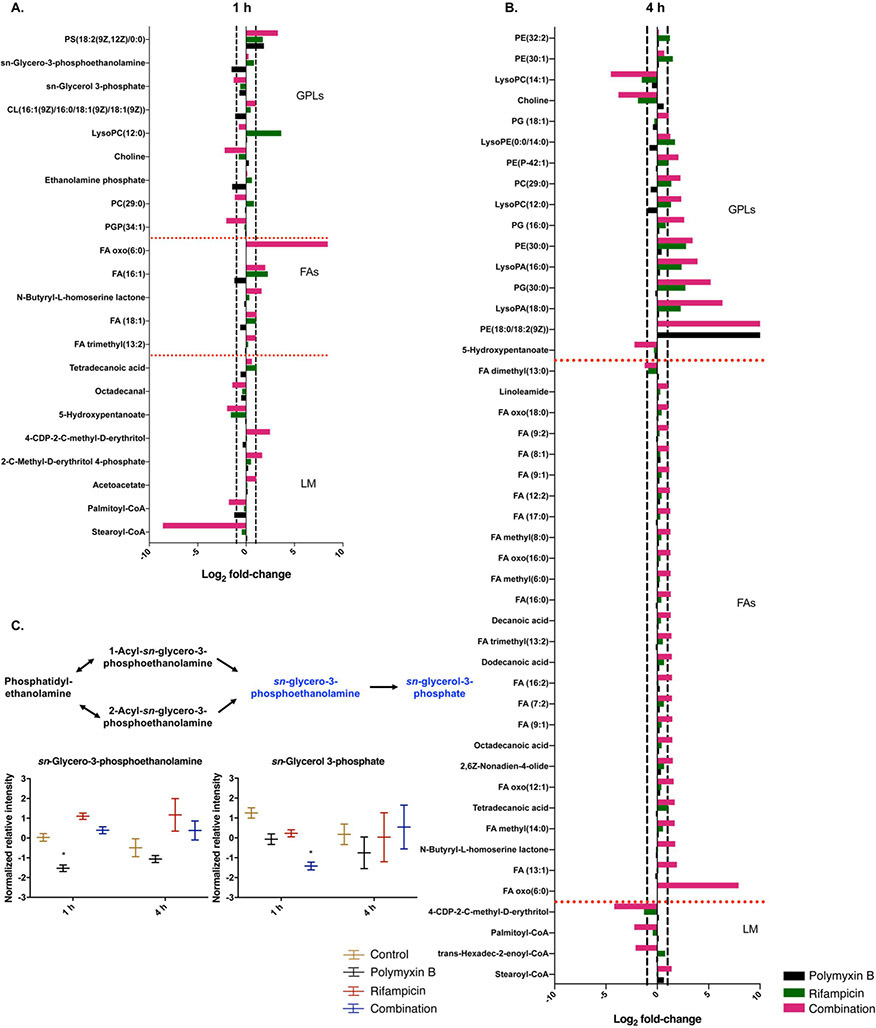
At 1 h, the combination significantly altered the levels of glycerophospholipids and fatty acids. Among them, phosphatidylethanolamine (PE [32:0]), phosphatidylglyerophosphate (PGP [34:1]) octadecanal, 5-hydroxypentanoate, sn-glycerol 3-phosphate and palmitoyl-CoA were decreased (log2FC = −2.05 to −1.17) by the combination. For polymyxin B alone, the levels of cardiolipin (CL [68:3]), sn-glycero-3-phosphoethanolamine and palmitoyl-CoA were significantly decreased (log2FC = −1.50 to −1.13) at 1 h; whereas the level of phosphatidylserine (PS [18:2]) (log2FC = 1.84 ) was increased at 1 h. Rifampicin alone resulted in increases (log2FC = 1.72 to 3.64) of the levels of PS [18:2], FA [16:1] and lysoPE [15:0], while the level of 5-hydroxypentanoate (log2FC = −1.57) was significantly decreased at 1 h (Fig. 4A, C).
Figure 4. Perturbations of lipids in A. baumannii AB5075 treated with polymyxin B alone, rifampicin alone and the combination at 1 and 4 h.
Bar charts show the significantly perturbed lipids in A. baumannii AB5075 following polymyxin B (black), rifampicin (green) and the combination (red) treatments at 1 and 4 h. Schematic diagram and box plots show the differences of normalized relative intensity of the metabolites in lipid biosynthesis pathways in the groups of control (yellow), polymyxin B (black), rifampicin (red) and the combination (blue) at 1 and 4 h. Significantly perturbed metabolites (log2FC ≤ −1 or ≥ 1 and FDR < 0.05) are shown in blue in the schematic diagram or marked with *.
At 4 h, significant increases of glycerophospholipid and fatty acid levels were observed by the combination, while levels of other associated metabolites were significantly decreased (log2FC = −4.53 to −2.12), including lysoPE [17:1], 5-hydroxypentanoate, 4-(cytidine 5′-diphospho)-2-C-methyl-D-erythritol, palmitoyl-CoA and trans-hexadec-2-enoyl-CoA. Rifampicin alone also induced significant changes in glycerophospholipids, including the increased (log2FC = 2.37 to 2.80) levels of PE [30:0], PG [30:0], lysoPA [16:0] and lysoPA [18:0]; and decreased levels of lysoPC [14:1] (log2FC = −1.51) and 4-(cytidine 5′-diphospho)-2-C-methyl-D-erythritol (log2FC = −1.30). Whereas polymyxin B alone had minimal effects on glycerophospholipids and fatty acid metabolisms at 4 h (Fig. 4B).
3.3. Rifampicin alone and its combination with polymyxin B significantly perturbed nucleotide metabolism
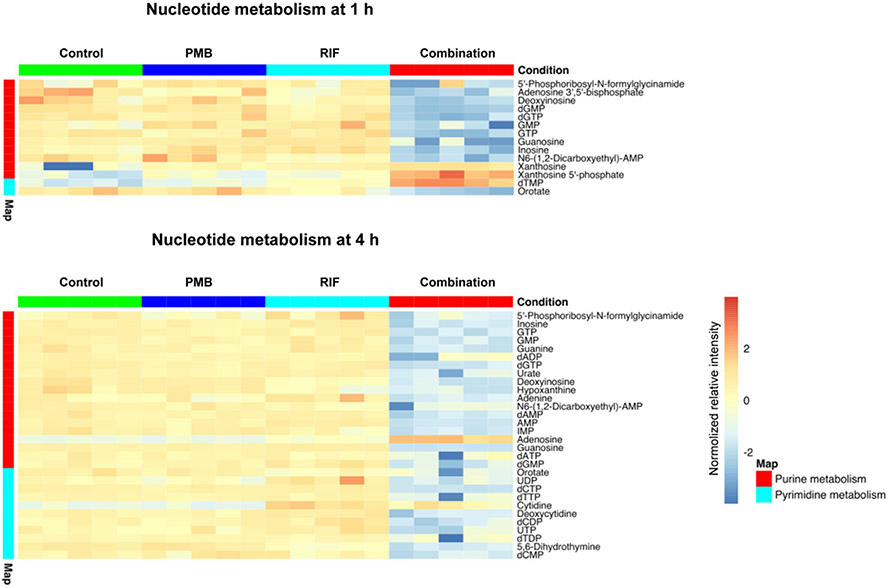
Nucleotide metabolism was significantly perturbed by the combination at both 1 and 4 h. At 1 h, in the pyrimidine metabolism the level of deoxythymidine monophosphate (dTMP) was significantly increased (log2FC = 1.07), whereas orotate was significantly depleted by the combination (log2FC = −3.60). Whereas, most intermediate metabolites of purine biosynthesis were depleted (log2FC = −5.49 to −1.04) by the combination, with the exception of xanthosine and xanthosine 5′-phosphate which were significantly increased (log2FC = 4.12 and 2.33, respectively) (Fig. 5). Interestingly, the level of xanthosine was also increased (log2FC = 3.07 and 3.22) under polymyxin B and rifampicin monotherapy at 1 h, respectively. At 4 h, it is notable that more metabolites from both pyrimidine and purine biosynthesis were significantly decreased by the combination compared to 1 h. Specifically, at 4 h, 10 metabolites from pyrimidine biosynthesis were significantly decreased by the combination, whereas only orotate was decreased by the combination at 1 h. The level of 5,6-dihydrothymine was decreased (log2FC = −1.02) by rifampicin alone at 4 h, while the level of cytidine was increased (log2FC = 1.16).
Figure 5. Heat map profiles of relative abundance of significantly perturbed metabolites in nucleotide metabolism in A. baumannii AB5075 at 1 h and 4 h.
Data represent normalized log2-transformed raw intensity. Significant metabolites were identified by FDR < 0.05 and log2FC ≥ 1 or ≤ −1.
3.4. Polymyxin B in combination with rifampicin significantly perturbed central carbon metabolism and inhibited cell wall biosynthesis, energy, amino acid and peptide metabolism
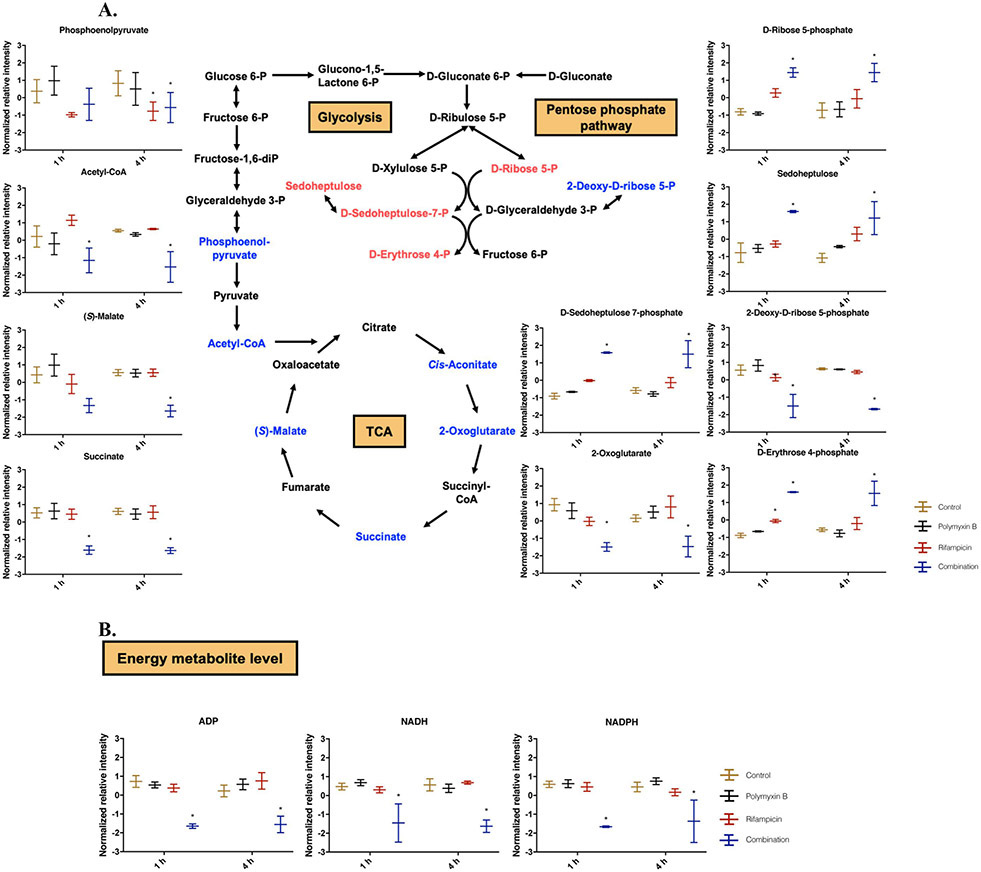
Several unique pathways were significantly perturbed only by the combination. Polymyxin B in combination with rifampicin significantly perturbed central carbon metabolism at 1 and 4 h (Fig. 6A). The levels of intermediate metabolites in TCA cycle (including acetyl-CoA, S-malate, succinate and 2-oxoglutarate) were significantly reduced by the combination at 1 and 4 h (Fig. 6A). Notably, the level of acetyl-CoA was drastically reduced (log2FC = −7.65) by the combination at 4 h. In glycolysis, rifampicin alone and the combination reduced the level of phosphoenolpyruvate (log2FC = −1.08 and −1.10) at 4 h, respectively. At the examined concentration, polymyxin B alone exerted a minimal impact on glycolysis and TCA cycle at both 1 and 4 h.
Figure 6. Perturbations of central carbon metabolism in A. baumannii AB5075 treated with polymyxin B alone, rifampicin alone and the combination at 1 and 4 h.
(A) Schematic diagram and box plots show the significantly perturbed intermediates of PPP, glycolysis and TCA cycle in A. baumannii AB5075. Significantly perturbed metabolites (log2FC ≤ −1 or ≥ 1 and FDR < 0.05) are colored blue (decrease) or red (increase) in the schematic diagram and marked with * in box plot. (B) Box plots for the main depleted energy metabolites in A. baumannii AB5075 in the groups of control (yellow), polymyxin B (black), rifampicin (red) and the combination (blue). Significantly perturbed metabolites (log2FC ≤ −1 or ≥ 1 and FDR < 0.05) are marked with *.
In pentose phosphate pathway (PPP) (Fig. 6A), at 1 and 4 h the combination significantly increased the levels of D-erythrose 4-phosphate (log2FC = 3.51 and 1.02, respectively), D-ribose 5-phosphate (log2FC = 1.69 and 1.06, respectively), and D-sedoheptulose 7-phosphate (log2FC = 3.08 and 1.03, respectively). D-ribose 5-phosphate is an essential precursor for nucleotide biosynthesis and D-erythrose 4-phosphate is the precursor of aromatic amino acid biosynthesis. Rifampicin alone only slightly increased the level of D-erythrose 4-phosphate (log2FC = 1.04) at 1 h. In addition to central carbon metabolism, the key metabolites related to energy metabolism were significantly depleted by the combination at 1 and 4 h, including ADP (log2FC = −1.54 and −1.40, respectively), NADH (log2FC = −6.46 and −8.38, respectively) and NADPH (log2FC = −4.69 and −7.52, respectively) (Fig. 6B).
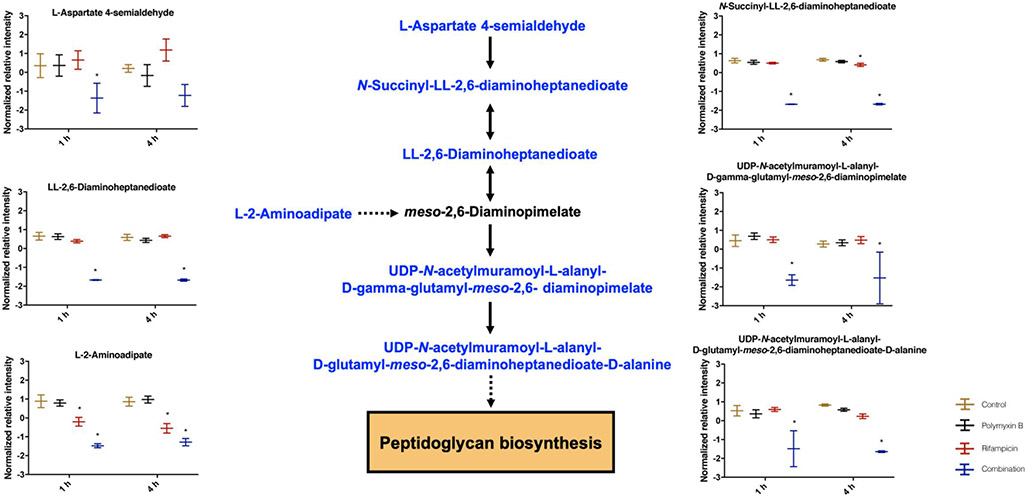
Different from each antibiotic alone, the combination remarkably depleted a number of key intermediate metabolites in cell wall biosynthesis at 1 and 4 h (Fig. 7). Specifically, the significantly depleted metabolites include L-aspartate 4-semialdehyde (log2FC = −1.11 and −0.97, 1 h and 4 h , respectively), N-succinyl-LL-2,6-diaminoheptanedioate (log2FC = −9.55 and −9.22), LL-2,6-diaminoheptanedioate (log2FC = −6.33 and −5.57), L-2-aminoadipate (log2FC = −2.19 and −3.93), UDP-N-acetylmuramoyl-L-alanyl-D-gamma-glutamyl-meso-2,6-diaminopimelate (log2FC = −2.62 and −2.46) and UDP-N-acetylmuramoyl-L-alanyl-D-glutamyl-meso-2,6-diaminoheptanedioate-D-alanine (log2FC = −4.68 and −8.27).
Figure 7. Perturbations of peptidoglycan biosynthesis in A. baumannii AB5075 treated with polymyxin B alone, rifampicin alone and the combination at 1 and 4 h.
Schematic diagram and box plots show the significantly perturbed intermediates of peptidoglycan biosynthesis in A. baumannii AB5075. Significantly perturbed metabolites (log2FC ≤ −1 or ≥ 1 and FDR < 0.05) are colored blue in the schematic diagram or marked with * in the box plot.
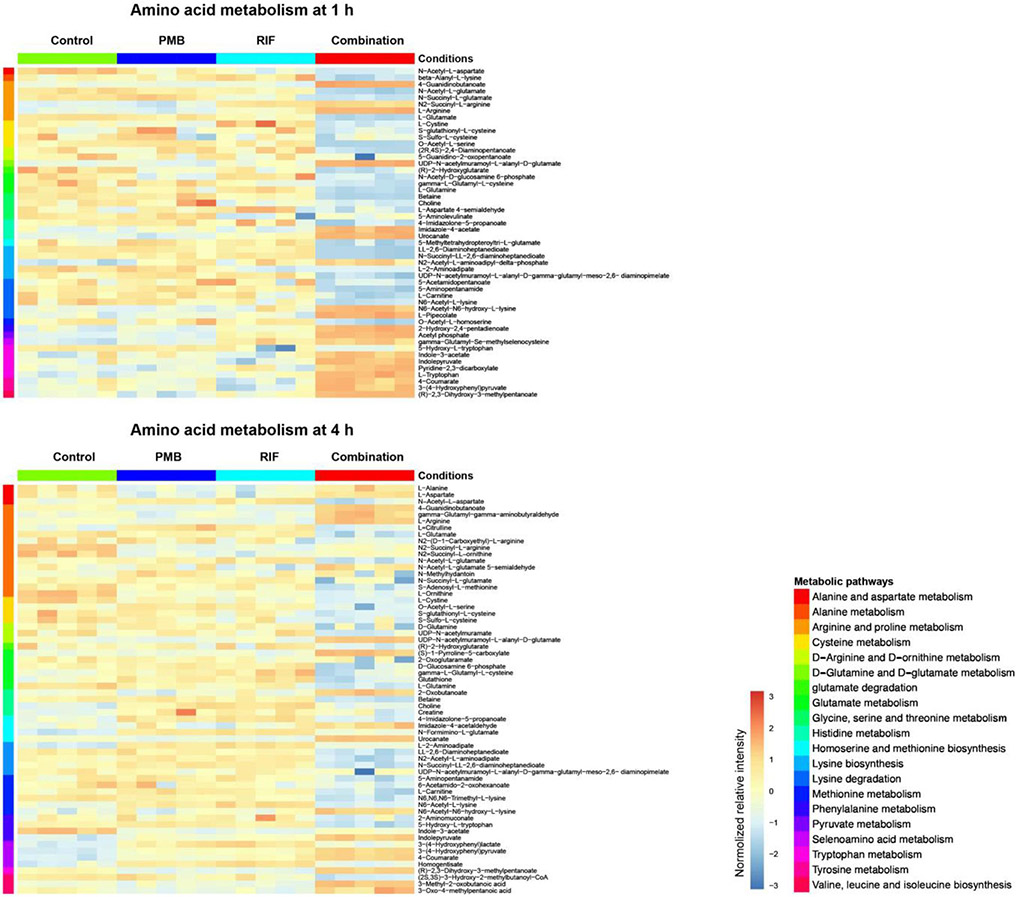
Polymyxin B in combination with rifampicin significantly perturbed amino acid and peptide levels at 1 and 4 h. Specifically, the combination significantly increased the levels of tryptophan (log2FC = 1.11, 1 h) and arginine (log2FC = 1.31 and 1.53, at 1 and 4 h, respectively). On the other hand, intermediate metabolites involved in the degradation of lysine, histidine, tryptophan, cysteine, arginine and proline were significantly depleted by the combination at 1 h (Fig. 8). At 4 h, 62 key metabolites in amino acid metabolism were significantly affected by the combination treatment. At the examined concentrations, both antibiotics alone had minimal impacts on amino acid metabolism at both time points. Rifampicin alone only perturbed 12 and 17 metabolites in amino acid metabolism at 1 and 4 h, respectively; whereas polymyxin B alone perturbed only 3 metabolites at 1 h and nil at 4 h.
Figure 8. Heat map profiles of relative abundance of significantly perturbed metabolites in amino acid metabolism in A. baumannii AB5075 at 1 h and 4 h.
Data represent normalized log2-transformed raw intensity. Significant metabolites were identified by FDR < 0.05 and log2FC ≥ 1 or ≤ −1.
4. Discussion
The emergence of resistance to last-resort polymyxins after monotherapy is frequently reported [34, 35]; thus, polymyxin combination therapy has been increasingly used in the clinic considering the pharmacokinetics/pharmacodynamics (PK/PD) [36, 37]. Among polymyxin combination therapies, the combination with rifampicin has been shown to produce highly bacterial killing and prevent the development of resistance in vitro [20]. Although use of the combination of polymyxin and rifampicin in patients is largely empirical, it is still highly effective against severe infections caused by MDR A. baumannii in the clinic [17, 20, 38]. Understanding the mechanism of this synergistic combination against A. baumannii is essential for optimizing this combination clinically. LC-MS/MS based metabolomics has been utilized to investigate metabolic changes in paired polymyxin-susceptible and -resistant A. baumannii strains in response to synergistic killing by polymyxin combinations with other antibiotics and non-antibiotic drugs [26, 39-41]. Synergistic activities of different antibiotic combinations are often explained by the parallel pathway inhibition model (i.e. both antibiotics inhibit different targets in parallel pathways) and the bioavailability model (i.e. action of one drug enhances the effect of the other drug on the target) [41, 42]. For example, the synergistic activity of colistin and doripenem/sulbactam against A. baumannii was due to the parallel pathway inhibition [39, 41]; while the bioavailability model explains the synergistic activity of polymyxin B and rifampicin against A. baumannii, which is well supported by our metabolomics data here. To the best of our knowledge, the present study is the first to demonstrate that the time-dependent synergistic killing of polymyxin B in combination with rifampicin, against A. baumannii, was initially driven by polymyxin B and subsequently by rifampicin.
Rifampicin is widely used to treat Mycobacterium infections and its average steady-state plasma concentration (Css, avg) varies from 8.2 mg/L to 22 mg/L in humans [43]. Over the last decade, polymyxins have become a last-line therapy for severe infections caused by MDR A. baumannii and the target average steady-state plasma concentration of polymyxin B is 2-4 mg/L [15]. Considering the plasma protein binding and average steady-state concentrations of both antibiotics in patients [24, 44], rifampicin at 1 mg/L and polymyxin B at 0.75 mg/L (3 × MIC) were examined in the present study to ensure clinical relevance [15, 43]. With 108 CFU/ml bacterial inoculum, neither polymyxin B (0.75 mg/L) nor rifampicin (1 mg/L) showed a killing effect on A. baumannii AB5075; while the combination exhibited a significantly synergistic killing at 1 and 4 h. A limitation of our study is that only one clinically-relevant concentration of each antibiotic was examined in this metabolomic study.
The results showed distinct metabolomic profiles by the combination compared to each antibiotic alone at both time points (Fig. 1). Metabolic pathway analyses proved that the synergistic killing of polymyxin B in combination with rifampicin was attributed to the perturbations of key metabolic pathways, including lipid, carbohydrate, nucleotide, energy and amino acid metabolism (Figs. 1, 2 and 3). Following 1-h treatment with polymyxin B alone and its combination, a large number of cellular metabolites associated with lipid metabolism; particularly fatty acids and phospholipids were significantly perturbed (Fig. 4). These findings are in line with the primary mode of action of polymyxins via disrupting the Gram-negative outer membrane, resulting in increased permeability and phospholipid exchange [4]. Specifically, following polymyxin B treatment for 1 h, sn-glycero-3-phosphoethanolamine and sn-glycerol-3-phosphate were significantly depleted. Both sn-glycero-3-phosphoethanolamine and sn-glycerol-3-phosphate are crucial intermediates in the synthesis of outer membrane glycerophospholipids [45]. Rifampicin is a hydrophobic antibiotic that is unable to easily penetrate Gram-negative outer membrane. Not surprisingly, rifampicin monotherapy resulted in minor metabolic perturbations as shown by PCA plots and Venn diagrams (Fig. 1); only a limited number of significantly changed metabolites in fatty acid and lipid metabolism were identified at 1 h. These results are consistent with the limited bacterial killing of rifampicin against A. baumannii AB5075. Overall, these findings demonstrate that the synergistic antimicrobial activity by the combination at 1 h was mainly driven by polymyxin B and is consistent with previous studies showing that polymyxins significantly disturbed gene expression and metabolites in fatty acid and lipid metabolism [41, 46].
Notably, polymyxin B in combination with rifampicin significantly perturbed nucleotide metabolism and the levels of nucleotides, which is in agreement with the mode of action of rifampicin, i.e. inhibition of bacterial RNA synthesis [47, 48]. Nucleotides are essential for energy transfer, RNA, DNA, lipid and protein biosynthesis [49, 50]. A number of purine metabolites were significantly decreased by the combination at 1 and 4 h, while the depletion of pyrimidine metabolites was only evident at 4 h (Fig. 5). A previous study showed that rifampicin significantly perturbed the levels of nucleotides in Staphylococcus aureus [51], which is consistent with our observations in A. baumannii (Fig. 5). We purport that A. baumannii cells were metabolically inactive owing to the inhibition of RNA biosynthesis by rifampicin. In support of this, a significantly enrichment in the levels of D-ribose 5-phosphate in PPP (a key precursor in nucleotide metabolism) was observed in the combination group (Fig. 6). As the outer membrane damage caused by polymyxin B led to the increased intracellular concentration of rifampicin [52], it was very likely that rifampicin was the driving force of the nucleotide metabolism perturbations at both time points.
In addition to disrupting nucleotide metabolism, several unique metabolic perturbations were also observed by the combination, including peptidoglycan biosynthesis, carbohydrate, energy and amino acid metabolism. In the biosynthesis of the peptidoglycan, meso-diaminopimelate is conjugated with UDP-N-acetylmuramoyl-L-alanyl-D-glutamate in the cytoplasm to form UDP-N-acetylmuramoyl-L-alanyl-γ-D-glutamyl-meso-2-6-diaminopimelate [53]. In response to the decreased peptidoglycan caused by the combination, intracellular choline levels were significantly increased to counteract the increased intracellular pressure from osmotic balance [54]. This is consistent with our previous findings in A. baumannii that choline is necessary to counteract imbalances of osmotic pressure [40, 46]. TCA cycle provides the bacterial cell with energy, reducing power and precursors for amino acid and nucleotide biosynthesis [55]. It is reported that antibiotic-induced bacterial death is mainly due to the altered cellular respiration and central metabolism via carbon flux in the TCA cycle [51]. Six key metabolites (i.e. phosphoenol-pyruvate, acetyl-CoA, cis-aconitate, 2-oxoglutarate, succinate, S-malate) in glycolysis and TCA cycle were significantly reduced by the combination (Fig. 6A). The levels of ADP, NADH and NADPH also significantly declined after 1 and 4 h in the combination group (Fig. 6B). Compared to the untreated control and each antibiotic alone, the lower abundances of TCA cycle intermediates and energy metabolism by the combination indicate that the inhibition of energy production also plays a key role in the synergistic killing. In addition, the combination also severely depleted amino acid and peptide levels (Figs. 2 and 8). These effects might be attributed to the inhibition of nucleotide and carbohydrate metabolism, as a result of the inhibition of RNA synthesis by rifampicin.
Overall, our findings demonstrated that the initial synergistic killing activity of polymyxin B-rifampicin combination was largely driven by the former which disrupted the outer membrane and facilitated the entry of rifampicin into bacteria. Subsequently, the synergistic activity was dominated by rifampicin which perturbed purine and pyrimidine nucleotide metabolism. Importantly, the combination synergistically perturbed peptidoglycan biosynthesis and central carbon metabolism, which is unique to the combination rather than the distinctive primary mode of action of either antibiotic. Our quantitative mechanistic data will facilitate mechanism-based PK/PD modelling, which provides a systems approach to optimize the synergistic combination of polymyxins and rifampicin against polymyxin-susceptible MDR A. baumannii in clinical practice.
Acknowledgements
The authors appreciate the technical support from Associate Professor Darren Creek and Monash Proteomics and Metabolomics Facility. The authors also appreciate the language editing assistance from Dr Phillip Bergen. J.L, Q.T.Z. and T.V. are supported by a research grant from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01 AI132681). J.L. is an Australian NHMRC Principal Research Fellow and T.V. is an Australian NHMRC Industry Career Development Level 2 Research Fellow. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
There is no conflict of interest to declare.
References
- [1].Antunes L, Visca P, Towner KJ. Acinetobacter baumannii: evolution of a global pathogen. Pathogens and Disease. 2014;71(3):292–301. 10.1111/2049-632x.12125. [DOI] [PubMed] [Google Scholar]
- [2].Willyard C The drug-resistant bacteria that pose the greatest health threats. Nature News. 2017;543(7643):15. 10.1038/nature.2017.21550. [DOI] [PubMed] [Google Scholar]
- [3].Asokan GV, Vanitha A. WHO global priority pathogens list on antibiotic resistance: an urgent need for action to integrate one health data. Perspectives in Public Health. 2018;138(2):87–88. 10.1177/1757913917743881. [DOI] [PubMed] [Google Scholar]
- [4].Velkov T, Roberts KD, Nation RL, Thompson PE, Li J. Pharmacology of polymyxins: new insights into an'old'class of antibiotics. Future Microbiology. 2013;8(6):711–724. 10.2217/fmb.13.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infectious Diseases. 2006;6(9):589–601. 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- [6].Zavascki AP, Goldani LZ, Li J, Nation RL. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. Journal of Antimicrobial Chemotherapy. 2007;60(6):1206–1215. 10.1093/jac/dkm357. [DOI] [PubMed] [Google Scholar]
- [7].Lim LM, Ly N, Anderson D, Yang JC, Macander L, Jarkowski A III, Forrest A, Bulitta JB, Tsuji BT. Resurgence of colistin: a review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacotherapy. 2010;30(12):1279–1291. 10.1592/phco.30.12.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Poirel L, Jayol A, Nordmann P. Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clinical Microbiology Reviews. 2017;30(2):557–596. 10.1128/CMR.00064-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Landman D, Georgescu C, Martin DA, Quale J. Polymyxins revisited. Clinical Microbiology Reviews. 2008;21(3):449–465. 10.1128/CMR.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Velkov T, Roberts KD, Nation RL, Wang J, Thompson PE, Li J. Teaching ‘old’ polymyxins new tricks: new-generation lipopeptides targeting Gram-negative ‘superbugs’. ACS Chemical Biology. 2014;9(5):1172. 10.1021/cb500080r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Velkov T, Thompson PE, Nation RL, Li J. Structure-activity relationships of polymyxin antibiotics. Journal of Medicinal Chemistry. 2009;53(5):1898–1916. 10.1021/jm900999h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jiang X, Yang K, Yuan B, Han M, Zhu Y, Roberts KD, Patil NA, Li J, Gong B, Hancock RE. Molecular dynamics simulations informed by membrane lipidomics reveal the structure𠀓interaction relationship of polymyxins with the lipid A-based outer membrane of Acinetobacter baumannii. Journal of Antimicrobial Chemotherapy. 2020. 10.1093/jac/dkaa376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Moffatt JH, Harper M, Boyce JD. Mechanisms of polymyxin resistance. In. Polymyxin Antibiotics: From Laboratory Bench to Bedside: Springer; 2019. p. 55–71. 10.1007/978-3-030-16373-0_5. [DOI] [PubMed] [Google Scholar]
- [14].Pogue JM, Cohen DA, Marchaim D. Editorial commentary: Polymyxin-resistant Acinetobacter baumannii: urgent action needed. Clinical Infectious Diseases. 2015;60(9):1304. 10.1093/cid/civ044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, Giacobbe DR, Viscoli C, Giamarellou H, Karaiskos I. International consensus guidelines for the optimal use of the polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy. 2019;39(1):10–39. 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Park HJ, Cho JH, Kim HJ, Han SH, Jeong SH, Byun MK. Colistin monotherapy versus colistin/rifampicin combination therapy in pneumonia caused by colistin-resistant Acinetobacter baumannii: a randomised controlled trial. Journal of Global Antimicrobial Resistance. 2019;17:66–71. 10.1016/j.jgar.2018.11.016. [DOI] [PubMed] [Google Scholar]
- [17].Aydemir H, Akduman D, Piskin N, Comert F, Horuz E, Terzi A, Kokturk F, Ornek T, Celebi G. Colistin vs. the combination of colistin and rifampicin for the treatment of carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia. Epidemiology and Infection. 2013; 141(6): 1214–1222. 10.1017/S095026881200194X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bassetti M, Repetto E, Righi E, Boni S, Diverio M, Molinari M, Mussap M, Artioli S, Ansaldi F, Durando P. Colistin and rifampicin in the treatment of multidrug-resistant Acinetobacter baumannii infections. Journal of Antimicrobial Chemotherapy. 2008;61(2):417–420. 10.1093/jac/dkm509. [DOI] [PubMed] [Google Scholar]
- [19].Motaouakkil S, Charra B, Hachimi A, Nejmi H, Benslama A, Elmdaghri N, Belabbes H, Benbachir M. Colistin and rifampicin in the treatment of nosocomial infections from multiresistant Acinetobacter baumannii. Journal of Infection. 2006;53(4):274–278. 10.1016/j.jinf.2005.11.019 [DOI] [PubMed] [Google Scholar]
- [20].Lee HJ, Bergen PJ, Bulitta JB, Tsuji B, Forrest A, Nation RL, Li J. Synergistic activity of colistin and rifampin combination against multidrug-resistant Acinetobacter baumannii in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrobial Agents and Chemotherapy. 2013;57(8):3738–3745. 10.1128/AAC.00703-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bratu S, Tolaney P, Karumudi U, Quale J, Mooty M, Nichani S, Landman D. Carbapenemase-producing Klebsiella pneumoniae in Brooklyn, NY: molecular epidemiology and in vitro activity of polymyxin B and other agents. Journal of Antimicrobial Chemotherapy. 2005;56(1):128–132. 10.1093/jac/dki175. [DOI] [PubMed] [Google Scholar]
- [22].Lim T-P, Tan T-Y, Lee W, Sasikala S, Tan T-T, Hsu L-Y, Kwa AL. In-vitro activity of polymyxin B, rifampicin, tigecycline alone and in combination against carbapenem-resistant Acinetobacter baumannii in Singapore. PLoS One. 2011;6(4):e18485. 10.1371/journal.pone.0018485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jacobs AC, Thompson MG, Black CC, Kessler JL, Clark LP, McQueary CN, Gancz HY, Corey BW, Moon JK, Si Y. AB5075, a highly virulent isolate of Acinetobacter baumannii, as a model strain for the evaluation of pathogenesis and antimicrobial treatments. mBio. 2014;5(3):e01076–01014. 10.1128/mBio.01076-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhão RC, Wang J, Forrest A. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clinical Infectious Diseases. 2013;57(4):524–531. 10.1093/cid/cit334. [DOI] [PubMed] [Google Scholar]
- [25].Ruslami R, Nijland HM, Alisjahbana B, Parwati I, van Crevel R, Aarnoutse RE. Pharmacokinetics and tolerability of a higher rifampin dose versus the standard dose in pulmonary tuberculosis patients. Antimicrobial Agents and Chemotherapy. 2007;51(7):2546–2551. 10.1128/AAC.01550-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tran TB, Bergen PJ, Creek DJ, Velkov T, Li J. Synergistic killing of polymyxin B in combination with the antineoplastic drug mitotane against polymyxin-susceptible and-resistant Acinetobacter baumannii: A metabolomic study. Frontiers in Pharmacology. 2018;9:359. 10.3389/fphar.2018.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mulani MS, Kamble EE, Kumkar SN, Tawre MS, Pardesi KR. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Frontiers in Microbiology. 2019;10:539. 10.3389/fmicb.2019.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang T, Creek DJ, Barrett MP, Blackburn G, Watson DG. Evaluation of coupling reversed phase, aqueous normal phase, and hydrophilic interaction liquid chromatography with Orbitrap mass spectrometry for metabolomic studies of human urine. Analytical Chemistry. 2012;84(4):1994–2001. 10.1021/ac2030738. [DOI] [PubMed] [Google Scholar]
- [29].Creek DJ, Jankevics A, Burgess KE, Breitling R, Barrett MP. IDEOM: an Excel interface for analysis of LC–MS-based metabolomics data. Bioinformatics. 2012;28(7):1048–1049. 10.1093/bioinformatics/bts069. [DOI] [PubMed] [Google Scholar]
- [30].Scheltema RA, Jankevics A, Jansen RC, Swertz MA, Breitling R. PeakML/mzMatch: a file format, Java library, R library, and tool-chain for mass spectrometry data analysis. Analytical Chemistry. 2011;83(7):2786–2793. 10.1021/ac2000994. [DOI] [PubMed] [Google Scholar]
- [31].Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, Wishart DS, Xia J. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic acids research. 2018. 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Research. 2016;45(D1):D353–D361. 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Karp PD, Billington R, Caspi R, Fulcher CA, Latendresse M, Kothari A, Keseler IM, Krummenacker M, Midford PE, Ong Q. The BioCyc collection of microbial genomes and metabolic pathways. Briefings in Bioinformatics. 2017. 10.1093/bib/bbx085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhao M, Bulman ZP, Lenhard JR, Satlin MJ, Kreiswirth BN, Walsh TJ, Marrocco A, Bergen PJ, Nation RL, Li J. Pharmacodynamics of colistin and fosfomycin: a ‘treasure trove’combination combats KPC-producing Klebsiella pneumoniae. Journal of Antimicrobial Chemotherapy. 2017;72(7):1985–1990. 10.1093/jac/dkx070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schneider EK, Azad MA, Han M-L, Zhou Q, Wang J, Huang JX, Cooper MA, Doi Y, Baker MA, Bergen PJ. An “unlikely” pair: the antimicrobial synergy of polymyxin B in combination with the cystic fibrosis transmembrane conductance regulator drugs KALYDECO and ORKAMBI. ACS Infectious Diseases. 2016;2(7):478–488. 10.1021/acsinfecdis.6b00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nation RL, Velkov T, Li J. Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clinical Infectious Diseases. 2014;59(1):88–94. 10.1093/cid/ciu213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cai B, Cai Y, Liew YX, Chua NG, Teo JQ-M, Lim T-P, Kurup A, Ee PLR, Tan TT, Lee W. Clinical efficacy of polymyxin monotherapy versus nonvalidated polymyxin combination therapy versus validated polymyxin combination therapy in extensively drug-resistant gram-negative Bacillus infections. Antimicrobial Agents and Chemotherapy. 2016;60(7):4013–4022. 10.1128/AAC.03064-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zusman O, Altunin S, Koppel F, Dishon Benattar Y, Gedik H, Paul M. Polymyxin monotherapy or in combination against carbapenem-resistant bacteria: systematic review and meta-analysis. Journal of Antimicrobial Chemotherapy. 2016;72(1):29–39. 10.1093/jac/dkw377. [DOI] [PubMed] [Google Scholar]
- [39].Han M-L, Liu X, Velkov T, Lin Y-W, Zhu Y, Creek DJ, Barlow CK, Yu HH, Zhou Z, Zhang J. Comparative metabolomics reveals key pathways associated with the synergistic killing of colistin and sulbactam combination against multidrug-resistant Acinetobacter baumannii. Frontiers in Pharmacology. 2019;10:754. 10.3389/fphar.2019.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Maifiah MHM, Cheah S-E, Johnson MD, Han M-L, Boyce JD, Thamlikitkul V, Forrest A, Kaye KS, Hertzog P, Purcell AW. Global metabolic analyses identify key differences in metabolite levels between polymyxin-susceptible and polymyxin-resistant Acinetobacter baumannii. Scientific Reports. 2016;6:22287. 10.1038/srep22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Maifiah MHM, Creek DJ, Nation RL, Forrest A, Tsuji BT, Velkov T, Li J. Untargeted metabolomics analysis reveals key pathways responsible for the synergistic killing of colistin and doripenem combination against Acinetobacter baumannii. Scientific Reports. 2017;7:45527. 10.1038/srep45527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pritchard JR, Bruno PM, Gilbert LA, Capron KL, Lauffenburger DA, Hemann MT. Defining principles of combination drug mechanisms of action. Proceedings of the National Academy of Sciences. 2013;110(2):E170–E179. 10.1073/pnas.1210419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Abulfathi AA, Decloedt EH, Svensson EM, Diacon AH, Donald P, Reuter H. Clinical pharmacokinetics and pharmacodynamics of rifampicin in human tuberculosis. Clinical Pharmacokinetics. 2019:1–27. 10.1007/s40262-019-00764-2. [DOI] [PubMed] [Google Scholar]
- [44].Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Research. 2018;46(D1):D1074–D1082. 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang Y-M, Rock CO. Membrane lipid homeostasis in bacteria. Nature Reviews Microbiology. 2008;6(3):222. 10.1038/nrmicro1839. [DOI] [PubMed] [Google Scholar]
- [46].Henry R, Crane B, Powell D, Deveson Lucas D, Li Z, Aranda J, Harrison P, Nation RL, Adler B, Harper M. The transcriptomic response of Acinetobacter baumannii to colistin and doripenem alone and in combination in an in vitro pharmacokinetics/pharmacodynamics model. Journal of Antimicrobial Chemotherapy. 2015;70(5):1303–1313. 10.1093/jac/dku536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Goldstein BP. Resistance to rifampicin: a review. The Journal of Antibiotics. 2014;67(9):625. 10.1038/ja.2014.107. [DOI] [PubMed] [Google Scholar]
- [48].McClure WR, Cech CL. On the mechanism of rifampicin inhibition of RNA synthesis. Journal of Biological Chemistry. 1978;253(24):8949–8956. [PubMed] [Google Scholar]
- [49].Armenta-Medina D, Segovia L, Perez-Rueda E. Comparative genomics of nucleotide metabolism: a tour to the past of the three cellular domains of life. BMC Genomics. 2014;15(1):800. 10.1186/1471-2164-15-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Moffatt BA, Ashihara H. Purine and pyrimidine nucleotide synthesis and metabolism. The Arabidopsis Book/American Society of Plant Biologists. 2002;1. 10.1199/tab.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lobritz MA, Belenky P, Porter CB, Gutierrez A, Yang JH, Schwarz EG, Dwyer DJ, Khalil AS, Collins JJ. Antibiotic efficacy is linked to bacterial cellular respiration. Proceedings of the National Academy of Sciences. 2015:201509743. 10.1073/pnas.1509743112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Khondker A, Rheinstädter MC. How do bacterial membranes resist polymyxin antibiotics? Communications Biology. 2020;3(1):1–4. 10.1038/s42003-020-0803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gordon E, Flouret B, Chantalat L, van Heijenoort J, Mengin-Lecreulx D, Dideberg O. Crystal structure of UDP-N-acetylmuramoyl-L-alanyl-D-glutamate: meso-diaminopimelate ligase from Escherichia coli. Journal of Biological Chemistry. 2001;276(14):10999–11006. 10.1074/jbc.M009835200. [DOI] [PubMed] [Google Scholar]
- [54].Romantsov T, Guan Z, Wood JM. Cardiolipin and the osmotic stress responses of bacteria. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2009;1788(10):2092–2100. 10.1016/j.bbamem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Fuchs TM, Eisenreich W, Heesemann J, Goebel W. Metabolic adaptation of human pathogenic and related nonpathogenic bacteria to extra-and intracellular habitats. FEMS Microbiology Reviews. 2012;36(2):435–462. 10.1111/j.1574-6976.2011.00301.x. [DOI] [PubMed] [Google Scholar]