Abstract
Hormones are key drivers of cancer development, and alteration of the intratumoral concentration of thyroid hormone (TH) is a common feature of many human neoplasias. Besides the systemic control of TH levels, the expression and activity of deiodinases constitute a major mechanism for the cell-autonomous, prereceptoral control of TH action. The action of deiodinases ensures tight control of TH availability at intracellular level in a time- and tissue-specific manner, and alterations in deiodinase expression are frequent in tumors. Research over the past decades has shown that in cancer cells, a complex and dynamic expression of deiodinases is orchestrated by a network of growth factors, oncogenic proteins, and miRNA. It has become increasingly evident that this fine regulation exposes cancer cells to a dynamic concentration of TH that is functional to stimulate or inhibit various cellular functions. This review summarizes recent advances in the identification of the complex interplay between deiodinases and cancer and how this family of enzymes is relevant in cancer progression. We also discuss whether deiodinase expression could represent a diagnostic tool with which to define tumor staging in cancer treatment or even a therapeutic tool against cancer.
Keywords: thyroid hormone action, deiodinase, cancer, proliferation
Thyroid hormones (THs) are implicated in the control of a variety of biological events including proliferation, apoptosis, differentiation, and metabolism in vertebrates (1, 2). Upon binding with its nuclear receptors (TRs), canonical action of active TH (triiodothyronine [T3]) determines enhancement or inhibition of the expression of target genes. The thyroid gland produces a large excess of the inactive hormone, thyroxine (T4), compared with the active hormone, T3. Thus, most of the circulating T3 and of the T3 intracellular availability derive from the action of 3 enzymes, the iodothyronine deiodinases D1, D2, and D3, that are expressed in a tissue-specific manner in fetal and adult life (3) and selectively catalyze the activation or inactivation of TH. D1 is an integral plasma membrane protein and is mainly expressed in the liver, thyroid, and kidney (4-6). It converts T4 to T3 primarily to provide T3 for the circulation and it also works as a scavenger enzyme that recycles iodine to replenish the thyroid’s iodine reservoir (3, 6). D2 is an endoplasmic reticulum resident protein expressed in many tissues including muscle, brain, heart, bone, and brown adipose tissue (4, 6, 7). Differently from D1, the main function of D2 is thought to be providing T3 to the nucleus to meet intracellular needs, which is a concept consistent with its subcellular localization (3). D3 is the major TH inactivating enzyme; in fact, it converts T4 and T3 into respectively rT3 and T2, which are inactive at nuclear level. D3 is widely expressed in fetal tissues and placenta, and protects developing tissues from excessive TH levels present in the maternal circulation (8). In adult life, D3 expression declines and persists essentially in skin, brain, and pregnant uterus (9, 10).
It is well established that intracellular regulation of TH concentration is important in cancer, and that this process occurs independently from the systemic control of TH plasma concentration (11). Importantly, different studies suggested that TH plays a crucial role in the neoplastic process. In fact, it has been shown to affect the various phases of tumor formation, growth, and metastasis both in experimental animal models and in humans (12, 13). The first studies to demonstrate a correlation between TH action and cancer date back to the 1980s, and report that TRs are the cellular homologs of v-erbA, which is a viral oncogene product involved in avian erythroblastosis (14, 15). V-erbA is a mutated TRα1 protein that is unable to bind T3 and acts as a dominant negative mutant on the wild-type receptor, thereby attenuating TH signaling. This mutated protein increases its oncogenic potential by cooperating with various oncoproteins to induce tumorigenesis (16).
Subsequently, deregulation of deiodinase expression was identified in diverse tumor contexts. Initial studies, in the late 1980s, showed that immortalized rat pituitary tumor cells (GH4C1) express elevated levels of the D3 enzyme (17). Since then, many in vitro and in vivo studies demonstrated that TH levels vary during the various steps of tumorigenesis, namely tumor formation, growth, migration, and invasiveness, and that the magnitude and specificity of such regulation is tissue and tumor dependent (18-20).
In this review, we focus on the role of deiodinases in the control of TH signaling in cancer. We also discuss the possibility that deiodinases may have diagnostic/therapeutical potential in the cancer field.
D3 and Cancer
Although its expression is barely detectable in adult tissues, the D3 enzyme has been found reactivated in several physiopathological conditions in which cell proliferation is enhanced, as in the case of chronic inflammation, myocardial infarction, tissue repair and critical illness (Fig. 1). In these conditions, D3 expression is increased in order to enable cell function and, in many cases, cell proliferation (21-23). This was demonstrated, in skin and skeletal muscle, to be the consequence of induced proliferation to ensure correct tissue turnover (24). Tissue-specific D3 knockout studies in these models indicate that D3 action is crucial in these conditions (24, 25). Indeed, D3 depletion in keratinocytes and in myoblasts resulted in drastic decreased cell proliferation and enhanced cell differentiation in keratinocytes (26) and in massive apoptosis of muscle stem cells (24). The mechanisms by which loss of D3 reduce cell proliferation were in both cases due to increased nuclear activity of TH and induction of prodifferentiation and proapoptotic gene expression.
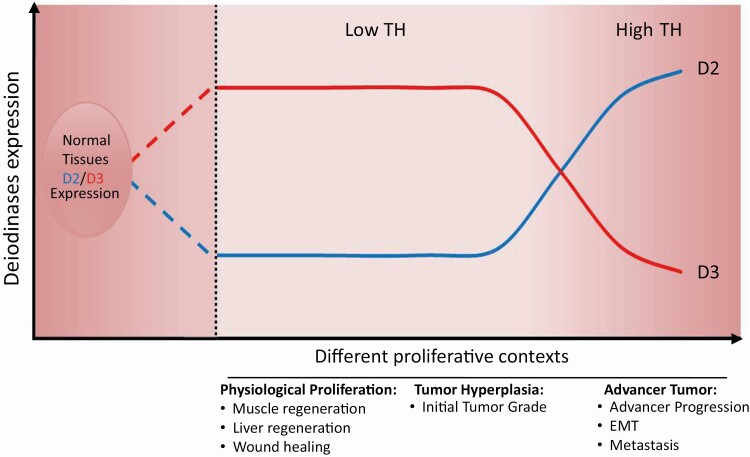
Figure 1.
The balance of deiodinase expression under normal conditions, in normal proliferative states and in cancer. Schematic illustration of D2 and D3 expression under various physiopathological conditions. The image shows the most common trend of Dio expression and how it is modified by various stimuli and in various contexts.
Interestingly, in adult life, D3 is also re-expressed in cancer. D3 was initially identified in various immortalized cell lines derived from adenocarcinoma (HCT116, Caco2, and SW280 cells), breast cancer (MCF-7 cells), endometrium carcinoma (ECC-1), neuroblastoma (SH-SY5Y cells) basal cell carcinoma (BCC), ovarian cancer (HGSO cells), and colon (23, 27-29). Accordingly, D3 is upregulated in many murine and human tumors tissues including the vascular tumors infantile hemangiomas and hepatic hemangioendothelioma (27, 30) as well as in various brain tumors, among which, gliosarcoma and glioblastoma multiforme (31-34). Conversely, D3 was found to be lower in all cases of astrocytoma, irrespective of their grade, than in the normal brain counterpart (31-34). Furthermore, D3 has been detected in pituitary tumors, especially those producing adrenocorticotropic hormone, thyroid-stimulating hormone, or growth hormone, as well as in nonfunctional hormones (35).
Various hormones and other factors, such as estrogens, progesterone, and epidermal and fibroblast growth factors, that promote cell proliferation are potent D3 inducers in a wide variety of cell types. Furthermore, D3 expression in diverse tumoral contexts has been associated to relevant oncogenic pathways namely Shh-Gli2, Wnt/β-catenin, tumor growth factor β (TGF-β) and hypoxia-inducible factor-1α) (11) (Table 1).
Table 1.
Intracellular pathways and main factors that regulate D2 and D3 expression and/or activity in the cancer context
| D3 regulators | Reference | |
|---|---|---|
| EGF | ↑ upregulated | Hernandez A, Endocrinology (1995) (36) |
| Estrogen | ↑ upregulated | Bates JM, Endocrinology (1999) (9) |
| FGF | ↑ upregulated | Hernandez A, Endocrinology (1995) (36) |
| GRHL3 | ↓ downregulated | Di Girolamo D, J Clin Invest (2016) (37) |
| Hypoxia-inducible factor-1α | ↑ upregulated | Simonides WS, J Clin Invest (2008) (38) |
| microRNA-21 (miR21) | ↑ upregulated | Di Girolamo D, J Clin Invest (2016) (37) |
| Phorbol compounds | ↑ upregulated | Courtin F, J Neurochem (1991) (39) |
| Progesteron | ↑ upregulated | Bates JM, Endocrinology (1999) (9) |
| Serum | ↑ upregulated | Courtin F, J Neurochem (1991) (39) |
| Shh-Gli2 | ↑ upregulated | Dentice M, Proc Natl Acad Sci U S A (2007) (21) |
| TGF-β | ↑ upregulated | Huang SA, Thyroid (2005) (10) |
| Wnt/β-catenin | ↑ upregulated | Dentice M, Gastroenterology (2012) (23) |
| D2 regulators | Reference | |
| cAMP | ↑ upregulated | Wang YY, Cardiovasc Res (2010) (40) |
| NANOG | ↑ upregulated | Nappi A, Cancers (2020) (41) |
| NF-κB | ↑ upregulated | Zeold A, Endocrinology (2006) (42) |
| Wnt/β-catenin | ↓ downregulated | Dentice M, Gastroenterology (2012) (23) |
Relevant to the understanding of the interplay between deiodinases and cancer is BCC. It is the most common skin cancer and accounts for approximately 80% of all nonmelanoma skin cancers (43, 44). Although its incidence is elevated, it has a low mortality rate (43, 44). The Sonic hedgehog (Shh) pathway is frequently involved and activated in many human tumors including BCC (45). We demonstrated that this pathway affects TH metabolism by a dual convergent mechanism that involves D2 and D3 activity differently. Indeed, we found that Shh induces D3 expression via the binding of Gli2 transcription factor (1 of the Shh effector proteins), to the human DIO3 promoter (21, 46). Furthermore, another Hedgehog family member, Ihh, degrades D2 by inducing WSB-1, which is an E3 ubiquitin ligase adaptor that accelerates D2 degradation (47). These effects exerted on D2 and D3 significantly reduce intracellular T3 concentration. The resulting local cellular hypothyroidism leads to an increase in Cyclin-D1, which in turn results in a sustained proliferative rate (48). Conversely, D3 depletion significantly reduced Cyclin-D1 and proliferation; vice versa when a functional D3 gene is reintroduced in D3-depleted cells, this leads to reincreased Cyclin-D1 levels and cell proliferation. Accordingly, T3 treatment or D3 depletion, in vivo, interferes with tumorigenesis, reducing tumor growth and survival of BCC xenografts in nude mice (21).
MiR21 is an oncomiR that promotes tumor development by inhibiting several tumor suppressor genes and plays a key role in promoting various human and murine tumors including BCC. We recently identified that TH and miR21 reciprocally regulate each other (37). In fact, while TH suppresses miR21 expression, it regulates TH metabolism by indirectly inducing D3 expression in BCC. MiR21 downregulates a tumor suppressor, GRHL3, which is an inhibitor of D3. The novel described functional axis in BCCs, namely the miR21–GRHL3–D3 axis, leads to reduced T3 concentrations in tumor microenvironments thereby favoring tumor growth (37). Conversely, D3 depletion was found to attenuate BCC cell proliferation and in vivo xenograft carcinogenesis, whereas miR21 overexpression enhances the oncogenic potential of BCC cells (46).
D3 overexpression has been detected in early tumorigenesis and its action has been correlated with tumor cell proliferation in colon cancer and in BCC (49). In the intestine, D3 expression has been found to be significantly higher in both adenomas and colon carcinomas than in normal tissues (23). Interestingly, its expression in colon carcinomas is negatively associated with advanced lesion grade. In fact, its expression was found to decrease from G1 to G3, which suggests that D3 is a marker of the early stages of tumorigenesis (23). This finding, which initially appeared counterintuitive and difficult to explain, was subsequently clarified by studies in skin cancer wherein D3 expression declines with cancer progression, and in the more metastatic lesions (see ‘D2 and Cancer’).
Deiodinases are also involved in the crosstalk between TH and the Wnt/β-catenin pathways (23). Indeed, the Wnt/β-catenin/T-cell factor (TCF) axis transcriptionally induces D3 overexpression and simultaneously represses D2 expression thereby leading to reduced TH signaling in tumors. In the opposite direction, β-catenin knockdown decreases D3 expression while simultaneously increasing D2 expression. Thus, increment of β-catenin signaling leads to D3 upregulation thereby decreasing the intracellular T3 level, and hence its growth-inhibitory effects. Vice versa, active TH can suppress Wnt signaling by inhibiting the β-catenin-TCF4 complex-mediated transcription of Cyclin-D1 in colon cancer (50). In addition, it can regulate the target genes of Wnt signaling by inducing the direct binding of TRs to β-catenin (51). Besides via the Shh and the Wnt pathways, D3 expression is transcriptionally stimulated by TGF-β in hemangioma and glioma cells (52). The local hypothyroidism induced by TGF-β could favor the expression of oncofetal genes and suppress the differentiative effects of TH or promote cell survival in such pathological conditions as cancer (53).
In some cancer cases, very high D3 activity affects plasma TH levels and cause a rare form of hypothyroidism, called “consumptive hypothyroidism” (27). This condition results from the accelerated rate of TH plasmatic degradation, which cannot be compensated for by the production of TH by the thyroid gland.
Overall, the studies available indicate that local attenuation of intracellular T3 occurs in many human tumors, and that this may be advantageous for cell proliferation and tumor growth. An understanding of the molecular basis of upregulated D3 might suggest avenues of research that might lead to new strategies to treat cancer.
D2 and Cancer
D2 expression has rarely been associated with neoplastic transformation. It has been found upregulated in benign hyperfunctioning thyroid nodules and in thyroid tumors including follicular thyroid carcinoma, anaplastic and medullary thyroid cancer (54, 55), whereas its expression was lower in papillary thyroid carcinoma than in normal thyroid tissues (56). This expression pattern is consistent with D2 being a cAMP-responsive gene whose expression increases in those tumoral contexts (thyroid adenoma and follicular carcinoma) in which there is a corresponding overstimulation of the cAMP pathway (Table 1).
At least 2 studies (57, 58) found that functional D2 activity is present in human anterior pituitary tissues, both in adenomas with different secretory activities and in normal tissues. Moreover, Tannahill et al. (35) reported that D2 expression is 2.6-fold higher in pituitary tumors than in normal pituitary tissues, and that the highest D2 expression (3.6-fold) occurred in nonfunctioning pituitary tumors.
Although neurons are thought to be a major target of THs in the brain, the TH-activating enzyme D2, rather than being expressed in the neurons themselves, is expressed in astrocytes and in adjacent glial cells that provide T3 availability in neurons (59). In most brain tumors, D2 expression is remarkably heterogeneous depending on the histological type of the tumor tissue. In fact, D2 expression is lower in astrocytomas and glioblastomas than in the normal counterpart (34) and it is higher in oligodendrogliomas, gliosarcomas, and glioblastoma multiforme (32). Bunevicius et al. (60) evaluated DIO polymorphisms in human brain tumors of various histological origin. They identified 5 single nucleotide polymorphisms in the DIO2 gene (rs225011, rs2267873, rs225015, rs225014, and rs12885300) that commonly occur in glioblastoma patients; however, only the genetic rs12885300 polymorphism was significantly associated with glioblastoma in all samples analyzed. In fact, rs12885300 had a prognostic significance and was associated with an increased mortality risk and 2-year survival.
D2 and D3 are both expressed in normal skin (61-63), suggesting that both activating and inactivating TH enzymes are required to ensure a balanced intracellular level of TH in the different epithelial skin compartment (64). While the role of D3 has been investigated in the growth and differentiation of keratinocytes (21, 26, 65), both in a pathological and skin cancer context (11, 21, 37, 46, 66), the role of D2 has only recently been partially clarified. Although BCC has been proposed as a paradigm of D3-overexpressing tumor (21, 37, 46), BCC cells and tumors also express D2 (48). Notably, while D2 depletion enhances the proliferative potential of cancer cells, D3 depletion attenuates it. (48).
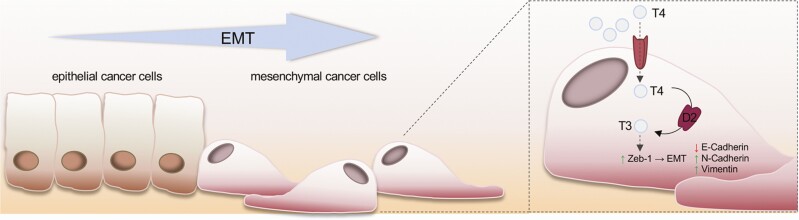
The role of D2 in skin cancer was further clarified in squamous cell carcinoma (SCC), which, similar to BCC, is a cutaneous cancer caused by keratinocyte transformation (67-69). Unlike BCC, SCC can be invasive and is associated with a substantial risk of metastasis and death (70). During the progression of SCC, D3 and D2 expression are coupled to the various phases of tumorigenesis. Indeed, while D3 is expressed and critical in the early phases of carcinogenesis up to the formation of benign papillomas, D2 is expressed in the late stages of neoplastic transformation, up to the formation of poorly differentiated and invasive SCC. The dynamic expression of D3 and D2 led to the concept that TH attenuation promotes tumor formation and amplification while high T3 levels are later required to ensure the invasiveness and metastatic propensity of cutaneous SCC (20) (Fig. 2). Notably, we reported that the D3 to D2 shift coincides with the EMT transition of SCC cells. Moreover, in the same context, D2 upregulation and the consequent increase in intracellular T3 induced the expression of the pro-EMT gene Zeb-1 and of its downstream targets Vimentin and N-Cadherin, while reducing the expression of the epithelial markers E-Cadherin and K14 (20) (Fig. 2), thus revealing that D2 is a prognostic marker in cutaneous SCC. Indeed, an analysis of databases deposited in the GEO DataSet Suite, GSE686 (71) and GSE10300 (72), showed that D2 expression is associated with a worse SCC prognosis and correlated with both postsurgical relapse and a shorter disease-free survival. Finally, in SCC and BCC, D2 expression is under the control of the transcriptional factor NANOG (Table 1) (41) which is a marker of stemness and also a pro-oncogenic gene in various epithelial tumors, including SCC (70).
Figure 2.
The deiodinase expression profile in cancer cell progression and the mechanism induced by thyroid hormone (TH) to promote the epithelial-to-mesenchymal transition (EMT). Illustration showing the progression of epithelial cancer toward a more aggressive stage and its correlation with an adaptive response of the intracellular TH concentration. The molecular mechanisms by which TH support the EMT are also depicted (right).
Does D1 Play a Role in Cancer?
D1 expression is often altered in cancer tissues (31). However, the role of D1 in cancer remains largely unexplored, and the studies that are available are in part discordant. In tissues normally expressing D1, such as the thyroid gland, D1 activity has been reported to be either up- or downmodulated in a tumoral context depending on the histological subtype (31, 56).
Meta-analyses of gene expression profiles in human thyroid neoplasia revealed that the DIO1 gene is underexpressed in both benign and malignant thyroid tumors versus normal tissue (56, 73). Notably, D1 levels were found to be decreased in some histological subtypes of thyroid neoplasms, including papillary thyroid carcinoma and anaplastic thyroid carcinoma, both at various clinical stages (74). By contrast, normal or increased D1 levels were found in follicular thyroid adenoma, follicular thyroid carcinoma, and Hurthle cell cancer versus adjacent normal thyroid tissues (73, 75).
D1 expression was altered also in nonthyroidal cancers (76). Baur et al. (57) provided the first evidence that D1 is expressed in the normal human pituitary gland and in pituitary adenomas, where D1 activity was found to be higher in about 50% of tumors analyzed than in normal pituitary samples (35). In studies on the clinical significance of DIO1 gene polymorphisms in patients affected by a brain tumor, no D1 activity was found in tumors of the central nervous system (60). Bunevicius et al. (60) demonstrated that a common variant in the C-allele of the DIO1 gene (rs2235544) reflects increased D1 activity and has a prognostic significance in glioblastoma patients where it is associated with overall survival.
Similar evidence of altered D1 expression has been found in clear cell renal adenocarcinoma (77), liver adenocarcinoma (78), squamous cell lung carcinoma (79), and prostate cancer (80) in which a remarkable reduction (versus normal tissue) or even no D1 activity was found (77, 81). In renal cancer cells loss of D1 seems to contribute to the carcinogenic process since restoration of D1 activity by ectopically DIO1 induction strongly inhibits the expression of genes and proteins involved in proliferation, migration and tumor progression (82, 83). Piekielko et al. identified various DIO1 splicing transcript variants that are specific to cancerous renal tissue and may thus serve as prognostic markers of kidney carcinogenesis (84). DIO1 is also regulated through a post-transcriptional mechanism by miR-224 and miR-383 (85). Downregulation of endogenous DIO1 expression due to overexpression of both miR-224 and miR-383 in clear cell renal cell carcinoma results in decreased intratumoral T3 concentration, which suggests that hypothyroidism may influence and, in particular promote, tumor growth (85).
Low D1 levels were also detected in gastric cancer in which mechanistic studies found that reduced DIO expression was related to selenium deficiency (86, 87). The impairment of DIO1 gene expression in gastric cancer suggests this selenoprotein plays a role in specific subgroups of gastric cancer in humans (88). D1 overexpression has been detected in mammary gland carcinoma (89), at levels at least 2 orders higher than that of intact mammary gland. Moreover, in mammary carcinoma, D1 expression was higher in the early phases than in the late phase of tumorigenesis. In mammary carcinoma, D1 was differentially expressed during tumor progression. In fact, D1 expression was higher in the early phases than in late phases of tumorigenesis (89-91). These results suggest that a progressive loss of D1 activity occurs during tumor progression, and also highlights that D1 expression could be associated with the loss of epithelial differentiation in breast cancer cells (92). Taken together, these studies show that D1 expression is highly heterogeneous in cancer (Table 2). Overall, in multiple tumoral contexts D1 acts like a typical differentiation marker in various tissues (eg, liver, kidney, brain) the expression of which is altered upon neoplastic transformation. Whether those variations are functionally relevant for tumors remains to be investigated.
Table 2.
Variable D1 activity and expression in various cancer settings
| Tissue | Type of cancer | D1 activity | Reference |
|---|---|---|---|
| Thyroid | Follicular thyroid adenoma | ↑ increased | Schrek R, J Clin Endocrinol Metab 1994 (75); Arnaldi LAT, Thyroid 2005 (56); de Souza Meyer EL 2005 (73) |
| Follicular thyroid carcinoma | ↑ increased | Souza Meyer EL, Clin Endocrinol 2005 (73) Arnaldi LAT, Thyroid 2005 (56) |
|
| Hurthle cell cancer | ↑ increased | Souza Meyer EL, Clin Endocrinol 2005 (73) | |
| Papillary thyroid carcinoma | ↓ decreased | Murakami M, J Clin Endocrinol Metab 2000 (34) Souza Meyer EL, Clin Endocrinol 2005 (73) Arnaldi LAT, Thyroid 2005 (56) |
|
| Anaplastic thyroid carcinoma | ↑ increased | Casula S, Front Endocrinol 2012 (31) | |
| Pituitary gland | Pituitary adenoma | ↑ increased | Tannahill LA, Clin Endocrinol 2002 (35) Baur A, Eur J Endocrinol 2002 (57) |
| Brain | Glioma | not detected | — |
| Astrocytoma | not detected | — | |
| Glioblastoma | not detected | — | |
| Oligodendroglioma | not detected | — | |
| Astrocytoma | not detected | — | |
| Gliosarcoma | not detected | — | |
| Glioblastoma multiforme | not detected | — | |
| Kidney | Clear cell renal adenocarcinoma | ↓ decreased | Pachucki J, J Endocrinol Invest 2001 (77) |
| Liver | Liver adenocarcinoma | ↓ decreased | Sabatino L, Life Sci 2000 (78) |
| Lung | Squamous cell lung carcinoma | ↓ decreased | Wawrzynska L, Monaldi Arch Chest Dis 2003 (79) |
| Prostate | Prostate cancer | ↓ decreased | Dutkiewicz S, Int Urol Nephrol 1995 (80) |
Conclusions
Research over the past 3 decades has challenged the concept that the central regulation of TH action is the principal cue determining TH availability in target cells. Not only do deiodinases allow tissue-specific modulation of TH intracellular signaling, but they are also dynamically exploited by target cells to achieve the optimal time- and spatiospecific TH concentration at intracellular level. This is true in physiological contexts as well as during embryonic development and tissue regeneration, and also in such pathological states as tumorigenesis (Fig. 1). Cancer cells are an example of cells that can finely tune TH concentration during the various stages of cancer progression. In skin models, early-stage tumorigenesis requires a high degree of proliferation and is sensitive to TH-mediated cell cycle arrest. To overcome this, cells have devised a strategy enabling attenuation of the TH signal mediated by the D3 enzyme that enables cells to proliferate. Conversely, progression to the invasive and prometastatic stages occurs through upregulation of the TH signal that is induced by the catalytic activity of D2. Why this occurs remains to the established. We have yet to define the events that determine the switch in the D3-D2 deiodinase expression during tumor progression in some cancers. Furthermore, is the switch of deiodinase expression a marker of mutated grading of a tumor? In other words, could the deiodinase profile serve as a marker of tumor staging/grading? This is still an open issue, although the correlations arising from the analysis of large in silico data sets between D2 expression and poor prognosis in skin cancer and reduced D3 expression and lower survival rates in breast cancer support this possibility (93).
Another important issue is the crosstalk between cancer cells and cells that constitute their microenvironment. While the role and significance of deiodinases in cancer cells is starting to be elucidated, the role of the tumor microenvironment in the control of “local” TH concentration is far from being established. It is reasonable that the regulation of intracellular TH concentration in the cells surrounding the tumor will also affect cancer growth and progression.
In this scenario, the finding that deiodinase manipulation in animal models potently affects tumor formation and progression opens the way to the therapeutic application of deiodinase modulators in cancer. Although the druggable control of specific deiodinase action in specific cells remains a mirage, the requirement of deiodinases to allow tumor maintenance may be an Achilles heel for tumor growth. Should this be the case, it may open new avenues of translational research at the crossroads between TH and cancer research fields.
Acknowledgments
We thank Jean Ann Gilder (Scientific Communication srl., Naples, Italy) for writing assistance.
Financial Support: This work was supported by a grant under the European Union’s Horizon 2020 Programme – EU FP7 contract Thyrage (grant number 666869) that was awarded to D.S.
Glossary
Abbreviations
- BCC
basal cell carcinoma
- EMT
epithelial to mesenchymal transition
- SCC
squamous cell carcinoma
- Shh
sonic hedgehog
- T3
triiodothyronine
- T4
thyroxine
- TH
thyroid hormone
- TGF-β
transforming growth factor β
- TR
thyroid hormone receptor
Additional Information
Disclosures: The authors have declared that no conflict of interest exists.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Oppenheimer JH, Schwartz HL, Mariash CN, Kinlaw WB, Wong NC, Freake HC. Advances in our understanding of thyroid hormone action at the cellular level. Endocr Rev. 1987;8(3):288-308. [DOI] [PubMed] [Google Scholar]
- 2. Yen PM. Molecular basis of resistance to thyroid hormone. Trends Endocrinol Metab. 2003;14(7):327-333. [DOI] [PubMed] [Google Scholar]
- 3. Dentice M, Marsili A, Zavacki A, Larsen PR, Salvatore D. The deiodinases and the control of intracellular thyroid hormone signaling during cellular differentiation. Biochim Biophys Acta. 2013;1830(7):3937-3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. St Germain DL, Galton VA. The deiodinase family of selenoproteins. Thyroid. 1997;7(4):655-668. [DOI] [PubMed] [Google Scholar]
- 5. Larsen PR, Silva JE, Kaplan MM. Relationships between circulating and intracellular thyroid hormones: physiological and clinical implications. Endocr Rev. 1981;2(1):87-102. [DOI] [PubMed] [Google Scholar]
- 6. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23(1):38-89. [DOI] [PubMed] [Google Scholar]
- 7. Sagliocchi S, Cicatiello AG, Di Cicco E, et al. The thyroid hormone activating enzyme, type 2 deiodinase, induces myogenic differentiation by regulating mitochondrial metabolism and reducing oxidative stress. Redox Biol. 2019;24:101228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van der Geyten S, Segers I, Gereben B, et al. Transcriptional regulation of iodothyronine deiodinases during embryonic development. Mol Cell Endocrinol. 2001;183(1-2):1-9. [DOI] [PubMed] [Google Scholar]
- 9. Bates JM, St Germain DL, Galton VA. Expression profiles of the three iodothyronine deiodinases, D1, D2, and D3, in the developing rat. Endocrinology. 1999;140(2):844-851. [DOI] [PubMed] [Google Scholar]
- 10. Huang SA. Physiology and pathophysiology of type 3 deiodinase in humans. Thyroid. 2005;15(8):875-881. [DOI] [PubMed] [Google Scholar]
- 11. Dentice M, Antonini D, Salvatore D. Type 3 deiodinase and solid tumors: an intriguing pair. Expert Opin Ther Targets. 2013;17(11):1369-1379. [DOI] [PubMed] [Google Scholar]
- 12. Sibilio A, Ambrosio R, Bonelli C, et al. Deiodination in cancer growth: the role of type III deiodinase. Minerva Endocrinol. 2012;37(4):315-327. [PubMed] [Google Scholar]
- 13. Dentice M, Salvatore D. Deiodinases: the balance of thyroid hormone: local impact of thyroid hormone inactivation. J Endocrinol. 2011;209(3):273-282. [DOI] [PubMed] [Google Scholar]
- 14. Weinberger C, Thompson CC, Ong ES, Lebo R, Gruol DJ, Evans RM. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986;324(6098):641-646. [DOI] [PubMed] [Google Scholar]
- 15. Sap J, Muñoz A, Damm K, et al. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986;324(6098):635-640. [DOI] [PubMed] [Google Scholar]
- 16. Sap J, Muñoz A, Schmitt J, Stunnenberg H, Vennström B. Repression of transcription mediated at a thyroid hormone response element by the v-erb-A oncogene product. Nature. 1989;340(6230):242-244. [DOI] [PubMed] [Google Scholar]
- 17. Koenig RJ. Regulation of thyroxine 5’-deiodinase by thyroid hormones and activators of protein kinase C in GH4C1 cells. Endocrinology. 1986;118(4):1491-1497. [DOI] [PubMed] [Google Scholar]
- 18. Dentice M. Hedgehog-mediated regulation of thyroid hormone action through iodothyronine deiodinases. Expert Opin Ther Targets. 2011;15(4):493-504. [DOI] [PubMed] [Google Scholar]
- 19. Catalano V, Dentice M, Ambrosio R, et al. Activated thyroid hormone promotes differentiation and chemotherapeutic sensitization of colorectal cancer stem cells by regulating Wnt and BMP4 signaling. Cancer Res. 2016;76(5):1237-1244. [DOI] [PubMed] [Google Scholar]
- 20. Miro C, Di Cicco E, Ambrosio R, et al. Thyroid hormone induces progression and invasiveness of squamous cell carcinomas by promoting a ZEB-1/E-cadherin switch. Nat Commun. 2019;10(1):5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dentice M, Luongo C, Huang S, et al. Sonic hedgehog-induced type 3 deiodinase blocks thyroid hormone action enhancing proliferation of normal and malignant keratinocytes. Proc Natl Acad Sci U S A. 2007;104(36):14466-14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hernandez A, Park JP, Lyon GJ, Mohandas TK, St Germain DL. Localization of the type 3 iodothyronine deiodinase (DIO3) gene to human chromosome 14q32 and mouse chromosome 12F1. Genomics. 1998;53(1):119-121. [DOI] [PubMed] [Google Scholar]
- 23. Dentice M, Luongo C, Ambrosio R, et al. β-Catenin regulates deiodinase levels and thyroid hormone signaling in colon cancer cells. Gastroenterology. 2012;143(4):1037-1047. [DOI] [PubMed] [Google Scholar]
- 24. Dentice M, Ambrosio R, Damiano V, et al. Intracellular inactivation of thyroid hormone is a survival mechanism for muscle stem cell proliferation and lineage progression. Cell Metab. 2014;20(6):1038-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hernandez A, Martinez ME, Fiering S, Galton VA, St Germain D. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest. 2006;116(2):476-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mancino G, Sibilio A, Luongo C, et al. The thyroid hormone inactivator enzyme, type 3 deiodinase, is essential for coordination of keratinocyte growth and differentiation. Thyroid. 2020;30(7):1066-1078. [DOI] [PubMed] [Google Scholar]
- 27. Huang SA, Tu HM, Harney JW, et al. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med. 2000;343(3):185-189. [DOI] [PubMed] [Google Scholar]
- 28. Kester MH, Kuiper GG, Versteeg R, Visser TJ. Regulation of type III iodothyronine deiodinase expression in human cell lines. Endocrinology. 2006;147(12):5845-5854. [DOI] [PubMed] [Google Scholar]
- 29. Moskovich D, Alfandari A, Finkelshtein Y, et al. DIO3, the thyroid hormone inactivating enzyme, promotes tumorigenesis and metabolic reprogramming in high grade serous ovarian cancer. Cancer Lett. 2020;501:224-233. [DOI] [PubMed] [Google Scholar]
- 30. Ruppe MD, Huang SA, Jan de Beur SM. Consumptive hypothyroidism caused by paraneoplastic production of type 3 iodothyronine deiodinase. Thyroid. 2005;15(12):1369-1372. [DOI] [PubMed] [Google Scholar]
- 31. Casula S, Bianco AC. Thyroid hormone deiodinases and cancer. Front Endocrinol (Lausanne). 2012;3:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nauman P, Bonicki W, Michalik R, Warzecha A, Czernicki Z. The concentration of thyroid hormones and activities of iodothyronine deiodinases are altered in human brain gliomas. Folia Neuropathol. 2004;42(2):67-73. [PubMed] [Google Scholar]
- 33. Mori K, Yoshida K, Kayama T, et al. Thyroxine 5-deiodinase in human brain tumors. J Clin Endocrinol Metab. 1993;77(5):1198-1202. [DOI] [PubMed] [Google Scholar]
- 34. Murakami M, Araki O, Morimura T, et al. Expression of type II iodothyronine deiodinase in brain tumors. J Clin Endocrinol Metab. 2000;85(11):4403-4406. [DOI] [PubMed] [Google Scholar]
- 35. Tannahill LA, Visser TJ, McCabe CJ, et al. Dysregulation of iodothyronine deiodinase enzyme expression and function in human pituitary tumours. Clin Endocrinol (Oxf). 2002;56(6):735-743. [DOI] [PubMed] [Google Scholar]
- 36. Hernández A, Obregón MJ. Presence of growth factors-induced type III iodothyronine 5-deiodinase in cultured rat brown adipocytes. Endocrinology. 1995;136(10):4543-4550. [DOI] [PubMed] [Google Scholar]
- 37. Di Girolamo D, Ambrosio R, De Stefano MA, et al. Reciprocal interplay between thyroid hormone and microRNA-21 regulates hedgehog pathway-driven skin tumorigenesis. J Clin Invest. 2016;126(6):2308-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simonides WS, Mulcahey MA, Redout EM, et al. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J Clin Invest. 2008;118(3):975-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Courtin F, Liva P, Gavaret JM, Toru-Delbauffe D, Pierre M. Induction of 5-deiodinase activity in astroglial cells by 12-O-tetradecanoylphorbol 13-acetate and fibroblast growth factors. J Neurochem. 1991;56(4):1107-1113. [DOI] [PubMed] [Google Scholar]
- 40. Wang YY, Morimoto S, Du CK, et al. Up-regulation of type 2 iodothyronine deiodinase in dilated cardiomyopathy. Cardiovasc Res. 2010;87(4):636-646. [DOI] [PubMed] [Google Scholar]
- 41. Nappi A, Di Cicco E, Miro C, et al. The NANOG transcription factor induces type 2 deiodinase expression and regulates the intracellular activation of thyroid hormone in keratinocyte carcinomas. Cancers (Basel). 2020;12(3):715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zešld A, Doleschall M, Haffner MC, et al. Characterization of the nuclear factor-kappa B responsiveness of the human dio2 gene. Endocrinology. 2006;147(9):4419-4429. [DOI] [PubMed] [Google Scholar]
- 43. Kricker A, Armstrong BK, English DR, Heenan PJ. Does intermittent sun exposure cause basal cell carcinoma? a case-control study in Western Australia. Int J Cancer. 1995;60(4):489-494. [DOI] [PubMed] [Google Scholar]
- 44. Kricker A, Armstrong BK, English DR, Heenan PJ. A dose-response curve for sun exposure and basal cell carcinoma. Int J Cancer. 1995;60(4):482-488. [DOI] [PubMed] [Google Scholar]
- 45. Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15(23):3059-3087. [DOI] [PubMed] [Google Scholar]
- 46. Luongo C, Ambrosio R, Salzano S, Dlugosz AA, Missero C, Dentice M. The sonic hedgehog-induced type 3 deiodinase facilitates tumorigenesis of basal cell carcinoma by reducing Gli2 inactivation. Endocrinology. 2014;155(6):2077-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dentice M, Bandyopadhyay A, Gereben B, et al. The Hedgehog-inducible ubiquitin ligase subunit WSB-1 modulates thyroid hormone activation and PTHrP secretion in the developing growth plate. Nat Cell Biol. 2005;7(7):698-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miro C, Ambrosio R, De Stefano MA, et al. The concerted action of type 2 and type 3 deiodinases regulates the cell cycle and survival of basal cell carcinoma cells. Thyroid. 2017;27(4):567-576. [DOI] [PubMed] [Google Scholar]
- 49. Cicatiello AG, Ambrosio R, Dentice M. Thyroid hormone promotes differentiation of colon cancer stem cells. Mol Cell Endocrinol. 2017;459:84-89. [DOI] [PubMed] [Google Scholar]
- 50. Natsume H, Sasaki S, Kitagawa M, et al. Beta-catenin/Tcf-1-mediated transactivation of cyclin D1 promoter is negatively regulated by thyroid hormone. Biochem Biophys Res Commun. 2003;309(2):408-413. [DOI] [PubMed] [Google Scholar]
- 51. Guigon CJ, Zhao L, Lu C, Willingham MC, Cheng SY. Regulation of beta-catenin by a novel nongenomic action of thyroid hormone beta receptor. Mol Cell Biol. 2008;28(14):4598-4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huang J, Li X, Maguire CA, Hilf R, Bambara RA, Muyan M. Binding of estrogen receptor beta to estrogen response element in situ is independent of estradiol and impaired by its amino terminus. Mol Endocrinol. 2005;19(11):2696-2712. [DOI] [PubMed] [Google Scholar]
- 53. Huang SA, Mulcahey MA, Crescenzi A, et al. Transforming growth factor-beta promotes inactivation of extracellular thyroid hormones via transcriptional stimulation of type 3 iodothyronine deiodinase. Mol Endocrinol. 2005;19(12):3126-3136. [DOI] [PubMed] [Google Scholar]
- 54. Kim BW, Daniels GH, Harrison BJ, et al. Overexpression of type 2 iodothyronine deiodinase in follicular carcinoma as a cause of low circulating free thyroxine levels. J Clin Endocrinol Metab. 2003;88(2):594-598. [DOI] [PubMed] [Google Scholar]
- 55. Meyer EL, Goemann IM, Dora JM, Wagner MS, Maia AL. Type 2 iodothyronine deiodinase is highly expressed in medullary thyroid carcinoma. Mol Cell Endocrinol. 2008;289(1-2):16-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Arnaldi LA, Borra RC, Maciel RM, Cerutti JM. Gene expression profiles reveal that DCN, DIO1, and DIO2 are underexpressed in benign and malignant thyroid tumors. Thyroid. 2005;15(3):210-221. [DOI] [PubMed] [Google Scholar]
- 57. Baur A, Buchfelder M, Köhrle J. Expression of 5’-deiodinase enzymes in normal pituitaries and in various human pituitary adenomas. Eur J Endocrinol. 2002;147(2):263-268. [DOI] [PubMed] [Google Scholar]
- 58. Itagaki Y, Yoshida K, Ikeda H, et al. Thyroxine 5’-deiodinase in human anterior pituitary tumors. J Clin Endocrinol Metab. 1990;71(2):340-344. [DOI] [PubMed] [Google Scholar]
- 59. Freitas BC, Gereben B, Castillo M, et al. Paracrine signaling by glial cell-derived triiodothyronine activates neuronal gene expression in the rodent brain and human cells. J Clin Invest. 2010;120(6):2206-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bunevicius A, Laws ER, Saudargiene A, et al. Common genetic variations of deiodinase genes and prognosis of brain tumor patients. Endocrine. 2019;66(3):563-572. [DOI] [PubMed] [Google Scholar]
- 61. Kaplan MM, Pan CY, Gordon PR, Lee JK, Gilchrest BA. Human epidermal keratinocytes in culture convert thyroxine to 3,5,3’-triiodothyronine by type II iodothyronine deiodination: a novel endocrine function of the skin. J Clin Endocrinol Metab. 1988;66(4):815-822. [DOI] [PubMed] [Google Scholar]
- 62. van Beek N, Bodó E, Kromminga A, et al. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93(11):4381-4388. [DOI] [PubMed] [Google Scholar]
- 63. Slominski A, Pisarchik A, Semak I, Sweatman T, Szczesniewski A, Wortsman J. Serotoninergic system in hamster skin. J Invest Dermatol. 2002;119(4):934-942. [DOI] [PubMed] [Google Scholar]
- 64. Antonini D, Sibilio A, Dentice M, Missero C. An intimate relationship between thyroid hormone and skin: regulation of gene expression. Front Endocrinol (Lausanne). 2013;4:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Huang MP, Rodgers KA, O’Mara R, et al. The thyroid hormone degrading type 3 deiodinase is the primary deiodinase active in murine epidermis. Thyroid. 2011;21(11):1263-1268. [DOI] [PubMed] [Google Scholar]
- 66. Dentice M, Ambrosio R, Salvatore D. Role of type 3 deiodinase in cancer. Expert Opin Ther Targets. 2009;13(11):1363-1373. [DOI] [PubMed] [Google Scholar]
- 67. Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26(10):1657-1667. [DOI] [PubMed] [Google Scholar]
- 68. Ratushny V, Gober MD, Hick R, Ridky TW, Seykora JT. From keratinocyte to cancer: the pathogenesis and modeling of cutaneous squamous cell carcinoma. J Clin Invest. 2012;122(2):464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sánchez-Danés A, Blanpain C. Deciphering the cells of origin of squamous cell carcinomas. Nat Rev Cancer. 2018;18(9):549-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344(13):975-983. [DOI] [PubMed] [Google Scholar]
- 71. Chung CH, Parker JS, Karaca G, et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell. 2004;5(5):489-500. [DOI] [PubMed] [Google Scholar]
- 72. Cohen EE, Zhu H, Lingen MW, et al. A feed-forward loop involving protein kinase Calpha and microRNAs regulates tumor cell cycle. Cancer Res. 2009;69(1):65-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. de Souza Meyer EL, Dora JM, Wagner MS, Maia AL. Decreased type 1 iodothyronine deiodinase expression might be an early and discrete event in thyroid cell dedifferentation towards papillary carcinoma. Clin Endocrinol (Oxf). 2005;62(6):672-678. [DOI] [PubMed] [Google Scholar]
- 74. Toyoda N, Nishikawa M, Mori Y, et al. Identification of a 27-kilodalton protein with the properties of type I iodothyronine 5’-deiodinase in human thyroid gland. J Clin Endocrinol Metab. 1992;74(3):533-538. [DOI] [PubMed] [Google Scholar]
- 75. Schreck R, Schnieders F, Schmutzler C, Köhrle J. Retinoids stimulate type I iodothyronine 5’-deiodinase activity in human follicular thyroid carcinoma cell lines. J Clin Endocrinol Metab. 1994;79(3):791-798. [DOI] [PubMed] [Google Scholar]
- 76. Maia AL, Goemann IM, Meyer EL, Wajner SM. Deiodinases: the balance of thyroid hormone: type 1 iodothyronine deiodinase in human physiology and disease. J Endocrinol. 2011;209(3):283-297. [DOI] [PubMed] [Google Scholar]
- 77. Pachucki J, Ambroziak M, Tanski Z, Luczak J, Nauman J, Nauman A. Type I 5’-iodothyronine deiodinase activity and mRNA are remarkably reduced in renal clear cell carcinoma. J Endocrinol Invest. 2001;24(4):253-261. [DOI] [PubMed] [Google Scholar]
- 78. Sabatino L, Iervasi G, Ferrazzi P, Francesconi D, Chopra IJ. A study of iodothyronine 5’-monodeiodinase activities in normal and pathological tissues in man and their comparison with activities in rat tissues. Life Sci. 2000;68(2):191-202. [DOI] [PubMed] [Google Scholar]
- 79. Wawrzynska L, Sakowicz A, Rudzinski P, Langfort R, Kurzyna M. The conversion of thyroxine to triiodothyronine in the lung: comparison of activity of type I iodothyronine 5’ deiodinase in lung cancer with peripheral lung tissues. Monaldi Arch Chest Dis. 2003;59(2):140-145. [PubMed] [Google Scholar]
- 80. Dutkiewicz S, Witeska A, Stepień K. Relationship between prostate-specific antigen, prostate volume, retention volume and age in benign prostatic hypertrophy (BPH). Int Urol Nephrol. 1995;27(6):763-768. [DOI] [PubMed] [Google Scholar]
- 81. Master A, Wójcicka A, Piekiełko-Witkowska A, et al. Untranslated regions of thyroid hormone receptor beta 1 mRNA are impaired in human clear cell renal cell carcinoma. Biochim Biophys Acta. 2010;1802(11):995-1005. [DOI] [PubMed] [Google Scholar]
- 82. Popławski P, Wiśniewski JR, Rijntjes E, et al. Restoration of type 1 iodothyronine deiodinase expression in renal cancer cells downregulates oncoproteins and affects key metabolic pathways as well as anti-oxidative system. PLoS One. 2017;12(12):e0190179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bogusławska J, Rodzik K, Popławski P, et al. TGF-β1 targets a microRNA network that regulates cellular adhesion and migration in renal cancer. Cancer Lett. 2018;412:155-169. [DOI] [PubMed] [Google Scholar]
- 84. Piekielko-Witkowska A, Master A, Wojcicka A, et al. Disturbed expression of type 1 iodothyronine deiodinase splice variants in human renal cancer. Thyroid. 2009;19(10):1105-1113. [DOI] [PubMed] [Google Scholar]
- 85. Boguslawska J, Wojcicka A, Piekielko-Witkowska A, Master A, Nauman A. MiR-224 targets the 3’UTR of type 1 5’-iodothyronine deiodinase possibly contributing to tissue hypothyroidism in renal cancer. PLoS One. 2011;6(9):e24541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Charalabopoulos K, Kotsalos A, Batistatou A, et al. Serum and tissue selenium levels in gastric cancer patients and correlation with CEA. Anticancer Res. 2009;29(8):3465-3467. [PubMed] [Google Scholar]
- 87. Steevens J, van den Brandt PA, Goldbohm RA, Schouten LJ. Selenium status and the risk of esophageal and gastric cancer subtypes: the Netherlands cohort study. Gastroenterology. 2010;138(5):1704-1713. [DOI] [PubMed] [Google Scholar]
- 88. Lan X, Xing J, Gao H, et al. Decreased expression of selenoproteins as a poor prognosticator of gastric cancer in humans. Biol Trace Elem Res. 2017;178(1):22-28. [DOI] [PubMed] [Google Scholar]
- 89. Macejová D, Líska J, Brtko J. Mammary gland carcinoma-related increase of type I iodothyronine 5’-deiodinase activity in Sprague-Dawley rats. Gen Physiol Biophys. 2001;20(3):293-302. [PubMed] [Google Scholar]
- 90. Vázquez-Landaverde LA, Rojas-Huidobro R, Alonso Gallegos-Corona M, Aceves C. Periodontal 5’-deiodination on forced-induced root resorption–the protective effect of thyroid hormone administration. Eur J Orthod. 2002;24(4):363-369. [DOI] [PubMed] [Google Scholar]
- 91. Aceves C, Rojas-Huidobro R. Effect of suckling and adrenergic stimulation on peripheral deiodination in lactating rats: differential expression of type 1 deiodinase mRNA forms. J Endocrinol. 2001;171(3):533-540. [DOI] [PubMed] [Google Scholar]
- 92. García-Solís P, Aceves C. 5’Deiodinase in two breast cancer cell lines: effect of triiodothyronine, isoproterenol and retinoids. Mol Cell Endocrinol. 2003;201(1-2):25-31. [DOI] [PubMed] [Google Scholar]
- 93. Goemann IM, Marczyk VR, Recamonde-Mendoza M, Wajner SM, Graudenz MS, Maia AL. Decreased expression of the thyroid hormone-inactivating enzyme type 3 deiodinase is associated with lower survival rates in breast cancer. Sci Rep. 2020;10(1):13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.