Abstract
Fibroblast growth factor 19 (FGF19) is a protein hormone that produces antidiabetic effects when administered intracerebroventricularly in the forebrain. However, no studies have examined how FGF19 affects hindbrain neurons that participate directly in autonomic control of systemic glucose regulation. Within the dorsal hindbrain, parasympathetic motor neurons of the dorsal motor nucleus of the vagus (DMV) express fibroblast growth factor receptors and their activity regulates visceral homeostatic processes, including energy balance. This study tested the hypothesis that FGF19 acts in the hindbrain to alter DMV neuron excitability and lower blood glucose concentration. Fourth ventricle administration of FGF19 produced no effect on blood glucose concentration in control mice, but induced a significant, peripheral muscarinic receptor-dependent decrease in systemic hyperglycemia for up to 12 h in streptozotocin-treated mice, a model of type 1 diabetes. Patch-clamp recordings from DMV neurons in vitro revealed that FGF19 application altered synaptic and intrinsic membrane properties of DMV neurons, with the balance of FGF19 effects being significantly modified by a recent history of systemic hyperglycemia. These findings identify central parasympathetic circuitry as a novel target for FGF19 and suggest that FGF19 acting in the dorsal hindbrain can alter vagal output to produce its beneficial metabolic effects.
Keywords: autonomic, diabetes, EPSC, fibroblast growth factor, hyperglycemia, vagus nerve
Fibroblast growth factor 19 (FGF19) is a postprandially released hormone that is closely linked to metabolic homeostasis. When administered to the lateral or third cerebral ventricles [intracerebroventricular (ICV)], FGF19 improves insulin sensitivity, decreases food intake, and decreases blood glucose concentration in an insulin-independent fashion in both type I and type II diabetes (T1DM and T2DM, respectively) models (1,2). Autonomic or neuroendocrine mechanisms have been proposed to mediate these effects (1,3,4). While previous studies have focused on the effects of FGF19 in the hypothalamus, studies identifying the direct actions of FGF19 in the brainstem dorsal vagal complex (DVC), the primary parasympathetic regulatory center, have not been performed.
The DVC is principally comprised of the area postrema, nucleus tractus solitarius (NTS), and DMV. Vagal afferents convey viscerosensory information to second-order sensory neurons in the NTS, which integrate this information with neural input from other brain areas and effects of humoral factors. NTS neurons make excitatory and inhibitory connections with DMV motor neurons, whose axons comprise the efferent vagus nerve and regulate visceral homeostatic processes that regulate blood glucose concentration (5-11).
The DVC contains fenestrated capillaries that allow diffusion of humoral components, potentially including peptides like FGF19, which might typically be excluded by the blood-brain barrier (12,13). DVC neurons respond to changing glucose concentration as well as several metabolic hormones, including leptin, insulin, glucagon, and GLP-1 (14-18). Importantly, FGF receptors 1 and 3 (FGFR1 and FGFR3, respectively) and β-klotho (an obligate co-receptor) are expressed in the DVC, which suggests that DVC neurons participate in endogenous FGF signaling (19-21). Manipulation of neuronal activity in the DVC is directly linked to changes in blood glucose concentration (6,9). Thus, alteration of neuronal activity in this area by FGF19 could contribute to regulation of systemic blood glucose. This study tests the hypothesis that FGF19 affects neural excitability in the DVC to lower blood glucose concentration. Identifying antidiabetic effects of FGF19 in the brainstem will improve understanding of how blood glucose can be regulated via central mechanisms.
Research Design and Methods
Animals
Experiments were performed on juvenile (3-8 weeks old) male and female FVB mice [FVB-Tg(GadGFP)4570Swn/J, FVB; Jackson Laboratory, Bar Harbor, ME, USA] housed in the University of Kentucky Division of Laboratory Animal Resources facilities under normal 14:10 light-dark conditions with food (Teklad 2018) and water available ad libitum, except where noted. Roughly equal numbers of males and females were used and results from both sexes were aggregated. The University of Kentucky Animal Care and Use Committee approved all animal procedures.
To induce necrosis of insulin-secreting pancreatic ß-cells, mice were fasted for 6 h prior to receiving an intraperitoneal injection (i.p.; 0.15 mL) of either citric acid vehicle (0.1 M) or streptozotocin in citric acid vehicle (STZ; 200 mg/kg; Sigma-Aldrich, St. Louis, MO, USA). After injection, mice were returned to their home cages. Blood glucose was monitored by tail lance (Nova Max Plus, Nova Diabetes Care, Billerica, MA, USA), and animals were used for experiments after ≥5 days (range: 5-19 days) of sustained hyperglycemia (≥300 mg/dL) (22,23). Similar periods of continuous hyperglycemia have been associated with persistent changes in intrinsic and synaptic properties of NTS and DMV neurons (22-27). Mice with sustained hyperglycemia after STZ injection were considered a model of early T1DM.
Intracranial injection
Fasted mice (2 h) were anesthetized using isoflurane (5% induction, 3% maintenance) and placed in a stereotaxic frame. Mice were kept on a heating pad during surgery to prevent hypothermia. The skull was exposed using a midline incision, and a ~2 mm diameter midline craniotomy was made 2 mm posterior to lambda. Vehicle [VEH; 1 µL, phosphate-buffered saline (PBS)] or FGF19 (3 µg) in VEH was delivered to the fourth ventricle (4V) using a 5 µL syringe equipped with a 26-gauge flat-tipped needle (Hamilton, Reno, NV, USA) over a period of 10 min. The needle was allowed to stay in place for 3 min before withdrawing. After surgery, mice were returned to their home cage and given buprenorphine (0.1 mg/kg, analgesic). Mice recovered rapidly from surgery and resumed normal feeding behavior within 10 min of anesthesia withdrawal. Blood glucose was measured 30 min prior to and at 10 min and 6, 12, and 24 h after 4V injection. The first postsurgery analysis time point (6 h) was chosen to allow for sufficient time to recover from surgery. Similar experiments were performed with the addition of (-)-scopolamine methyl bromide [methylscopolamine (MSA); 1 mg/kg; i.p.] to block peripheral muscarinic receptors and consequent parasympathetic output (9). MSA was administered 30 min prior to and every 2 h after surgery until the conclusion of the experiment (6 h postsurgery).
Electrophysiology
Mice were anesthetized with isoflurane inhalation to effect (ie, lack of foot pinch response) and decapitated. The brain was rapidly removed and submerged in ice-cold, oxygenated (2°C-4°C; 95% O2/5% CO2) artificial cerebrospinal fluid (ACSF) composed of (in mM): 124 NaCl, 3 KCl, 26 NaHCO3, 1.4 NaH2PO4, 11 glucose, 1.3 CaCl2, and 1.3 MgCl2. The hindbrain was mounted to a sectioning stage and coronal brainstem slices (300 µm) were cut using a vibratome (Series 1000; Technical Products International, St. Louis, MO, USA). Slices were transferred to a holding chamber and incubated for 1 h in warmed (30°C-33°C), oxygenated ACSF. For experiments, a single slice was transferred to the recording chamber on a fixed-stage, upright microscope (BX51WI; Olympus, Melville, NY, USA) and continuously superfused with warmed (30°C-33°C) ACSF, identical to the slicing ACSF except when drugs were added, as described.
Whole-cell, patch-clamp recordings were performed under visual control using infrared illumination and differential interference contrast optics. Glass recording pipettes (1.65 mm OD, 1.2 mm ID; King Precision Glass, Claremont, CA, USA) were filled with a solution containing (in mM): 130 K-gluconate (or Cs-gluconate), 1 NaCl, 5 EGTA, 10 HEPES, 1 MgCl2, 1 CaCl2, 3 KOH, and 2 Mg-ATP; pH = 7.2-7.3, adjusted with 5 M KOH (or CsOH). In some experiments, Cs+ was used as the primary cation charge carrier in the recording pipette, which prevents K+ current-dependent drug effects. Open tip resistance was 3 to 5 MΩ; seal resistance was 1 to 7 GΩ. For cell-attached recordings, pipettes were filled with 150 mM NaCl. Neuronal activity was recorded using a Multiclamp 700B amplifier, Digidata 1440A digitizer, and pClamp 10.6 software (Molecular Devices, Axon Instruments, Sunnyvale, CA, USA). Data were recorded at 20 kHz and filtered at 3 kHz.
FGF19 (ProspecBio, Ness Ziona, Israel) was bath applied at 230 pM. This concentration was shown to stimulate approximately half-maximal glucose uptake and phosphorylated extracellular signal-related kinase (pERK) induction in cell culture assays (28). FGF19 was applied only once per slice to prevent the influence of potential long-term effects. Added to the ACSF for specific experiments were the following: tetrodotoxin (TTX; 2µM; Alomone Labs, Jerusalem, Israel), kynurenic acid (1 mM; Sigma-Aldrich), 4-aminopyridine (4-AP; 5µM; Sigma-Aldrich), tetraethylammonium chloride (TEA; 10µM; Sigma-Aldrich), and picrotoxin (100 µM; Alomone Labs). Incubation time for FGF19 was 5 min; antagonists or channel blockers were applied for ≥10 min prior to and during agonist application.
DMV neurons were identified by their morphology (>20 µm soma width, multipolar) and location in the slice (29,30). Once in whole-cell configuration, neurons were held near the resting membrane potential (RMP) for 10 min to allow equilibration of the pipette solution and cytoplasm. Voltage values were corrected post-hoc to account for the liquid junction potential (−15 mV). Excitatory postsynaptic currents (EPSCs) were recorded at a holding potential of −85 mV and inhibitory postsynaptic currents (IPSCs) were recorded at a holding potential of −15 mV. For event-based recordings (eg, action potentials, EPSCs), 3 min of continuous activity (typically 300-3000 events) was examined. In these recordings, neurons were rejected if the initial event frequency was less than 0.4 Hz to ensure an adequate number of events for analysis. Input resistance (Rin) was measured in current-clamp mode from responses to 500 ms current steps ranging from −20 to 0 pA in 5 pA increments; Rin was calculated as the slope of the line that best fit these points using linear regression. A peptide-induced change in input resistance of more than 20% was considered responsive.
Potassium currents were measured in neurons voltage-clamped at −75 mV using a voltage step protocol. The protocol consisted of a 500 ms step to −125 mV, followed by a 3000 ms depolarizing step to the test potential, after which the cell was returned to −75 mV. The test potentials ranged from −75 mV to +35 mV in 10 mV increments. Peak current was measured at the first 50 ms of the depolarizing step and steady-state current was measured over the last 500 ms of the step, once the current had saturated. Peak values were recorded as the difference between peak and steady-state amplitudes within the same step. When the peak current was blocked (ie, 4-AP or intracellular Cs+), only the steady-state value was measured. Acceptable series resistance was considered to be <25 MΩ (range = 3.48-23.57 MΩ; mean = 12.88 ± 0.32 MΩ) and was regularly monitored; recordings were discarded if series resistance or cell capacitance changed by ≥20% during recording.
Immunofluorescence
DMV neurons were recovered by staining for biocytin (0.2% added to the internal solution; Sigma-Aldrich). After recording, brainstem slices were fixed overnight in 4% paraformaldehyde in 0.15 M phosphate buffer. Slices were rinsed 3 times with 0.01 M PBS (Sigma-Aldrich) and immersed in avidin conjugated to AMCA Avidin-D (1:400; Vector Laboratories) in PBS containing 1% Triton X-100 for 3 to 4 h at room temperature. Slices were then rinsed 3 times with PBS and then visualized to identify slices that contained biocytin-filled neurons. Slices containing biocytin-filled neurons were placed in a 30% sucrose solution in PBS overnight for cryoprotection, sectioned on a cryostat at 20 µm thickness, and rinsed with PBS. The sections were then immersed in 5% normal goat serum in PBS for 30 min and then in PBS containing 1% normal goat serum, 0.5% Triton X-100, and FGFR1 antibody (rabbit monoclonal antibody; 1:500; Cell Signaling Technologies 9740T) (31) for 24 h; rinsed for 15 min 3 times with PBS; and immersed in a goat antirabbit secondary antibody (1:400; Alexafluor 568; Invitrogen, Carlsbad, CA, USA) (32). Sections were washed again for 15 min 3 times with PBS and then mounted on slides and air dried, covered with Vectashield H-1000 (Vector Laboratories, Burlingame, CA, USA), and coverslipped. Sections were visualized using epifluorescence (BX-41; Olympus) and imaged using a Spot RT camera and software (Diagnostic Instruments, Sterling Heights, MI, USA). Final images were adjusted for brightness and contrast for illustrative clarity only. Omission of either the primary or secondary antibody resulted in no labeling (n = 4).
Statistics and analysis
Recordings were analyzed using pClamp 10.6 (Axon Instruments), Minianalysis 6.0.7 (Synaptosoft, Decatur, GA, USA), and Prism 8 (GraphPad Software, San Diego, CA, USA). Within-cell analysis of multi-event recordings (eg, EPSCs before and after drug application) was performed using the 2-sample Kolmogorov-Smirnov (K-S) test. Grouped analyses were performed using a Student’s t-test, Wilcoxon matched pairs test, or 1-way analysis of variance, as appropriate. In vivo glucose measurements were analyzed using a repeated-measures 2-way analysis of variance with Tukey multiple comparisons test. Significance was set at P < 0.05 for all analyses.
Results
Hindbrain application of FGF19 decreases blood glucose concentration in hyperglycemic mice via a parasympathetic mechanism
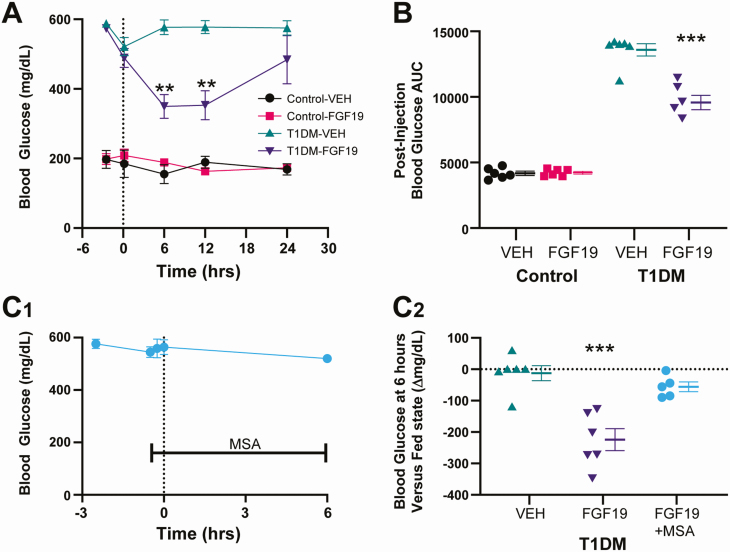
To determine whether FGF19 alters blood glucose concentration by interacting with the dorsal hindbrain, FGF19 (3 µg) was administered via 4V infusion in fasted control or STZ-treated mice with sustained (≥ 5 days), continuous hyperglycemia (ie, T1DM mice). Blood glucose concentration was measured 2 h before injection (ie, before fasting) and at 10 min and 6, 12, and 24 h after 4V administration. In control mice, FGF19 did not significantly alter blood glucose concentration at any time point after injection, compared to VEH (n = 12; P > 0.05) (Fig. 1A). In T1DM mice, FGF19 significantly decreased blood glucose concentration at 6 and 12 h post-4V administration, compared to VEH injection (6 h: VEH, 577.17 ± 21.273 mg/dL; FGF19, 349.66 ± 33.98 mg/dL; n = 12; P < 0.01; 12 h: VEH, 577.67 ± 18.45 mg/dL; FGF19, 353.00 ± 41.763 mg/dL; n = 12; P < 0.01) (Fig. 1A). Blood glucose returned to pre-injection concentration by the 24-h time point and was not statistically significant relative to VEH (P > 0.05). FGF19 also decreased the postinjection glucose area under the curve in T1DM mice (VEH, 13 588 ± 467 mg·h/dL; FGF19, 9576 ± 544 mg·h/dL; P < 0.0001) (Fig. 1B). FGF19 did not significantly alter area under the curve in control mice (VEH, 4170 ± 166 mg·h/dL; FGF19, 4233 ± 99 mg·h/dL; P > 0.05). FGF19 infusion into 4V therefore significantly reduced blood glucose concentration in hyperglycemic, but not normoglycemic mice.
Figure 1.
Hindbrain application of FGF19 decreases blood glucose concentration in diabetic mice through a parasympathetic mechanism. (A) Blood glucose concentration measured 2 h before injection (before fasting), and at 10 min and 6, 12, and 24 h after 4V microinjection of VEH (1 µL PBS) or FGF19 (3 µg in VEH) in normoglycemic (control) and hyperglycemic mice with T1DM. Asterisk indicates significance vs VEH for T1DM mice (**P < 0.01; n = 24; repeated measures 2-way analysis of variance with Tukey’s multiple comparison test). (B) Postinjection blood glucose area under the curve values calculated for the data shown in (A). Asterisk indicates significance vs VEH (***P < 0.001; n = 24; 1-way analysis of variance). (C1) Blood glucose concentrations measured at −2.5 h (fed), −30 min(fasted), −15 min (after MSA injection), 0 min, and 6 h in relation to FGF19 injection (C2) Change in blood glucose from fed state to 6-h time point. Pretreatment with systemic MSA prevented the glucose lowering effect of FGF19. VEH and FGF19 group data were calculated from (A). Asterisk indicates significance vs VEH (***P < 0.001; n = 17; unpaired t-test).
Since 4V administration of FGF19, but not VEH, significantly reduced blood glucose concentration in T1DM mice, the involvement of parasympathetic output in the response was determined in a cohort of T1DM mice that were pretreated with MSA (1 mg/kg; i.p.) prior to FGF19 application (n = 5) (Fig. 1C). MSA blocks peripheral muscarinic receptors mediating parasympathetic output to the viscera but does not cross the blood-brain barrier to block central muscarinic receptors (33). MSA may also prevent activation of sympathetic superior cervical ganglion neurons (34), and sympathetic activation can be associated with an increase in blood glucose concentration (35). Considering this, a peripheral sympathetic blockade might be expected to decrease blood glucose. However, pretreatment with MSA in fasted T1DM mice was not found to significantly alter blood glucose concentration (fasted, 544 ± 20.1 mg/dL; fasted+MSA, 558 ± 35.7 mg/dL; n = 5; P > 0.05) (Fig. 1C1). The effect of 4V administration of FGF19 on blood glucose was abolished in mice pre-treated with MSA (P > 0.05 (Fig. 1C), indicating that the glucose-lowering effects of 4V infused FGF19 were most likely mediated by parasympathetic activity.
Differential effects of FGF19 on synaptic excitability
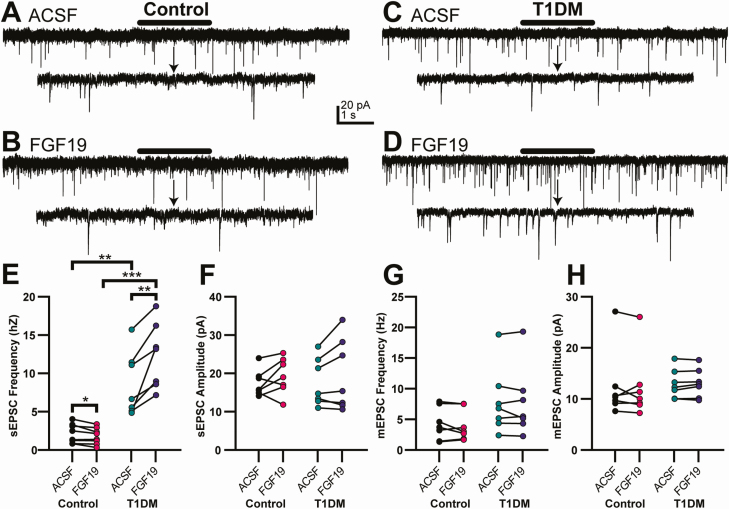
Whole-cell patch-clamp recordings were made from DMV neurons in acutely prepared brainstem slices to assess the effects of FGF19 on excitatory synaptic transmission. DMV neurons were voltage-clamped at −85 mV and spontaneous EPSCs (sEPSCs) were recorded while bath-applying FGF19 (230 pM) (Fig. 2). In neurons from control mice, FGF19 significantly decreased sEPSC frequency in 4 out 8 neurons, determined by within-recording K-S test. Overall, mean sEPSC frequency was modestly, but significantly reduced (19%) by FGF19 (ACSF, 2.152 ± 0.44 Hz; FGF19, 1.807 ± 0.37 Hz; n = 8; P < 0.05) (Fig. 2E). There was no significant effect of FGF19 on sEPSC amplitude (ACSF, 21.45 ± 4.28 pA; FGF19, 22.91 ± 2.49 pA; n = 7; P > 0.05) (Fig. 2F).
Figure 2.
FGF19 decreases sEPSC frequency in neurons of the DMV in slices from normoglycemic control mice and increases sEPSC frequency in DMV neurons from hyperglycemic T1DM mice. (A) Voltage clamp recordings of sEPSCs in a DMV neuron from a control mouse and (B) after addition of FGF19 (230 pM). (C) Recording of sEPSCs in a DMV neuron from a T1DM mouse and (D) in FGF19. Lower traces indicated by arrows in (A-D) are expanded from the area indicated with a bar in the top traces in each set; all sEPSC measurements were from neurons voltage-clamped at −85 mV. (E) FGF19 significantly decreased mean sEPSC frequency in control mice (n = 8) and increased mean sEPSC frequency in T1DM mice (n = 7). sEPSC frequency was significantly greater in neurons from T1DM mice than from control mice (*P < 0.05; **P < 0.01; ***P < 0.001). (F) FGF19 did not affect mean sEPSC amplitude in control (n = 8) or T1DM mice (n = 7; P > 0.05). sEPSCs were recorded from control, 2 male and 2 female, and T1DM, 3 male and 2 female mice. In the presence of TTX (2 µM), FGF19 did not affect mean mEPSC frequency (G) or amplitude (H) in control (n = 7; P > 0.05) or T1DM mice (n = 7; P > 0.05). mEPSCs were recorded from control, 2 male and 2 female, and T1DM, 2 male and 2 female mice.
Consistent with previous reports, sEPSC frequency was significantly greater in DMV neurons from T1DM mice than in controls (P < 0.05) (24,26). In DMV neurons from T1DM mice, FGF19 consistently and significantly increased sEPSC frequency in each of 7 neurons (P < 0.02; K-S test) (Fig. 2E). Mean sEPSC frequency was significantly increased by 43% in the presence of FGF19 (ACSF, 8.63 ± 1.57 Hz; FGF19, 12.33 ± 1.62 Hz; n = 7; P < 0.02) (Fig. 2E). There was no significant change in sEPSC amplitude (ACSF, 17.70 ± 2.34 pA; FGF19, 19.55 ± 3.52 pA; P > 0.05) (Fig. 2F). Thus, FGF19 led to a modest, but significant decrease in excitatory synaptic input to DMV neurons in slices from normoglycemic mice, but the peptide further increased the already enhanced excitatory synaptic drive to DMV neurons from T1DM mice.
To determine whether the effects of FGF19 on EPSC frequency were due to activation of receptors located on presynaptic terminals, miniature EPSCs (mEPSCs) were recorded in the presence of TTX (2 µM), a blocker of action potential-dependent synaptic activity. In control mice, there was no significant change in mean mEPSC frequency during FGF19 application (ACSF, 4.26 ± 1.00 Hz; FGF19, 4.00 ± 0.95 Hz; n = 7; P > 0.05) (Fig. 2G), although significant changes in frequency could be detected in four of seven neurons (increase, n = 2; decrease, n = 2; P < 0.02; K-S test). There was no significant difference in mean mEPSC amplitude (ACSF, 12.4 ± 2.51 pA; FGF19, 12.2 ± 2.39 pA; n = 7; P > 0.05) (Fig. 2H).
FGF19 also produced no significant change in overall mEPSC frequency in T1DM mice (ACSF, 7.93 ± 2.06 Hz; FGF19, 7.77 ± 2.14 Hz; n = 7; P > 0.05) (Fig. 2G). In this group, only 1 neuron produced a significant change of mEPSC frequency as measured by the K-S test (P < 0.01). Similarly, there was no significant difference in mEPSC amplitude (ACSF, 12.9 ± 1.08 pA; FGF19, 13.1 ± 1.05 pA; P > 0.05) (Fig. 2H). FGF19 therefore modestly decreased excitatory synaptic input to DMV neurons in control mice but significantly increased sEPSC frequency in DMV neurons from T1D mice, and these effects were mostly prevented when action potential-dependent synaptic activity was blocked.
To assess possible effects of FGF19 on inhibitory synaptic activity, spontaneous IPSCs (sIPSCs) were recorded. No effects on sIPSC frequency were observed in control (ACSF, 4.19 ± 1.06 Hz; FGF19, 3.87 ± 1.16 Hz; n = 5; P > 0.05) or T1DM mice (ACSF, 2.72 ± 1.07 Hz; FGF19, 2.53 ± 0.96 Hz; n = 5; P > 0.05). Additionally, baseline sIPSC frequency was not significantly different between the two groups (control; 4.19 ± 1.06 Hz; T1DM; 2.72 ± 1.07 Hz; P > 0.05; n = 10). Similarly, FGF19 did not change sIPSC amplitude in control (ACSF, 29.5 ± 3.67 pA; FGF19, 25.7 ± 4.56 pA; n = 5; P > 0.05) or T1DM mice (ACSF, 33.5 ± 1.74 pA; FGF19, 27.4 ± 3.56 pA; n = 5; P > 0.05). FGF19 therefore selectively modulated glutamate release in the DMV, being decreased in control and increased in T1DM mice, with no significant effect on inhibitory synaptic activity.
Effects on resting membrane potential and input resistance
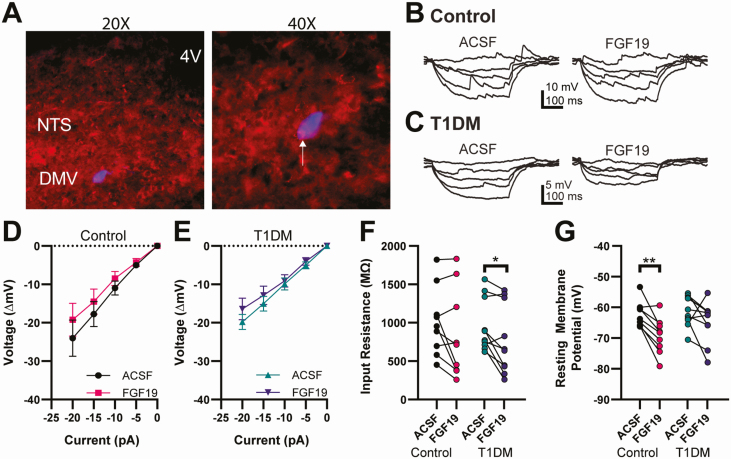
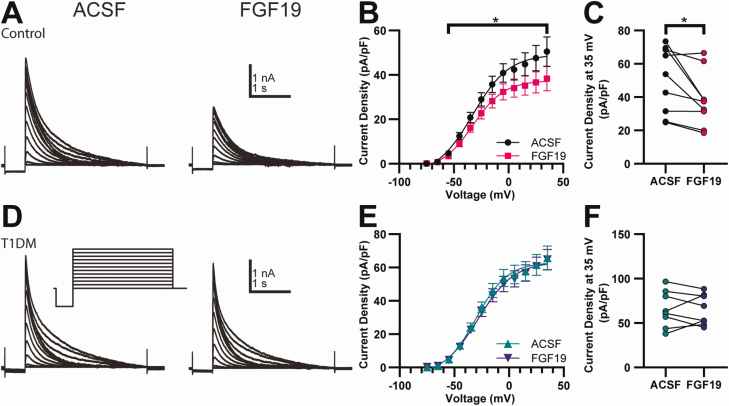
To confirm the presence of FGF receptors in DMV neurons, immunolabeling of FGFR1 was performed. Figure 3A shows an example of a biocytin filled DMV neuron and FGFR1-like immunoreactivity in the DVC. To investigate the effects of FGF19 on membrane properties of DMV neurons, input resistance (Rin) and RMP were recorded in current-clamp mode in the presence of TTX (2 µM) to prevent spontaneous action potentials (Fig. 3B-3G). Neither RMP nor Rin differed significantly between control and T1DM mice (P > 0.05). In control mice, FGF19 significantly hyperpolarized the RMP whereas in T1DM mice, FGF19 did not significantly alter mean RMP (Table 1). Despite the lack of an overall effect, 7/10 T1DM neurons were hyperpolarized by >3 mV, suggesting inhibition of most neurons. In control mice, the overall effect of FGF19 on Rin was not significant, whereas in T1DM FGF19 significantly decreased overall Rin (Table 1). In DMV neurons from both control and T1DM mice, FGF19 induced a >20% decrease Rin in approximately half of neurons in each group. Excluding 1 neuron in the T1DM group that depolarized in response to FGF19, a decrease in Rin was always accompanied by a concomitant hyperpolarization of RMP, suggesting the opening of a channel at RMP. The predominant effects of FGF19 on RMP and Rin were therefore inhibitory, consisting of a membrane hyperpolarization and decrease in Rin, in DMV neurons from both normoglycemic and hyperglycemic mice.
Figure 3.
Effects on input resistance and RMP. (A) Fluorescence images showing a recorded, biocytin-filled DMV neuron (blue) and immunofluorescence staining for FGFR1 (red) at 2 magnifications. Arrow indicates an area of label overlap (purple), suggestive of FGFR1 localization on the recorded DMV neuron. (B) Current injection recordings of DMV neurons from a control mouse and (C) T1DM mouse. (D) I-V plots for responses of DMV neurons to FGF19 application from control mice (n = 9) and (E) T1DM mice (n = 10). The slope of the lines in (D) and (E) reflect input resistance. (F) FGF19 did not alter mean input resistance in control mice (n = 9; P > 0.05) but significantly decreased input resistance in T1DM mice (n = 10; *P < 0.05). (G) FGF19 significantly hyperpolarized neurons from control mice (n = 9; **P < 0.01) but did not alter mean RMP in T1DM mice (n = 10; P > 0.05). All recordings in TTX (2 µM). Recorded from control, 2 male and 2 female, and T1DM, 4 male and 2 female mice.
Table 1.
FGF19 effects on intrinsic membrane properties
| Control mice | T1DM mice | |||||||
|---|---|---|---|---|---|---|---|---|
| ACSF | ACSF+FGF19 | P-value | n | ACSF | ACSF+FGF19 | P-value | n | |
| RMP (mV) | −62.3 ± 1.40 | −69.3 ± 1.92 | P < 0.01 | 9 | −61.2 ± 1.55 | −64.7 ± 2.10 | n.s. | 10 |
| Rin (MΩ) | 1013 ± 148 | 844 ± 193 | n.s. | 9 | 964 ± 109 | 771 ± 142 | P < 0.05 | 10 |
| Action potential frequency (Hz) | 0.99 ± 0.112 | 1.12 ± 0.171 | n.s. | 14 | 1.46 ± 0.277 | 1.06 ± 0.274 | P < 0.05 | 8 |
Action potential frequency
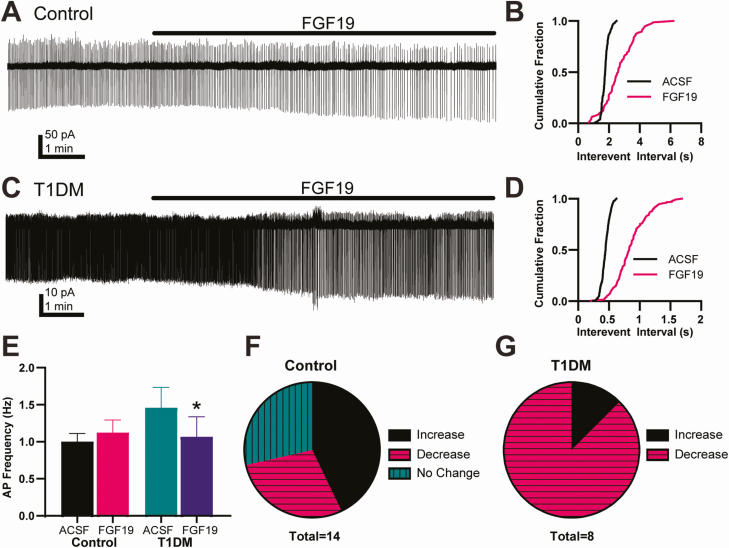
To investigate the effects of FGF19 on spontaneous action potential current (IAP) frequency, neurons were recorded in cell-attached configuration (Fig. 4). In control mice, there was no significant change in mean IAP frequency during FGF19 application (Table 1). However, IAP frequency in individual neurons was significantly altered (increased, n = 6; decreased n = 4; P < 0.02 K-S test). In T1DM mice, there was a significant decrease in mean IAP frequency during FGF19 application (Table 1). IAP frequency in individual neurons was predominantly decreased (n = 7; P < 0.02; K-S test), with an increase in frequency in one neuron.
Figure 4.
FGF19 variably affects action potential frequency. (A) Representative traces showing effects of FGF19 on spontaneous sodium-dependent action potential currents (IAP) in cell-attached recordings in a DMV neuron from a control mouse. (B) Cumulative probability plot of the traces in (A). (C) Representative traces showing effects of FGF19 on IAP frequency in a DMV neuron from a T1DM mouse. (D) Cumulative probability plot of the traces in (C). (E) FGF19 did not alter mean IAP frequency in control mice (n = 14; P > 0.05) but significantly decreased IAP frequency in T1DM mice (n = 8; P < 0.05). Asterisk indicates significance vs ACSF. (F) Relative proportion of neurons with responses to FGF19 application in neurons from control mice indicate variable responses. (G) Proportions of responses in T1DM mice suggests a that FGF19 decreases IAP frequency in most neurons. Recorded from control, 2 male and 2 female, and T1DM, 3 male and 2 female mice.
FGF19 decreases A-type K+ current amplitude in control but not in T1DM mice
Since inconsistent effects of FGF19 were observed on IAP firing, we investigated the possibility that FGF19 altered voltage-gated K+-current amplitude, in addition to its effects on passive membrane properties of DMV neurons. To determine the effects of FGF19 on voltage-gated K+ current amplitudes, a step protocol was performed in voltage-clamp mode in the presence of TTX (2 µM), picrotoxin, a γ-aminobutyric acid (GABA) type A receptor blocker (100 µM) and kynurenic acid, an ionotropic glutamate receptor blocker (1 mM). To either block or help isolate the A-type current, ACSF included either 4-AP, an A-type current blocker (5 mM) or TEA, a delayed-rectifier current blocker (10 mM). To adjust for any potential differences in cell size, current values were normalized to cell capacitance to yield current density (pA/pF).
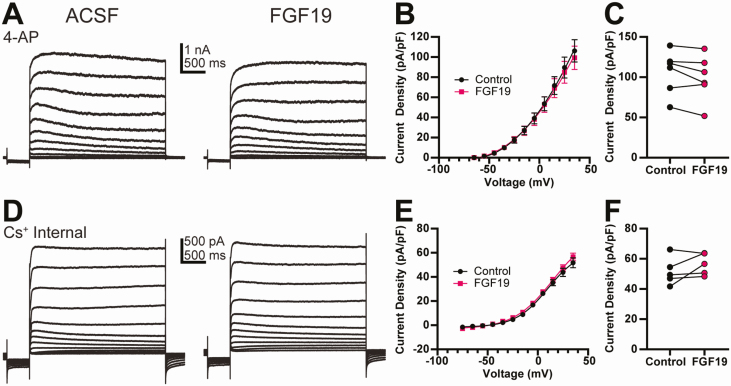
In the presence of TEA, FGF19 significantly decreased the amplitude of the peak current density measured at 50 ms in DMV neurons from control mice (ACSF, 50.5 ± 6.61 pA/pF; FGF19, 38.3 ± 5.46 pA/pF; n = 9; P < 0.05) (Fig. 5). Unlike in controls, FGF19 produced no significant change in peak current density in neurons from T1DM mice (ACSF, 65.7 ± 7.22 pA/pF; FGF19, 64.8 ± 6.04 pA/pF; n = 8; P > 0.05). Since FGF19 only altered voltage-dependent K+ currents in DMV neurons from control mice, additional experiments to further identify the nature of this effect were restricted to this group. The effects on FGF19 on K+ current amplitude were blocked by 4-AP (ACSF, 106.0 ± 11.1 pA/pF; FGF19, 99.2 ± 11.6 pA/pF; n = 6; P > 0.05) (Fig. 6A-6C) or when recording pipettes contained Cs+ (ACSF, 51.7 ± 4.16 pA/pF; FGF19, 56.6 ± 3.20 pA/pF; n = 5; P > 0.05) (Fig. 6D-6F). FGF19 therefore reduced the peak current amplitude of the 4-AP sensitive, putative A-type K+ current in control, but not T1DM mice.
Figure 5.
FGF19 decreases A-type K+ current amplitude in DMV neurons from control, but not in T1DM mice. (A) Voltage activation step recording a from control mouse in ACSF and in the presence of FGF19 (230 pM). Traces shown are peak data only (ie, steady state current has been subtracted). (B) Graphed peak current density data from control mice (n = 9) indicate an FGF19-mediated reduction in peak current density at potentials at and above −45 mV (*P < 0.05). (C) Effect of FGF19 on peak current density for the +35 mV step for individual neurons (n = 9; *P < 0.05). (D) Voltage activation step recording from T1DM mouse in ACSF and in the presence of FGF19. (E) Peak current density in DMV neurons from T1DM mice (n = 8). (F) The effect of FGF19 on peak current density for the +35 mV step was not significant at any potential (n = 8; P > 0.05). All recordings in TTX (2 µM), picrotoxin (100 µM), kynurenic acid (1 mM), and TEA (10 µM). Recorded from control, 4 male and 3 female, and T1DM 2, male and 2 female mice.
Figure 6.
The effect of FGF19 on the A-type K+ current in normoglycemic mice is prevented by 4-AP or intracellular Cs+. (A) Representative voltage activation step recording in the presence of 4-AP (5 mM). (B) Steady-state current density in DMV neurons from in the presence of 4-AP (5 µM; n = 6). (C) FGF19 effect on steady-state current density plotted for individual neurons from the 35 mV step in (B) (n = 6; P > 0.05). 4-AP recordings were from 2 male and 1 female mouse. (D) Representative voltage activation step recording using a Cs+ internal solution. (E) Steady-state current density in DMV neurons using a Cs+ internal solution (n = 5). (F) FGF19 effect on steady-state current density plotted for individual neurons from the 35 mV step in E (n = 5; P > 0.05). Cs+ recordings were from 1 male and 1 female mouse. All recordings are from normoglycemic mice and performed in TTX (2 µM), picrotoxin (100 µM), and kynurenic acid (1 mM).
Discussion
This report identifies the brainstem DVC as a previously underappreciated target for the antidiabetic effects of FGF19. Previous research on the metabolic effects of centrally applied FGF19 has focused on hypothalamic circuits, since third ventricle administration led to lower blood glucose levels that were correlated with decreased plasma ACTH and suppression of agouti-related peptide/neuropeptide Y neuron activity (2,4). The DVC contains FGFR1, FGFR3, and ß-klotho, suggesting that DVC neurons can respond to exogenous FGF and engage in endogenous FGF signaling (19-21). Importantly, DVC neurons regulate autonomic control of hepatic glucose production, food intake, and hepatic enzyme expression (9,36-39), all of which are mechanisms proposed to mediate the antidiabetic actions of ICV FGF19 (1-3). While the findings from previous studies are consistent with the hypothesis that FGF19 regulates metabolism and interacts with hypothalamic neurons, the present data suggest that actions of the peptide in the DVC may be sufficient to produce significant effects on systemic glucose concentration under hyperglycemic conditions.
The effects of FGF on hypothalamic neurons could also function to lower blood glucose levels by signaling through the DVC and vagus nerve (36,40). ICV FGF19 activation of hypothalamic neurons decreases DVC c-Fos expression and vagus nerve activity in response to neuroglucopenia (41). Furthermore, some reports suggest that hypothalamic regulation of hepatic glucose production works through DVC neuron activation and requires an intact vagus nerve (36,42). Consequently, interactions of FGF19 with DVC circuits may provide a more direct path for FGF19-mediated antidiabetic effects.
Acute 4V administration of FGF19 produced a significant, reversible decrease in blood glucose concentration in hyperglycemic mice for at least 12 h. Consistent with the effects of FGF19 application in the diencephalon, the glucose-lowering ability of FGF19 applied to the hindbrain was only observed in hyperglycemic mice (43,44). The effects on blood glucose are unlikely to the result of anesthesia or recovery from surgery, as all mice resumed normal feeding behavior rapidly after surgery and returned to baseline glucose levels, except the T1DM-FGF19 group. Diffusion of FGF19 to the hypothalamus is unlikely, as cerebrospinal fluid flows rostrocaudally and FGF19 was injected slowly into 4V to prevent backflow rostrally (45). To wit, 4V application is often used as a substitute for direct DVC parenchymal injections (38,46-48). Altered activity of DMV motor neurons has been linked with blood glucose regulation, which is mediated by parasympathetic regulation of pancreatic and hepatic vagal activity (6,9,11). Moreover, the effect of 4V FGF19 on blood glucose was prevented when peripheral muscarinic receptors were blocked by MSA, suggesting a parasympathetic mechanism. Previous studies did not reach a consensus regarding the mechanism by which FGF19 decreases blood glucose in diabetic mice. Taken together, they suggest that FGF19 in the brain may work by altering hepatic metabolism with no effects on insulin or glucagon levels (1,2,49). However, no further investigation into this mechanism was performed here. While insulin is unlikely to play a role due to the use of a T1DM model, we cannot rule out the potential involvement of glucagon in the response to FGF19.
A previous report found that third-ventricular FGF19 was able to decrease blood glucose concentration by >200 mg/dL in T1DM rats—similar in magnitude to the results found here—and this was associated with a 50% decrease in hepatic glucose production (HGP) (2). Manipulation of DVC neuron excitability has been shown to decrease HGP by ~50% (6,36,38,42), suggesting that FGF19 modulation of DVC excitability can alter HGP sufficiently to produce the decrease in blood glucose shown in Figure 1. Notably, the largest proportion of DMV neurons project to the stomach (50). Since FGF19 altered excitability of most DMV neurons, this suggests effects on gastrointestinal function, which may alter blood glucose indirectly and warrants further research. While this study was performed in a T1DM mouse model, similar results would likely be found in a T2DM model, since previous reports indicate that FGF19 functions independently of insulin to lower blood glucose concentration in both T1DM and T2DM models (1,2). The reduced insulin availability in this model of hyperglycemia is consistent with an insulin-independent effect of FGF19 acting in the DVC. The FGF19 effects in the hindbrain neurons are therefore sufficient to lower systemic glucose concentration in hyperglycemic mice.
FGF19 produced a complex set of electrophysiological responses in DMV neurons that differed as a function of disease state (ie, presence or absence of hyperglycemia). While FGF19 modestly decreased action potential-dependent glutamate release onto DMV neurons from control mice, it produced a robust and consistent increase in synaptic excitation of DMV neurons from T1DM mice. The FGF19-mediated effects on glutamate release are mainly due to activity of the peptide on action potential firing of neurons with intact projections to the DMV, since blockade of action potentials with TTX prevented the overall effect of FGF19 on glutamate release. Interestingly, analysis of IPSC frequency and amplitude revealed no significant effect of FGF19 on GABA release, suggesting relatively selective effects of the peptide on upstream excitatory circuits. Because intact NTS neuron projections to the DMV are contained within the slice (51) and FGFRs are present in the NTS (19,21), it is likely that FGF19 increases activity of premotor, glutamatergic NTS neurons to affect increased synaptic excitability of vagal motorneurons in the DMV of T1DM mice, but effects on other local circuits cannot be discounted. Thus, the effects of FGF19 appear to impinge on central vagal circuitry that participates in vago-vagal reflexes, possibly including the gut-brain-liver glucose regulatory circuit (52). Phasic glutamatergic input profoundly affects vagal motor activity in vitro (7,53), injection of glutamatergic agonists into the DVC lowers hepatic glucose production (6), and blockade of glutamate receptors in the NTS prevents vagally mediated, reflexive modulation of hepatic gluconeogenesis (52). Glutamate release is persistently increased in the DMV of T1DM mice, and N-methyl-D-aspartate (NMDA) receptor function in glutamatergic NTS neurons is enhanced (24,26). Glutamate system plasticity in the DVC could also underlie the differences in FGF19 effects on EPSC frequency between control and T1DM mice. Consequently, the effect of FGF19 on EPSC frequency in T1DM mice may prove to be the primary determinant of its effects on blood glucose.
The effect of FGF19 on intrinsic excitability in DMV neurons was inconsistent and modest. In control mice, FGF19 produced mixed effects on action potential firing, whereas it mainly decreased firing in T1DM mice. Most neurons from both groups displayed a decrease in Rin and membrane hyperpolarization in response to FGF19 application. While these effects tend to be inhibitory, FGF19 also decreased the magnitude of an A-type K+ current in control mice. Where the decreased synaptic excitation and generally inhibitory effect on RMP and Rin by FGF19 in control mice suggest a decrease in cellular excitability, the decrease in A-type K+ current might contribute to increased AP firing during periods of membrane depolarization (54). Due to recording constraints, effects on postsynaptic currents, intrinsic membrane properties, action potentials, and A-type K+ channels were recorded separately. Thus, opposing pre-and postsynaptic effects were not observed within the same neuron. DMV neurons display considerable heterogeneity regarding morphological and electrophysiological properties that may also contribute to their considerable variation in response to FGF19 (30,55). Notably, the inhibitory effects on DMV EPSC frequency and Rin in control mice are shared with both leptin and insulin (15,56). The marked FGF19-induced increase in synaptic excitability in DMV neurons from T1DM mice suggests that this factor may underlie the differential effects of the peptide on blood glucose in T1DM mice.
It is not fully understood how DMV activity corresponds to changes in blood glucose. The increase in sEPSC frequency in DMV neurons from T1DM mice reported here and previously may represent a compensatory response to hyperglycemia (24,26). This suggests a model where an increase in synaptic excitation of DMV motor neurons results in decreased blood glucose concentration. Correspondingly, microinjection of NMDA in the DVC decreases hepatic glucose production (6). This model is also consistent with our previous findings showing that increased synaptic inhibition of DMV neurons by depolarization of GABAergic afferents results in increased blood glucose in mice (9). Here, FGF19 increased the already elevated sEPSC frequency in T1DM mice, which would be predicted to decrease blood glucose in this model.
Diabetic hyperglycemia leads to profound changes in synaptic activity and postsynaptic responsiveness in the DVC, including increased glutamate release in the DMV and increased NMDA receptor function in the NTS (24-26). This plasticity of excitatory circuitry in the DVC may contribute to the different metabolic and neuronal responses in hyper- and normoglycemic mice. The nature of the differences in electrophysiological effects between disease groups (eg, diabetes reversed and amplified effects on sEPSCs while abolishing effects on A-type K+ currents) suggests that hyperglycemia may differentially regulate discrete intracellular pathways downstream from the FGFR. Indeed, FGFRs signal via mitogen-activated protein kinases, phospholipase C-γ, and protein kinase C (57), all of which are also modulated by hyperglycemia/diabetes (58-60). In addition, FGFRs signal through the PI3K-Akt pathway, which is dysregulated in the brains of diabetic rats (61). Moreover, the insulin receptor and FGFR share several intracellular signaling pathways that likely exhibit significant crosstalk (57,62,63). Since the DVC responds to insulin (15,38) and changing glucose concentrations (10,18) and has access to circulating insulin and glucose via local fenestrated capillaries (13), it is to be expected that the changes in peripheral metabolism in T1DM mice would produce profound effects on FGFR signal transduction in the DVC. Thus, differential responses to FGFs between disease groups may be linked to diabetes-induced changes in the intracellular pool of signaling machinery. As such, future study is warranted to investigate this possibility.
The cellular responses of DMV neurons confirm that FGF19 modulates DMV neuron excitability, consistent with the hypothesis that FGF19 normalizes blood glucose in T1DM mice by altering autonomic output to the viscera. Despite this, it cannot be discounted that the antidiabetic effects found here could be partially mediated through interactions with other brain areas, since the NTS communicates with neurons in hypothalamic and ventral brainstem areas that regulate sympathetic and neuroendocrine functions, in addition to the DMV (40,64,65). However, MSA blocked the antidiabetic actions of FGF19, suggesting a predominantly parasympathetic mechanism.
In conclusion, this study identified the dorsal hindbrain as a novel target tissue for the glucose-lowering effects of centrally acting FGF19 in hyperglycemic mice. The cellular and synaptic effects of FGF19 on DMV neurons were consistent with a parasympathetically mediated effect on blood glucose in T1DM mice. This research highlights the importance of the DVC in FGFR-mediated glucoregulation and suggests that understanding FGF activity in central vagal circuitry may reveal new targets for brain-centered therapies to relieve diabetic hyperglycemia.
Acknowledgments
Parts of this work were presented in abstract form at Annual Meetings of the Society for Neuroscience in 2018 and 2019.
Author contributions: JBW designed experiments, collected data, analyzed data, prepared figures, and wrote and edited the manuscript. BNS designed experiments and edited the manuscript. BNS is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial support: This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK056132, R01 DK122811, and National Institute of General Medical Sciences T32 GM118292.
Additional Information
Disclosure: No conflicts of interest, financial or otherwise, are declared by the authors.
Data Availability
The data sets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- 1. Morton GJ, Matsen ME, Bracy DP, et al. FGF19 action in the brain induces insulin-independent glucose lowering. J Clin Invest. 2013;123(11):4799-4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perry RJ, Lee S, Ma L, Zhang D, Schlessinger J, Shulman GI. FGF1 and FGF19 reverse diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat Commun. 2015;6:6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ryan KK, Kohli R, Gutierrez-Aguilar R, Gaitonde SG, Woods SC, Seeley RJ. Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology. 2013;154(1):9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marcelin G, Jo YH, Li X, et al. Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol Metab. 2014;3(1):19-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okumura T, Taylor IL, Pappas TN. Microinjection of TRH analogue into the dorsal vagal complex stimulates pancreatic secretion in rats. Am J Physiol. 1995;269(3 Pt 1):G328-G334. [DOI] [PubMed] [Google Scholar]
- 6. Lam CK, Chari M, Su BB, et al. Activation of N-methyl-D-aspartate (NMDA) receptors in the dorsal vagal complex lowers glucose production. J Biol Chem. 2010;285(29):21913-21921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Travagli RA, Anselmi L. Vagal neurocircuitry and its influence on gastric motility. Nat Rev Gastroenterol Hepatol. 2016;13(7):389-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boychuk CR, Smith KC, Peterson LE, et al. A hindbrain inhibitory microcircuit mediates vagally-coordinated glucose regulation. Sci Rep. 2019;9(1):2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferreira M Jr, Browning KN, Sahibzada N, Verbalis JG, Gillis RA, Travagli RA. Glucose effects on gastric motility and tone evoked from the rat dorsal vagal complex. J Physiol. 2001;536(Pt 1):141-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamy CM, Sanno H, Labouèbe G, et al. Hypoglycemia-activated GLUT2 neurons of the nucleus tractus solitarius stimulate vagal activity and glucagon secretion. Cell Metab. 2014;19(3):527-538. [DOI] [PubMed] [Google Scholar]
- 12. Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am J Physiol. 1990;259(6 Pt 2):R1131-R1138. [DOI] [PubMed] [Google Scholar]
- 13. Merchenthaler I. Neurons with access to the general circulation in the central nervous system of the rat: a retrograde tracing study with fluoro-gold. Neuroscience. 1991;44(3):655-662. [DOI] [PubMed] [Google Scholar]
- 14. Williams KW, Smith BN. Rapid inhibition of neural excitability in the nucleus tractus solitarii by leptin: implications for ingestive behaviour. J Physiol. 2006;573(Pt 2):395-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blake CB, Smith BN. Insulin reduces excitation in gastric-related neurons of the dorsal motor nucleus of the vagus. Am J Physiol Regul Integr Comp Physiol. 2012;303(8):R807-R814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. LaPierre MP, Abraham MA, Yue JT, Filippi BM, Lam TK. Glucagon signalling in the dorsal vagal complex is sufficient and necessary for high-protein feeding to regulate glucose homeostasis in vivo. EMBO Rep. 2015;16(10):1299-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wan S, Browning KN, Travagli RA. Glucagon-like peptide-1 modulates synaptic transmission to identified pancreas-projecting vagal motoneurons. Peptides. 2007;28(11):2184-2191. [DOI] [PubMed] [Google Scholar]
- 18. Boychuk CR, Gyarmati P, Xu H, Smith BN. Glucose sensing by GABAergic neurons in the mouse nucleus tractus solitarii. J Neurophysiol. 2015;114(2):999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Belluardo N, Wu G, Mudo G, Hansson AC, Pettersson R, Fuxe K. Comparative localization of fibroblast growth factor receptor-1, -2, and -3 mRNAs in the rat brain: in situ hybridization analysis. J Comp Neurol. 1997;379(2):226-246. [PubMed] [Google Scholar]
- 20. Bookout AL, de Groot MH, Owen BM, et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med. 2013;19(9):1147-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hultman K, Scarlett JM, Baquero AF, et al. The central fibroblast growth factor receptor/beta klotho system: comprehensive mapping in Mus musculus and comparisons to nonhuman primate and human samples using an automated in situ hybridization platform. J Comp Neurol. 2019;527(12):2069-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Halmos KC, Gyarmati P, Xu H, et al. Molecular and functional changes in glucokinase expression in the brainstem dorsal vagal complex in a murine model of type 1 diabetes. Neuroscience. 2015;306:115-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boychuk CR, Smith BN. Glutamatergic drive facilitates synaptic inhibition of dorsal vagal motor neurons after experimentally induced diabetes in mice. J Neurophysiol. 2016;116(3):1498-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bach EC, Halmos KC, Smith BN. Enhanced NMDA receptor-mediated modulation of excitatory neurotransmission in the dorsal vagal complex of streptozotocin-treated, chronically hyperglycemic mice. PloS One. 2015;10(3):e0121022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boychuk CR, Halmos KC, Smith BN. Diabetes induces GABA receptor plasticity in murine vagal motor neurons. J Neurophysiol. 2015;114(1):698-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zsombok A, Bhaskaran MD, Gao H, Derbenev AV, Smith BN. Functional plasticity of central TRPV1 receptors in brainstem dorsal vagal complex circuits of streptozotocin-treated hyperglycemic mice. J Neurosci. 2011;31(39):14024-14031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boychuk CR, Smith KC, Smith BN. Functional and molecular plasticity of γ and α1 GABAA receptor subunits in the dorsal motor nucleus of the vagus after experimentally induced diabetes. J Neurophysiol. 2017;118(5):2833-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adams AC, Coskun T, Rovira AR, et al. Fundamentals of FGF19 & FGF21 action in vitro and in vivo. PloS One. 2012;7(5):e38438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao H, Glatzer NR, Williams KW, Derbenev AV, Liu D, Smith BN. Morphological and electrophysiological features of motor neurons and putative interneurons in the dorsal vagal complex of rats and mice. Brain Res. 2009;1291:40-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Browning KN, Renehan WE, Travagli RA. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. J Physiol. 1999;517(Pt 2):521-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. RRID:AB_11178519, https://scicrunch.org/resolver/AB_11178519. [Google Scholar]
- 32. RRID:AB_143157, https://scicrunch.org/resolver/AB_143157. [Google Scholar]
- 33. Ayer A, Antic V, Dulloo AG, Van Vliet BN, Montani JP. Hemodynamic consequences of chronic parasympathetic blockade with a peripheral muscarinic antagonist. Am J Physiol Heart Circ Physiol. 2007;293(2):H1265-H1272. [DOI] [PubMed] [Google Scholar]
- 34. Lechner SG, Mayer M, Boehm S. Activation of M1 muscarinic receptors triggers transmitter release from rat sympathetic neurons through an inhibition of M-type K+ channels. J Physiol. 2003;553(Pt 3):789-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carnagarin R, Lambert GW, Kiuchi MG, et al. Effects of sympathetic modulation in metabolic disease. Ann N Y Acad Sci. 2019;1454(1):80-89. [DOI] [PubMed] [Google Scholar]
- 36. Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 2005;1(1):53-61. [DOI] [PubMed] [Google Scholar]
- 37. Filippi BM, Bassiri A, Abraham MA, Duca FA, Yue JT, Lam TK. Insulin signals through the dorsal vagal complex to regulate energy balance. Diabetes. 2014;63(3):892-899. [DOI] [PubMed] [Google Scholar]
- 38. Filippi BM, Yang CS, Tang C, Lam TK. Insulin activates Erk1/2 signaling in the dorsal vagal complex to inhibit glucose production. Cell Metab. 2012;16(4):500-510. [DOI] [PubMed] [Google Scholar]
- 39. Browning KN, Verheijden S, Boeckxstaens GE. The vagus nerve in appetite regulation, mood, and intestinal inflammation. Gastroenterology. 2017;152(4):730-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J Comp Neurol. 2010;518(9):1460-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Picard A, Soyer J, Berney X, et al. A genetic screen identifies hypothalamic Fgf15 as a regulator of glucagon secretion. Cell Rep. 2016;17(7):1795-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lam CK, Chari M, Rutter GA, Lam TK. Hypothalamic nutrient sensing activates a forebrain-hindbrain neuronal circuit to regulate glucose production in vivo. Diabetes. 2011;60(1):107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scarlett JM, Muta K, Brown JM, et al. Peripheral mechanisms mediating the sustained anti-diabetic action of FGF1 in the brain. Diabetes. 2019;68(3):654-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scarlett JM, Rojas JM, Matsen ME, et al. Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents. Nat Med. 2016;22(7):800-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lun MP, Monuki ES, Lehtinen MK. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat Rev Neurosci. 2015;16(8):445-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Swartz EM, Browning KN, Travagli RA, Holmes GM. Ghrelin increases vagally mediated gastric activity by central sites of action. Neurogastroenterol Motil. 2014;26(2):272-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Browning KN, Babic T, Toti L, Holmes GM, Coleman FH, Travagli RA. Plasticity in the brainstem vagal circuits controlling gastric motor function triggered by corticotropin releasing factor. J Physiol. 2014;592(20):4591-4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143(1):239-246. [DOI] [PubMed] [Google Scholar]
- 49. Fu L, John LM, Adams SH, et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145(6):2594-2603. [DOI] [PubMed] [Google Scholar]
- 50. Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol. 1991;260(1 Pt 2):R200-R207. [DOI] [PubMed] [Google Scholar]
- 51. Davis SF, Derbenev AV, Williams KW, Glatzer NR, Smith BN. Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res. 2004;1017(1-2):208-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rasmussen BA, Breen DM, Luo P, et al. Duodenal activation of cAMP-dependent protein kinase induces vagal afferent firing and lowers glucose production in rats. Gastroenterology. 2012; 142:834-843.e833. [DOI] [PubMed] [Google Scholar]
- 53. Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol. 1991;260(3 Pt 1):G531-G536. [DOI] [PubMed] [Google Scholar]
- 54. Sutton GM, Patterson LM, Berthoud HR. Extracellular signal-regulated kinase 1/2 signaling pathway in solitary nucleus mediates cholecystokinin-induced suppression of food intake in rats. J Neurosci. 2004;24(45):10240-10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Travagli RA, Gillis RA. Hyperpolarization-activated currents, IH and IKIR, in rat dorsal motor nucleus of the vagus neurons in vitro. J Neurophysiol. 1994;71(4):1308-1317. [DOI] [PubMed] [Google Scholar]
- 56. Williams KW, Zsombok A, Smith BN. Rapid inhibition of neurons in the dorsal motor nucleus of the vagus by leptin. Endocrinology. 2007;148(4):1868-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4(3):215-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Purves T, Middlemas A, Agthong S, et al. A role for mitogen-activated protein kinases in the etiology of diabetic neuropathy. Faseb J. 2001;15(13):2508-2514. [DOI] [PubMed] [Google Scholar]
- 59. Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, Nogueira-Machado JA. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018;9(2):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Das Evcimen N, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res. 2007;55(6):498-510. [DOI] [PubMed] [Google Scholar]
- 61. Bathina S, Das UN. Dysregulation of PI3K-Akt-mTOR pathway in brain of streptozotocin-induced type 2 diabetes mellitus in Wistar rats. Lipids Health Dis. 2018;17(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Haeusler RA, McGraw TE, Accili D. Biochemical and cellular properties of insulin receptor signalling. Nat Rev Mol Cell Biol. 2018;19(1):31-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7(2):85-96. [DOI] [PubMed] [Google Scholar]
- 64. Bailey TW, Hermes SM, Whittier KL, Aicher SA, Andresen MC. A-type potassium channels differentially tune afferent pathways from rat solitary tract nucleus to caudal ventrolateral medulla or paraventricular hypothalamus. J Physiol. 2007;582(Pt 2):613-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Browning KN, Travagli RA. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol. 2014;4(4):1339-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.