Abstract
Purpose
Acute Respiratory Distress Syndrome (ARDS) secondary to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has demonstrated variable oxygenation and respiratory-system mechanics without investigation of transpulmonary and chest-wall mechanics. This study describes lung, chest wall and respiratory-system mechanics in patients with SARS-CoV-2 and ARDS.
Methods
Data was collected from forty patients with confirmed SARS-CoV-2 and ARDS at Beth Israel Deaconess Medical Center in Boston, Massachusetts. Esophageal balloons were placed to estimate pleural and transpulmonary pressures. Clinical characteristics, respiratory-system, transpulmonary, and chest-wall mechanics were measured over the first week.
Results
Patients had moderate-severe ARDS (PaO2/FiO2 123[98–149]) and were critically ill (APACHE IV 108 [94–128] and SOFA 12 [11–13]). PaO2/FiO2 improved over the first week (150 mmHg [122.9–182] to 185 mmHg [138–228] (p = 0.035)). Respiratory system (30–35 ml/cm H2O), lung (40–50 ml/cm H2O) and chest wall (120–150 ml/cm H2O) compliance remained similar over the first week. Elevated basal pleural pressures correlated with BMI. Patients required prolonged mechanical ventilation (14.5 days [9.5–19.0]), with a mortality of 32.5%.
Conclusions
Patients displayed normal chest-wall mechanics, with increased basal pleural pressure. Respiratory system and lung mechanics were similar to known existing ARDS cohorts. The wide range of respiratory system mechanics illustrates the inherent heterogeneity that is consistent with typical ARDS.
Keywords: ARDS, Acute respiratory distress syndrome, COVID-19, SARS-CoV-2, Coronavirus, Transpulmonary pressure, Chest wall mechanics, Mechanics, Mechanical ventilation
1. Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic has led to an unprecedented number of mechanically ventilated patients with Acute Respiratory Distress Syndrome (ARDS) [1]. Lung protective ventilation [2] improves mortality in ARDS by limiting tidal volume and lung stress, while providing sufficient positive end-expiratory pressure (PEEP) to prevent collapse. The optimal approach to mechanical ventilation is determined largely by patient lung mechanics and gas exchange to limit total airway pressures, optimize PEEP levels and safe ranges for delivered tidal volumes and driving pressures. Therefore, a detailed investigation of the physiology of SARS-CoV-2 ARDS is important in order to characterize parameters that affect clinical decision making and outcomes. Questions have been raised as to whether the lung disease associated with SARS-CoV-2 should be considered a “typical” form of ARDS and whether standard treatment, optimal targets for ventilation and PEEP management should be reconsidered [3,4]. Indeed it has been proposed that two phenotypes of ARDS secondary to SARS-CoV-2 exist with an early “L” phenotype with high compliance and low recruitability, and a later “H” phenotype with low compliance and higher recruitability [5] with the proposal that patients with the “L” phenotype can be given high tidal volumes and low PEEP strategies. Interestingly, other case series of patients with SARS-CoV-2 have reported varying degrees of impairment in oxygenation and respiratory system mechanics [3,4,6] between patients raising questions as to the validity of these proposed phenotypes while another study was unable to differentiate SARS-CoV-2 patients based upon the proposed phenotypes [7]. Additionally, the proposed SARS-CoV-2 ARDS phenotypes could be found in ARDS patients in the LUNG SAFE study, suggesting that these phenotypes are not novel or different from pre-SARS-CoV-2 related ARDS [8].
Notably, previous case series in patients with ARDS from SARS-CoV-2 were limited to measurement of respiratory system mechanics without description of lung (transpulmonary) and chest-wall mechanics. Due to widely variable chest wall between patients secondary to body habitus, type and severity of ARDS, and other baseline patients factors [9], respiratory system pressure measurements may be quite different from the pressures across the lung (transpulmonary pressures –PL). Esophageal manometry is used for clinical and research purposes to measure pleural pressures, estimate chest wall mechanics and isolate the effects of the chest wall and determine the distending pressures across the lungs [10]. Esophageal manometry can be used clinically for PEEP titration with a recent study showing equivalence to an empiric high PEEP strategy using ARDSnet tables [10], and to determine safe levels of total and cyclic distending pressures on the lungs for clinical care [11]. It can also be used to demonstrate the usual wide variability in chest wall mechanics which might lead to under or over-estimation of the effect of respiratory system pressures on the lungs. As such, a comprehensive understanding of lung and chest wall physiology in SARS-CoV-2 associated ARDS may be of critical importance both for general understanding of this unique disease process, as well as to inform personalized clinical decision making and ventilator management. This case series provides a description of the early respiratory system, lung and chest wall mechanics profiles of ventilated patients with SARS-CoV-2 and ARDS.
2. Methods
Observational data were collected from patients admitted to Beth Israel Deaconess Medical Center(BIDMC) in Boston, Massachusetts between March 11 and April 12, 2020, with laboratory confirmed SARS-CoV-2 (RT-PCR) and ARDS defined per Berlin criteria. All data was collected from routine clinical care without specific measurements performed for this investigation. Mechanics data was not collected in patients with spontaneous efforts as per standard protocol at BIDMC with data validated during breath holds. All patients had esophageal balloons (Cooper Surgical and Viasys) placed for early positive end-expiratory pressure (PEEP) titration as part of routine clinical care for moderate-severe ARDS (as is typically performed at BIDMC). Balloon position was confirmed via cardiac oscillations and chest/abdomen pushes during breath holds. PEEP titration was performed to target a positive end expiratory transpulmonary pressure to overcome the collapsing pressures of the chest wall as measured by estimation of the basal end-expiratory pleural pressures. Proning was performed as per standard of care at BIDMC with decision to prone at the discretion of the primary clinical team. The typical protocol targets patients with PaO2/FiO2 ratio of <150 mmHg, with proning for at least 16 h of every 24 h period as per prior studies [12] however this remained a clinical team decision. Respiratory system (PAO), lung/transpulmonary (PL), and chest wall (PCW) mechanics were recorded on days 1, 3 and at 1 week (day 7 or 8 as available). Additionally mechanics and oxygenation were recorded before and immediately following proning in the 19 patients who underwent this maneuver during their first proning session. Without clear definitions of mechanics severity in the literature, the degree of impairment was determined based upon our standard clinical practice at BIDMC and to demonstrate the essentially arbitrary nature of these cutoffs with mild-preserved mechanics defined in patients as a respiratory system compliance of >40 ml/cm H2O (The “L-type” per Gattinoni et al. [3]). In addition, moderately impaired mechanics were defined as a compliance from 25 to 40 ml/cm H2O and severely impaired mechanics defined by a respiratory system compliance of <25 ml/cm H2O (together being similar to the “H-type” per Gattinoni et al. [3]). Patients were followed until death, tracheostomy placement, or extubation and discharge from the hospital to determine outcomes. Descriptive statistics were used to summarize the data and statistical comparisons (repeated measures ANOVA with post-hoc Sidak test) were made for PaO2/FiO2 ratio as well as compliance of the respiratory system, the lung, and chest wall over time. Linear regression was used for univariate analysis. A sensitivity analysis was performed to account for the effects of proning. Results were reported as medians (interquartile ranges) or frequencies (%). Analyses were performed using STATA 14.2 (STATAcorp). This study was determined to be exempt by the Institutional Review Board.
3. Results
Forty patients with moderate to severe ARDS were included. Demographics were the following: median age 57 [46–66.75] years, 21 [55%] male, 28 white [70%], 12 Hispanic [30%], BMI of 33.5 [29.6–37.6]. Median APACHE IV score was 108 [94–128] and SOFA score was 12 [11–13], with baseline PaO2/FiO2 ratio of 123 [98–149].
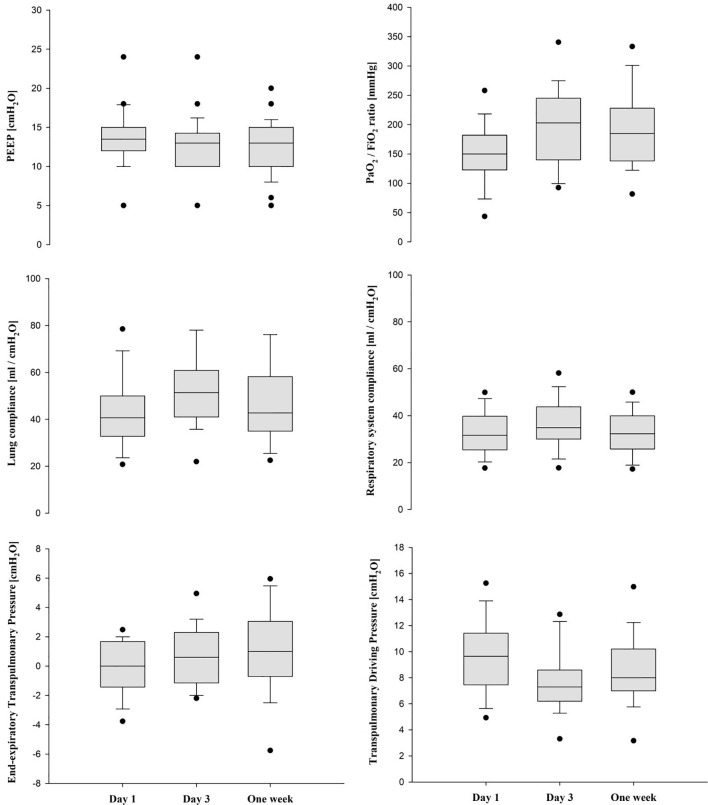
After initial PEEP titration using esophageal manometry on day 1, PaO2/FiO2 ratio improved from 150 [122.9–182] to 185 mmHg [138–228] by one week (p = 0.035). RASS and GCS scores indicated deep sedation over the initial week (Table 1 ). Respiratory system compliance increased from day 1 (31.6 [25.8–39.4] ml/cm H2O) to day 3 (34.9 [30–43.8] ml/cm H2O), and then decreased by one week (32.3 (25.8–38.9) ml/cm H2O, p = 0.010, Fig. 1 ). Lung and chest wall compliance were similar over the observation period (day 1 to one week 40.7 [33.5–50.0] to 42.7 [37.2–56.5] ml/cm H2O, p = 0.23 and 154.4 [109.4–307.5] to 121.4 [61.4–184.2] ml/cm H2O, p = 0.07, respectively). Lung and respiratory system compliance were widely variable over the entire cohort (Fig. 2A) and although correlation was overall strong (slope = 1.49, R2 0.6745, p < 0.001), there was wide variability in PL and lung compliance for a given Pao and respiratory system compliance (Fig. 2B and C). Additionally, there was no correlation between PaO2/FiO2 ratio and respiratory system (p = 0.129) or lung (p = 0.257) compliance values at baseline (Fig. 3 ). Sensitivity analyses excluding eight patients who were in prone position at the time of recordings on day 3 and at one week yielded robust results: PaO2/FiO2 ratio increased from 159 [122–182] to 185 mmHg [154–210] (p = 0.049) by one week, and respiratory system compliance increased from day 1 (30.8 [25.0–38.9] ml/cm H2O) to day 3 (36.0 [30.8–43.8] ml/cm H2O), and then decreased again by one week (32.2 [26.2–38.7] ml/cm H2O, p = 0.013). Lung and chest wall compliance remained similar over the observation period (p = 0.26 and 0.17, respectively).
Table 1.
Clinical parameters over the first week of measurements.
| Day 1 |
Day 3 |
1 Week |
|
|---|---|---|---|
| n = 40 | n = 38 | n = 35 | |
| Ventilator Settings | |||
| Pressure regulated volume control - n% | 39 (97.5) | 36 (95) | 32 (91) |
| Other Mode - n% | 1 (2.5) | 2 (5) | 3 (8.5) |
| Tidal volume - ml | 360 (325–405) | 390 (330–450) | 380 (330–450) |
| Tidal volume – ml/kg IBW | 6.2 (5.8–6.7) | 6.2 (6–6.7) | 6.1 (6.0–6.9) |
| Set PEEP – cm H2O | 13.5 (12–15) | 13 (10–14) | 13 (10–15) |
| Set respiratory rate - breaths/min | 26 (21–28) | 26 (22–28) | 26 (22−30) |
| Observed respiratory rate - breaths/min | 26 (22–28) | 26 (22–28) | 26 (22–30) |
| Fraction of inspired oxygen | 0.7 (0.58–0.85) | 0.5 (0.4–0.6) | 0.5 (0.4–0.55) |
| Mechanics | |||
| Plateau pressure – cm H2O | 25 (23.2–27) | 24 (23–26) | 25.5 (23–28) |
| Total PEEP – cm H2O | 13.6 (12–15) | 13.5 (10–15) | 13.5 (10–15.8) |
| Respiratory system driving pressure – cm H2O | 11.9 (9.5–13.7) | 10.7 (10−13) | 12 (10–14) |
| Respiratory system compliance – ml/cm H2O | 31.6 (25.8–39.4) | 34.9 (30–43.8) | 32.3 (25.8–38.9) |
| End inspiratory PL – cm H2O | 9.9 (7.1–11.5) | 8 (6.5–10) | 9.5 (7–13) |
| End expiratory PL – cm H2O | 0.0 (−1.35–1.55) | 0.6 (−1.1–2.3) | 1.0 (−0.6–3.1) |
| Transpulmonary driving pressure – cm H2O | 9.7 (7.5–11.4) | 7.3 (6.3–8.5) | 8.5 (7–9.6) |
| Lung compliance – ml/cm H2O | 40.7 (33.5–50.0) | 51.4 (41.4–60.6) | 42.7 (37.2–56.5) |
| End inspiratory Pes – cm H2O | 16.1 (13.4–18.9) | 15.9 (13.6–17.7) | 15.8 (14–18) |
| End expiratory Pes – cm H2O | 13.7 (10.9–15.3) | 13 (9–15.2) | 12.1 (9.7–14.1) |
| Chest wall driving pressure – cm H2O | 2.3 (1.4–3.5) | 3.1 (2.3–4.5) | 3 (1.9–5.1) |
| Chest wall compliance – ml/cm H2O | 154.4 (109.4–307.5) | 120.2 (87.1–155.8) | 121.4 (61.4–184.2) |
| Change Gas Exchange | |||
| pH | 7.36 (7.3–7.39) | 7.38 (7.36–7.42) | 7.39 (7.33–7.44) |
| PaCO2 – mmHg | 48 (43–53) | 44 (38–54.5) | 49 (44.5–54.5) |
| PaO2 – mmHg | 100.5 (90–121) | 87 (80–110) | 86 (75.5–107.5) |
| SpO2 - % | 96.5 (94–98) | 96 (94–98) | 95.5 (94–99) |
| PaO2:FiO2 ratio – mmHg | 150 (122.9–182) | 203 (140–245) | 185 (138–228) |
| Sedation Level | |||
| Glasgow coma scale | 3 (3–4) | 3 (3–3.5) | 3 (3–4) |
| Richmond agitation sedation scale | −5 (−5- −4) | −5 (−5- −4) | −5 (−5- −4) |
PEEP denotes the positive end expiratory pressure, PL denotes transpulmonary pressure, Pes esophageal pressure, PaCO2 partial pressure of carbon dioxide, PaO2 partial pressure of oxygen, SpO2 percent of oxygen-saturated hemoglobin, and FiO2 fraction of inspired oxygen.
Fig. 1.
Median (IQR) physiological and mechanical parameters over first week of measurement.
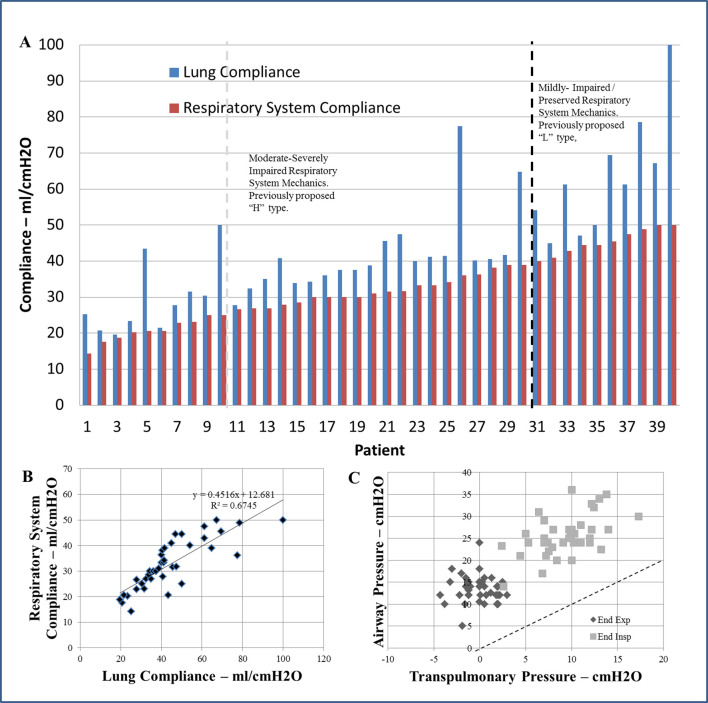
Fig. 2.
Patients with SARS-CoV-2 and Acute Respiratory Distress Syndrome (ARDS). Lung mechanics were measured using esophageal manometry to remove the component of the chest wall from the respiratory system values. (A) Respiratory system (red bars) and lung compliance (blue bars) measurements in each of the 40 patients. There was wide variability in the distribution of lung and respiratory system mechanics overall without clear differentiation of phenotypes by degree of compliance. The distribution of mechanics appears similar to other known cohorts of non-SARS-CoV-2 related ARDS. With mechanics appearing more as a continuum as opposed to unique phenotypes, cutoffs appear arbitrary in nature. The black dotted line differentiates the proposed “L” from “H” types, and the light grey dotted line differentiates the equally arbitrary cutoff from severe (compliance <25 ml/cmH2O) to moderately impaired mechanics (compliance 25-40 ml/cmH2O) (B) Comparison between respiratory system and lung compliance illustrating the expected excellent correlation. The solid line represents the slops of this correlation. (C) Comparison of end inspiratory (light grey) and end expiratory (dark grey) airway and transpulmonary pressures. This illustrates that despite the good correlation, there is wide variability for the corresponding transpulmonary pressure illustrating the variability inherent to the chest wall mechanics. The dotted line illustrates the line of identity. There was an unpredictable and inconsistent and underestimation of transpulmonary pressure by the corresponding airway pressure as seen by the variable offset from the line of identity. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
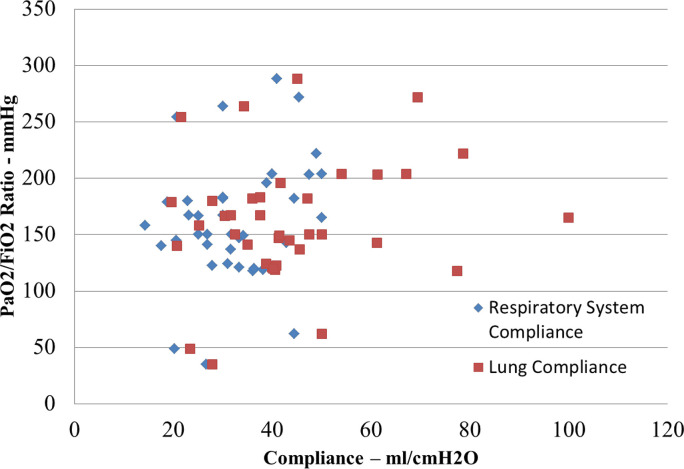
Fig. 3.
Comparison of the PaO2/FiO2 ratio with respiratory system and lung compliance on day 1 of measurements. There is no correlation between PaO2/FiO2 ratio and either measurement of compliance illustrating the inherent and expected variability in this cohort.
Ten patients (25%) had mild-preserved mechanics that would be consistent with the postulated “L” phenotype (median lung compliance 61.3 ml/cm H2O [50–69.4] and respiratory system compliance 44.9 ml/cm H2O [42.9–48.9]). Thirty patients (75%) had moderate-severely impaired mechanics consistent with the postulated “H” phenotype with 8 patients displaying severely impaired respiratory system mechanics (median lung compliance 24.3 ml/cm H2O [21.1–29.7] and respiratory system compliance 20.4 ml/cm H2O [18.2–21.8]) and 22 patients displaying moderately impaired respiratory system mechanics (median lung compliance 40.1 ml/cm H2O [35–41.7] and respiratory system compliance 30.5 ml/cm H2O [27.9–34.2. Basal pleural pressure (end-expiratory esophageal pressure) was elevated in the majority of patients at baseline 13.7 [10.9–15.3] cm H2O and were correlated with increased BMI on day 1 (slope = 0.2271, R2 0.2237, p = 0.002), and at one week (slope = 0.2148, R2 0.3687, p < 0.001). Hypercarbia was uncommon in early disease (pCO2 47 mmHg [40–53]). Further characteristics are listed in Table 1.
Nineteen patients [47.5%] were proned with initiation at a median of 2 days [[1], [2], [3], [4]], for a median duration of 37 h [17–80]. Zero patients were proned during the baseline measurements, two patients were proned during day 3 measurements, and five patients were proned during the one week measurements. Respiratory system compliance was similar before (30 ml/cm H2O [26.7–35]) and after proning (31.8 ml/cm H2O [27.7–40.8]; p = 0.12). PaO2/FiO2 ratio also significantly increased from 131.7 [109–144.3] to 225 [141.2–272.9]. In the ten patients with transpulmonary pressure measurements immediately before and after proning, lung compliance significantly increased from 35.8 [25.2–42.1] to 48.7 [29.9–63.5] after proning (p = 0.05). Fourteen of the proned patients were extubated [74%], one underwent tracheostomy [5%] and four died [21%]. Patients required prolonged mechanical ventilation for persistent hypoxemia with a median duration of mechanical ventilation of 14.5 days [9.5–19.0]. Thirteen patients [32.5%] died during their hospitalization after a median of 10 days [[7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]]. One patient [2.5%] underwent tracheostomy at day 33. 26 patients [65%] were extubated and discharged from the hospital.
4. Discussion
This represents the largest study to date of lung and chest wall mechanics in mechanically ventilated patients with SARS-CoV-2. The use of esophageal manometry allowed for separation of lung and chest wall mechanics, providing a detailed understanding of mechanical derangements.
Prior case series report a large range of compliance in patients with SARS-CoV-2: Bhatraju et al. reported a mean respiratory system compliance of 29 ml/cm H2O [6], Gattinoni et al. of 51 ml/cm H2O [3], and Pan et al. of 20 ml/cm H2O [4]. The respiratory system mechanics in our study cohort fall within these prior studies suggesting heterogeneity of SARS-CoV-2 related ARDS. While it has been suggested that lung injury associated with SARS-CoV-2 differs from “typical” ARDS [3,5], the mechanics in our case series resembled those typical of ARDS from other etiologies [10,12,13]: patients enrolled in the ART [13] and PROCEVA [12] studies had comparable baseline respiratory system compliance (~30 ml/H2O and ~35 ml/H2O respectively), despite lower PaO2/FiO2 ratios (~118 mmHg vs 100 mmHg) while receiving mean PEEP levels of 16 cm H20 and 10 cm H2O respectively. Additionally when compared with patients without SARS-CoV-2, two studies found similar overall mechanics in patients with SARS-CoV-2 without discernible phenotypes or differences and an expected degree of heterogeneity [7,8].
Notably, prior case series describe respiratory system mechanics without accounting for chest wall variability and the effect on lung/transpulmonary mechanics [10]. This variability can occur in patients with ARDS due to differences in chest wall compliance and basal pleural pressure elevation secondary to obesity, abdominal hypertension, edema and critical illness [9,10]. Increased chest wall weight and stiffness leads to dissipation of delivered pressures into the chest wall (thereby decreasing the delivered pressures to the lungs), while elevated basal pressures may result in collapsing pressures and increased atelectasis at end expiration with functional effects on mechanics and oxygenation [10]. Of note, the cohort of patients in this study displayed relatively normal chest wall compliance, with increased basal pleural pressure elevations likely secondary to a higher median BMI with good correlation over the first week between basal pressure elevation and BMI. These findings are expected based upon our understanding of obesity on chest wall mechanics. Obese patients may have preserved chest wall compliance, but with a right-shifted compliance curve to a higher basal (end-expiratory) pleural pressure [[14], [15], [16]]. Normal chest wall compliance is expected in a clinical cohort with the primarily pulmonary parenchymal injury associated with SARS-CoV-2 pneumonia and these data confirm this. The physiologic approach to PEEP titration in this case series resulted in a range of PEEP from 10 to 24 cm H2O applied to offload the collapsing pressures of the chest wall at end expiration (due to elevated basal pleural pressures from obesity), emphasizing the heterogeneity inherent in ARDS and the need for an individualized approach. Additionally as would be expected in a cohort of ARDS patients, the chest wall mechanics were heterogeneous with corresponding expected variability between the transpulmonary and respiratory system derived measurements which is in agreement with prior work in typical ARDS [9]. When examining the mechanics profiles of patients in this cohort, there are no clearly defined phenotypes with the majority of patients having moderately impaired compliance, and essentially arbitrary cutoffs using either “H” vs “L” phenotypes or the cutoffs used in this paper. Notably there appeared to be little change in lung or respiratory system compliance over the first week while receiving individualized lung protective ventilation and deep sedation even after accounting for the population of patients who were proned. Compared with patients from the EPVent-2 study [10], the respiratory system compliance, lung compliance and basal end-expiratory pleural pressures were surprisingly similar in our SARS-CoV-2 study group despite a worse PaO2/FiO2 (median 90 mmHg) and higher PEEP levels (~17 cm H2O) in the EPVent-2 cohort, again suggestive of a similar overall profile to typical ARDS patients. Importantly, the “typical” ARDS cohort in EPVent-2 displayed a similar distribution of “H” phenotypes (74% of patients) and “L” phenotypes (26%) again reiterating these similarities, and a similar overall distribution of mechanics.
Although the mechanics in this study population appear similar to “typical” ARDS cohorts, the disease course in our study and others [6] resulted in prolonged illness and there has been suggestion that these patients may be sensitive to developing patient self-inflicted lung injury (P-SILI) with difficulty in weaning, possible injury during weaning attempts and prolongation of mechanical ventilation [17,18]. While P-SILI in SARS-CoV-2 remains an unproven hypothesis that has justifiably generated significant debate [19,20], the principles of P-SILI [21] as a potential driver of the transition from “L” to “H” phenotype [5] warrants some discussion in our cohort. Due to a BIDMC policy to prevent aerosolization with high flow nasal cannula and non-invasive positive pressure ventilation, patients were generally intubated early in their clinical course, however the duration of other non-invasive support and measurements of respiratory effort were not documented and cannot be assessed. While patients were not specifically intubated to prevent P-SILI [20], the timing of intubation certainly could have implications on the mechanics and ultimate outcome. The prolonged recovery phase of these patients and the possible sensitivity to P-SILI or other mechanisms of decompensation with lightened sedation and spontaneous breathing [18] reiterates the time needed for lung rest and recovery during the prolonged period of persistent infection, while applying lung protective ventilation strategies.
Prone position which has demonstrated mortality benefit in non-SARS-CoV-2 [12] was initiated in nineteen patients in this cohort resulting in improved oxygenation and minimal changes in respiratory system compliance after proning. While consistent with prior observations of proning in patients with ARDS [22], the implications of proning specific to SARS-CoV-2 remain unclear based upon these data. Notably however the discussion of “L” and “H” phenotypes has led to some postulation that ideal treatment in these patients could be different from “typical” ARDS with higher acceptable tidal volumes, lower PEEP and even the avoidance of proning [3,5]. Our results do not support this postulation at this time and are in concurrence with other studies showing overlapping and similar patterns and distribution of mechanics with non-SARS-CoV-2 ARDS [7,8]. Indeed any cutoffs based upon mechanics are arbitrary (without clear points of differentiation) and an oversimplification of a complex disease with unknown consequences in changes to clinical care. As best clinical practices are based upon large multi-center studies from patients without SARS-CoV-2 with populations that have similar heterogeneity and overall mechanics profiles with SAR-CoV-2 related ARDS, there appears to be minimal evidence at this time to change clinical practices based upon compliance phenotypes.
5. Conclusions
Our data and the variability within other case series [1,3,6,7] suggest that SARS-CoV-2 associated ARDS may have similar mechanics to what is thought of as “typical” ARDS [10,12,13]. While SARS-CoV-2 may cause a distinct pattern of lung injury with prolonged recovery, and lung mechanics alone do not illustrate the entire picture, patients remain at risk for developing further lung injury if not ventilated appropriately. There appears to be little evidence for a change in practice to liberate volumes, lower PEEP levels and avoid proning [3,5] as we do not know if indeed these practices themselves could inherently lead to further ventilator induced lung injury. Similar mechanics to prior studies of non-SARS-CoV-2 ARDS [10,12,13] and the heterogeneity reported among other case series emphasizes the need for individualized mechanical ventilation based on a physiological strategy, lung protective tidal volumes and close monitoring of driving pressures and respiratory efforts to prevent further lung injury, while not abandoning our best data driven practices [2,12,23,24]. By definition ARDS represents a syndrome of many different forms of lung injury with variable etiologies, disease manifestations and mechanics. While we must continue to keep an open mind as to whether such differentiation matters for clinical care of patients with or without SARS-CoV-2 [25], at this time our data suggest that ARDS secondary to SARS-CoV-2 may not be as atypical as has been suggested.
Author statement and contributions
Conceptualization: E.B.K, A.M. and D.T.. Data Curation: E.BK, L.Y and B.H.. Formal analysis: E.BK, J.H.M, M.S.·S and A.M.. Writing-Original Draft and Writing-review & editing: E.B.K., L.Y. M.M.H. B.H., J.H.M, M.S.S., A.M. D.T..
Sources of support
This work was conducted with the support of a KL2 award (an appointed KL2 award) from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award KL2 TR002542). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Declaration of Competing Interest
Dr. Baedorf Kassis and Dr. Talmor have received speaking fees for educational conferences from Hamilton Medical Inc. There are no conflicts related to the submitted research manuscript. As above, this work was conducted under the support of the NIH KL2 TR002542 award.
References
- 1.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brower R.G., Matthay M.A., Morris A., Schoenfeld D., Thompson B.T., Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000 doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 3.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. Covid-19 does not Lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan C., Chen L., Lu C., Zhang W., Xia J.-A., Sklar M.C., et al. Lung Recruitability in SARS-CoV-2 associated acute respiratory distress syndrome: a single-center, observational study. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202003-0527le. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L., et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020 doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020 doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haudebourg A.-F., Perier F., Tuffet S., de Prost N., Razazi K., Mekontso Dessap A., et al. Respiratory mechanics of COVID-19 vs. non-COVID-19 associated acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202004-1226LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panwar R., Madotto F., Laffey J.G., Van Haren F.M.P. Compliance phenotypes in early ARDS before the COVID-19 pandemic. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202005-2046oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talmor D., Sarge T., O’Donnell C.R., Ritz R., Malhotra A., Lisbon A., et al. Esophageal and transpulmonary pressures in acute respiratory failure. Crit Care Med. 2006 doi: 10.1097/01.CCM.0000215515.49001.A2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beitler J.R., Sarge T., Banner-Goodspeed V., Gong M.N., Cook D.J., Novack V., et al. 2019. Lung mechanics to guide positive end-expiratory pressure in acute respiratory distress syndrome: the EPVent-2 randomized clinical trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baedorf Kassis E., Loring S.H., Talmor D. Mortality and pulmonary mechanics in relation to respiratory system and transpulmonary driving pressures in ARDS. Intensive Care Med. 2016;42 doi: 10.1007/s00134-016-4403-7. [DOI] [PubMed] [Google Scholar]
- 12.Guérin C., Reignier J., Richard J.C., Beuret P., Gacouin A., Boulain T., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013 doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 13.Cavalcanti A.B., Suzumura É.A., Laranjeira L.N., De Moraes Paisani D., Damiani L.P., Guimarães H.P., et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome - a randomized clinical trial. JAMA. 2017 doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behazin N., Jones S.B., Cohen R.I., Loring S.H. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol. 2010 doi: 10.1152/japplphysiol.91356.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suratt P.M., Wilhoit S.C., Hsiao H.S., Atkinson R.L., Rochester D.F. Compliance of chest wall in obese subjects. J Appl Physiol Respir Environ Exerc Physiol. 1984 doi: 10.1152/jappl.1984.57.2.403. [DOI] [PubMed] [Google Scholar]
- 16.Loring S.H., Topulos G.P., Hubmayr R.D. Transpulmonary pressure: the importance of precise definitions and limiting assumptions. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201512-2448CP. [DOI] [PubMed] [Google Scholar]
- 17.Gattinoni L., Marini J.J., Camporota L. The respiratory drive: an overlooked tile of COVID-19 pathophysiology. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202008-3142ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esnault P., Cardinale M., Hraiech S., Goutorbe P., Baumstrack K., Prud’homme E., et al. High respiratory drive and excessive respiratory efforts predict relapse of respiratory failure in critically ill patients with COVID-19. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202005-1582le. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tobin M.J., Laghi F., Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann Intensive Care. 2020 doi: 10.1186/s13613-020-00692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tobin M.J., Laghi F., Jubran A. P-SILI is not justification for intubation of COVID-19 patients. Ann Intensive Care. 2020 doi: 10.1186/s13613-020-00724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brochard L. Ventilation-induced lung injury exists in spontaneously breathing patients with acute respiratory failure: yes. Intensive Care Med. 2017;43:250–252. doi: 10.1007/s00134-016-4645-4. [DOI] [PubMed] [Google Scholar]
- 22.Guerin C., Baboi L., Richard J.C. Mechanisms of the effects of prone positioning in acute respiratory distress syndrome. Intensive Care Med. 2014 doi: 10.1007/s00134-014-3500-8. [DOI] [PubMed] [Google Scholar]
- 23.Amato M.B.P., Meade M.O., Slutsky A.S., Brochard L., Costa E.L.V., Schoenfeld D.A., et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2014 doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 24.Briel M., Meade M., Mercat A. Higher vs lower positive end-expiratory pressure in patients with acute lung injury. JAMA. 2010 doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 25.Tobin M.J. Does making a diagnosis of ARDS in patients with coronavirus disease 2019 matter? Chest. 2020 doi: 10.1016/j.chest.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]