Abstract
The disease severity of COVID-19, especially in the elderly and patients with co-morbidities, is characterized by hypercytokinemia, an exaggerated immune response associated with an uncontrolled and excessive release of proinflammatory cytokine mediators (cytokine storm). Flavonoids, important secondary metabolites of plants, have long been studied as therapeutic interventions in inflammatory diseases due to their cytokine-modulatory effects. In this review, we discuss the potential role of flavonoids in the modulation of signaling pathways that are crucial for COVID-19 disease, particularly those related to inflammation and immunity. The immunomodulatory ability of flavonoids, carried out by the regulation of inflammatory mediators, the inhibition of endothelial activation, NLRP3 inflammasome, toll-like receptors (TLRs) or bromodomain containing protein 4 (BRD4), and the activation of the nuclear factor erythroid-derived 2-related factor 2 (Nrf2), might be beneficial in regulating the cytokine storm during SARS-CoV-2 infection. Moreover, the ability of flavonoids to inhibit dipeptidyl peptidase 4 (DPP4), neutralize 3-chymotrypsin-like protease (3CLpro) or to affect gut microbiota to maintain immune response, and the dual action of angiotensin-converting enzyme 2 (ACE-2) may potentially also be applied to the exaggerated inflammatory responses induced by SARS-CoV-2. Based on the previously proven effects of flavonoids in other diseases or on the basis of newly published studies associated with COVID-19 (bioinformatics, molecular docking), it is reasonable to assume positive effects of flavonoids on inflammatory changes associated with COVID-19. This review highlights the current state of knowledge of the utility of flavonoids in the management of COVID-19 and also points to the multiple biological effects of flavonoids on signaling pathways associated with the inflammation processes that are deregulated in the pathology induced by SARS-CoV-2. The identification of agents, including naturally occurring substances such as flavonoids, represents great approach potentially utilizable in the management of COVID-19. Although not clinically investigated yet, the applicability of flavonoids against COVID-19 could be a promising strategy due to a broad spectrum of their biological activities.
Keywords: SARS-CoV-2, COVID-19, Inflammation, Cytokine storm, Phytochemicals, Flavonoids, Immunomodulation, Anti-inflammatory effects
Graphical Abstract
Nomenclature
- 3CLpro
3-Chymotrypsin-like protease
- ACE-2
Angiotensin-converting enzyme 2
- Ang1-7
Angiotensin1-7
- AngII
AngiotensinII
- APC
Antigen-presenting cells
- ARDS
Acute respiratory distress syndrome
- AT1R
Angiotensin II receptor type 1
- BET
Bromodomain and extra terminal domain
- BRD4
Bromodomain containing protein 4
- CCL2
Monocyte chemo-attractant protein-1
- CRP
C-reactive protein
- CXCL10
C-X-C motif chemokine ligand 10
- DPP4
Dipeptidyl peptidase 4
- EGCG
Epigallocatechin-3-gallate
- IL
Interleukin
- JAK
Janus kinase
- KEAP1
Kelch-like ECH-associated protein 1
- Mas
Mitochondrial assembly receptor
- MCP-1
Monocyte chemoattractant protein 1
- MERS-CoV
Middle East respiratory syndrome coronavirus
- NF-κB
Nuclear factor kappa B
- NK
Natural killer
- Nrf2
Nuclear factor erythroid-derived 2-related factor 2
- RAAS
Renin-angiotensin-aldosterone system
- RNA
Ribonucleic acid
- SARS-CoV
Severe acute respiratory syndrome coronavirus
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- STAT-2
Signal transducer and activator of transcription 2
- TCM
Traditional Chinese medicine
- TLRs
Toll-like receptors
- TMPRSS
Transmembrane serine protease
- TNF-α
Tumor necrosis factor-alpha
- VEGF
Vascular endothelial growth factor
1. Introduction
The ongoing COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is currently one of the most discussed research topics worldwide [1]. The clinical manifestation of COVID-19 occurs after an incubation period of approximately 5–7 days and usually includes cough, fever, fatigue, headache, diarrhea, dyspnoea, and/or lymphopenia [2]. Most patients are asymptomatic or experience only mild to moderate symptoms. However, some patients may develop respiratory failure, acute respiratory distress syndrome (ARDS), or multiple organ failure [1], [3]. Advanced age and co-morbidities such as diabetes, cardiovascular disease, hypertension, chronic kidney disease, and chronic obstructive pulmonary disease are highly associated with increased risk of severe course of COVID-19. Complications of severe COVID-19 includes ARDS, septic shock, coagulation dysfunction, metabolic acidosis, cardiac arrhythmia, kidney damage, liver dysfunction, heart failure, or secondary infections [2]. There is ample evidence that lung damage and multiple organ failure in COVID-19 result from systemic hyper-inflammation [1]. Similar to patients with SARS-CoV and MERS-CoV, the subgroup of COVID-19 patients with severe disease have a high level of serum ferritin, D-dimer, fibrinogen, C-reactive protein, interleukin 6 (IL-6) and procalcitonin and are in increased risk of thrombosis and disseminated intravascular coagulation. These clinical and laboratory traits are linked to hyper-inflammation and are associated with macrophage activation syndrome [3]. Therefore, the identification of relevant anti-inflammatory agents applicable to COVID-19 patients could support strategies to overcome the current pandemic [1]. Reducing uncontrolled inflammation should be a therapeutic strategy, especially in patients with exaggerated immune responses demonstrated by over-production of proinflammatory mediators also known as a cytokine storm. Cytokine hyper-production is frequently followed by the development or worsening of acute respiratory failure, acute cardiac damage, or multiple organ failure [2]. Recently, the therapeutic use of naturally occurring phytochemicals has been revived, building on the traditional use of herbs for medicinal purposes [4], [5]. Flavonoids are secondary plant metabolites with a polyphenolic structure [6] that exhibit a wide range of biological effects, including anti-viral, anti-inflammatory, and immunomodulatory activities [7], [8], [9], [10], [11], [12]. In addition, flavonoids are relatively cheap and environmentally-friendly substances with minimal or no side effects [7], [8]. During the pandemic that limits people's lives worldwide, it is necessary to investigate all potential treatment options for COVID-19. Flavonoids, which are among the most important plant substances found in nature, might advance the treatment of excessive inflammation in COVID-19.
2. Pathogenesis of SARS-CoV-2 infection
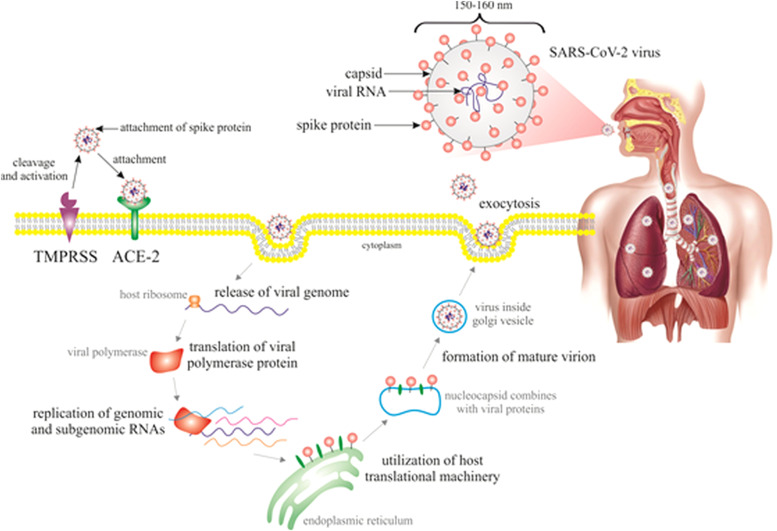
The primary immunogenic components of coronaviruses are spike glycoproteins that bind to host cells' receptors to enter them. The human receptor of SARS-CoV-2, angiotensin-converting enzyme-2 (ACE-2), is intensely expressed on the surface of alveolar epithelial type II, renal, cardiac, intestinal, endothelial, and brain cells, which is in a consistency with target organs of COVID-19 [3], [13]. ACE-2 is crucial for the entry and replication of SARS-CoV-2; nevertheless, once attached, viral translocation and replication is followed by depletion of membrane ACE-2 [14]. However, the role of ACE-2 is to cleave the angiotensinII (AngII) into angiotensin1-7 (Ang1-7) [15]. Therefore, the binding of SARS-CoV-2 prevents the production of anti-inflammatory Ang1-7 and leads to the accumulation of proinflammatory AngII. This mechanism is believed to be crucial in the pathogenesis of acute lung injury (ALI) in COVID-19 [16]. The protective effects of ACE-2 on ALI were observed in animal models; after infection by SARS-CoV, these injuries were more severe in mice with inactivated ACE-2 [17]. Dipeptidyl peptidase 4 (DPP4) has also been suggested as a co-receptor for SARS-CoV-2 [18], [19]. However, the evidence of a potential role of DPP4 in COVID-19 is still unclear. While Pitocco et al. have not so far supported the inhibition of DPP4 as a credible approach to mitigate COVID-19 [20], other authors discuss the potential role of DPP4 as a target of therapeutic strategies in the pathology of SARS-CoV-2 [19], [21]. As is shown in Fig. 1 [18], [19], [22], the primary route of SARS-CoV-2 transmission includes direct contact through droplets of saliva or discharge from the respiratory tract when a person sneezes or coughs. After the binding of spike glycoprotein to ACE-2 receptor on the cell surface, SARS-CoV-2 enters the cell, releases RNA genome, and replicates [3]. When the cell is invaded by many viral particles and the viral load is high, the whole protein synthesis apparatus is dedicated to the replication of the virus. The new viral particles are assembled and released by exocytosis and the cell is up to the death by apoptosis or as a result of energetic-metabolic chaos. The released copies of the virus spread and infect other cells and organs in a chain expansion. Eventually, an inflammatory reaction develops in tissues with many dead cells, initially in the lungs and then systematically (lymph, blood, immune system, coagulation, liver, kidney) while the reaction can be clinically severe, especially in patients with co-morbidities [13].
Fig. 1.
SARS-CoV-2 infection of host cells. Abbreviations: ACE-2, angiotensin-converting enzyme-2; TMPRSS, transmembrane serine protease. Explanatory notes: The spike glycoprotein of SARS-CoV-2 is composed of two subunits: S1 mediates the binding of the virus to the ACE-2 receptor and S2 drives host cell membrane fusion allowing viral entry. After the binding of S1 region of the virus to the receptor (ACE-2), the S protein is cleaved by host proteases such as TMPRSS (more specifically TMPRSS2) to be functional and to activate fusogenicity. Then, the fusion of the viral envelope and host plasma membrane and acidified endosomes results in the release of viral genome into the cytoplasm. The next process is facilitated by low pH of endosomes and S2 functional subunit of spike protein. SARS-CoV-2 takes advantage of host endoplasmatic reticulum to form numerous double-membrane vesicles that shield the viral genome and enable replication through the replication-transcription complex. The viral genome is translated into viral polyproteins by the protein translation machinery of the host cell that split by viral proteases into structural and non-structural viral proteins. The assembly of viral particles takes place in the endoplasmatic reticulum/Golgi compartment, and then the assembled virions are carried to the cell surface and are discharged from the cell via exocytosis [18], [19], [22].
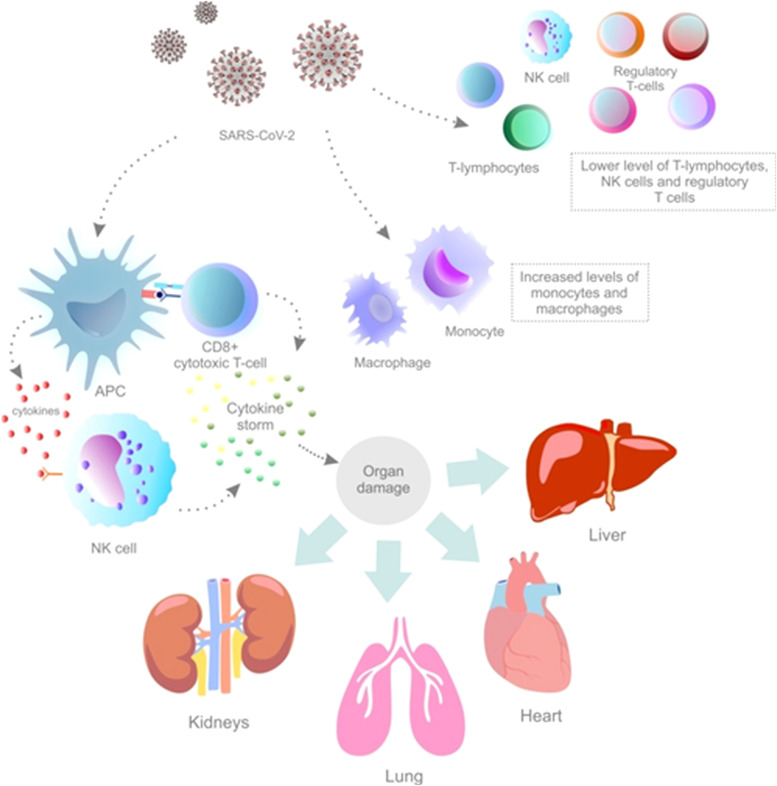
After the recognition of viral antigens by the immune system, antigen-presenting cells present viral antigens to natural killer (NK) cells and CD8-positive cytotoxic T-cells, which activate innate and adaptive immunity leading to the production of proinflammatory cytokines and chemokines [3]. Although the appropriate cytokine release is crucial for the defense against viral infection, an aberrant immune response can lead to organ injury [1]. Cytokines are secreted proteins with specific effects on the development, differentiation, and regulation of immune cells [23]. The immune activation by SARS-CoV-2 infection [24], especially in the elderly or co-morbid patients [25], can become so intense that it may cause deregulated inflammatory processes and uncontrolled systemic inflammatory responses, also known as a cytokine storm [24]. The cytokine storm, which has also been observed in other viral diseases (such as influenza, MERS, and SARS) is defined as an exaggerated immune response demonstrated by the overproduction of proinflammatory cytokines such as IL-6, tumor necrosis factor-alpha (TNF-α), and IL-1β. Currently, there is a clear correlation between elevated pro-inflammatory cytokines as a surrogate marker of cytokine storm and the severity of COVID-19 as well as unfavorable outcomes in hospitalized patients. Elevated IL-6 is recognized as one of the most important marker of unfavorable outcomes [1], [23]. The cytokine storm could lead to thrombotic events, ARDS, multiple organ failure, and death [3], [26]. The direct cause of death from acute COVID-19 includes cytokine storm-induced damage predominantly to the lungs. However, other organs, including heart, kidney, and liver are also affected. ARDS is characterized by the cytokine storm as one of its main features [24]. Therefore, the over-stimulated immune response may cause more damage to host cells than the foreign invader (SARS-CoV-2) [23]. Other immunologic features of COVID-19 include a lower level of T-lymphocytes, NK cells, and regulatory T-cells in patients with severe manifestations of the disease. On the contrary, an increased level of monocytes and macrophages in COVID-19 patients may explain the elevation of proinflammatory cytokines associated with the cytokine storm [3]. The above-discussed immunologic features of COVID-19 are illustrated in Fig. 2 [1], [3], [23], [24]. Also, the normal gut microbiota can maintain immune response to a viral infection and improve respiratory symptoms [18].
Fig. 2.
Immunologic features of SARS-CoV-2-associated pathology. Abbreviations: NK cells, natural killer cells; APC, antigen-presenting cells. Explanatory notes: APC presents viral antigens to NK cells and CD8-positive cytotoxic cells to activate innate and adaptive immunity and to produce proinflammatory mediators (cytokines) [3]. The immune activation might become so intense that it can lead to exaggerated immune response (cytokine storm) [1], [23]. The cytokine storm can result in the damage of lungs, kidneys, heart, and/or liver [24]. The immunologic features of COVID-19 include also lower levels of T-lymphocytes, NK cells, and regulatory T-cells in patients with severe disease progression. An increased level of monocytes and macrophages in COVID-19 patients can also explain the elevation of proinflammatory cytokines [3].
2.1. Inflammatory pathways associated with SARS-CoV-2
Elevated levels of cytokines and inflammatory markers including IL-6, IL-10, IL-1β, and D-dimer have been observed in severely ill COVID-19 patients compared to those with moderate symptoms [1]. Also, the examination of bronchoalveolar lavage fluid revealed the excessive release of chemokines, including C-X-C motif chemokine ligand 10 (CXCL10) and monocyte chemo-attractant protein-1 (CCL2), due to SARS-CoV-2 infection [26]. Table 1 [27], [28], [29], [30], [31] provides a detailed overview of the clinical features of COVID-19 patients associated with the inflammatory chaos.
Table 1.
Excessive levels of cytokines and chemokines in COVID-19 patients.
| The level of inflammation-associated molecules in COVID-19 patients | Reference |
|---|---|
| Increased IL-1B, IL-1RA, IL-7, IL-8, IL-9, IL-10, FGF, GM-CSF, IFNγ, G-CSF, IP10, MCP1, MIP1A, PDGF, TNFα, VEGF in COVID-19 patients (among which IL-2, IL-7, IL-10, G-CSF, IP10, MCP1, MIP1A, TNFα higher in severe patients) | [27] |
| Increased plasmatic concentration of IL-2, IL-7, IFN-γ, GCSF, IP-10, MCP1, MIP, and TNF-α in severe COVID-19 patients | [28] |
| Increased IL-6 in patients with ARDS who died in comparison with patients with ARDS who survived | [29] |
| Increased IL-6 in patients with pneumonia | [30] |
| Increased IL-6 associated with death | [31] |
Abbreviations: ARDS, acute respiratory distress syndrome; FGF, fibroblast growth factor; G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFNγ, interferon-gamma; IL, interleukin; IP10, interferon-γ-inducible protein 10; MCP1, monocyte chemo-attractant protein 1; MIP, macrophage inflammatory proteins; MIP1A, macrophage inflammatory protein 1 alpha; PDGF, platelet-derived growth factor; TNFα, tumor necrosis factor-alpha; VEGF, vascular endothelial growth factor.
Inflammatory pathways associated with SARS-CoV-2 during innate immune response include toll-like receptors (TLRs), which represent a subfamily of pattern recognition receptors (PRR). PRR are capable to recognize viruses including SARS-CoV-2 in the extracellular milieu or endosomes and mediate the inflammatory signaling leading to the activation and production of inflammatory cytokines [23]. Another important inflammatory mediator of the SARS-CoV-2-related exaggerated immune response might be the inflammasome triggered by NLRP3 that also belong s to PRR family. It is expressed in various cell types, including innate immune, endothelial, lung epithelial, hematopoietic, kidney, or cardiac cells [32]. Another mediator of innate immune reaction to SARS-CoV-2 is the bromodomain-containing protein 4 (BRD4). It is an epigenetic reader of acetylated lysines belonging to the bromodomain and extra terminal domain (BET) family that play an important role in epithelial-driven and nuclear factor kappa B (NF-κB)-dependent innate inflammation during viral infection [1]. Furthermore, respiratory viral infections are associated with inflammatory processes and oxidative stress of the epithelium lining cells that activate the transcription factor nuclear factor erythroid-derived 2-related factor 2 (Nrf2), which protects cells from oxidative damage and inflammation. The severity of COVID-19 disease is related to pre-existing conditions, including impaired immune responses, obesity, or age. These conditions are associated with decreased Nrf2 levels [25].
2.2. Targeting inflammation in COVID-19 patients
Based on the known mechanisms of SARS-CoV-2 infection, substances with potentially beneficial effects may act at various stages such as preventing the binding of the virus to the receptors or inhibiting the function of the receptor, suppressing viral replication, helping cells to resist viral attack via inhibition of cytotoxicity processes (potentially slowed down by natural antioxidants), and blocking the virus spread in the body [13]. Moreover, due to the association between over-inflammation or exaggerated immune response and SARS-CoV-2 infection, the cytokine is necessary to be early identified and appropriate anti-inflammatory treatment must be applied. Corticosteroids are currently anti-inflammatory agents with most solid evidence of benefit in the treatment of COVID-19 [1].
However, their applicability is still associated with many unknowns [1]. Notably, as SARS-CoV-2 mainly increases IL-6, and therefore the administration of corticosteroids acting against a wide range of cytokines might be excessive [26]. Therefore, in addition to the global targeting of inflammation, the neutralization of a single key inflammatory mediator is a currently evaluated therapeutical approach in COVID-19 management [33]. Anti-cytokine therapies such as IL-6, TNFα, and IL-1 cytokine antagonists are suggested to alleviate the hyper-inflammation associated with COVID-19 [23]. Potential treatment strategies targeting the cytokine storm in COVID-19 include the recombinant humanized anti-human IL-6 receptor monoclonal antibody tocilizumab. Nevertheless, the efficacy and safety of tocilizumab in COVID-19 patients needs to be evaluated in large samples and high quality studies and its benefits are yet to be established [26]. In addition, anti-TNF antibodies (infliximab, adalimumab) are suggested to modulate hyper-inflammation and cause a rapid decrease in proinflammatory cytokines in COVID-19 patients. As mentioned above, NLRP3 inflammasome plays a crucial role in the activation of IL-1β. Therefore, drugs targeting NLRP3 inflammasome and IL-1β are promising agents to mitigate hyper-inflammation and cytokine storm induced by NLRP3 inflammasome in severely ill COVID-19 patients [23]. Other potential therapeutic strategies against COVID-19 include cytokine-adsorption device [26], JAK inhibitors, chloroquine, hydroxychloroquine [27], or interferon [34]. Despite the lack of any reported cases of COVID-19 patients treated with BRD4 inhibitors, these drugs can be also considered as potential candidates to ameliorate the excessive inflammation and cytokine storm associated with COVID-19 [1]. Nevertheless, before implementing anti-inflammatory strategies in COVID-19 patients, it is highly important to balance the risks and benefits of specific therapeutic modalities due to the impairment of host immune system which might lead to secondary infections [27].
3. Flavonoids in COVID-19
Flavonoids are defined as important naturally occurring compounds with a phenolic structure [7]. Chemically, flavonoids are composed of fifteen-carbon skeleton that consists of two benzene rings connected through a pyrane ring [35]. Flavonoids are classified by their chemical structure, level of oxidation, and the pattern of the substitution of their heterocyclic pyrane ring also known as C ring. The classification of individual compounds within each class is based on the substitution of benzene rings (A and B rings) [7]. Table 2 provides an overview of the classification and food sources of flavonoids [7], [8], [9], [36], [37], [38], [39], [40], [41].
Table 2.
| Flavonoid classification | Members of the flavonoids subgroup | Food sources |
|---|---|---|
| Flavanones | Hesperidin, naringenin, naringin, taxifolin, eriodictyol, naringenin | Oranges, lemons, oregano, grapes, medicinal plants |
| Flavonols | Kaempferol, quercetin, fisetin, myricetin, morin, rutin | Onion, apples, tomatoes, kale, grapes, berries, lettuce, tea, red wine, olive oil, medicinal plants |
| Flavanols | Catechin, epicatechin, epigallocatechin-3-gallate | Green tea, apples, bananas, blueberries, cacao beans, peaches, pears, medicinal plants |
| Flavones | Apigenin, luteolin, hispidulin, wogonin, oroxylin, scutellarin, rhamnocitrin baicalein, chrysin, morusin, tangeretin, pectolinarigenin, scutellarin | Chamomile, mint, celery, parsley, Ginkgo biloba, tomatoes, fruit skin, red wine, medicinal plants |
| Isoflavonoids | Genistein, glycitein, daidzein | Soya, medicinal plants |
| Chalcones | Phloretin, xanthohumol, isoliquiritigenin, velutone F | Strawberries, apples, medicinal plants |
| Anthocyanidins | Cyanidin, delphinidin, apigenidin, malvidin | Black/cran/rasp/straw/blue-berries, grapes, cherries, blackcurrants, nuts, medicinal plants |
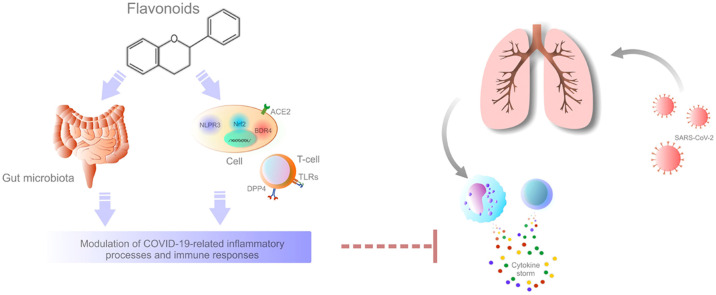
Flavonoids are secondary plant metabolites responsible for their color, flavor, and are also related to plants’ pharmacological activities. Flavonoids possess significant anti-bacterial, anti-oxidant, anti-cancer, anti-inflammatory, and immunomodulatory abilities [7], [8], [42]. In addition, flavonoids exert a strong anti-viral capacity in numerous pathologies [43], [44], [45], [46]. More importantly, flavonoids demonstrated anti-viral and immunomodulatory activities against coronaviruses [47]. Therefore, the anti-viral properties of flavonoids might be applicable also in the current COVID-19 pandemic. The potentially beneficial role of flavonoids or flavonoid-rich whole plants in COVID-19 pandemic is currently a widely discussed topic [48], [49], [50], [51]. One of the suggested targets of SARS-CoV-2 therapies is the ACE-2 receptor [52]. The applicability of flavonoids is associated with the SARS-CoV-2 spike-protein that engages ACE-2 receptors to entry into host cells as was demonstrated by bioinformatics and molecular docking in case of tea flavonoids, especially epigallocatechin-3-gallate (EGCG) and theaflavin gallate [53], fisetin, quercetin, kaempferol [54], quercetin, luteolin, or naringenin [55]. Moreover, the biological activity of flavonoids predetermines them to be effective also in terms of the modulation of inflammatory and immune pathways of SARS-CoV-2-associated pathology.
3.1. Flavonoids as potential inflammatory modulators in COVID-19
The anti-inflammatory and immunomodulatory properties of flavonoids are well-described [7], [8], [9], [10], [11], [12]. Thus, flavonoids could potentially be useful in the modulation of COVID-19-related inflammatory processes and immune responses. Due to the excessive immune responses that trigger cytokine release and can result in the overproduction of proinflammatory cytokines, the modulation of systemic immune responses and reversion of hyper-inflammation are suggested to possess a potential role in the management of COVID-19 patients [23]. Generally speaking, flavonoids can modulate the production of inflammatory mediators. Luteolin inhibited IL-1β-induced inflammation in rat chondrocytes [56]. Similarly, apigetrin, a glucoside conjugate of apigenin [57], reduced inflammatory factors including IL-lβ, TNF-α, IL-6, and VEGF in mice with acute otitis media [58]. Also, Smilax campestris aqueous extract, which contains catechin and glycosylated derivatives of quercetin (quercetin-3-O-glucoside, quercetin-3-O-galactoside, rutin, and quercetin-3-rhamnoside) as its main constituents, reduced the production of proinflammatory cytokines TNF-α, IL-1β, IL-6, IL-8, and MCP-1 in lipopolysaccharide (LPS)-activated macrophages derived from the monocytic cell line THP-1 [59]. In addition, apigenin alleviated inflammation demonstrated through reduced plasma levels of IL-6, TNF-α, and interferon-γ (IFN-γ) in vivo [60]. Furthermore, flavonoids can modulate the switch of macrophages from proinflammatory to anti-inflammatory phenotype [61]. As was demonstrated by et al, flavonoids (quercetin, naringenin and naringin) induced metabolic variations opposite to proinflammatory metabolic reprogramming elicited by LPS and IFN-γ stimulation in in vitro cultured human macrophages [62]. Also, flavonoids regulate the immune cell functions through the enhancement of the activity of NK cells and cytotoxic T lymphocytes and also through the macrophage functions via modulation of lysosomal activity and the release of nitric oxide [63]. Moreover, potent modulatory properties of hesperidin on systemic immunity (demonstrated by enhanced NK cell cytotoxicity and proportion of phagocytic monocytes, amelioration of the secretion of cytokines stimulated by macrophages, or increased T helper cells) were observed in rats following an intensive training and exhausting exercise [64]. Therefore, a significant benefit of flavonoids is associated with their potent immunomodulatory properties [11], [62], [65].
Based on the immunomodulatory properties of flavonoids discussed above, we can assume their significant effects on the production of proinflammatory cytokines also within COVID-19. Despite the high affinity to the spike protein, helicase and protease sites on ACE-2 demonstrated in in silico studies, the flavonoid-based phytomedicine caflanone also exhibited the ability to inhibit the production of cytokines including IL-1β, IL-6, IL-8, Mip-1α, TNF-α [47]. Moreover, Bellavite and Donzelli [13] recently discussed the nutraceutical properties of citrus fruits, primarily focusing on its flavonoid component hesperidin as a potential substance against SARS-CoV-2 due to its anti-viral, anti-oxidant, and inflammation-modulatory activities [13]. Hesperidin ameliorated altered level of inflammatory mediators in ischemia/reperfusion-induced kidney injury in rats [66] and also triggered anti-inflammatory responses resulting in a decreased level of IL-33 and TNF-α in mice co-treated with hesperidin and LPS [67]. Nevertheless, the hypothetical role of citrus flavonoid hesperidin against COVID-19 requires corroboration in further pre-clinical, epidemiological, and clinical studies [13].
Moreover, vascular endothelial activation has a crucial role in the excessive cytokine production leading to the cytokine storm and severe pathologies in infectious diseases such as SARS or COVID-19. Therefore, rhamnocitrin, a flavonoid extracted from Nervilia fordii, which has been identified as a potent inhibitor of endothelial activation, might be suggested as a potential modulator of cytokine storm in the management of these diseases [68].
Notably, the traditional Chinese medicine (TCM) with its main active ingredients including flavonoids (such as quercetin, kaempferol, luteolin, baicalein, naringenin, and wogonin) could also exert beneficial effects in the management of COVID-19 via the inhibition of viral adsorption and replication as well as the regulation of inflammatory mediators, anti-inflammatory, and immune-regulatory effects to prevent cytokine storm and to protect the target organs [69]. Indeed, Niu et al. [70] recently evaluated chemical constituents of three TCM formulas that were proven to be effective in COVID-19. Eventually, the network pharmacology research revealed decreased IL-6 through several TCM compounds, including but not restricted to quercetin, luteolin, and rutin. These observations suggest the positive association between TCM efficiency in the prevention and rehabilitation of at-risk COVID-19 [70]. Similarly, other TCM formula, which includes quercetin among others, could inhibit COVID-19 through ACE2 downregulation [71]. Moreover, Table 3 [72], [73], [74], [75] briefly summarizes the applicability of other TCM formulas with defined core compounds (represented mostly by flavonoids among others) in COVID-19 through the modulation of inflammatory/immune pathways, based on studies of network pharmacology and molecular docking.
Table 3.
Effects of TCM (mostly flavonoids among core compounds) in COVID-19 evaluated through network pharmacology and molecular docking.
| TCM | Potential effects in COVID-19 | Reference |
|---|---|---|
| TCM prescription Dayuanyin | Suppression of the inflammatory storm and regulation of immune function. | [72] |
| Observed affinity between the core compounds of Dayuanyin (kaempferol, quercetin, 7-Methoxy-2-methyl isoflavone, naringenin, formononetin) and its target genes such as IL-6, IL1β, and CCL2. | ||
| Maxingyigan decoction | Recognized and verified gene targets (including IL-6) and three components of Maxingyigan (quercetin, formononetin, luteolin). | [73] |
| The potential role of Maxingyigan in the prevention and treatment of COVID-19 could be based on its anti-inflammatory and immunity-based actions including the activation of T-cells, lymphocytes, leukocytes, cytokine-cytokine-receptor, and chemokine signaling pathways. | ||
| Toujie Quwen granule | The potential role of Toujie Quwen granule and its key active ingredients (including quercetin, kaempferol, luteolin, and oroxylin A, among others) in the treatment of COVID-19 associated with the mechanisms that elevate immunity, suppress inflammatory stress, and regulate inflammatory responses among others. | [74] |
| Qing-Fei-Pai-Du decoction | Observed immuno-regulatory, anti-inflammatory and multi-organ protective abilities (attributed to four compounds including also flavonoids baicalin and hesperidin and its targets) that could be applicable in COVID-19 management (thrombin and TLR signaling suggested as essential pathways of its anti-inflammatory effects). | [75] |
Abbreviations: CCL2, monocyte chemo-attractant protein-1; IL, interleukin; TCM, Traditional Chinese medicine; TLR, Toll-like receptor.
Thus, flavonoids are currently a widely discussed source of agents potentially applicable in the management of COVID-19, as demonstrated by network pharmacology and molecular docking experiments.
3.2. The potential of flavonoids to modulate specific inflammatory pathways deregulated in SARS-CoV-2 infection
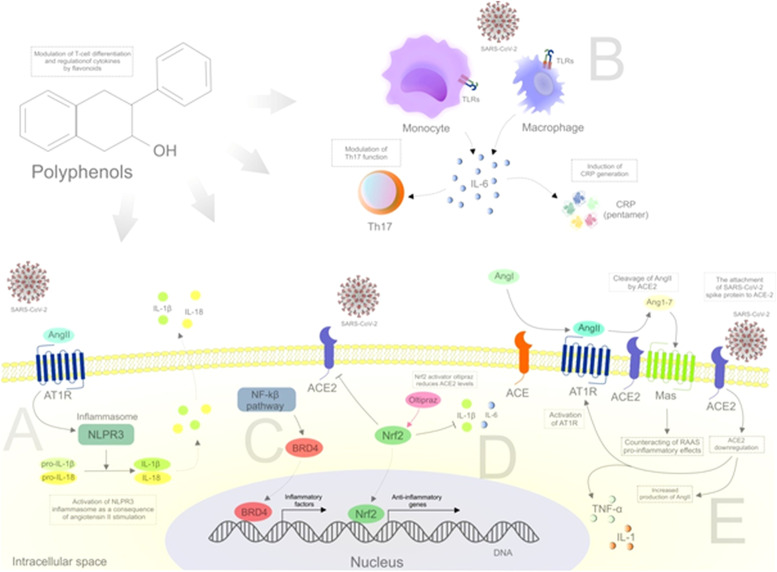
Apart from the above-discussed effects of flavonoids against the SARS-CoV-2, research databases offer a wide range of results that could indirectly point to flavonoids’ role in targeting inflammatory mediators that are altered in COVID-19. Based on the above-described inflammatory processes associated with COVID-19 and with diverse biological effects of the flavonoids taken into account, it is possible to hypothesize their significant effects on SARS-CoV-2-associated pathways such as the modulation of NLRP3 inflammasome, TLRs, or BRD4, and activation of Nrf2, or the effects on ACE-2 ( Fig. 3) [1], [16], [23], [32], [76], [77], [78].
Fig. 3.
Inflammatory pathways associated with SARS-CoV-2 that can be potentially targeted by flavonoids. Abbreviations: AngII, angiotensin II; ACE, angiotensin-converting enzyme; ACE-2, angiotensin-converting enzyme 2; Ang1-7, angiotensin 1-7; AngII, angiotensin II; AT1R, angiotensin II receptor type 1; BRD4, bromodomain-containing protein 4; CRP, c-reactive protein; IL, interleukin; Mas, mitochondrial assembly receptor; NF-κB, nuclear factor kappa B; Nrf2, nuclear factor erythroid 2-related factor 2; RAAS, renin-angiotensin-aldosterone system; TLRs, toll-like receptors. Explanatory notes: (A) An essential determinant of the inflammatory response is the cleavage and secretion of pro-IL-1β and pro-IL-18 into bioactive cytokines activated by the NLRP3 inflammasome [23]. The NLRP3 inflammasome is activated in response to AngII stimulation [32]. (B) TLR activation followed by viral infection can induce the production of IL-6 by macrophages and monocytes. TLRs, TNFα, and IL-1β are considered as the most important stimulators of IL-6. IL-6 is the main regulator of T-cells and can modulate the function of Th17 cells to serve as proinflammatory self-reactive T-cells. IL-6 can also induce the production of acute phase proteins such as CRP [23]. (C) The recruitment of BRD4 by NF-κB leads to the activation of NF-κB-mediated proinflammatory signaling while BRD4 inhibitors decrease the recruitment of macrophages and infiltration of T-cells. The transmembrane E protein of SARS-CoV-2 has been recently demonstrated to bind to BRD4 [1]. (D) The activity of Nrf2 is associated with the modulation of execution and resolution of inflammation through the repression of proinflammatory signals such as IL-6 or IL-1β [76]. (E) Despite the crucial role for viral entry, ACE-2 paradoxically exerts protective effects via conversing AngII to Ang1-7 [77]. SARS-CoV-2 spike protein attachment to ACE-2 leads to ACE-2 downregulation (increase in the level of AngII and augmentation of AngII/AT1R axis activation that are associated with proinflammatory responses). RAAS activation can promote proinflammatory responses through AT1R in kidney and vascular system [16]. The ACE-2-cleaved protein Ang1-7 bind to Mas that is followed by a decrease in proinflammatory cytokine production (TNF-α, IL-6) [78]. Therefore, the binding of SARS-CoV-2 to ACE-2 prevents the production of anti-inflammatory Ang1-7 and leads to the accumulation of proinflammatory AngII [16].
3.2.1. Flavonoids potentially targeting NLRP3 inflammasomes and TLRs in COVID-19
Flavonoids demonstrated reasonable effects in the modulation of inflammatory mediators or signaling cascades including TLRs and NLRP3 inflammasomes ( Table 4) [41], [79], [80], [81], [82], [83], [84], [85], [86], [87]. Altogether, these results highlight flavonoids’ capacity to target inflammatory processes associated with TLRs and NLRP3 inflammasome, the deregulation of which is discussed also in terms of SARS-CoV-2 pathology. Therefore, it can be assumed that flavonoids exert significant anti-viral and immunomodulatory effects mediated through TLRs or NLRP3 inflammasomes in COVID-19 patients while these effects need to be precisely evaluated in well-defined pre-clinical and clinical studies.
Table 4.
Effects of flavonoids on inflammatory cascades TLRs and NLRP3 inflammasomes.
| Target of inflammatory pathway | Flavonoid | Aim of the study | Effects | Reference |
|---|---|---|---|---|
| TLR | Epigallocatechin-3-gallate | BALB/C mice (lipopolysaccharide-induced acute lung injury) | Ameliorated lipopolysaccharide-induced acute lung injury by suppression of TLR4/NF-κB signaling. | [79] |
| Decreased proinflammatory cytokines TNF-α, IL-1β, and IL-6 in lung, serum, and bronchoalveolar lavage fluid. | ||||
| Luteolin | C57BL/6J mice (inflammation-mediated metabolic diseases) | TLR signaling modulation. | [80] | |
| Reduction of macrophage infiltration and modulation of the inflammatory response. | ||||
| Nobiletin | Prostate cancer cells (anti-inflammatory activities) | Anti-inflammatory effects (inhibition of TLR4 and TL9-dependent signaling). | [81] | |
| Pycnogenol® (extract of French maritime pine bark rich in flavonoids) | TLR-dependent immunomodulatory activities | TLRs inhibition (after gastrointestinal metabolization). | [82] | |
| Flavonoids from Houttuynia cordata | Effects and mechanism of flavonoid glycosides from H. cordata on influenza A virus-induced acute lung injury in mice | Attenuation of H1N1-induced acute lung injury (inhibition of TLR signaling). | [83] | |
| NLRP3 inflammasomes | Apigenin | Effects on NLRP3 inflammasome pathways – measurement of active IL-1β (differentiated THP-1 cells) | Inhibition of IL-1β. | [84] |
| Scutellarin | Effects on NLRP4 inflammasome activation (macrophages) | Suppression of NLRP3 inflammasome activation in macrophages. | [85] | |
| Myricetin | Effects on NLRP3-driven inflammatory diseases | Inhibition of NLRP3 inflammasome assembly. | [86] | |
| Baicalin | Effects on neuroinflammation (amyloid beta precursor protein/presenilin-1 mice) | Protection of neurons from microglia-mediated neuroinflammation via suppression of NLRP3 inflammasomes and the TLR4/NF-κB signaling pathway. | [87] | |
| Flavonoids isolated from Millettiavelutina (velutone F) | Effects on NLRP3 inflammasome activation (THP1 cells) | Suppression of NLRP3 inflammasome activation and serum IL-1β release. | [41] |
Abbreviations: IL, interleukin; NF-κB, nuclear factor kappa B; TLRs, Toll-like receptors; TNFα, tumor necrosis factor.
3.2.2. Flavonoids potentially targeting BRD4 in COVID-19
As mentioned above, the transmembrane E protein of SARS-CoV-2 has been recently demonstrated to bind to BRD4 while its recruitment by NF-κB activates proinflammatory signaling [1]. Importantly, fisetin and amentoflavone are putative ligands of BRD4 and amentoflavone can establish contacts with non-canonical residues for BET inhibition [88]. Moreover, Yokoyama [89] recently discussed the structural and thermodynamic characteristics of isoliquiritigenin interactions with BRD4 and suggested it as a novel template for BDR inhibitors [89]. Hence, BRD4 inhibition represents another approach potentially applicable to overcome COVID-19 associated inflammatory chaos.
3.2.3. Flavonoids potentially targeting Nrf2 in COVID-19
The Nrf2-response has been recently demonstrated to be suppressed in COVID-19 patient biopsies while the Nrf2 agonists 4-octyl-itaconate and the clinically approved dimethyl fumarate inhibited SARS-CoV-2 replication across cell lines in vitro [90]. The poor reproducibility of COVID-19 in animal models limits the effectiveness of the development of therapies against SARS-CoV-2. Nevertheless, genetic or pharmacological activation of Nrf2 demonstrated anti-inflammatory and anti-viral effects in other pathologies while the most relevant mechanisms of its action are associated with targeting specific cysteine receptors within KEAP1 [76]. Although the evaluation of Nrf2 inducers for the reduction of oxidative stress and inflammation in SARS-CoV-2 infections has not been yet performed, a wide range of compounds including flavonoids can activate Nrf2 within other pathologies (7-O-methylbiochanin A, biochanin A, flavonoids of Abelmoschus esculentus L. flowers, cyanidin chloride) [91], [92], [93], [94], [95]. Moreover, xanthohumol protected LPS-induced acute lung injury against inflammatory damage and oxidative stress via induction of the AMPK/GSK3β-Nrf2 signaling axis in vivo and in vitro [96]. Also, EGCG was observed to protect endothelial cells against inflammation induced by environmental pollutants while the mechanisms of action included the induction of Nrf2-regulated genes [97]. Moreover, Crateva nurvala Buch. Ham extracts containing flavonoids as the major class of bioactive phytochemicals activated Nrf2 and decreased proinflammatory TNF-α, NF-κβ, and IL-6 in vivo [98]. Besides, flavonoids from Apios americana Medikus leaves reduced inflammatory cytokines and activated Nrf2-KEAP1 pathways in RAW264.7 cells that are, when induced by LPS, accepted as a classic inflammatory model [99]. Furthermore, a study evaluating flavonoids’ role in the inhibition of Influenza A viral replication demonstrated that 6-demethoxy-4′-O-methylcapillarisin, a flavonoid derivative of Artemisia rupestris L., activated Nrf2/heme oxygenase pathway [100]. It can therefore be hypothesized that flavonoids could decrease the severity of SARS-CoV-2 via the activation of Nrf2 and subsequent modulation of inflammatory and immune processes [25].
3.2.4. Flavonoids potentially targeting ACE-associated pathways in COVID-19
The dual impact of ACE-2 in COVID-19 is associated with its ability to convert AngII to Ang1-7, thus counteracting the inflammatory action of AngII [77]. The attachment of SARS-CoV-2 spike protein to ACE-2 is followed by down-regulation of ACE-2 through its intracellular binding site and then an increase in the level of AngII and augmentation of AngII/AT1R axis activation. Increased production of AngII and activation of angiotensin II receptor type 1 (AT1R) are processes associated with proinflammatory response. The activation of NF-κB by AngII also leads to the production of TNF-α, IL-6, IL-1β. AT1R activation is followed by the regulation of mitogen-activated protein kinase (MAPK) by AngII, which also affects the release of cytokines (TNF-α, IL-1). Furthermore, the activation of the renin-angiotensin-aldosterone system (RAAS) can be associated with proinflammatory responses through AT1R in the kidney and vascular system. In fact, increased circulatory levels of AngII have been observed in COVID-19 patients with the association of its plasma level and lung injury [16]. However, the ACE-2-cleaved protein Ang1-7 is hypothesized to possess beneficial effects on immune regulation and its low expression during SARS-CoV-2 infection can be associated with COVID-19 severity. The antagonist effects of AngII-derived pathway is associated with the binding of Ang1-7 to the mitochondrial assembly (Mas) receptor and consequent decrease in proinflammatory cytokine production (TNF-α, IL-6) [78]. Therefore, the binding of SARS-CoV-2 to ACE-2 decreases its anti-inflammatory Ang1-7 production and promotes the accumulation of proinflammatory AngII [16].
In 1982, Agarwal demonstrated anti-inflammatory effects of a flavonoid nepitrin that could be mediated through its anti-angiotensin action [101]. More recently, in hypertensive rats models, high levels of AngII were attenuated by hesperidin [102] and RAS activation was inhibited by tangeretin [103]. Similarly, kaempferol exerted inflammation-inhibitory effects mediated by a decrease in AngII-induced collagen accumulation in cardiac fibroblasts [104]. A precise analysis of the effects of ACE-2 on the overall course of SARS-CoV-2 pathogenesis may contribute to the identification of key agents, potentially even among flavonoids, targeting the discussed ACE-2 and related signaling mechanisms.
3.2.5. Other potential mechanisms of flavonoids targeting inflammation as a strategy against COVID-19
Other mechanisms that could potentially be modulated by flavonoids in COVID-19 include the inhibition of DPP4, the neutralization of 3CLpro, or the effects on gut microbiota.
3.2.5.1. Dipeptidyl peptidase 4
In addition to ACE-2, recently suggested interaction between SARS-CoV-2 and dipeptidyl peptidase 4 (DPP4) as a co-receptor could lead to the development of novel therapeutic COVID-19 strategies. DPP4 is a ubiquitous membrane-bound aminopeptidase with multiple roles in metabolism, nutrition, and the endocrine and immune system [105]. A large interface was observed between the SARS-CoV-2 spike glycoprotein and DPP4 using a docked complex model. The capacity of DPP4 to cleave numerous substrates, such as chemokines or growth factors, is associated with its ability to regulate numerous physiological processes [18] and diseases of the immune system. DPP4 is expressed on epithelia and endothelia of the systemic vasculature, lung, small intestine, kidney, and heart. Accordingly, DPP4 distribution may contribute to the virus’s entry through the respiratory tract and may also facilitate the development of cytokine storm and immune-pathologies associated with fatal COVID-19 consequences [19]. Therefore, some investigators suggest the inhibition of DPP4 as a therapeutic strategy to slow the progression of COVID-19 or to hamper cytokine storm and inflammation [18], [19]. DPP-4 inhibitors, generally known as anti-diabetic drugs, may possess immunomodulatory functions and could be beneficial in inflammatory diseases [106], [107] and could act beneficially also in COVID-19 patients through the reduction of inflammation [108]. Natural compounds, including flavonoids such as EGCG also target DPP4 [109], [110], [111], [112]. Indeed, citrus flavonoids [113], epicatechin [114], and chrysin [115] were demonstrated to be potent DPP4 inhibitors. Overall, flavonoids may represent a source of DPP4 inhibitors with potential efficacy against COVID-19.
3.2.5.2. 3-Chymotrypsin-like protease
3-Chymotrypsin-like protease (3CLpro) is a non-structural protein of coronaviruses. The 3CLpro of SARS-CoV-2 shares 96.1% of its sequence with other SARS-CoV family members such as SARS-CoV or MERS-CoV. 3CLpro cleaves polyproteins into viral replication-related proteins, a process essential for viral replication and maturation. Another crucial function of 3CLpro is to cleave host proteins related to innate immune responsiveness such as the signal transducer and activator of transcription 2 (STAT-2) and NF-κB transcription factor. Therefore, the neutralization of 3CLpro can avert viral maturation and restore the natural immune response [18]. As in the case of SARS-CoV [116], [117] and MERS-CoV [118], the promising role of flavonoids has been recently suggested also in SARS-CoV-2 pathology. Interestingly, quercetin has been demonstrated as a potent inhibitor of SARS-CoV-2 3CLpro in vitro and can be considered a proper candidate for further optimization and development [119]. Similarly, the docking study revealed quercetin, scutellarin, and myricetin as potent candidates to target 3CLpro [120]. Despite quercetin’s promising efficiency in COVID-19 targeting 3CLpro, its anti-viral properties may be challenged by its poor oral bioavailability. Therefore, Di Pierro et al. have recently demonstrated an increased bioavailability of quercetin’s phospholipid complex (Quercetin Phytosome®) in humans [121]. Furthermore, a 3CLpro inhibitory activity of quercetin-3-O-rutinoside (rutin), kaempferol-3-O-rutinoside (nicotiflorin), and their human metabolites has been demonstrated using molecular docking approach [122]. Thus, the neutralization of 3CLpro by flavonoids to restore immune response represents another potential strategy against COVID-19.
3.2.5.3. Gut microbiota
Despite that SARS-CoV-2 is associated with acute respiratory syndrome, there are also gastrointestinal manifestations that may precede respiratory events. Therefore, due to the capacity of normal gut microbiota restoration to maintain immune response to viral diseases and improve respiratory symptoms, the prebiotic properties of polyphenolic compounds may represent a therapeutic strategy for COVID-19, primarily due to the possibility to affect the gut microbiota of patients [18]. Flavonoids can modulate intestinal immune function through the modulation of T-cell differentiation, alteration of gut microbiota as well as the regulation of cytokines [123], [124]. Also, Estruel-Amades [125] demonstrated the immunomodulatory activity of hesperidin on gut-associated lymphoid tissue and reinforced its prebiotic role [125]. Hence, the restoration of the immune responses through gut microbiota may potentially support the organism to overcome COVID-19.
Above all, flavonoids modulate the synthesis or activity of a plethora of inflammatory mediators and immunomodulatory signals [51], [74], [113], [114], [118], [121], [123], [126], [127]. However, pre-clinical evidence might be limited by the utilization of non-physiological concentrations in in vitro models while flavonoids are extensively metabolized in vivo [126]. Low bioavailability of flavonoids can limit or hinder their activity. Generally speaking, the bioavailability of flavonoids is affected by several factors including molecular weight, glycosylation, or metabolic conversion [128]. The metabolism of flavonoids occurs in small and large intestine, and liver. Also, the absorption, distribution, and metabolism of flavonoids and their circulating concentrations, elimination, and tissue exposure are also affected by age, sex, genotype, habitual diet, prescribed medicine, and gut microbiome [7]. Gut microbiota plays an essential role in the metabolism of flavonoids [129]. However, some metabolites were observed to paradoxically exert more robust physiological functions than their precursors [7]. Nevertheless, the increase in flavonoids bioavailability as well as the safety evaluation of their application are the goals of ongoing research [7], [9], [130], [131], [132]. Furthermore, human studies are necessary to clarify the anti-inflammatory properties of flavonoids [126].
4. Conclusion
The well-known capacity of flavonoids to regulate anti-viral, anti-inflammatory, and immunomodulatory responses underscores their potential importance also in the treatment of COVID-19. Although the results of studies utilizing flavonoids against COVID-19 published so far are promising, the body of literature on this topic as a whole remains partial and does not provide sufficient evidence for its applicability in COVID-19 patients. Nevertheless, current intensive research suggests the great potential of flavonoids as natural substances promoting the prevention or overcoming the SARS-CoV-2 infection due to the wide range of their biological effects including the modulation of inflammatory processes and immune responses. Due to the extensive biological effects of flavonoids demonstrated in various pathologies, promising results are hypothesized also in the association of their usefulness in the management of COVID-19. Based on the findings discussed in this review, the beneficial effects of flavonoids in the modulation of inflammatory and immune processes in COVID-19 can be expected. Nevertheless, the precise and detailed evaluation of flavonoid effects in well-defined research are necessary to analyze the exact mechanisms of their action, to specify the population that could benefit from such treatment, and to evaluate their bioavailability or the potentialities to improve their efficacy as a single compound or in combination with other SARS-CoV-2-targeted agents. COVID-19 as a new pathological process represents a unique challenge in the search for and identification of therapeutic substances enabling the overcoming of this pandemic, which is a severe current problem paralyzing the whole society.
Conflict of interest statement
All authors declare that they have no conflict of interest.
Funding
This work was supported by the Scientific Grant Agency of the Ministry of Education of the Slovak Republic under the Contract No. VEGA 1/0136/19 awarded to Professor Dr. Peter Kubatka and by a National Priorities Research Program grant (NPRP 11S-1214-170101; awarded to Professor Dr. Dietrich Büsselberg, June 2019-Current) from the Qatar National Research Fund (QNRF, a member of Qatar Foundation).
References
- 1.Huang Q., Wu X., Zheng X., Luo S., Xu S., Weng J. Targeting inflammation and cytokine storm in COVID-19. Pharmacol. Res. 2020;159 doi: 10.1016/j.phrs.2020.105051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soy M., Keser G., Atagündüz P., Tabak F., Atagündüz I., Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020;39:2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrovska B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012;6:1–5. doi: 10.4103/0973-7847.95849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Che C.T., Zhang H. Plant natural products for human health. IJMS. 2019;20:830. doi: 10.3390/ijms20040830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginwala R., Bhavsar R., Chigbu D.G.I., Jain P., Khan Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants. 2019:35. doi: 10.3390/antiox8020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liskova A., Koklesova L., Samec M., Smejkal K., Samuel S.M., Varghese E., Abotaleb M., Biringer K., Kudela E., Danko J., Shakibaei M., Kwon T.K., Büsselberg D., Kubatka P. Flavonoids in cancer metastasis. Cancers. 2020;12:1498. doi: 10.3390/cancers12061498. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abotaleb M., Samuel S.M., Varghese E., Varghese S., Kubatka P., Liskova A., Büsselberg D. Flavonoids in cancer and apoptosis. Cancers. 2018;11:28. doi: 10.3390/cancers11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liskova A., Koklesova L., Samec M., Varghese E., Abotaleb M., Samuel S.M., Smejkal K., Biringer K., Petras M., Blahutova D., Bugos O., Pec M., Adamkov M., Büsselberg D., Ciccocioppo R., Adamek M., Rodrigo L., Caprnda M., Kruzliak P., Kubatka P. Implications of flavonoids as potential modulators of cancer neovascularity. J. Cancer Res. Clin. Oncol. 2020;146:3079–3096. doi: 10.1007/s00432-020-03383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L., Wei Y., Zhao S., Zhang M., Yan X., Gao X., Li J., Gao Y., Zhang A., Gao Y. Antitumor and immunomodulatory activities of total flavonoids extract from persimmon leaves in H 22 liver tumor-bearing mice. Sci. Rep. 2018;8:10523. doi: 10.1038/s41598-018-28440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosseinzade A., Sadeghi O., Naghdipour Biregani A., Soukhtehzari S., Brandt G.S., Esmaillzadeh A. Immunomodulatory effects of flavonoids: possible induction of T CD4+ regulatory cells through suppression of mTOR pathway signaling activity. Front. Immunol. 2019;10:51. doi: 10.3389/fimmu.2019.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahn-Jarvis J.H., Parihar A., Doseff A.I. Dietary flavonoids for immunoregulation and cancer: food design for targeting disease. Antioxidants. 2019;8:202. doi: 10.3390/antiox8070202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellavite P., Donzelli A. Hesperidin and SARS-CoV-2: new light on the healthy function of citrus fruits. Antioxidants. 2020;9:742. doi: 10.3390/antiox9080742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abassi Z., Higazi A.A.R., Kinaneh S., Armaly Z., Skorecki K., Heyman S.N. ACE2, COVID-19 infection, inflammation, and coagulopathy: missing pieces in the puzzle. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.574753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seltzer S. Linking ACE2 and angiotensin II to pulmonary immunovascular dysregulation in SARS-CoV-2 infection. Int. J. Infect. Dis. 2020;101:42–45. doi: 10.1016/j.ijid.2020.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahmudpour M., Roozbeh J., Keshavarz M., Farrokhi S., Nabipour I. COVID-19 cytokine storm: the anger of inflammation. Cytokine. 2020;133 doi: 10.1016/j.cyto.2020.155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horne J.R., Vohl M.-C. Biological plausibility for interactions between dietary fat, resveratrol, ACE2, and SARS-CoV illness severity. Am. J. Physiol. Endocrinol. Metab. 2020;318:E830–E833. doi: 10.1152/ajpendo.00150.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy E., Delvin E., Marcil V., Spahis S. Can phytotherapy with polyphenols serve as a powerful approach for the prevention and therapy tool of novel coronavirus disease 2019 (COVID-19)? Am. J. Physiol. Endocrinol. Metab. 2020;319:E689–E708. doi: 10.1152/ajpendo.00298.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solerte S.B., Di Sabatino A., Galli M., Fiorina P. Dipeptidyl peptidase-4 (DPP4) inhibition in COVID-19. Acta Diabetol. 2020;57:779–783. doi: 10.1007/s00592-020-01539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitocco D., Tartaglione L., Viti L., Di Leo M., Pontecorvi A., Caputo S. SARS-CoV-2 and DPP4 inhibition: is it time to pray for Janus Bifrons? Diabetes Res. Clin. Pract. 2020;163 doi: 10.1016/j.diabres.2020.108162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y., Zhang Z., Yang L., Lian X., Xie Y., Li S., Xin S., Cao P., Lu J. The MERS-CoV receptor DPP4 as a candidate binding target of the SARS-CoV-2 spike. Science. 2020;23 doi: 10.1016/j.isci.2020.101400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mollica V., Rizzo A., Massari F. The pivotal role of TMPRSS2 in coronavirus disease 2019 and prostate cancer. Future Oncol. 2020;16:2029–2033. doi: 10.2217/fon-2020-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roshanravan N., Seif F., Ostadrahimi A., Pouraghaei M., Ghaffari S. Targeting cytokine storm to manage patients with COVID-19: a mini-review. Arch. Med. Res. 2020;51:608–612. doi: 10.1016/j.arcmed.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nile S.H., Nile A., Qiu J., Li L., Jia X., Kai G. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendonca P., Soliman K.F.A. Flavonoids activation of the transcription factor Nrf2 as a hypothesis approach for the prevention and modulation of SARS-CoV-2 infection severity. Antioxidants. 2020;9:659. doi: 10.3390/antiox9080659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front. Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X., Zeng X., Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratajczak M.Z., Kucia M. SARS-CoV-2 infection and overactivation of Nlrp3 inflammasome as a trigger of cytokine “storm” and risk factor for damage of hematopoietic stem cells. Leukemia. 2020:1–4. doi: 10.1038/s41375-020-0887-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahmati M., Moosavi M.A. Cytokine-targeted therapy in severely ill COVID-19 patients: options and cautions. Eurasia J. Med. Oncol. 2020;4:179–181. [Google Scholar]
- 34.Zhou Q., Chen V., Shannon C.P., Wei X.-S., Xiang X., Wang X., Wang Z.-H., Tebbutt S.J., Kollmann T.R., Fish E.N. Interferon-α2b treatment for COVID-19. Front. Immunol. 2020;11:1061. doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferraz C.R., Carvalho T.T., Manchope M.F., Artero N.A., Rasquel-Oliveira F.S., Fattori V., Casagrande R., Verri W.A. Therapeutic potential of flavonoids in pain and inflammation: mechanisms of action, pre-clinical and clinical data, and pharmaceutical development. Molecules. 2020;25:762. doi: 10.3390/molecules25030762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang M.H., Li L.Z., Sun J.B., Wu F.H., Liang J.Y. A new antioxidant flavone glycoside from Scutellaria baicalensis Georgi. Nat. Prod. Res. 2014;28:1772–1776. doi: 10.1080/14786419.2014.931391. [DOI] [PubMed] [Google Scholar]
- 37.Zhao T.T., Xu Y.Q., Hu H.M., Gong H.B., Zhu H.L. Isoliquiritigenin (ISL) and its formulations: potential antitumor agents. Curr. Med. Chem. 2019;26:6786–6796. doi: 10.2174/0929867325666181112091700. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z.L., Wang S., Kuang Y., Hu Z.M., Qiao X., Ye M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018;56:465–484. doi: 10.1080/13880209.2018.1492620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X., Zhang Z.S., Zhang X.H., Yang S.N., Liu D., Diao C.R., Wang H., Zheng F.P. Cyanidin inhibits EMT induced by oxaliplatin via targeting the PDK1-PI3K/Akt signaling pathway. Food Funct. 2019;10:592–601. doi: 10.1039/c8fo01611a. [DOI] [PubMed] [Google Scholar]
- 40.Wang K., Lv Q., Miao Y.M., Qiao S.M., Dai Y., Wei Z.F. Cardamonin, a natural flavone, alleviates inflammatory bowel disease by the inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway. Biochem. Pharmacol. 2018;155:494–509. doi: 10.1016/j.bcp.2018.07.039. [DOI] [PubMed] [Google Scholar]
- 41.Ma X., Zhao M., Tang M.H., Xue L.L., Zhang R.J., Liu L., Ni H.F., Cai X.Y., Kuang S., Hong F., Wang L., Chen K., Tang H., Li Y., Peng A.H., Yang J.H., Pei H.Y., Ye H.Y., Chen L.J. Flavonoids with inhibitory effects on NLRP3 inflammasome activation from Millettia velutina. J. Nat. Prod. 2020;83:2950–2959. doi: 10.1021/acs.jnatprod.0c00478. [DOI] [PubMed] [Google Scholar]
- 42.Kopustinskiene D.M., Jakstas V., Savickas A., Bernatoniene J. Flavonoids as anticancer agents. Nutrients. 2020;12:457. doi: 10.3390/nu12020457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Senthilvel P., Lavanya P., Kumar K.M., Swetha R., Anitha P., Bag S., Sarveswari S., Vijayakumar V., Ramaiah S., Anbarasu A. Flavonoid from Carica papaya inhibits NS2B-NS3 protease and prevents Dengue 2 viral assembly. Bioinformation. 2013;9:889–895. doi: 10.6026/97320630009889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang S.Y., Kang J.Y., Oh M.J. Antiviral activities of flavonoids isolated from the bark of Rhus verniciflua stokes against fish pathogenic viruses in vitro. J. Microbiol. 2012;50:293–300. doi: 10.1007/s12275-012-2068-7. [DOI] [PubMed] [Google Scholar]
- 45.Fukuchi K., Okudaira N., Adachi K., Odai-Ide R., Watanabe S., Ohno H., Yamamoto M., Kanamoto T., Terakubo S., Nakashima H., Uesawa Y., Kagaya H., Sakagami H. Antiviral and antitumor activity of licorice root extracts. In Vivo (Athens, Greece) 2016;30:777–785. doi: 10.21873/invivo.10994. [DOI] [PubMed] [Google Scholar]
- 46.Roschek B., Fink R.C., McMichael M.D., Li D., Alberte R.S. Elderberry flavonoids bind to and prevent H1N1 infection in vitro. Phytochemistry. 2009;70:1255–1261. doi: 10.1016/j.phytochem.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 47.Ngwa W., Kumar R., Thompson D., Lyerly W., Moore R., Reid T.E., Lowe H., Toyang N. Potential of flavonoid-inspired phytomedicines against COVID-19. Molecules. 2020;25:2707. doi: 10.3390/molecules25112707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song J.W., Long J.Y., Xie L., Zhang L.L., Xie Q.X., Chen H.J., Deng M., Li X.F. Applications, phytochemistry, pharmacological effects, pharmacokinetics, toxicity of Scutellaria baicalensis Georgi. and its probably potential therapeutic effects on COVID-19: a review. Chin. Med. 2020;15:102. doi: 10.1186/s13020-020-00384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korkmaz H. Could sumac be effective on COVID-19 treatment? J. Med. Food. 2020 doi: 10.1089/jmf.2020.0104. [DOI] [PubMed] [Google Scholar]
- 50.Hamza M., Ali A., Khan S., Ahmed S., Attique Z., Ur Rehman S., Khan A., Ali H., Rizwan M., Munir A., Khan A.M., Siddique F., Mehmood A., Nouroz F., Khan S. nCOV-19 peptides mass fingerprinting identification, binding, and blocking of inhibitors flavonoids and anthraquinone of Moringa oleifera and hydroxychloroquine. J. Biomol. Struct. Dyn. 2020:1–11. doi: 10.1080/07391102.2020.1778534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solnier J., Fladerer J.P. Flavonoids: a complementary approach to conventional therapy of COVID-19? Phytochem. Rev. 2020;25:1–23. doi: 10.1007/s11101-020-09720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muchtaridi M., Fauzi M., Khairul Ikram N.K., Mohd Gazzali A., Wahab H.A. Natural flavonoids as potential angiotensin-converting enzyme 2 inhibitors for Anti-SARS-CoV-2. Molecules. 2020;25:3980. doi: 10.3390/molecules25173980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maiti S., Banerjee A. Epigallocatechin gallate and theaflavin gallate interaction in SARS-CoV-2 spike-protein central channel with reference to the hydroxychloroquine interaction: bioinformatics and molecular docking study. Drug Dev. Res. 2020;82:86–96. doi: 10.1002/ddr.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pandey P., Rane J.S., Chatterjee A., Kumar A., Khan R., Prakash A., Ray S. Targeting SARS-CoV-2 spike protein of COVID-19 with naturally occurring phytochemicals: an in silico study for drug development. J. Biomol. Struct. Dyn. 2020:1–11. doi: 10.1080/07391102.2020.1796811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maurya V.K., Kumar S., Prasad A.K., Bhatt M.L.B., Saxena S.K. Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor. VirusDisease. 2020;31:179–193. doi: 10.1007/s13337-020-00598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fei J., Liang B., Jiang C., Ni H., Wang L. Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed. Pharmacother. 2019;109:1586–1592. doi: 10.1016/j.biopha.2018.09.161. [DOI] [PubMed] [Google Scholar]
- 57.Hadrich F., Sayadi S. Apigetrin inhibits adipogenesis in 3T3-L1 cells by downregulating PPARγ and CEBP-α. Lipids Health Dis. 2018;17:95. doi: 10.1186/s12944-018-0738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo H., Li M., Xu L.J. Apigetrin treatment attenuates LPS-induced acute otitis media though suppressing inflammation and oxidative stress. Biomed. Pharmacother. 2019;109:1978–1987. doi: 10.1016/j.biopha.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 59.Salaverry L.S., Parrado A.C., Mangone F.M., Dobrecky C.B., Flor S.A., Lombardo T., Sotelo A.D., Saccodossi N., Rugna A.Z., Blanco G., Canellada A., Rey-Roldán E.B. In vitro anti-inflammatory properties of Smilax campestris aqueous extract in human macrophages, and characterization of its flavonoid profile. J. Ethnopharmacol. 2020;247 doi: 10.1016/j.jep.2019.112282. [DOI] [PubMed] [Google Scholar]
- 60.Jung U.J., Cho Y.Y., Choi M.S. Apigenin ameliorates dyslipidemia, hepatic steatosis and insulin resistance by modulating metabolic and transcriptional profiles in the liver of high-fat diet-induced obese mice. Nutrients. 2016;8:305. doi: 10.3390/nu8050305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zaragozá C., Villaescusa L., Monserrat J., Zaragozá F., Álvarez-Mon M. Potential therapeutic anti-inflammatory and immunomodulatory effects of dihydroflavones, flavones, and flavonols. Molecules. 2020;25:1017. doi: 10.3390/molecules25041017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mendes L.F., Gaspar V.M., Conde T.A., Mano J.F., Duarte I.F. Flavonoid-mediated immunomodulation of human macrophages involves key metabolites and metabolic pathways. Sci. Rep. 2019;9:14906. doi: 10.1038/s41598-019-51113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sassi A., Mokdad Bzéouich I., Mustapha N., Maatouk M., Ghedira K., Chekir-Ghedira L. Immunomodulatory potential of hesperetin and chrysin through the cellular and humoral response. Eur. J. Pharmacol. 2017;812:91–96. doi: 10.1016/j.ejphar.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 64.Ruiz-Iglesias P., Estruel-Amades S., Camps-Bossacoma M., Massot-Cladera M., Franch À., Pérez-Cano F.J., Castell M. Influence of hesperidin on systemic immunity of rats following an intensive training and exhausting exercise. Nutrients. 2020;12:1291. doi: 10.3390/nu12051291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Samec M., Liskova A., Koklesova L., Samuel S.M., Murin R., Zubor P., Bujnak J., Kwon T.K., Büsselberg D., Prosecky R., Caprnda M., Rodrigo L., Ciccocioppo R., Kruzliak P., Kubatka P. The role of plant-derived natural substances as immunomodulatory agents in carcinogenesis. J. Cancer Res. Clin. Oncol. 2020;146:3137–3154. doi: 10.1007/s00432-020-03424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meng X., Wei M., Wang D., Qu X., Zhang K., Zhang N., Li X. The protective effect of hesperidin against renal ischemia-reperfusion injury involves the TLR-4/NF-κB/iNOS pathway in rats. Physiol. Int. 2020;107:82–91. doi: 10.1556/2060.2020.00003. [DOI] [PubMed] [Google Scholar]
- 67.Al-Rikabi R., Al-Shmgani H., Dewir Y.H., El-Hendawy S. In vivo and in vitro evaluation of the protective effects of hesperidin in lipopolysaccharide-induced inflammation and cytotoxicity of cell. Molecules. 2020;25:478. doi: 10.3390/molecules25030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin T., Luo W., Li Z., Zhang L., Zheng X., Mai L., Yang W., Guan G., Su Z., Liu P., Li Z., Xie Y. Rhamnocitrin extracted from Nervilia fordii inhibited vascular endothelial activation via miR-185/STIM-1/SOCE/NFATc3. Phytomedicine. 2020;79 doi: 10.1016/j.phymed.2020.153350. [DOI] [PubMed] [Google Scholar]
- 69.Huang Y.F., Bai C., He F., Xie Y., Zhou H. Review on the potential action mechanisms of Chinese medicines in treating Coronavirus Disease 2019 (COVID-19) Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niu W.H., Wu F., Cao W.Y., Wu Z.G., Chao Y.C., Liang C. Network pharmacology for the identification of phytochemicals in traditional Chinese medicine for COVID-19 that may regulate interleukin-6. Biosci. Rep. 2021;41 doi: 10.1042/BSR20202583. BSR20202583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niu W., Wu F., Cui H., Cao W., Chao Y., Wu Z., Fan M., Liang C. Network pharmacology analysis to identify phytochemicals in traditional chinese medicines that may regulate ACE2 for the treatment of COVID-19. Evid. Based Complement. Altern. Med. 2020;2020:1–14. doi: 10.1155/2020/7493281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruan X., Du P., Zhao K., Huang J., Xia H., Dai D., Huang S., Cui X., Liu L., Zhang J. Mechanism of Dayuanyin in the treatment of coronavirus disease 2019 based on network pharmacology and molecular docking. Chin. Med. 2020;15:62. doi: 10.1186/s13020-020-00346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang M., Fu D., Yao L., Li J. Theoretical study of the molecular mechanism of maxingyigan decoction against COVID-19: network pharmacology-based strategy. Comb. Chem. High Throughput Screen. 2020 doi: 10.2174/1386207323666200806164635. [DOI] [PubMed] [Google Scholar]
- 74.Huang Y., Zheng W.J., Ni Y.S., Li M.S., Chen J.K., Liu X.H., Tan X.H., Li J.Q. Therapeutic mechanism of Toujie Quwen granules in COVID-19 based on network pharmacology. BioData Min. 2020;13:15. doi: 10.1186/s13040-020-00225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao J., Tian S., Lu D., Yang J., Zeng H., Zhang F., Tu D., Ge G., Zheng Y., Shi T., Xu X., Zhao S., Yang Y., Zhang W. Systems pharmacological study illustrates the immune regulation, anti-infection, anti-inflammation, and multi-organ protection mechanism of Qing-Fei-Pai-Du decoction in the treatment of COVID-19. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cuadrado A., Pajares M., Benito C., Jiménez-Villegas J., Escoll M., Fernández-Ginés R., Garcia Yagüe A.J., Lastra D., Manda G., Rojo A.I., Dinkova-Kostova A.T. Can activation of NRF2 be a strategy against COVID-19? Trends Pharmacol. Sci. 2020;41:598–610. doi: 10.1016/j.tips.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Behl T., Kaur I., Bungau S., Kumar A., Uddin M.S., Kumar C., Pal G., Sahil, Shrivastava K., Zengin G., Arora S. The dual impact of ACE2 in COVID-19 and ironical actions in geriatrics and pediatrics with possible therapeutic solutions. Life Sci. 2020;257 doi: 10.1016/j.lfs.2020.118075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Méry G., Epaulard O., Borel A.-L., Toussaint B. COVID-19: underlying adipokine storm and angiotensin 1-7 umbrella. Front. Immunol. 2020;11:1714. doi: 10.3389/fimmu.2020.01714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang J., Fan S.M., Zhang J. Epigallocatechin-3-gallate ameliorates lipopolysaccharide-induced acute lung injury by suppression of TLR4/NF-κB signaling activation. Braz. J. Med. Biol. Res. 2019;52 doi: 10.1590/1414-431X20198092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kwon E.Y., Choi M.S. Luteolin targets the toll-like receptor signaling pathway in prevention of hepatic and adipocyte fibrosis and insulin resistance in diet-induced obese mice. Nutrients. 2018;10:1415. doi: 10.3390/nu10101415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deveci Ozkan A., Kaleli S., Onen H.I., Sarihan M., Guney Eskiler G., Kalayci Yigin A., Akdogan M. Anti-inflammatory effects of nobiletin on TLR4/TRIF/IRF3 and TLR9/IRF7 signaling pathways in prostate cancer cells. Immunopharmacol. Immunotoxicol. 2020;42:93–100. doi: 10.1080/08923973.2020.1725040. [DOI] [PubMed] [Google Scholar]
- 82.Verlaet A., van der Bolt N., Meijer B., Breynaert A., Naessens T., Konstanti P., Smidt H., Hermans N., Savelkoul H.F.J., Teodorowicz M. Toll-like receptor-dependent immunomodulatory activity of pycnogenol. Nutrients. 2019;11:214. doi: 10.3390/nu11020214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ling L.J., Lu Y., Zhang Y.Y., Zhu H.Y., Tu P., Li H., Chen D.F. Flavonoids from Houttuynia cordata attenuate H1N1-induced acute lung injury in mice via inhibition of influenza virus and Toll-like receptor signalling. Phytomedicine. 2020;67 doi: 10.1016/j.phymed.2019.153150. [DOI] [PubMed] [Google Scholar]
- 84.Lim H., Min D.S., Park H., Kim H.P. Flavonoids interfere with NLRP3 inflammasome activation. Toxicol. Appl. Pharmacol. 2018;355:93–102. doi: 10.1016/j.taap.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 85.Liu Y., Jing Y.Y., Zeng C.Y., Li C.G., Xu L.H., Yan L., Bai W.J., Zha Q.B., Ouyang D.Y., He X.H. Scutellarin suppresses NLRP3 inflammasome activation in macrophages and protects mice against bacterial sepsis. Front. Pharmacol. 2018;8:975. doi: 10.3389/fphar.2017.00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen H., Lin H., Xie S., Huang B., Qian Y., Chen K., Niu Y., Shen H.M., Cai J., Li P., Leng J., Yang H., Xia D., Wu Y. Myricetin inhibits NLRP3 inflammasome activation via reduction of ROS-dependent ubiquitination of ASC and promotion of ROS-independent NLRP3 ubiquitination. Toxicol. Appl. Pharmacol. 2019;365:19–29. doi: 10.1016/j.taap.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 87.Jin X., Liu M.Y., Zhang D.F., Zhong X., Du K., Qian P., Yao W.F., Gao H., Wei M.J. Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway. CNS Neurosci. Ther. 2019;25:575–590. doi: 10.1111/cns.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prieto-Martínez F.D., Medina-Franco J.L. Flavonoids as putative epi-modulators: insight into their binding mode with BRD4 bromodomains using molecular docking and dynamics. Biomolecules. 2018;8:61. doi: 10.3390/biom8030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yokoyama T., Matsumoto K., Ostermann A., Schrader T.E., Nabeshima Y., Mizuguchi M. Structural and thermodynamic characterization of the binding of isoliquiritigenin to the first bromodomain of BRD4. FEBS J. 2019;286:1656–1667. doi: 10.1111/febs.14736. [DOI] [PubMed] [Google Scholar]
- 90.Olagnier D., Farahani E., Thyrsted J., Blay-Cadanet J., Herengt A., Idorn M., Hait A., Hernaez B., Knudsen A., Iversen M.B., Schilling M., Jørgensen S.E., Thomsen M., Reinert L.S., Lappe M., Hoang H.-D., Gilchrist V.H., Hansen A.L., Ottosen R., Nielsen C.G., Møller C., van der Horst D., Peri S., Balachandran S., Huang J., Jakobsen M., Svenningsen E.B., Poulsen T.B., Bartsch L., Thielke A.L., Luo Y., Alain T., Rehwinkel J., Alcamí A., Hiscott J., Mogensen T., Paludan S.R., Holm C.K. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat. Commun. 2020;11:4938. doi: 10.1038/s41467-020-18764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Y.R., Li G.H., Zhou M.X., Xiang L., Ren D.M., Lou H.X., Wang X.N., Shen T. Discovery of natural flavonoids as activators of Nrf2-mediated defense system: structure-activity relationship and inhibition of intracellular oxidative insults. Bioorg. Med. Chem. 2018;26:5140–5150. doi: 10.1016/j.bmc.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 92.Liang F., Cao W., Huang Y., Fang Y., Cheng Y., Pan S., Xu X. Isoflavone biochanin A, a novel nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element activator, protects against oxidative damage in HepG2 cells. Biofactors. 2019;45:563–574. doi: 10.1002/biof.1514. [DOI] [PubMed] [Google Scholar]
- 93.Luo Y., Cui H.X., Jia A., Jia S.S., Yuan K. The protective effect of the total flavonoids of Abelmoschus esculentus L. flowers on transient cerebral ischemia-reperfusion injury is due to activation of the Nrf2-ARE pathway. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/8987173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee D.Y., Yun S.M., Song M.Y., Jung K., Kim E.H. Cyanidin chloride induces apoptosis by inhibiting NF-κB signaling through activation of Nrf2 in colorectal cancer cells. Antioxidants. 2020;9:285. doi: 10.3390/antiox9040285. (Basel, Switzerland) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zinovkin R.A., Grebenchikov O.A. Transcription factor Nrf2 as a potential therapeutic target for prevention of cytokine storm in COVID-19 patients. Biochemistry. 2020;85:833–837. doi: 10.1134/S0006297920070111. (Mosc.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lv H., Liu Q., Wen Z., Feng H., Deng X., Ci X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017;12:311–324. doi: 10.1016/j.redox.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han S.G., Han S.S., Toborek M., Hennig B. EGCG protects endothelial cells against PCB 126-induced inflammation through inhibition of AhR and induction of Nrf2-regulated genes. Toxicol. Appl. Pharmacol. 2012;261:181–188. doi: 10.1016/j.taap.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Choucry M.A., Khalil M.N.A., El Awdan S.A. Protective action of Crateva nurvala Buch. Ham extracts against renal ischaemia reperfusion injury in rats via antioxidant and anti-inflammatory activities. J. Ethnopharmacol. 2018;214:47–57. doi: 10.1016/j.jep.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 99.Chu Q., Yu X., Jia R., Wang Y., Zhang Y., Zhang S., Liu Y., Li Y., Chen W., Ye X., Zheng X. Flavonoids from Apios americana Medikus leaves protect RAW264.7 cells against inflammation via inhibition of MAPKs, Akt-mTOR pathways, and Nfr2 activation. Oxid. Med. Cell. Longev. 2019;2019:1–14. doi: 10.1155/2019/1563024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhong M., Wang H., Ma L., Yan H., Wu S., Gu Z., Li Y. DMO-CAP inhibits influenza virus replication by activating heme oxygenase-1-mediated IFN response. Virol. J. 2019;16:21. doi: 10.1186/s12985-019-1125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Agarwal O.P. The anti-inflammatory action of nepitrin, a flavonoid. Agents Actions. 1982;12:298–302. doi: 10.1007/BF01965393. [DOI] [PubMed] [Google Scholar]
- 102.Wunpathe C., Potue P., Maneesai P., Bunbupha S., Prachaney P., Kukongviriyapan U., Kukongviriyapan V., Pakdeechote P. Hesperidin suppresses renin-angiotensin system mediated NOX2 over-expression and sympathoexcitation in 2K–1C hypertensive rats. Am. J. Chin. Med. 2018;46:751–767. doi: 10.1142/S0192415X18500398. [DOI] [PubMed] [Google Scholar]
- 103.Wunpathe C., Maneesai P., Rattanakanokchai S., Bunbupha S., Kukongviriyapan U., Tong-Un T., Pakdeechote P. Tangeretin mitigates l-NAME-induced ventricular dysfunction and remodeling through the AT1R/pERK1/2/pJNK signaling pathway in rats. Food Funct. 2020;11:1322–1333. doi: 10.1039/c9fo02365h. [DOI] [PubMed] [Google Scholar]
- 104.Du Y., Han J., Zhang H., Xu J., Jiang L., Ge W. Kaempferol prevents against Ang II-induced cardiac remodeling through attenuating Ang II-induced inflammation and oxidative stress. J. Cardiovasc. Pharmacol. 2019;74:326–335. doi: 10.1097/FJC.0000000000000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bassendine M.F., Bridge S.H., McCaughan G.W., Gorrell M.D. COVID-19 and comorbidities: a role for dipeptidyl peptidase 4 (DPP4) in disease severity? J. Diabetes. 2020;12:649–658. doi: 10.1111/1753-0407.13052. [DOI] [PubMed] [Google Scholar]
- 106.Katsiki N., Ferrannini E. Anti-inflammatory properties of antidiabetic drugs: a “promised land” in the COVID-19 era? J. Diabetes Complicat. 2020;34 doi: 10.1016/j.jdiacomp.2020.107723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Drucker D.J. Coronavirus infections and type 2 diabetes-shared pathways with therapeutic implications. Endocr. Rev. 2020;41 doi: 10.1210/endrev/bnaa011. bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mozafari N., Azadi S., Mehdi-Alamdarlou S., Ashrafi H., Azadi A. Inflammation: a bridge between diabetes and COVID-19, and possible management with sitagliptin. Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pereira A.S.P., Banegas-Luna A.J., Peña-García J., Pérez-Sánchez H., Apostolides Z. Evaluation of the anti-diabetic activity of some common herbs and spices: providing new insights with inverse virtual screening. Molecules. 2019;24:4030. doi: 10.3390/molecules24224030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.González-Abuín N., Martínez-Micaelo N., Blay M., Pujadas G., Garcia-Vallvé S., Pinent M., Ardévol A. Grape seed-derived procyanidins decrease dipeptidyl-peptidase 4 activity and expression. J. Agric. Food Chem. 2012;60:9055–9061. doi: 10.1021/jf3010349. [DOI] [PubMed] [Google Scholar]
- 111.González-Abuín N., Martínez-Micaelo N., Margalef M., Blay M., Arola-Arnal A., Muguerza B., Ardévol A., Pinent M. A grape seed extract increases active glucagon-like peptide-1 levels after an oral glucose load in rats. Food Funct. 2014;5:2357–2364. doi: 10.1039/c4fo00447g. [DOI] [PubMed] [Google Scholar]
- 112.Hou H., Wang Y., Li C., Wang J., Cao Y. Dipeptidyl peptidase-4 is a target protein of epigallocatechin-3-gallate. Biomed. Res. Int. 2020;2020 doi: 10.1155/2020/5370759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gupta A., Jacobson G.A., Burgess J.R., Jelinek H.F., Nichols D.S., Narkowicz C.K., Al-Aubaidy H.A. Citrus bioflavonoids dipeptidyl peptidase-4 inhibition compared with gliptin antidiabetic medications. Biochem. Biophys. Res. Commun. 2018;503:21–25. doi: 10.1016/j.bbrc.2018.04.156. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Y., Yang Z., Liu G., Wu Y., Ouyang J. Inhibitory effect of chestnut (Castanea mollissima Blume) inner skin extract on the activity of α-amylase, α-glucosidase, dipeptidyl peptidase IV and in vitro digestibility of starches. Food Chem. 2020;324 doi: 10.1016/j.foodchem.2020.126847. [DOI] [PubMed] [Google Scholar]
- 115.Kalhotra P., Chittepu V.C.S.R., Osorio-Revilla G., Gallardo-Velázquez T. Structure–activity relationship and molecular docking of natural product library reveal chrysin as a novel dipeptidyl peptidase-4 (DPP-4) inhibitor: an integrated in silico and in vitro study. Molecules. 2018;23:1368. doi: 10.3390/molecules23061368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen L., Li J., Luo C., Liu H., Xu W., Chen G., Liew O.W., Zhu W., Puah C.M., Shen X., Jiang H. Binding interaction of quercetin-3-β-galactoside and its synthetic derivatives with SARS-CoV 3CLpro: structure–activity relationship studies reveal salient pharmacophore features. Bioorg. Med. Chem. 2006;14:8295–8306. doi: 10.1016/j.bmc.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jo S., Kim S., Shin D.H., Kim M.-S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzym. Inhib. Med. Chem. 2020;35:145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jo S., Kim H., Kim S., Shin D.H., Kim M. Characteristics of flavonoids as potent MERS‐CoV 3C‐like protease inhibitors. Chem. Biol. Drug Des. 2019;94:2023–2030. doi: 10.1111/cbdd.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abian O., Ortega-Alarcon D., Jimenez-Alesanco A., Ceballos-Laita L., Vega S., Reyburn H.T., Rizzuti B., Velazquez-Campoy A. Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening. Int. J. Biol. Macromol. 2020;164:1693–1703. doi: 10.1016/j.ijbiomac.2020.07.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sharma A., Goyal S., Yadav A.K., Kumar P., Gupta L. In-silico screening of plant-derived antivirals against main protease, 3CLpro and endoribonuclease, NSP15 proteins of SARS-CoV-2. J. Biomol. Struct. Dyn. 2020:1–15. doi: 10.1080/07391102.2020.1808077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Di Pierro F., Khan A., Bertuccioli A., Maffioli P., Derosa G., Khan S., Khan B.A., Nigar R., Ujjan I., Devraian B.R. Quercetin phytosome® as a potential drug for Covid-19. Minerva Gastroenterol. Dietol. 2020 doi: 10.23736/S1121-421X.20.02771-3. [DOI] [PubMed] [Google Scholar]
- 122.da Silva F.M.A., da Silva K.P.A., de Oliveira L.P.M., Costa E.V., Koolen H.H., Pinheiro M.L.B., de Souza A.Q.L., de Souza A.D.L. Flavonoid glycosides and their putative human metabolites as potential inhibitors of the SARS-CoV-2 main protease (Mpro) and RNA-dependent RNA polymerase (RdRp) Mem. Inst. Oswaldo Cruz. 2020;115 doi: 10.1590/0074-02760200207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pei R., Liu X., Bolling B. Flavonoids and gut health. Curr. Opin. Biotechnol. 2020;61:153–159. doi: 10.1016/j.copbio.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 124.Oteiza P.I., Fraga C.G., Mills D.A., Taft D.H. Flavonoids and the gastrointestinal tract: local and systemic effects. Mol. Asp. Med. 2018;61:41–49. doi: 10.1016/j.mam.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 125.Estruel-Amades S., Massot-Cladera M., Pérez-Cano F., Franch À., Castell M., Camps-Bossacoma M. Hesperidin effects on gut microbiota and gut-associated lymphoid tissue in healthy rats. Nutrients. 2019;11:324. doi: 10.3390/nu11020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Serafini M., Peluso I., Raguzzini A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010;69:273–278. doi: 10.1017/S002966511000162X. [DOI] [PubMed] [Google Scholar]
- 127.Chen L., Teng H., Jia Z., Battino M., Miron A., Yu Z., Cao H., Xiao J. Intracellular signaling pathways of inflammation modulated by dietary flavonoids: the most recent evidence. Crit. Rev. Food Sci. Nutr. 2018;58:2908–2924. doi: 10.1080/10408398.2017.1345853. [DOI] [PubMed] [Google Scholar]
- 128.Thilakarathna S.H., Rupasinghe H.P.V. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients. 2013;5:3367–3387. doi: 10.3390/nu5093367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bondonno N.P., Dalgaard F., Kyrø C., Murray K., Bondonno C.P., Lewis J.R., Croft K.D., Gislason G., Scalbert A., Cassidy A., Tjønneland A., Overvad K., Hodgson J.M. Flavonoid intake is associated with lower mortality in the Danish diet cancer and health cohort. Nat. Commun. 2019;10:3651. doi: 10.1038/s41467-019-11622-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dabeek W.M., Marra M.V. Dietary quercetin and kaempferol: bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients. 2019;11:2288. doi: 10.3390/nu11102288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kawabata K., Yoshioka Y., Terao J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules. 2019;24:370. doi: 10.3390/molecules24020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Williamson G., Kay C.D., Crozier A. The bioavailability, transport, and bioactivity of dietary flavonoids: a review from a historical perspective. Compr. Rev. Food Sci. Food Saf. 2018;17:1054–1112. doi: 10.1111/1541-4337.12351. [DOI] [PubMed] [Google Scholar]