Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that was diagnosed in late 2019 in Wuhan (China)[1]. It has spread rapidly worldwide, affecting millions of people, acquiring the category of a pandemic in early April 2020 by the World Health Organization. The patients most vulnerable to the disease are those with chronic diseases, including cardiovascular disease [heart failure (HF), ischemic heart disease, hypertension, diabetes mellitus (DM)][2,3]. Recent publications indicate that patients with prior HF represent a subgroup of higher risk of short-term adverse events[4]. However, the prognostic impact of a previous HF diagnosis beyond 1-month follow-up in patients with COVID-19 remains unknown. In this study, we aimed to evaluate the relationship between a prior diagnosis of HF and adverse clinical at mid-term follow-up in patients hospitalized for COVID-19.
This is a retrospective observational study of a single center in Spain (Manises Hospital, Valencia-Spain, attending 250,000 inhabitants). This study included 225 consecutive patients admitted with COVID-19 in this center from March 12th to May 10th, 2020. The diagnosis of COVID-19 was made based on the WHO recommendations and based on a positive chain reaction of the polymerase (PCR).
Demographic data, medical history, physical examination, blood tests, chest X-ray, prior and in-hospital treatments (antivirals, antibiotics, corticosteroids, ventilatory support) were collected. The previous diagnosis of HF was based on the definition proposed by the current European Society of Cardiology guidelines.
The endpoint of this study was the composite of death or readmission at mid-term follow-up. Readmission definition required any unplanned in-hospital stay longer than 24 h. The follow-up started on admission, and events included those ascertained during the follow-up (including in-hospital deaths) and post-discharge.
Continuous variables were shown as mean ± standard deviation (SD) or median [interquartile range (IQR)], depending on their distribution. The categorical variables were expressed as percentages. Differences across prior HF status across categorical variables were compared by the Fisher exact test or chi-square test. For continuous variables, we used the Student's t-test or Wilcoxon rank-sum test appropriate.
To determine the impact of HF history on outcomes, univariate and multivariable Cox proportional hazard regression models were built. Estimates of risk were presented as hazard ratios (HR). All covariates shown in Table 1 were evaluated for predictive purposes. A final model was derived by using backward stepwise selection. Well-established prognosticators and potential confounders were included in the final model regardless of their p-value. During this selection process, the linearity assumption for all continuous variables was simultaneously tested, and the variable transformed, if appropriate, with fractional polynomials. We built a main multivariate model (Model 1) that included age, sex, and covariates independently associated with the outcome (non-invasive mechanical ventilation, C-reactive protein, lactate dehydrogenase, D-dimer, estimated glomerular filtration rate, and troponin I). In a sensitivity analysis, a second model was built including the same covariates included in model 1 plus potential confounders regardless of their p-value: hypertension, type 2 diabetes mellitus (DM2), coronary artery disease, atrial fibrillation, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), cancer, and stroke (Model 2). A second sensitivity analysis included only post-discharge events and incorporated into the multivariate analysis the following covariates: age, sex, C-reactive protein, hemoglobin, and COPD (Model 3). The discriminative ability of the three models evaluated by the Harrell's C statistics were 0.828, 0.833, and 0.786 for model 1, 2, and 3, respectively.
Table 1.
Baseline characteristics across prior HF
| Total (N=225) | HF (N=71; 31.5%) | Non-HF (N=154;68,5%) | P value | |
|---|---|---|---|---|
| Age, yrs | 68±17 | 76±13 | 65±17 | <0,001 |
| Women, n (%) | 97 (43) | 25 (35) | 72 (47) | 0,14 |
| Cardovascular risk factors and comorbidities | ||||
| Hypertension, n (%) | 123 (55) | 56(79) | 67 (43,5) | <0,001 |
| Diabetes mellitus, N (%) | 63 (28) | 31(44) | 32 (21) | <0,001 |
| COPD, n (%) | 10 (4,4) | 6(8,5) | 4(2,6) | 0,048 |
| Coronary artery disease, n (%) | 31 (14) | 27(38) | 4(3) | <0,001 |
| Stroke, n (%) | 11 (4,4) | 8(11,3) | 3(2) | 0,003 |
| Atrial Fibrilation, n (%) | 20(9) | 15(21,1) | 5(3,2) | <0,001 |
| Chronic kidney disease, n (%) | 19 (8,4) | 12 (16,9) | 7(4,5) | 0,002 |
| Cáncer, n (%) | 20(8,9) | 7(9,9) | 13(8,4) | 0,728 |
| Background treatment | ||||
| Inhibitors RAAS, n (%) | 64(28,4) | 33(46,5) | 31(20,1) | <0,001 |
| Beta-Blockers, n (%) | 34(15,1) | 27(38) | 7(4,5) | <0,001 |
| MRA, n (%) | 6(2,6) | 6(8,5) | 0(0) | <0,001 |
| Diuretics, n (%) | 38(16,9) | 27(38) | 11(7,14) | <0,001 |
| Clinical presentation | ||||
| Temperature, °C | 37±1,1 | 37±1,0 | 37±1,2 | 0,782 |
| Headhache, n (%) | 15 (6,7) | 2(2,8) | 13 (8,4) | 0,116 |
| Cough, n (%) | 140 (62,2) | 41 (57,7) | 99 (64,3) | 0,347 |
| Dyspnoea, n (%) | 93 (41,3) | 33 (46,5) | 60 (38,9) | 0,287 |
| Diarrhea, n (%) | 15 (6,7) | 4 (5,6) | 11 (7,1) | 0,665 |
| Myalgia, n (%) | 21 (9,3) | 6 (8,4) | 15 (9,7) | 0,757 |
| Blood test on admission | ||||
| Leukocytes | 6.920(5.150-9.870) | 7.650(5.710-10.150) | 6.765(5.070-9.480) | 0,089 |
| Lynphocytes | 1.000(680-1.570) | 920(660-1.460) | 1.025(710-1.610) | 0,119 |
| PCR, mg/L | 58,89(22,06-137,89) | 55,98(13,73-155,76) | 59,59(23,73-125,97) | 0,559 |
| LDH, U/L | 530(398-688) | 521(386-790) | 536(398-678) | 0,755 |
| AST/ GOT, U/ml | 37(25-54) | 34(27-54) | 37(25-53) | 0,947 |
| ALT/GPT, U/ml | 26(18-45) | 23(14-36) | 30(18-50) | 0,012 |
| D-dimer, ng/ml | 900(500-1600) | 1100(500-1860) | 900(500-1400) | 0,049 |
| Hemoglobin, gr/dl | 13,4 (12,3-14,6) | 12,7(11,6-14,5) | 13,6(12,5-14,7) | 0,058 |
| Sodium, mmol/L | 140(137-142) | 140(137-142) | 139(137-141) | 0,297 |
| eFGR, ml/min/1.73m2 | 87(63-91) | 74(43-88) | 90(74-91) | <0,001 |
| Troponin I, ng/ml | 9 (4-22) | 20,3 (9-64,9) | 6.9 (3,2-14,3) | <0.001 |
| Chest X-ray | ||||
| Phatological, n (%) | 172(76,4) | 51(72) | 121(78,6) | 0,268 |
| Bilateral intersticial inf., n (%) | 36(16) | 10(14) | 26(16,9) | 0,587 |
| Uneven opacity, n (%) | 101(44,9) | 29(40,8) | 72(46,7) | 0,412 |
| Pneumonic infiltrate, n (%) | 33(14,6) | 11(15,5) | 22(14,3) | 0,808 |
| In-hospital treatment | ||||
| Antibiotics, n (%) | 205 (91) | 63(88,7) | 142 (92,2) | 0,577 |
| Hidroxichloroquine, n (%) | 159 (70,6) | 42(59) | 117 (75) | 0,011 |
| Lorinavir/Ritonavir, n (%) | 8 (3,55) | 1(1,4) | 7 (4,5) | 0,243 |
| Steroids, n (%) | 94 (41,7) | 31(43,7) | 63 (40,9) | 0,688 |
| NIMV, n (%) | 51(22,6) | 18(25,3) | 33(21,4) | 0,509 |
| Respiratory insufficiency,n(%) | 162(72) | 56(78,9) | 106(68,8) | 0,123 |
| Length of stay, days | 9 (5-15) | 11 (6-18) | 8 (5-13) | 0.022 |
| Endpoints | ||||
| Death, n (%) | 52 (23,1) | 27(38) | 25(16,2) | <0,001 |
| Death or readmission, n (%) | 78 | 41(57,7) | 37(24) | <0,001 |
Values are mean ± SD, n (%), or median (interquartile range). COPD: chronic obstructive pulmonary disease; HF: heart failure; RAAS: renin angiotensin aldosterone system; MRA: mineralocorticoid receptor antagonists; PCR: polymerase chain reaction; LDH: lactate dehydrogenase; AST/GOT: aspartate aminotransferase; ALT/GPT: alanine aminotransferase; NIMV: non-invasive mechanical ventilation
A two-tailed p-value of <0.05 was considered statistically significant for all analyzes. All analyzes were performed with STATA 15.1 (StataCorp, 2017, Stata Statistical Software: Release 15; StataCorp, LLC, College Station, TX).
The mean age of the study sample was 68±17 years, and 43% were women. Prior diagnosis of HF was present in 71 (31,5%) of the sample. In order of frequency, the most prevalent other comorbidities were hypertension (55%), DM2 (28%), ischemic heart disease (14%), atrial fibrillation (9%), cancer (8,9%), CKD (8,4%), COPD (4,4%) and stroke (4,4%). The clinical characteristics of the study population stratified by history of HF are listed in table 1. Patients with prior HF were older and showed a higher prevalence of other comorbidities, higher D-Dimer, troponin I, and lower hemoglobin and glomerular filtration rates. The mean left ventricular ejection fraction (LVEF) (last available measurement before hospitalization) and the median of N-terminal pro-brain natriuretic peptide (NT-proBNP) and troponin I on admission were among those with HF were 62%±12, 4294 pg/ml (2140-6448), and 111,4 ng/ml (49,2-173,5), respectively. A total of 64 patients (90.1%) had LVEF≥50%. There were no differences in COVID-19 clinical presentation between both groups. Also, there were no differences in non-invasive mechanical ventilation or oxygen supplementation with a nasal cannula. The median length of stay was higher in the HF-group (Table 1).
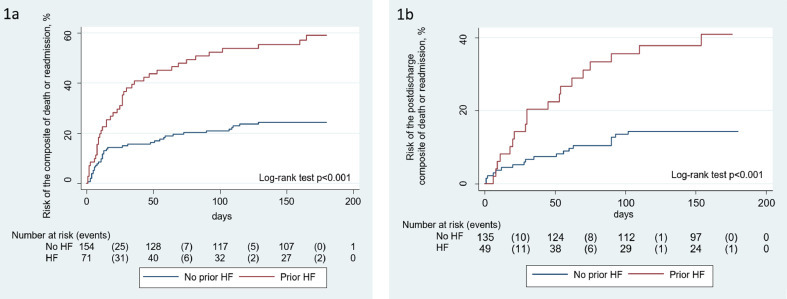
At a median follow-up of 169 days (48-186), we registered 52 deaths, 35 readmission, and 78 combined endpoints. Most of the deaths occurred during index hospitalizations (n=40). During the post-discharge follow-up, we registered 38 endpoints (35 readmissions and 12 deaths). Patients with prior HF showed higher composite endpoints rates (57,7 % vs. 24%, p< 0,001). These differences were found for in-hospital and post-discharge events (Table 1). Kaplan-Meier curves showed a progressive separation of curves during the entire follow-up and were not limited to hospitalization (Figure 1 ).
Figure 1.
Risk of the composite of death or readmission across heart failure status. (a) Risk of the combined endpoint during the whole follow-up. (b) Risk of the combined endpoint during the post-discharge follow-up.
HF: heart failure
After multivariate adjustment, prior HF remained associated with a higher risk of the combined endpoint (HR: 2.47; 95% CI: 1.48 to 4.08; p=0.001). In a sensitivity analysis forcing into the multivariate analysis, other comorbidities and potential confounders (model 2) HF was maintained as an independent risk factor for the endpoint (HR: 2.57; 95% CI: 1.42 to 4.61; p=0.002). A second sensitivity analysis (Model 3), including only patients who survived to index hospitalization (n=187), revealed that prior HF diagnosis persisted as an independent risk factor for the endpoint (HR: 2.43; 95% CI: 1.21 to 4.88; p=0.012).
In this retrospective study of a cohort of patients hospitalized for COVID-19 with a high prevalence of prior HF (31% of the study sample), patients with HF had more than the two-fold increased risk of the composite of death or readmission at mid-term follow-up. Interestingly, this excess of risk was not limited to hospitalization but extended to the post-discharge period, especially regarding a higher risk of new rehospitalizations.
HF has been evaluated as an independent risk factor for death in other studies, analyzing in-hospital mortality, or with follow-up of less than one month. Inciardi et al. [4]. retrospectively analyzed 99 patients hospitalized for COVID-19 pneumonia, 53 patients with previous CVD, including 21 patients with HF. Hospital mortality was 26% and higher in patients with cardiac disease than the others (36% vs. 15%, log-rank p= 0.019; relative risk: 2.35; 95% CI:1.08–5.09). In the same line, a large cohort of 6,349 patients admitted for COVID-19, 422 patients had prior HF (6.2%), and the risk of mortality among patients with HF was two-fold higher (40.0% vs. 24.9%; Odds Ratio: 2.02; 95% CI: 1.65 to 2.48; p<0.001)[5]. These data agree with our findings. However, we extended the persistence of higher risk at mid-term follow-up.
Interestingly, the risk associated with prior HF persisted after discharge, mainly by an increased risk of subsequent readmissions. The prevalence of HF in our study was higher than others[4,5,8]. This fact may reflect the older age and the increased cardiovascular burden of the sample here evaluated. There are no prior studies in the literature evaluating the prognostic impact of COVID-19 infection across HF status at a mid-term follow-up to the best of our knowledge.
With the present data, we cannot unravel the underlying mechanisms behind these findings: however, we may envision some of them. The baseline risk of HF patients is extremely high, especially in the first months after a decompensation. Thus, COVID-19, as it occurs with other viruses and infections, might be playing a role as a stressor and precipitating the HF decompensation, increasing the vulnerability and worse outcomes. On the other hand, there is abundant evidence demonstrating the tropism of SARS-CoV-2 for vascular/cells with a subsequent higher risk of thromboembolic event[6]. More recently, some data suggest SARS-CoV-2 may also infect cardiomyocytes as has been demonstrated in autopsies[7], and indicated by studies reflecting and increased levels of biomarkers of myocardial injury, such as troponins[8,9], and the presence of persistent myocardial inflammatory activity measured with magnetic resonance imaging in patients who had recovered from COVID-19[10]. Thus, further studies are warranted to confirm or not the causative role of COVID-19 on myocardial function and HF progression.
The clinical implications of this study are that COVID-19 patients with prior HF constitutes a very high-risk subgroup of adverse events at mid-term follow-up (not only limited to early in-hospital events). These findings suggest these patients should require close monitoring during the index hospitalization but also at post-discharge.
Important limitations should be acknowledged. First, this is an observational retrospective and single centre study in which the risk of meaningful selection bias and residual confounding cannot be ruled out. Indeed, this is a sample of the first wave of the pandemic in which only the most severe patients were identified. Second, natriuretic peptide levels were only available in those with prior HF (based on the protocol of the centre). Third, echocardiograms were not routinely performed during hospitalization. We reported the last LVEF available prior to COVID-19 infection.
In conclusion, in patients hospitalized with COVID-19, the prior diagnosis of HF significantly increases the risk of death or readmissions at mid-term follow-up.
Funding
This work was supported by CIBER Cardiovascular (grant numbers 16/11/00420).
Conflict of interest
The authors declared no potential conflicts of interest concerning the research, authorship, and publication of this article
References
- 1.Li Q, Guan X, Wu P. Early Transmission Dynamics in Wu han China, of Novel Coronavirus–Infected Pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruan Q, Yang K, Wang W. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo T, Fan Y, Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inciardi RM, Adamo M, Lupi L. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur. Heart J. 2020;41:1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez-Garcia J, Lee S, Gupta A. Prognostic Impact of Prior Heart Failure in Patients Hospitalized With COVID-19. J Am Coll Cardiol. 2020;76:2334–2348. doi: 10.1016/j.jacc.2020.09.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atri D, Siddiqi HK, Lang JP. COVID-19 for the cardiologist: basic virology, epidemiology, cardiac manifestations, and potential therapeutic strategies. J Am Coll Cardiol Basic Trans Science. 2020;5:518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindner D, Fitzek A, Brauninger H. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lala A, Johnson KW, Januzzi JL. prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lombardi CM, Carubelli V, Iorio A. Association of Troponin Levels With Mortality in Italian Patients Hospitalized With Coronavirus Disease 2019Results of a Multicenter Study. JAMA Cardiol. 2020;5:1274–1280. doi: 10.1001/jamacardio.2020.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puntmann VO, Carerj ML, Wieters I. Outcomes of cardiovascular magnetic resonance in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]