Abstract
CAPA (COVID-19 associated pulmonary aspergillosis) is an important complication of COVID-19. It has been reported that the incidence of CAPA is as high as 19%–33% worldwide. However, its onset has not been reported in Japan. A 72-year-old Japanese man was diagnosed with COVID-19 and was transferred to our hospital due to deterioration of respiratory condition. Treatment with remdesivir, dexamethasone (DEXA), and antibiotics was performed under mechanical ventilation. Although the condition improved temporarily, a new shadow appeared in the lung, and Aspergillus fumigatus was cultured from sputum. The patient was clinically diagnosed with CAPA and treated with voriconazole. However, his progress deteriorated and he died. High-risk COVID-19 patients should be tested for Aspergillus to ensure early diagnosis of CAPA.
Keywords: Coronavirus disease, COVID-19-Associated pulmonary aspergillosis, Invasive pulmonary aspergillosis
1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The first case in Japan was confirmed in January 2020 and the number of cases has increased. As of December 2020, there had been more than 180,000 cases of COVID-19 in Japan [1]. COVID-19 may have various complications including acute respiratory distress syndrome (ARDS) [2], thromboembolism [3], and cardiac complications including acute myocardial injury, arrhythmias, cardiogenic shock, and sudden death [4]. In addition, fungal infection, including COVID-19-associated pulmonary aspergillosis (CAPA) is an important complication of COVID-19 [[5], [6], [7], [8], [9], [10]]. It has been reported that 19%–33% of COVID-19 patients with severe disease develop CAPA [[5], [6], [7], [8]]. However, there have been no reports of CAPA in Japan to date, and the incidence of CAPA in Japan is unknown. We report the first known case of CAPA in Japan.
1.1. Case report
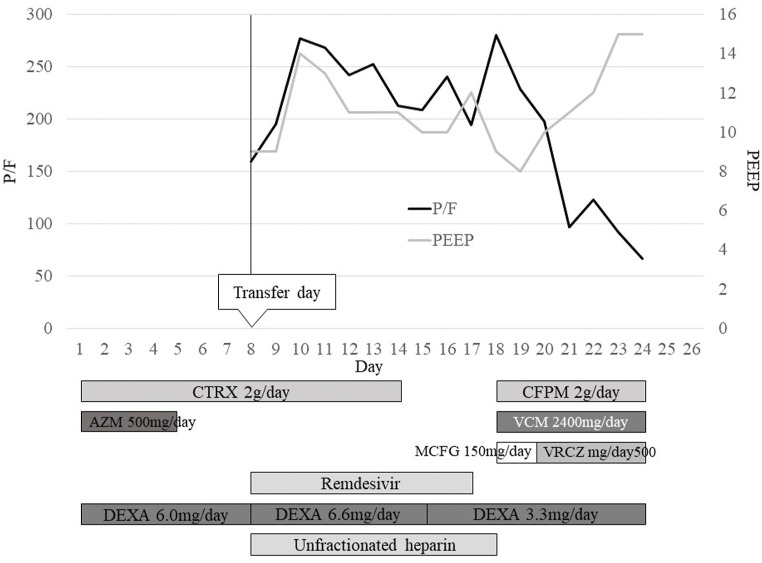
A 72-year-old Japanese man with dysarthria due to a previous stroke, presented with a staggering gait and exacerbation of his dysarthria. He was transferred to another hospital as an emergency and diagnosed with cerebral infarction from the left putamen to the external capsule (day 1). An antigen test for SARS-CoV-2 was performed upon admission as a screening measure for COVID-19 detection during the pandemic and the result was positive. The loop-mediated isothermal amplification of SARS-CoV-2, performed as a confirmatory test, was also positive, and the patient was diagnosed with COVID-19. He had a history of hypertension, atrial fibrillation, and chronic obstructive pulmonary disease (COPD) in addition the stroke, and was taking anticoagulants, antiplatelet drugs, an inhaled long-acting β2-agonist, and a long-acting muscarinic antagonist. He was 175 cm tall, weighed 61 kg, and had been a smoker until about a year before hospitalization with a cigarette consumption of about 50 packs/year. Antiplatelet drug infusion was started for cerebral infarction and DEXA was started for COVID-19. Ceftriaxone (2g/day) and azithromycin (AZM: 500mg/day, the total dose of AZM was 1500mg) were administered empirically for possible bacterial infection. However, his respiratory condition deteriorated from day 6, and he was transferred to our hospital on day 8. Computed tomography (CT) showed ground glass shadows throughout both lungs (Fig. 1 a). He was intubated and started on assisted ventilation. The ceftriaxone and DEXA were continued, and remdesivir was added for COVID-19. Unfractionated heparin (10,000 U/day) was started for cerebral infarction and COVID-19. His respiratory condition improved from day 10. A CT scan performed on day 13 showed that the ground glass shadows had improved slightly but a collection of nodular shadows had appeared in the right lower lobe (Fig. 1b). His treatment was not changed because there had been no deterioration of respiratory condition or abnormal laboratory data. Ceftriaxone and remdesivir were stopped on days 14 and 17, respectively. However, on day 18 he developed a fever and his laboratory data deteriorated. A catheter-related blood stream infection was suspected. Vancomycin and cefepime were started and the central venous catheter was replaced. As the shadows on X-ray had worsened, micafungin (150 mg/day) was added because of the possibility of fungal infection. The next day (day 19), a CT scan showed that the collection of nodular shadows in the right lower lobe had grown and become a massive shadow. Additional nodules had appeared in other parts of the right lower lobe and he had developed consolidation in both lower lobes (Fig. 1c). As his β-D glucan level was high (30.4 pg/mL, cut-off index ≤20 pg/mL) and Aspergillus fumigatus was detected in sputum culture collected on day 14 using a tracheal intubation tube submitted on day 14, he was diagnosed with probable invasive pulmonary aspergillosis (IPA) or CAPA [11,12]. The antimicrobial sensitivity profile is shown in Table 1 [13]. Micafungin was changed to voriconazole, which is more efficacious in the treatment of IPA according to Japanese guidelines, until the susceptibility of A. fumigatus was confirmed [14]. Aspergillus fumigatus was also cultured from sputum samples submitted on days 17 and 20, and an Aspergillus serum galactomannan test was positive (0.6, cut-off index ≤0.50). His respiratory condition continued to worsen (Fig. 2 ), and he developed frequent episodes of paroxysmal supraventricular tachycardia. On day 23 his tachycardia could not be controlled with medication and his blood pressure dropped and did not respond to catecholamine administration. The patient’s family was informed of the treatment options, and after obtaining consent, he was moved to palliative care and died on day 26. The total dose of DEXA until death was about 100 mg (treatment was skipped a few days at the hospital where the patient was hospitalized before being transferred). Although no autopsy could be performed, perhaps the patient’s death was associated with CAPA, as his respiratory condition deteriorated sharply after CAPA onset.
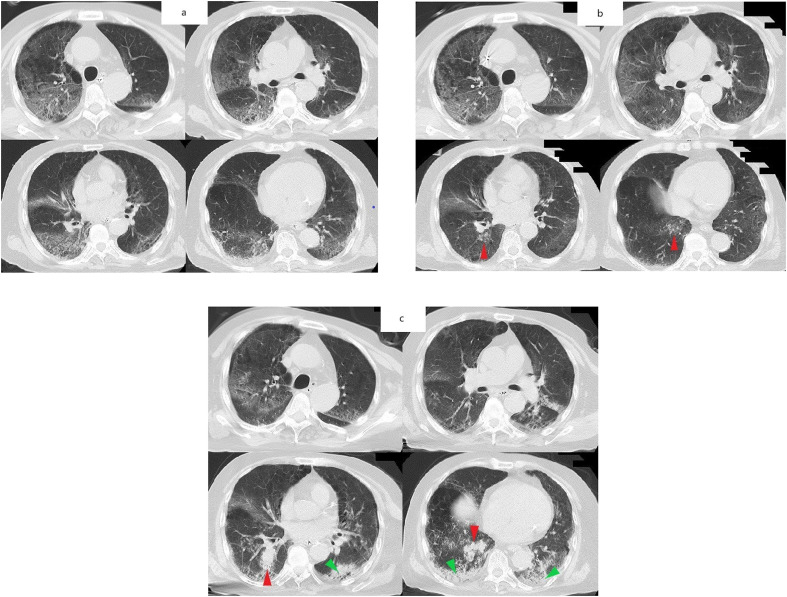
Fig. 1.
Serial chest computed tomography (CT) images showing the progression of the patient’s lung disease.
(a) Chest CT on day 8. Ground glass shadows are present in the periphery of all lung lobes.
(b) Chest CT on day 13. The ground glass shadows have improved but new nodular shadows have appeared in the right lower lobe (red arrow). (c) Chest CT on the day 20. The nodular shadows in the right lower lobe have enlarged and coalesced to resemble a tumor shadow (red arrow). In addition, new consolidation has appeared in both lower lobes (green arrow).
Table 1.
Antifungal susceptibility profile of Aspergillus fumigatus cultured from a tracheal aspirate specimen.
| Antifungal drug | MIC breakpoint (mg/L)† | MIC (mg/L) |
|---|---|---|
| Amphotericin B | 1 | 0.5 |
| Micafungin | IE | ≤0.015 |
| Caspofungin | IE | 0.25 |
| Itraconazole | 1 | 0.5 |
| Voriconazole | 1 | 1 |
Abbreviations: IE, insufficient evidence; MIC, minimum inhibitory concentration.
†Concentrations below the breakpoint indicate that the organism is susceptible to the agent, and concentrations above the breakpoint indicate that the organism is resistant.
Fig. 2.
The patient’s clinical course and timing of treatment.
Abbreviations: AZM, azithromycin; CFPM, cefepime; CTRX, ceftriaxone; DEXA, dexamethasone; MCFG, micafungin; P/F, PaO2/FiO2 ratio; PEEP, positive end expiratory pressure; VCM, vancomycin; VRCZ, voriconazole.
2. Discussion
Invasive pulmonary aspergillosis (IPA) has been identified as a complication of immunosuppression [[15], [16], [17]], but may occur in patients who are not immunosuppressed. Risk factors include short- or long-term steroid use; use of broad-spectrum antibiotics; organ failure; underlying chronic or acute pulmonary disease, including COPD; severe influenza; and ARDS [18]. CAPA is an important complication of COVID-19 [[5], [6], [7], [8], [9], [10]]. The risk factors for CAPA in patients with COVID-19 have not been determined, but a previous report identified immunomodulatory drugs and treatment in facilities that do not meet standards for appropriate room ventilation or air changes as possible risk factors [10]. Dellière et al. [19] reported that administration of a cumulative dose of azithromycin (AZM) ≥1500mg was associated with CAPA. They attributed this to decreased serum interleukin-6 and delays in the downregulation of the neutrophil oxidative burst, which is the most important immune response mechanism to aspergillosis, increased apoptosis, and changes in the lung microbiome that promote Aspergillus colonization. Our patient had underlying COPD and ARDS caused by COVID-19. In addition, he was treated with short-term steroids and a 3-day course of AZM, which may have increased his susceptibility to CAPA. Furthermore, due to the rapid increase in the incidence of COVID-19 and a shortage of intensive care unit beds in Osaka, Japan, a ward in our hospital for severe COVID-19 patients was expanded one week before the patient’s admission. An inadequate air-conditioning system may increase the risk of CAPA. Furthermore, hospital construction has been reported to be associated with IPA [20,21]. Attention to IPA is very important because COVID-19 treatment may be performed in temporary facilities or in urgently expanded wards due to a shortage of beds. Treatment at temporary facilities due to the rapid spread of COVID-19 has been linked to increased exposure to dust and construction-related materials that increase air spore counts and facilitate Aspergillus colonization [22]. Furthermore, it can cause nosocomial infection of SARS-CoV-2 due to improper air conditioning, inappropriate exhaust systems, or inadequate negative pressure devices in the room. Therefore, it is necessary to consider room design and ventilation systems for COVID-19 wards. We inspected the exhaust system and negative pressure in our facility and confirmed that there were no problems associated with room ventilation. However, the link between construction work and Aspergillus infection cannot be ruled out, although no other CAPA cases have been found in our hospital at present.
There are two possible reasons why there have been no previous reports of CAPA in Japan. First, the incidence of COVID-19 in Japan is very low compared to other countries [1,23]. Due to the relatively low incidence of COVID-19 in Japan, there are a limited number of patients with severe COVID-19, and so the incidence of CAPA is likely to be very low. Second, clinicians in Japan may not be aware of CAPA, so there may have been cases that were undiagnosed because of a lack of specific diagnostic testing, even if the patient’s clinical condition and laboratory findings were suggestive of CAPA. IPA has a high mortality rate [24]; furthermore, the mortality of patients with CAPA is considerably higher than that of COVID-19 patients without aspergillosis [25]. Although CAPA is more likely to occur in patients in poor condition, early diagnosis and treatment are necessary to reduce the mortality, and it is necessary to consider the possibility of CAPA among COVID-19 patients in Japan with risk factors or suggestive clinical signs.
Serum galactomannan and β-D glucan have low sensitivity for diagnosing IPA, and culture or galactomannan testing of bronchoalveolar lavage (BAL) specimens may be more sensitive for CAPA diagnosis than serum galactomannan [10]. However, considering the risk of aerosol generation and the ease of inspection, serum galactomannan and β-D glucan, rather than BAL, should be performed first. If a patient is clinically suspected to have CAPA clinically despite negative serum galactomannan and β-D glucan tests, the serum galactomannan and β-D glucan tests should be repeated and BAL should be considered.
In conclusion, CAPA is an important complication of COVID-19. Patients with aspergillosis-like sputum cultures should be screened using β-D glucan, and Aspergillus serum galactomannan testing. BAL should be considered in COVID-19 patients who develop a new shadow in the lung and who have risk factors such as steroid use, AZM use, ARDS, COPD, and being treated at a recently constructed or renovated hospital.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
Waki Imoto: Conceptualization, Investigation, Resources, Writing – Original Draft; Hoshi Himura: Resources, Writing – Review & Editing; Kenji Matsuo: Resources, Writing – Review & Editing; Sae Kawata: Resources, Writing – Review & Editing; Ayako Kiritoshi: Resources, Writing – Review & Editing; Ryo Deguchi: Resources, Writing – Review & Editing; Masahiro Miyashita: Resources, Writing – Review & Editing; Shinichiro Kaga: Resources, Writing – Review & Editing; Tomohiro Noda: Resources, Writing – Review & Editing; Katsumi Yamamoto: Resources, Writing – Review & Editing; Koichi Yamada: Investigation, Resources, Writing – Review & Editing; Kenichiro Uchida: Resources, Writing – Review & Editing; Tetsuro Nishimura: Resources, Writing – Review & Editing; Hiromasa Yamamoto: Resources, Writing – Review & Editing; Yasumitsu Mizobata: Resources, Writing – Review & Editing; Hiroshi Kakeya: Supervision, Project administration.
Declaration of competing InterestCOI
None.
Permission to publish
The patient’s next of kin has provided permission to publish this report and the accompanying images.
Acknowledgment
We would like to thank Editage (www.editage.com) for assistance with English language editing.
References
- 1.Ministry of Health, Labour and Welfare Novel coronavirus (COVID-19) 2020. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000164708_00079.html
- 2.Li X., Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit Care. 2020;24:198. doi: 10.1186/s13054-020-02911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imoto W., Kaga S., Noda T., Oshima K., Mizobata Y., Kakeya H. Coronavirus disease with multiple infarctions. QJM. 2020 doi: 10.1093/qjmed/hcaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhakal B.P., Sweitzer N.K., Indik J.H., Acharya D., William P. SARS-CoV-2 infection and cardiovascular disease: COVID-19 heart. Heart Lung Circ. 2020;29:973–987. doi: 10.1016/j.hlc.2020.05.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koehler P., Cornely O.A., Böttiger B.W., Dusse F., Eichenauer D.A., Fuchs F., et al. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63:528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasir N., Farooqi J., Mahmood S.F., Jabeen K. COVID-19-associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia: an observational study from Pakistan. Mycoses. 2020;63:766–770. doi: 10.1111/myc.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alanio A., Dellière S., Fodil S., Bretagne S., Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020;8:e48–e49. doi: 10.1016/S2213-2600(20)30237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Arkel A.L.E., Rijpstra T.A., Belderbos H.N.A., van Wijngaarden P., Verweij P.E., Bentvelsen R.G. COVID-19-associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020;202:132–135. doi: 10.1164/rccm.202004-1038LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arastehfar A., Carvalho A., van de Veerdonk F.L., Jenks J.D., Koehler P., Krause R., et al. COVID-19 associated pulmonary aspergillosis (CAPA)-from immunology to treatment. J Fungi (Basel) 2020;6:91. doi: 10.3390/jof6020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong-James D., Youngs J., Bicanic T., Abdolrasouli A., Denning D.W., Johnson E., et al. Confronting and mitigating the risk of COVID-19 associated pulmonary aspergillosis. Eur Respir J. 2020;56 doi: 10.1183/13993003.02554-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Pauw B., Walsh T.J., Donnelly J.P., Stevens D.A., Edwards J.E., Calandra T., et al. Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blot S.I., Taccone F.S., van den Abeele A.M., Bulpa P., Meersseman W., Brusselaers N., et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186:56–64. doi: 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]
- 13.European Society of Clinical Microbiology and Infectious Diseases . 2020. EUCAST. (European committee on antimicrobial susceptibility testing), clinical breakpoints for fungi v. 10.0.https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/AFST_BP_v10.0_200204_updatd_links_200924.pdf updated. [Google Scholar]
- 14.Kohno S., Tamura K., Niki Y., Izumikawa K., Oka S., Ogawa K., et al. Executive summary of Japanese domestic guidelines for management of deep-seated mycosis 2014. Med Mycol J. 2016;57:E117–E163. doi: 10.3314/mmj.16-00010. [DOI] [PubMed] [Google Scholar]
- 15.Gerson S.L., Talbot G.H., Hurwitz S., Strom B.L., Lusk E.J., Cassileth P.A. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345–351. doi: 10.7326/0003-4819-100-3-345. [DOI] [PubMed] [Google Scholar]
- 16.Soubani A.O., Chandrasekar P.H. The clinical spectrum of pulmonary aspergillosis. Chest. 2002;121:1988–1999. doi: 10.1378/chest.121.6.1988. [DOI] [PubMed] [Google Scholar]
- 17.Segal B.H. Aspergillosis. N Engl J Med. 2009;360:1870–1884. doi: 10.1056/NEJMra0808853. [DOI] [PubMed] [Google Scholar]
- 18.Tudesq J.J., Peyrony O., Lemiale V., Azoulay E. Invasive pulmonary aspergillosis in nonimmunocompromised Hosts. Semin Respir Crit Care Med. 2019;40:540–547. doi: 10.1055/s-0039-1696968. [DOI] [PubMed] [Google Scholar]
- 19.Dellière S., Dudoignon E., Fodil S., Voicu S., Collet M., Oillic P.A., et al. Risk factors associated with Covid-19-associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.12.005. 2030756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oren I., Haddad N., Finkelstein R., Rowe J.M. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: before and after chemoprophylaxis and institution of HEPA filters. Am J Hematol. 2001;66:257–262. doi: 10.1002/ajh.1054. [DOI] [PubMed] [Google Scholar]
- 21.Talento A.F., Fitzgerald M., Redington B., O’Sullivan N., Fenelon L., Rogers T.R. Prevention of healthcare-associated invasive aspergillosis during hospital construction/renovation works. J Hosp Infect. 2019;103:1–12. doi: 10.1016/j.jhin.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 22.Thompson G.R., Iii, Cornely O.A., Pappas P.G., Patterson T.F., Hoenigl M., Jenks J.D., et al. Invasive aspergillosis as an under-recognized superinfection in COVID-19. Open Forum Infect Dis. 2020;7:ofaa242. doi: 10.1093/ofid/ofaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int 2020/12/20 data last updated [accessed.
- 24.Cadena J., Thompson G.R., 3rd, Patterson T.F. Invasive aspergillosis: current strategies for diagnosis and management. Infect Dis Clin. 2016;30:125–142. doi: 10.1016/j.idc.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Bartoletti M., Pascale R., Cricca M., Rinaldi M., Maccaro A., Bussini L., et al. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1065. ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]