Abstract
Pulmonary vein isolation (PVI) is widely used for the ablation of atrial fibrillation, with prior reports suggesting good efficacy. Due to the widespread use of three-dimensional electroanatomic mapping systems and advances in intracardiac echocardiography, fluoroless ablation has been made possible. Fluoroless ablation with a cryoballoon (CB), however, has not been widely performed because of the need to prove occlusion of the vein with contrast dye and fluoroscopy. The objective of this study is to show that CB ablation can be performed safely and effectively without fluoroscopy. A dual-center, case–control study was performed of patients undergoing CB PVI with a fluoroless approach and a control group with traditional fluoroscopic techniques. The absence of color-flow Doppler signals around the periphery of the CB on intracardiac echocardiography and an increase in mean pressure by 5 mmHg, loss of the A-wave, and an increase in the V-wave as measured with continuous-wave pressure monitoring were adopted as indicators of vein occlusion in the absence of fluoroscopy. Temperature at 30 seconds, minimum temperature, time to isolation, procedure length, and complications were evaluated. During the study period of November 15, 2018 to November 15, 2019, a total of 100 patients underwent CB PVI at the participating centers. A total of 50 patients were enrolled in the fluoroless arm [35 men (70%), mean age: 64.9 ± 12 years, mean left atrium size: 44.2 ± 16 mL/m2, left ventricular ejection fraction: 61% ± 5%], while 50 patients were enrolled in the control arm with similar characteristics. Four hundred forty-one 441 PVs were evaluated in the study cohort compared to 339 PVs in the control arm. When comparing fluoroless and traditional techniques, the mean temperature at 30 seconds was −31.7°C ± 6°C versus −32.8°C ± 5°C (p = 0.037), the minimum temperature was −47.4°C ± 6°C versus −47.7°C ± 9°C (p = 0.677), the time to isolation was 56.8 ± 28 seconds versus 74.8 ± 45 seconds (p = 0.212), and the procedure time was 102.2 ± 27.3 seconds versus 104.5 ± 16.9 seconds (p = 0.6436). Ultimately, this proof-of-concept study revealed that fluoroless ablation can be performed with success and efficiency outcomes similar to those of a traditional ablation approach. This suggests that the ablation of atrial fibrillation with CB can be performed safely and effectively without the use of fluoroscopy by experienced operators.
Keywords: Ablation, atrial fibrillation, cryoballoon, fluoroless
Introduction
Pulmonary vein isolation (PVI) with either radiofrequency (RF) energy or cryoballoon (CB) technology is a well-established ablative approach for the management of atrial fibrillation (AF), with good reported efficacy rates. With widespread use of three-dimensional (3D) electroanatomic mapping systems and advances with intracardiac echocardiography (ICE), fluoroless ablation has become possible. This is desirable given the benefits of avoiding ionizing radiation as well as due to the potential orthopedic and other benefits operators and staff might experience. Fluoroless ablation using an RF energy source has been well-described, with a similar safety profile and outcomes as those of standard approaches.1–5 Fluoroless ablation with CB, however, has not been widely performed because of the perceived need to prove circumferential occlusion of the PV ostium with fluoroscopic evaluation of contrast dye injections into the targeted vein.
The reduction of fluoroscopy time during CB ablation has been achieved with the use of alternative imaging and hemodynamic modalities. Transesophageal echocardiography (TEE) has been shown to be an effective tool for reducing fluoroscopy exposure.6–8 Similarly, recent reports have demonstrated that, with the use of ICE, fluoroscopy can be reduced.9 A reduction in fluoroscopy has also been achieved with the use of continuous-wave pressure monitoring (CWPM) as a hemodynamic measure of vein occlusion.10–13 The aim of this proof-of-concept study was to show that, by employing imaging with ICE and hemodynamic measures with CWPM, fluoroscopy use during CB procedures can be safely and effectively eliminated. We present the first proof-of-concept, case–control study comparing the use of traditional fluoroscopic methods of CB ablation with a completely fluoroless approach.
Methods
Study participants
Study enrollment occurred from November 15, 2018 to November 15, 2019 at two high-volume AF ablation centers. All patients enrolled in the trial had drug-refractory, symptomatic paroxysmal AF or recent-onset (< 6 months) persistent AF. All patients received general anesthesia during their treatment and were monitored overnight and discharged home the next day.
Our study was a retrospective analysis of cases performed by four operators. All operators were experienced and had performed more than 100 CB ablation procedures to date. Abbott (Chicago, IL, USA) and Medtronic (Minneapolis, MN, USA) employees assisted with the data collection but did not participate in the data analysis nor the writing of this manuscript. This study was approved by the local institutional review board, who waived the need for participant consent due to the retrospective nature of this investigation.
Ablation procedure
All patients were anticoagulated for one month prior to their procedure either with a direct oral anticoagulant or with warfarin to achieve an international normalized ratio range of 2.0 to 3.0. For patients treated with the former, their dose was held the morning of the procedure, while those on warfarin maintained their dosing schedule. Antiarrhythmic medication management prior to ablation was left to the discretion of the operator. All procedures were performed with general anesthesia. A preoperative computed tomography (CT) scan to evaluate the PV anatomy and TEE imaging to evaluate for left atrial appendage thrombus were conducted at the discretion of the operator.
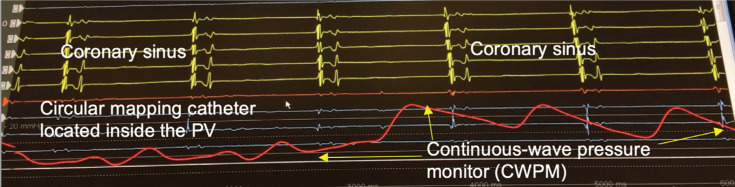
Vascular access was obtained under ultrasound guidance. Heparin was infused to maintain an activated clotting time goal of 300 to 350 seconds. An ICE catheter (ViewFlex Xtra ICE catheter; Abbott, Chicago, IL, USA or Accuson, AcuNav ultrasound catheter; Biosense Webster, Diamond Bar, CA, USA) was advanced into the right atrium and used for the visualization of all cardiac chambers throughout each case. One transseptal puncture was performed with a fixed sheath (SL-1; Abbott) and a transseptal needle (BRK-1; Abbott) using ICE or fluoroscopic guidance. A guidewire was advanced through the SL-1 and then exchanged for a steerable sheath (Flexcath Advance Steerable Sheath; Medtronic). Using an impedance-based electroanatomic 3D mapping system (Ensite Precision™; Abbott) geometry of the left atrium (LA) and PVs were acquired using a circular mapping catheter (Reflexion Spiral; Abbott). If CT was performed, the geometry was displayed along with the anatomy from the CT scan that was acquired prior to ablation. An esophageal temperature probe (Level 1 Acoustascope 12-French; Smiths Medical ASD, Inc., St. Paul, MN, USA) was placed and monitored for changes. The temperature probe was visualized with ICE or fluoroscopy and was moved inferiorly and superiorly in accordance with the location of the CB. In the fluoroless cohort, the ICE catheter was rotated to show the posterior aspects of the LA. With this view, the esophagus can be visualized and the temperature probe can be visualized as it is placed at the level of the PVs. Ablation lesions were stopped at an esophageal temperature threshold of 25°C. Ablation was performed using an Arctic Front Cryocath Advance and Advance Pro (Medtronic) 28-mm diameter balloon in all cases. The balloon has an inner lumen guidewire that is usually used to inject contrast fluid through the distal tip of the balloon. For the nonfluoroscopic cases, the inner lumen was connected to a pressure transducer to record CWPM. A multipolar mapping catheter (Achieve; Medtronic) was also placed through the inner lumen and used for the detection of PV signals as well as to assess the entrance and exit block at the end of the case. The vein was considered occluded in the fluoroscopic arm if there was no retrograde flow into the LA with the injection of contrast through the distal port. In the nonfluoroscopic arm, the vein was considered occluded if there was a change in the mean CWPM of 5 mmHg or greater in patients in AF and an increase in the V-wave amplitude. For patients in sinus rhythm, the vein was considered occluded if there was a change in CWPM of 5 mmHg or greater and an increase in the V-wave and decrease in the A-wave to achieve a pattern consistent with the transcapillary pulmonary arterial pressure (Figure 1). In addition, for the vein to be considered occluded, an absence of high-velocity Doppler color flow around the periphery of the CB on interrogation with ICE was necessary. The ICE catheter was manipulated to show all aspects of the PV. If leakage was detected, the CB, sheath, or both were adjusted until the CWPM and Doppler color-flow interrogation showed no evidence of flow.
Figure 1:
Change in CWPM with occlusion of the PV. Note the loss of the A-wave, increase in V-wave, and overall increase in the mean pressure. CWPM: continuous-wave pressure monitoring; PV: pulmonary vein.
In rare instances, complete occlusion could not be achieved with a single CB application. These veins were segmentally ablated to achieve isolation by focusing contact on different aspects of the PV (Figures 2A and 2B). Each CB freeze was applied for either 180 seconds total or until 120 seconds beyond isolation. If isolation was not achieved or less than 120 seconds of ablation were delivered following isolation, a second ablation lesion was delivered for either 120 seconds or 180 seconds, at the operator’s discretion. During ablation of the right-sided veins, the phrenic nerve was paced with a catheter in the superior vena cava or with compound motor action potential (CMAP), at the discretion of the operator. Phrenic nerve injury was particularly monitored. The location of the CB was monitored on ICE to make sure that it was ostial and not too deep in the PV. In addition, CMAP and/or palpation of the right hemidiaphragm was monitored throughout. At the completion of ablation of all the PVs, cardioversion was performed for patients that remained in AF. Afterward, all of the veins were reevaluated for the presence of PV potentials and with pacing maneuvers that showed the entrance and exit block. If necessary, further ablation lesions were delivered to achieve isolation and block. The temperature at 30 seconds, minimum temperature, time to isolation, thaw time to 20°C, and procedural complications were recorded.
Figure 2:
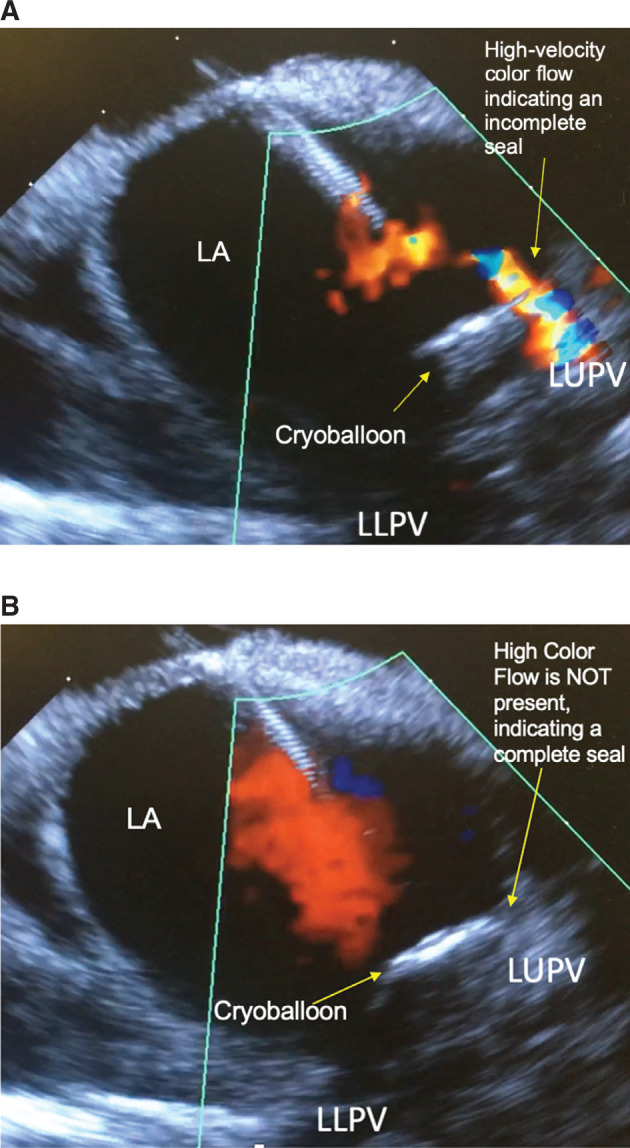
A: ICE image of the LA. The CB partially occluded the flow around the periphery of the vein. Note the presence of Doppler color flow around the right side of the CB. B: ICE image of the LA. CB fully occluded flow around the periphery of the vein. Note the absence of Doppler color flow around the right side of the balloon. CB: cryoballoon; ICE: intracardiac echo; LA: left atrium; LLPV: left lower pulmonary vein; LUPV: left upper pulmonary vein.
Statistical analysis
All values are reported as mean ± standard deviation. Study and control groups were compared using parametric (difference in means) and nonparametric (Wilcoxon rank-sum test) tests for continuous variables as appropriate and Fisher’s exact test for categorical data (SAS version 9.4; SAS Institute, Cary, NC, USA). A p-value of less than 0.05 was considered to be statistically significant.
Results
Total study cohort
During the study period of November 15, 2018 to November 15, 2019, a total of 100 patients underwent CB PVI at participating centers. Fifty consecutive patients who underwent fluoroless ablation were compared to 50 matched controls. A retrospective analysis was performed of the two cohorts. The baseline characteristics are summarized in Table 1. The cohort’s average age was 64.9 ± 11.5 years and 62% of participants were male. Moreover, 77% of patients had paroxysmal AF and 23% had early persistent AF. Overall, the average LA volume was 40.6 ± 14 mL/m2, the left ventricular ejection fraction (LVEF) was 60.5% ± 6.2%, and the CHA2DS2-VASc score was 1.82 ± 1.4 points.
Table 1:
Demographics
| Fluoroless Cohort | Control Cohort | p-value | |
|---|---|---|---|
| N | 50 | 50 | |
| Male sex | 35 (70%) | 27 (54%) | 0.8939 |
| Age | 64.9 ± 12.0 years | 64.9 ± 10.6 years | 0.993 |
| LA volume (ms/m2) | 44.2 ± 16 | 37.0 ± 12 | 0.0236 |
| LVEF | 61.2% ± 5.6% | 59.7% ± 6.7% | 0.1174 |
| CHA2DS2-VASc score | 1.54 ± 1.4 points | 2.1 ± 1.3 points | 0.0371 |
LA: left atrium; LVEF; left ventricular ejection fraction.
Fluoroless cohort
Fifty consecutive patients were enrolled in the fluoroless CB arm. Fluoroscopy was not used for any portion of the ablation procedure, including during transseptal access to the LA. Of the study participants, 35 were male (70%), with an age of 64.9 ± 8.1 years, LA volume of 44.2 ± 17 mL/m2, LVEF of 61.2% ± 5.5%, and CHA2DS2-VASC score of 1.51 ± 1.4 points recorded (Table 1). A total of 198 PVs were treated with 444 CB applications. There were two patients with left common ostia that were segmentally ablated. The treated veins were divided into four categories: those with both ICE and the CWPM evidence of occlusion (n = 213; category 1), those with only CWPM evidence (n = 121; category 2), those with only ICE evidence (n = 13; category 3), and those without evidence of occlusion with either modality (n = 97; category 4). The veins in categories 1 through 3 did show isolation, while none of the veins in category 4 were isolated. Measures of CB lesion efficacy are summarized in Table 2. Importantly, in terms of performance, category 1 veins were the strongest, followed by category 2 and category 3 veins. Veins in category 4 were segmentally ablated and thus had inferior metrics.
Table 2:
Fluoroless Cohort
| Category 1 | Category 2 | Category 3 | Category 4 | |
|---|---|---|---|---|
| Category details | Occlusion with CWPM Occlusion with ICE |
Occlusion with CWPM No occlusion with ICE |
No occlusion with CWPM Occlusion with ICE |
No occlusion with CWPM No occlusion with ICE |
| No. of freezes | 213 | 121 | 13 | 97 |
| Temperature at 30 seconds | −31.7°C ± 5.9°C Category 1 vs. 2: p = 0.0038 Category 1 vs. 3: p = 0.0378 Category 1 vs. 4: p ≤ 0.001 |
−29.9°C ± 4.0°C Category 2 vs. 3: p = 0.164 Category 2 vs. 4: p = 0.025 |
−28.83°C ± 2.8°C Category 3 vs. 4: p = 0.7142 |
−27.78°C ± 6.6°C |
| Minimum temperature | −47.4°C ± 6.3°C Category 1 vs. 2: p ≤ 0.0001 Category 1 vs. 3: p ≤ 0.0001 Category 1 vs. 4: p ≤ 0.0001 |
−41.9°C ± 5.9°C Category 2 vs. 3: p ≤ 0.0001 Category 2 vs. 4: p = 0.197 |
−40.33°C ± 4.7°C Category 3 vs. 4: p = 0.412 |
−38.9°C ± 4.6°C |
| Thaw time to 20°C | 50.4 ± 3.6 seconds Category 1 vs. 2: p = 0.0069 Category 1 vs. 3: p = 0.0278 Category 1 vs. 4: p = 0.1189 |
28.3 ± 13.6 seconds Category 2 vs. 3: p = 0.0206 Category 2 vs. 4: p = 0.4978 |
12.8 ± 6.3 seconds Category 3 vs. 4: p = 0.0475 |
31.28 ± 17.9 seconds |
| Time to isolation | 56.2 ± 28.1 seconds Category 1 vs. 2: p = 0.9504 Category 1 vs. 3: p = 0.0042 Category 1 vs. 4: p = N/A |
56.25 ± 18.87 seconds Category 2 vs. 3: p = 0.05 Category 2 vs. 4: p = N/A |
124 ± 43.0 seconds Category 3 vs. 4: p = N/A |
N/A (isolation not achieved) |
CWPM: continuous-wave pressure monitoring; ICE: intracardiac echo; N/A: not applicable.
Control cohort
Fifty consecutive matched patients were also enrolled in the traditional CB arm. Among these participants, 27 were male (54%), with an age of 64.9 ± 10.6 years, LA volume of 37.0 ± 12 mL/m2, LVEF of 59.7% ± 6.7%, and CHA2DS2VASc score of 2.1 ± 1.3 points recorded (Table 1). The veins in this cohort were divided into those with fluoroscopic evidence of occlusion (n = 187; category 1) and those without (n = 152; category 2). Veins in category 2 were segmentally ablated. Measures of CB lesion efficacy are summarized in Table 3. Veins with fluoroscopic evidence of occlusion performed superiorly in all of the following metrics: temperature at 30 seconds (p < 0.0001), minimum temperature (p < 0.0001), and thaw time (p < 0.0001).
Table 3:
Fluoroscopy Cohort
| Category 1 | Category 2 | p-value | |
|---|---|---|---|
| Category details | Occlusion with visual Inspection | No occlusion with visual inspection (segmental ablation was performed) | |
| No. of freezes | 187 | 152 | |
| Temp at 30 seconds | 32.8°C ± 4.7°C | 28.0°C ± 4.8°C | < 0.0001 |
| Minimum temperature | 47.7°C ± 9.2°C | 39.63°C ± 6.6°C | < 0.0001 |
| Thaw time to 20°C | 50.03 ± 32.7 seconds | 26.0 ± 16.8 seconds | < 0.0001 |
| Time to isolation | 46.79 ± 32.0 seconds | N/A (isolation not achieved) | N/A |
Comparing the fluoroless and control cohorts
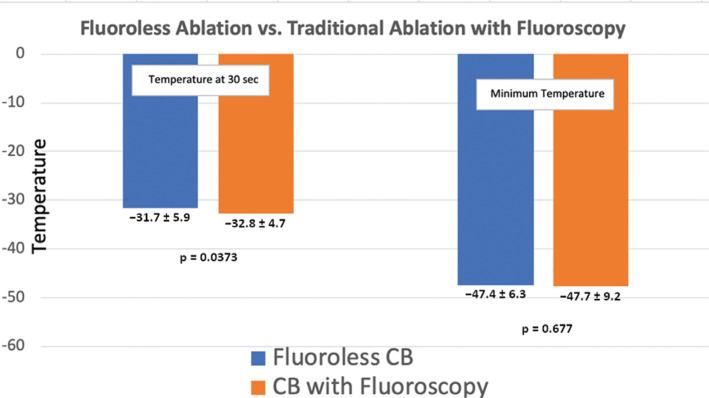
When comparing patients with complete fluoroless evidence of occlusion (fluoroless category 1) and those with fluoroscopic evidence of occlusion (control category 1), the mean temperature at 30 seconds was slightly lower in the traditional approach at −31.7°C ± 5.8°C versus −32.8°C ± 4.7°C (p = 0.03), while the maximum temperature (p = 0.6755) and the time to isolation (p = 0.2121) were not different (Figures 3 and 4). The full details of these comparisons are summarized in Table 4. When comparing veins without fluoroless ablation (category 4) and traditional evidence of occlusion (category 2), there also was no difference in the mean temperature at 30 seconds (p = 0.65), maximum temperature (p = 0.8762), or thaw time (p = 0.116) revealed.
Figure 3:
Comparison of fluoroless ablation (category 1) and traditional ablation with fluoroscopy (category 1). The results show that the temperature at 30 seconds was lower when using the traditional method but the overall absolute temperature was similar.
Figure 4:
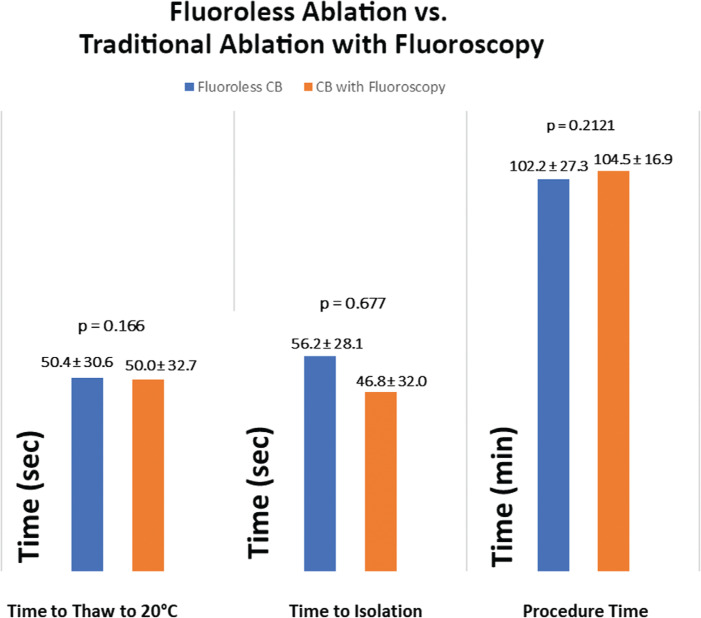
Comparison of fluoroless ablation (category 1) and traditional ablation with fluoroscopy (category 1). The results show that the thaw time to 20°C, time to vein isolation, and procedure time were similar.
Table 4:
Fluoroless Cohort with CWPM and ICE Showing Occlusion (Category 1) Versus Traditional Ablation with Fluoroscopy (Category 1)
| Fluoroless Ablation (Category 1) | Traditional Ablation with Fluoroscopy (Category 1) | p-value | |
|---|---|---|---|
| Category details | Ablation with both CWPM and ICE | Ablation with visual occulusion | |
| No. of freezes | 213 | 187 | |
| Temp at 30 seconds | −31.7°C ± 5.9°C | −32.8°C ± 4.7°C | 0.0373 |
| Minimum temperature | −47.4°C ± 6.3°C | −47.7°C ± 9.2°C | 0.677 |
| Thaw time to 20°C | 50.4 ± 30.6 seconds | 50.0 ± 32.7 seconds | 0.116 |
| Time to Isolation | 56.2 ± 28.1 seconds | 46.8 ± 32.0 seconds | 0.2121 |
| Procedure time | 102.2 ± 27.3 minutes | 104.5 ± 16.9 minutes | 0.6436 |
| Fluoroscopy time | 0.00 minutes | 17.0 ± 7.4 minutes | < 0.0001 |
CWPM: continuous wave pressure monitoring; ICE: intracardiac echo.
When comparing veins with only one of the two nonfluoroscopic measures of occlusion—either ICE or CPWM alone (fluoroless categories 2 and 3)—to controls with clear evidence of occlusion (control category 1), the fluoroless cohort performed inferiorly. When comparing between fluoroless category 2 and category 1 of the control group, the temperature at 30 seconds was −30.0°C ± 4.0°C versus −32.8°C ± 4.7°C (p ≤ 0.0001), the minimum temperature was 41.9°C ± 5.9°C versus 47.7°C ± 9.1°C (p ≤ 0.0001), and the time to isolation was 56.3 ± 18.7 seconds versus 50.3 ± 32.7 seconds (p = 0.5707). In comparing fluoroless category 3 with control category 1, the temperature at 30 seconds was −28.8°C ± 2.8°C (p = 0.0007), the minimum temperature was 40.3°C ± 1.7°C (p ≤ 0.0001), and the time to isolation was 124 ± 44 seconds (p = 0.0019).
Overall, there were more CB applications delivered (444 vs. 339 applications) with the fluoroless approach, although the procedure time was not different between groups (102.2 ± 27.3 vs. 104.5 ± 16.9 seconds; p = 0.6436). As data emerged demonstrating that dosing with single lesions was durable, fewer lesions were delivered in the control arm.14 Four of 198 (2.0%) PVs in the fluoroless arm and two of 200 (1.0%) PVs in the control arm could not be isolated with CB alone and required a touch-up with RF energy. Complications such as phrenic nerve injury, pericardial effusion, tamponade, stroke, and death were monitored but did not occur in either study group.
Discussion
This is a first-of-its-kind, proof-of-concept study of fluoroless CB AF ablation that has important implications. We present an approach that employs both imaging and hemodynamic measures as a viable alternative to the fluoroscopic evaluation of PV occlusion with CB. Our fluoroless technique performed similarly in terms of many of the traditional measures of CB lesion quality and was equivalent in both safety and procedure time. Importantly, the tools of ICE and CWPM are widely available in most laboratories and, thus, this technique is readily accessible for many operators. To our knowledge, this is the first study to combine ICE color Doppler and CWPM as a technique to completely eliminate fluoroscopy. There was no fluoroscopy used for any portion of the procedure in the fluoroless arm. This study constitutes an important step forward in a movement to eliminate fluoroscopy in electrophysiology (EP) procedures that has key implications for procedural radiation and orthopedic complications among operators and staff.
Lesion quality and durability
The achievement of high-quality and durable lesions is very important in any technique for AF ablation. In the case of CB ablation, it important to achieve certain critical endpoints to ensure that a patient’s PVs are durably isolated. For this reason, we elected to include many of the most important metrics in our study. These metrics include the absolute nadir of temperature during the cryoablation, 30-second temperature, time to isolation, and thaw time.
In our study, the fluoroscopy-free cohort had a slightly higher 30-second temperature but similar values of maximum temperature, thaw time, and time to isolation. Importantly, although the fluoroless cohort performed slightly worse with respect to the 30-second temperature metric, this has not been shown to be a reliable indicator of durable long-term lesions, while the other markers are better correlated with long-term lesion durability15 (Figure 3). With regard to the importance of absolute temperature during a freeze application, temperatures below −20°C to −50°C have also been shown to have an increased incidence of durable isolation.16,17 The absolute temperatures achieved in this study were similar in both arms, indicating that there is likely a similar degree of contact with the CB and the PV ostial tissue achieved with nonfluoroscopic techniques. A highly important CB ablation target is the time to isolation or effect, which, when achieved, indicates durable isolation.14 There was no statistically significant difference in the time to effect in patients who had either nonfluoroscopic or traditional evidence of vein occlusion (56.26 ± 28.1 vs. 46.6 ± 32.6 seconds; p = 0.212) (Figure 4). Another important aspect of this finding was that nonfluoroscopic techniques also achieved isolation within the traditional time frame of one minute on average.
It is important to point out that, based upon our study results, attaining evidence of occlusion via both ICE and CPWM is important for confirming durable lesions. When comparing veins with either ICE or CPWM evidence of occlusion alone with those with fluoroscopic evidence of occlusion, the fluoroless ablation cohort performed inferiorly in all metrics. Based on these results, we must conclude that, without both markers, a successful fluoroscopy-free procedure will not be achieved.
Adaptability to other operators
It is important to note that procedure time was similar between the two groups and major complications were absent. This suggests that, with experienced operators, a transition to a fluoroless approach is safe, efficacious, and possible with most laboratories’ existing equipment. While this concept was not studied, we would suggest that transitioning to a fluoroless approach may be undertaken by experienced electrophysiologists who have completed at least 100 CB ablation procedures. While this number is not rigorously studied, it is a reasonable experiential estimate.
The impetus for transitioning to fluoroless procedures
There are many reasons supporting why EP procedures should move to reduce and eliminate the use of fluoroscopy. According to the “as low as reasonably achievable” principle, radiation exposure during medical procedures should be limited as there is no known safe procedural dose of radiation.18 EP laboratory staff and operators, in comparison with the general population, exhibit two times the risk of skin lesions, three times the risk of cancer, 6.3 times the risk of cataracts, and 7.1 times the risk of orthopedic injuries.19–21 In addition, EP patients are exposed to an average of approximately 15 millisieverts during an average AF ablation.12 This is an equivalent radiation exposure of 750 chest X-rays. Given the increased risks associated with ionizing radiation, the need for fluoroscopy reduction in cardiac EP procedures is clearly apparent.
With the introduction of CB technology, however, the traditional dogma is that the use of fluoroscopy has remained necessary to assess for the complete occlusion of the PV—a concept we would like to challenge with our study findings. With the improvement in resolution with 3D anatomic mapping systems, ICE imaging, and PV hemodynamic monitoring, fluoroscopy can be safely and effectively eliminated as evidenced by our proof-of-concept study. As EP procedures are appropriately being expanded and offered at higher volumes, the potential burden of fluoroscopy of these procedures similarly increases. In our view, the concepts outlined in our study are critical to improving how the EP community offers these important procedures.
Limitations
This was a retrospective study of cases performed at two high-volume AF ablation centers and serves as a proof-of-concept study. More multicenter prospective and randomized data are needed with long-term clinical follow-up before this method can be widely adopted. It is important to note that the procedures in this study were performed by experienced operators. Fluoroscopy-free procedures require familiarity with the equipment and the ablation technique and further investigations are needed to understand the optimal time and process an electrophysiologist should take to transition to fluoroless CB ablation. For these reasons, we must conclude that this technique is not able to be used by inexperienced operators. In addition, the fluoroless procedure relies predominantly on the use of an ICE catheter. While the use of ICE and 3D mapping is the standard of care in the United States, this is not the case in other countries. The additional expense associated with using an ICE catheter or 3D mapping system may also be a barrier to widespread adoption of a fluoroless approach. Finally, an additional minor limitation is that the two study cohorts were slightly different, in that the fluoroless cohort had a larger LA volume and the control cohort had an elevated CHA2DS2-VASc score; however, this minor limitation likely did not contribute to any significant variation in the outcomes of this study.
Conclusion
In comparison with traditional CB ablation with fluoroscopy, this proof-of-concept study reveals fluoroless CB ablation can be performed similarly to the traditional approach with regard to the metrics of procedure success and efficiency. Safety was comparable between the ablation approaches as there were no major complications in either arm of the study. This suggests that the ablation of AF using CB technology can be conducted safely and effectively without the use of fluoroscopy by experienced operators.
References
- 1.Razminia M, Willoughby MC, Demo H, et al. Fluoroless catheter ablation of cardiac arrhythmias: a 5-year experience. Pacing Clin Electrophysiol. 2017;40(4):425–433. doi: 10.1111/pace.13038. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Razminia M, Zei PC. Fluoroscopic Reduction Techniques for Catheter Ablation of Cardiac Arrhythmias. Minneapolis, MN: Cardiotext; 2019. [Google Scholar]
- 3.Canpolat U, Faggioni M, Rocca DGG, et al. State of fluoroless procedures in cardiac electrophysiology practice. J Innov Card Rhythm Manag. 2020;11(3):4018–4029. doi: 10.19102/icrm.2020.110305. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razminia M, Zei P. Fluoroless catheter ablation of cardiac arrhythmias: change is inevitable. J Innov Card Rhythm Manag. 2020;11(4):4076–4078. doi: 10.19102/icrm.2020.110406. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doshi RN. Atrial fibrillation ablation without fluoroscopy: because we can. J Innov Card Rhythm Manag. 2018;9(11):3391–3394. doi: 10.19102/icrm.2018.091103. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun YJ, Yin XM, Cong T, et al. Comparison of cryoballoon ablation for atrial fibrillation guided by real-time three-dimensional transesophageal echocardiography vs. contrast agent injection. Chin Med J. 2019;132(3):285–293. doi: 10.1097/CM9.0000000000000076. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baykaner T, Quadros K, Thosani A, et al. Safety and efficacy of zero fluoroscopy transseptal puncture with different approaches. Pacing Clin Electrophysiol. 2020;43(1):12–18. doi: 10.1111/pace.13841. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siklody CH, Minners J, Allgeier M, et al. Cryoballoon pulmonary vein isolation guided by transesophageal echocardiography; novel aspects on an emerging ablation technique. J Cardiovasc Electrophysiol. 2009;20(11):1197–1202. doi: 10.1111/j.1540-8167.2009.01524.x. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Maalouf J, Whiteside HL, Pillai A, et al. Reduction of radiation and contrast agent exposure in a cryoballoon ablation procedure with integration of electromagnetic mapping and intracardiac echocardiography: a single center experience. J Interv Card Electrophysiol. 2019 Dec 23; doi: 10.1007/s10840-019-00667-z. [Epub ahead of print]. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Raizada A, Gedela M, Shaikh KA, Apte N, DeHaan M, Stanton C. Pressure-guided cryoablation of pulmonary veins in atrial fibrillation: a fast and effective strategy. Indian Heart J. 2017;69(2):223–225. doi: 10.1016/j.ihj.2016.11.330. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siklody CH, Minners J, Allgeier M, et al. Pressure-guided cryoballoon isolation of the pulmonary veins for the treatment of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21(2):120–125. doi: 10.1111/j.1540-8167.2009.01600.x. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Kosmidou I, Wooden S, Jones B, Deering T, Wickliffe A, Dan D. Direct pressure monitoring accurately predicts pulmonary vein occlusion during cryoballoon ablation. J Vis Exp. 2013;72(72):e50247. doi: 10.3791/50247. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safavi-naeini P, Shanoon F, Nzeri A. Cryoballoon pressure waveform change during balloon inflation is not a reliable predictor of adequate pulmonary vein occlusion. Pacing Clin Electrophysiol. 2014;37(12):1702–1707. doi: 10.1111/pace.12491. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.Aryana A, Kenigsberg DN, Kowalski M, et al. Verification of a novel atrial fibrillation cryoablation dosing algorithm guided by time-to-pulmonary vein isolation: results from the Cryo-DOSING Study (Cryoballoon-ablation DOSING Based on the Assessment of Time-to-Effect and Pulmonary Vein Isolation Guidance) Heart Rhythm. 2017;14(9):1319–1325. doi: 10.1016/j.hrthm.2017.06.020. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.Aryana A, Mugnai G, Singh SM, et al. Procedural and biophysical indicators of durable pulmonary vein isolation during cryoballoon of atrial fibrillation. Heart Rhythm. 2016;13(2):424–432. doi: 10.1016/j.hrthm.2015.10.033. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 16.Avitall B, Kalinski A. Cryotherapy of cardiac arrhythmia: from basic science to the bedside. Heart Rhythm. 2015;12(10):2195–2203. doi: 10.1016/j.hrthm.2015.05.034. [CrossRef] https://www.ncbi.nlm.nih.gov/pubmed/ext-link26031374">[PubMed] [DOI] [PubMed] [Google Scholar]
- 17.Gage AA, Baust JM, Baust JG. Experimental cryosurgery investigations in vivo. Cryobiology. 2009;59(3):229–243. doi: 10.1016/j.cryobiol.2009.10.001. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lickfett L, Mahesh M, Vasamreddy C, et al. Radiation exposure during catheter ablation of atrial fibrillation. Circulation. 2004;110(19):3003–3010. doi: 10.1161/01.CIR.0000146952.49223.11. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 19.Heidbuchel H, Wittkampf FH, Vano E, et al. Practical ways to reduce radiation dose for patients and staff during device implantations and electrophysiological procedures. Europace. 2014;16(7):946–964. doi: 10.1093/europace/eut409. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 20.Ahmed TAN, Taha S. Radiation exposure, the forgotten enemy: toward implementation of national safety program. Egypt Heart J. 2017;69(1):55–62. doi: 10.1016/j.ehj.2016.10.001. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreassi MG, Piccaluga E, Guagliumi G, Del Greco M, Gaita F, Picano E. Occupational health risks in cardiac catheterization laboratory workers. Circ Cardiovasc Interv. 2016;9(4):e003273. doi: 10.1161/CIRCINTERVENTIONS.115.003273. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]