Abstract
BACKGROUND:
Unanticipated respiratory compromise that lead to unplanned intubations is a known phenomenon in hospitalized patients. Most events occur in patients at high risk in well-monitored units; less is known about the incidence, risk factors, and trajectory of patients thought at low risk on lightly monitored general care wards. The aims of our study were to quantify demographic and clinical characteristics associated with unplanned intubations on general care floors and to analyze the medications administered, monitoring strategies, and vital-sign trajectories before the event.
METHODS:
We performed a multicenter retrospective cohort study of hospitalized subjects on the general floor who had unanticipated, unplanned intubations on general care floors from August 2014 to February 2018.
RESULTS:
We identified 448 unplanned intubations. The incidence rate was 0.420 per 1,000 bed-days (95% CI 0.374–0.470) in the academic hospital and was 0.430 (95% CI 0.352–0.520) and 0.394 per 1,000 bed-days (95% CI 0.301–0.506) at our community hospitals. Extrapolating these rates to total hospital admissions in the United States, we estimate 64,000 events annually. The mortality rate was 49.1%. Within 12 h preceding the event, 35.3% of the subjects received opiates. All received vital-sign assessments. Most were monitored with pulse oximetry. In contrast, 2.5% were on cardiac telemetry, and only 4 subjects used capnography; 53.7% showed significant vital-sign changes in the 24 h before the event. However, 46.3% had no significant change in any vital signs.
CONCLUSIONS:
Our study showed unanticipated respiratory compromise that required an unplanned intubation of subjects on the general care floor, although not common, carried a high mortality. Besides pulse oximetry and routine vital-sign assessments, very little monitoring was in use. A significant portion of the subjects had no vital-sign abnormalities leading up to the event. Further research is needed to determine the phenotype of the different etiologies of unexpected acute respiratory failure to identify better risk stratification and monitoring strategies.
Keywords: intubation, respiratory insufficiency, epidemiology, lung monitoring, airway management
Introduction
Respiratory compromise is defined as deterioration in respiratory function in which there is a high likelihood of respiratory failure or death.1 Once respiratory compromise has progressed to respiratory failure, the only measures that can provide necessary support are intubation and mechanical ventilation. Respiratory compromise is common in the hospital and a frequent reason for urgent intervention.2,3 Although most of these events occur in intensively monitored units and in patients thought to be at risk for respiratory compromise based on various prediction models4,5; they can also occur on lightly monitored general care floors.
General care floors are staffed at a lower nurse-to-patient ratio, and vital signs are conducted at less-frequent intervals compared with ICUs or step-down units.6 There are no guidelines to define admission criteria to general care floors, but, typically, patients who do not require intensive monitoring or who are thought by clinicians to be at low risk for physiologic decompensation are housed on general care floors. The literature about respiratory compromise on general care floors, however, is scant, and, to our knowledge, there is only one study from a resuscitation registry that estimated the incidence of these events at 44,500 per year in the United States.7
Respiratory compromise that leads to an unplanned intubation in a general care ward is a particularly challenging situation for clinicians. The event is generally unanticipated, respiratory deterioration is rapid, the emergency response is often slow, access to emergency equipment may be limited, and efforts to avoid an intubation may thus be unsuccessful. A better understanding of risk profiling and appropriate monitoring strategies for the broad general care population of patients who go on to experience an unplanned intubation is important.
The specific aims of this study were to (1) quantify demographic and clinical characteristics associated with unanticipated respiratory compromise and unplanned intubations on general medicine and/or surgery floors; and (2) analyze the medications administered, the monitoring strategies used, and the vital-sign trajectories of these subjects in the 12–24 h before the unplanned intubation. We specifically focused on the most severe form of respiratory compromise in patients on the general care floor: the requirement for an artificial airway and mechanical ventilatory support. It is in this population that the system may have failed the most in both early warning and in subsequent detection and/or management. By understanding the characteristics of these patients and the support structure that surrounds them, we hope to inform processes that may improve monitoring, prediction, and treatment of respiratory failure in this population.
QUICK LOOK.
Current knowledge
Respiratory compromise is a life-threatening event that can occur in any care setting. Demographics and clinical characteristics of patients who develop unanticipated respiratory compromise that requires an unplanned intubation during hospitalization are not well understood.
What this paper contributes to our knowledge
Unanticipated respiratory compromise leads to high mortality. Commonly used monitoring modalities may not be sufficient to detect respiratory compromise.
Methods
We performed a retrospective, health-system–wide study that analyzed electronic health record data from the Duke University Health System. Duke University Health System is composed of one tertiary-care academic facility and 2 community hospitals. The academic hospital had 957 acute care beds and >42,000 admissions per year. Community hospital 1 had 369 acute care beds and >16,000 admissions per year, whereas community hospital 2 had 186 acute care beds and >9,400 admission per year.
Cohort Definition
We used a nested case-control study design to account for the potentially large sample, with a relatively rare outcome. Patients were eligible if they were hospitalized from August 1, 2014, to February 14, 2018, and were ages ≥19 y. Subjects were defined by a documented endotracheal tube insertion or death within 1 h of cardiopulmonary resuscitation on a general care floor by any member of the care team in the flowsheet data within our electronic health record. In our institutions, emergency endotracheal intubations are performed by physicians, advanced practice professionals, and advanced respiratory therapists.
Death within 1 h of cardiopulmonary resuscitation was selected as a surrogate marker because of the high rates of intubation during cardiopulmonary resuscitation,8 which was likely not documented because the patient expired quickly. The event had to be at least 24 h after admission or surgical procedure. A patient was eligible to be a control if he or she was hospitalized for at least 24 h on a general care floor. Patients were excluded if they had an endotracheal intubation within 24 h of admission to the hospital, 24 h after an operation, or 24 h after transferring out of an ICU or a step-down unit. Endotracheal intubation before admission was also an exclusion criterion. Sample scenarios are illustrated in Supplemental Figure 1 (see the supplementary materials at http://www.rcjournal.com).
Outcome
The outcome for our study was an intubation defined as an endotracheal tube placement during the encounter, as shown in the lines, drains, and airways data or flowsheet data, or death within 1 h of cardiopulmonary resuscitation.
Variables of Interest
For all the subjects, we abstracted demographics, encounters, and comorbidities. We abstracted vital signs and medication data for all subjects and a random subset of controls. Medication data during the encounter and the year before the encounter were grouped according to therapeutic and pharmaceutical class. Device use during the encounter was obtained from flowsheets. Vital signs, including telemetry and pulse oximetry, could be visualized centrally or at the bedside.
Statistical Analysis
By using the sampled cohort, we compared baseline characteristics of subjects and controls, stratified by hospital. We calculated the incident ratio ratio for unplanned intubation and associated 95% CI by using the full eligible cohort. By using the sampled cohort, we assessed medication usage during the year preceding the encounter of interest and the medication use during hospitalizations. For subjects, we assessed device use in the 1–12 h preceding the unplanned intubation. We compared changes in vital signs between subjects and controls before intubation by using a method described previously.9
In brief, the time at admission was considered time 0; at the time each subject had his or her unplanned intubation, we sampled 4 eligible controls who were still at risk of being intubated. We then abstracted vital signs during the preceding 24 h. For each vital sign, we fit a linear mixed model, regressing the vital sign onto time and adjusting for the patient's age, sex, and race. To allow for flexibility in the change in the vital sign, we included a time as cubic spline and used a likelihood ratio test to determine the degrees of freedom for each vital sign. We fit an interaction term between case-control status and time, which allows the curves to vary between case status.
The model had the form of
where represents the random effects; represents a flexible function, that is, spline, of time; and are patient demographics. The parameter of interest, , is a parameter vector based on the spline of time. We performed a likelihood ratio test on this interaction term (β3) to assess whether the curves differed between the 2 groups. With each subject, for each vital sign, we also fit a simple linear model to assess whether the individual's vital signs were increasing or decreasing when leading up to the event. All analyses were conducted by using SAS 9.4 (SAS Institute, Cary, North Carolina) and R version 3.5.2 (R Foundation, Vienna, Austria). This study was approved by the Duke University Institutional Review Board.
Results
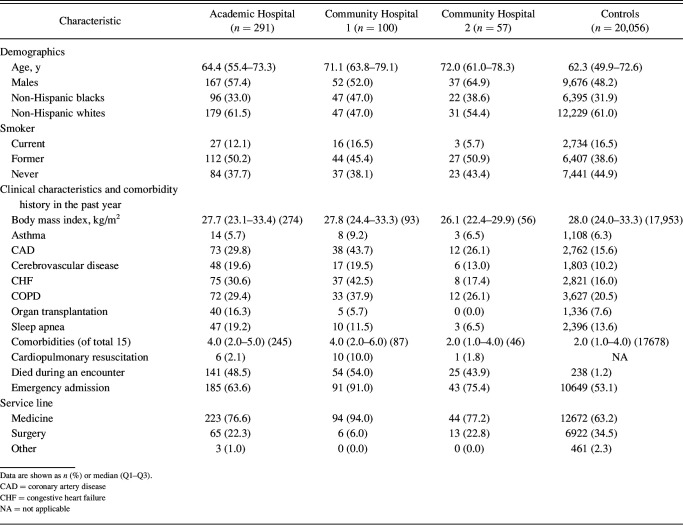
We identified a total of 448 unplanned intubations: 291 at our academic facility, and 100 and 57 at community hospitals 1 and 2, respectively. There were 202,910 eligible controls across all 3 hospitals; 20,056 sampled controls were included in the analyses. Baseline characteristics of subjects and sampled controls are presented in Table 1. Of the 448 unplanned intubations on general ward floors from our 3 facilities, the median age was 65.5 (quartiles, 56.6, 75.4) y, 57.1% were men, and the median body mass index was 27.4 (quartiles 23.4, 32.8) kg/m2. The median age for the controls were 62.3 (quartiles, 49.9, 72.6) y, 48.2% were men, and the median body mass index was 28 (quartiles, 24.0, 33.3) kg/m2.
Table 1.
Baseline Characteristics for Subjects With Unplanned Intubations and Sampled Controls
Based on our analyses, we found the incidence rate of unplanned intubations to be 0.420 per 1,000 (95% CI 0.374–0.470) bed-days in the academic hospital; 0.430 per 1,000 (95% CI 0.352–0.520) bed-days in community hospital 1 and 0.394 per 1,000 (95% CI 0.301–0.506) bed-days at community hospital 2. The mortality rate of the subjects with unplanned intubation was 49.1% during the event hospitalization. Seventeen subjects were defined by cardiopulmonary arrest. Subjects had higher rates of coronary artery disease, cerebrovascular disease, congestive heart failure, COPD, and organ transplantations compared with the controls. The medication use during the past year for subjects and controls is illustrated in Supplemental Table 1 (see the supplementary materials at http://www.rcjournal.com). Notably, subjects had higher rates of anti-hyperglycemics, cardiac and cardiovascular drugs, and diuretics.
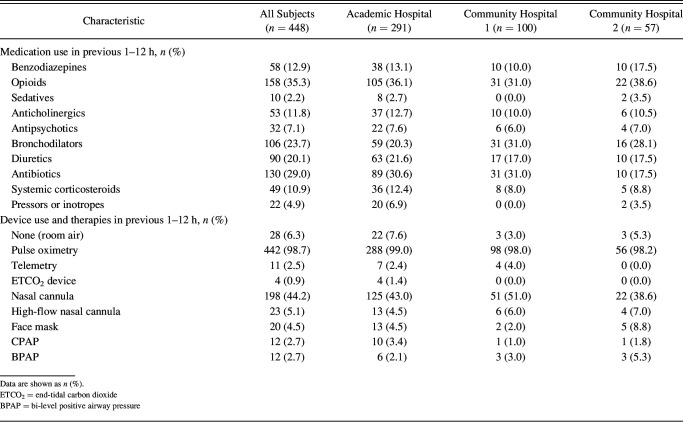
The use of medication and devices in the 1–12 h before the event is presented in Table 2. Of the subjects, 12.9% received a benzodiazepine, 35.3% received an opiate, 9.3% received either a sedative or antipsychotic, 23.7% received a bronchodilator, 20.1% received a diuretic, and 29.0% were on antibiotics. The vast majority were receiving pulse oximetry and had received oxygen supplementation. A small minority were on telemetry (2.5%), whereas only 4 cases had used an end-tidal CO2 monitor before the event; 5.4% used noninvasive ventilation, and 5.1% used high-flow nasal cannula oxygen therapy.
Table 2.
Medications and Devices in Subjects With Unplanned Intubations 1–12 h Before an Event
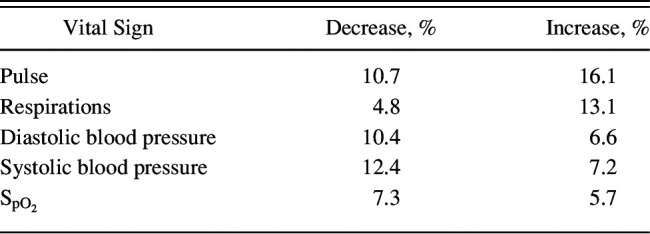
The average vital-sign trajectories of pulse, breathing frequency, systolic and diastolic blood pressure, and pulse oximetry in leading up to the events for subjects compared with time-matched controls are illustrated in Figure 1. All vital signs except diastolic blood pressure showed significantly different average trajectories (P < .01, likelihood ratio test). However, there was heterogeneity in the individual trajectories (Table 3). For example, with respect to respiration, 4.8% of the subjects showed a significant decrease in leading up to the event, indicative of bradypnea, whereas 13% showed a significant increase indicative of tachypnea. Importantly, 46.3% of the subjects had no significant change in any vital sign in leading up to the event.
Fig. 1.
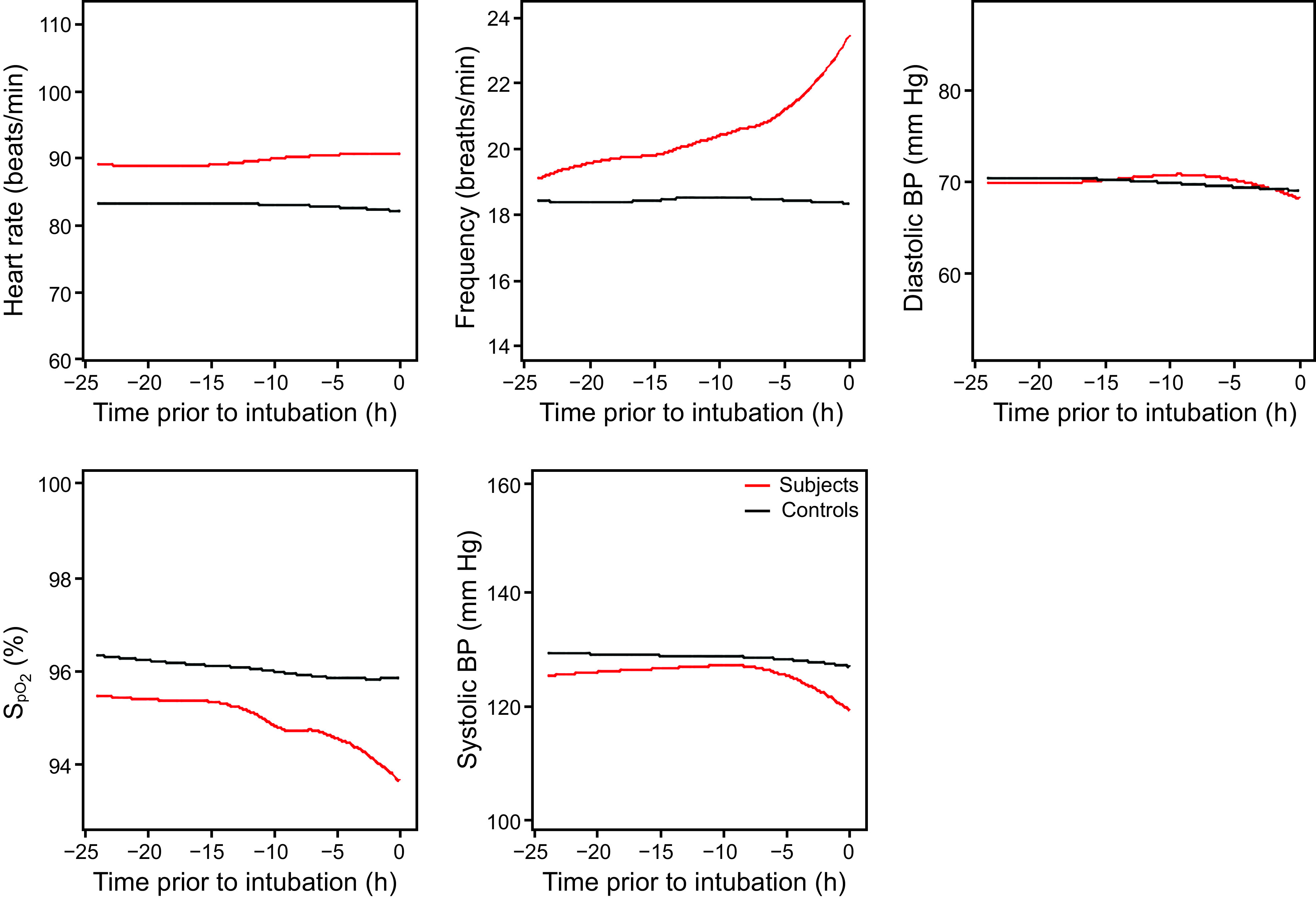
Vital sign trajectories heart rate, breathing frequency, diastolic blood pressure (BP), pulse oximetry, and systolic BP.
Table 3.
Percentage of Subjects Who Had a Significant Increase or Decrease in a Vital Sign in the 24 h Before the Event
Discussion
To our knowledge, this was the first study that focused solely on unplanned emergent intubations, the severest form of respiratory compromise, in subjects who were lightly monitored and in seemingly stable general floor care. Over our 43-month study period, we identified 448 such events in 3 hospitals, with >67,000 combined admissions per year. Extrapolating that to American Hospital Association data (https://www.aha.org/statistics/2020-01-07-archived-fast-facts-us-hospitals-2019, Accessed January 5, 2020), which reported >36 million admissions in 2017 in the United States, analysis of our results suggested that >64,000 unplanned intubations/year occur on general care services, a number comparable with that observed in the aforementioned study by Andersen et al7 in subjects outside the operating room, emergency department, and ICU.
Not surprisingly, we found that subjects who received an unplanned intubation had a significantly high mortality rate compared with the control subjects who were hospitalized and in general care, 49% of the subjects who required an unplanned intubation expired either at the time of intubation or during the hospitalization. This mortality rate was significantly higher than was found in other general care cohorts, including sepsis,10 COPD exacerbation,11 pneumonia,12 and heart failure.13 Compared with controls, our cases had higher rates of coronary disease, diabetes, COPD, and congestive heart failure. Furthermore, the cases had higher rates of multiple comorbidities compared with the controls. Interestingly, there was little difference in the rates of sleep apnea between the cases and the controls. The lack of difference runs contrary to the current literature because previous research has shown obstructive sleep apnea to be a risk factor for respiratory failure.14,15
In the 12 h leading up to the intubation event, the rate of opiate and sedative use was high in the cases that required intubations. Both of these medication classes have been shown to impact ventilatory drive.16–18 This likely suggests that the addition of sedative medications on a cohort of patients with comorbidities while acutely ill would play a factor in their decompensation. This warrants further study. Vital sign monitoring is a cornerstone of current general floor care management, and although designed to detect decompensation, monitoring is only done intermittently.19 Important vital sign changes were observed in the h before the unplanned intubation in a number of our cases. However, there were a significant number of subjects who had no vital sign abnormalities before their respiratory decompensation, which illustrates the shortcomings of current general floor vital sign monitoring modalities. The considerable heterogeneity in these vital sign patterns underscores the range of etiologies and trajectories of in-hospital respiratory compromise.
These “phenotypes” of respiratory compromise would include sudden (catastrophic) events versus gradual deteriorations that involve neurologic, cardiovascular, or respiratory function. Thus, there is no single approach to predicting, monitoring, or preventing these occurrences.20 For each of these phenotypes, risk prediction models, monitoring strategies, and intervention potential are likely to be different. Future research can be directed to better characterize these phenotypes and to group patients accordingly. Patients with specific patterns could then benefit from more-focused monitoring and intervention strategies.
In addition to vital-sign measurements, 2 monitors are generally available for patients in general care: exhaled gas capnography and pulse oximetry (). Capnography can offer a noninvasive means of accurately measuring the breathing frequency and is generally available in most hospitals. Capnography has also been shown to be effective in identifying respiratory compromise in patients in general care.21 However, despite the high use of opioids and sedatives, the rate of capnography monitoring in our subjects was very low. This underscores the likelihood that providers truly thought that these subjects were at low risk for further cardiopulmonary decompensation. Although guidelines exist for capnography usage for operating rooms, patients who are moderately sedated and on advanced life care support, there are no guidelines to direct capnography usage on patients on the general floor.22
Pulse oximetry is a widely used monitor that is noninvasive, simple to operate, and often is a part of admission order sets. Indeed, nearly all of our subjects had pulse oximetry monitors in use but, as noted above, recorded changes in before the unplanned intubation were uncommon. Whether this is because adequate supplemental oxygen was being adjusted to achieve the normal range of oxygen saturation or these subjects truly had no aberrancy in their oxygen saturation before respiratory failure remains unclear. The limitations of pulse oximetry are well known. The reported values are time-weighted averages and thus delays in recognizing acute events occur. Hypotension, vasoconstriction, motion artifacts, and low cardiac outputs can all lead to poor signal quality.23–25
Given the heterogeneity of respiratory compromise and the limited available data to inform clinicians, it should not be surprising that evidence-based guidelines on general floor care monitoring are lacking. Decisions and selection are largely left up to providers. Furthermore, initiatives such as the Choosing Wisely Campaign, have adopted recommendations in minimizing device monitoring in certain clinical settings.26 Our results strongly suggest that “one size does not fit all” and that much more research is needed to understand cost-effective strategies for general floor care monitoring.
The findings of this study should be interpreted in the context of the study design and the limitations of the data sets. Our definition of unplanned intubation, defined a 24-h time limit for inclusion after admission, ICU transfer, or operation, and does not truly reflect a point in time reflective of clinical stability. We also delineated the time frame for medication and device usage as 1-12 h before the event. From the data available, it is not possible to assess if the medications or devices were being used for routine treatment and/or monitoring (eg, noninvasive ventilation for obstructive sleep apnea) or directed toward impending respiratory failure.
Another limitation of our study was the reliance of data captured by documentation in the electronic health record. Electronic health record data can be incomplete27 and due to data entry discrepancies as well as back-end data transformation, not always reflective of the true clinical experience. Furthermore, although vital signs have been shown to be predictive of deterioration,28 recordings may be incomplete.29,30 Because we censored the subjects after the time stamp of intubation or cardiopulmonary arrest, data that were time stamped after the event but could have occurred before the event would not have been included.
Also, we used subjects who died 1 h after cardiopulmonary arrest as a surrogate marker for unplanned intubation since, patients generally need endotracheal intubation who develop cardiopulmonary arrest. Because we censored the subjects after the time stamp of intubation or cardiopulmonary arrest, data that were time stamped after the event but could have occurred before the event would not have been included.
Conclusions
Respiratory compromise that requires an unplanned intubation of patients on the general care floor, although uncommon, carries a high mortality and is potentially preventable. Not surprisingly, the subjects in general care who were older and sicker were at higher risk. Importantly, besides pulse oximetry and routine vital sign assessments, very little other monitoring was in use in our patient population. There clearly were multiple etiologies and clinical trajectories for acute respiratory compromise that occurred in this population. Further research is needed to effectively determine the phenotype of the different etiologies of acute de novo respiratory failure on the general care floors to identify better risk stratification, monitoring processes, and interventional strategies for these patients.
Footnotes
This research was supported by an investigator-initiated grant from the Respiratory Compromise Institute (RCI). RCI had no role in the design, analysis, or interpretation of the results in this study. RCI was given the opportunity to review the manuscript for medical and scientific accuracy before publication.
Dr Bhavsar: National Heart, Lung, and Blood Institute (K01HL140146).
Dr Goldstein: National Institute of Diabetes and Digestive and Kidney Disease (K25DK097279).
Supplementary material related to this paper is available at http://www.rcjournal.com.
Dr MacIntyre discloses a relationship with the Respiratory Compromise Institute. The remaining authors have disclosed no conflicts of interest.
See the Related Editorial on Page 1414
References
- 1. Morris TA, Gay PC, MacIntyre NR, Hess DR, Hanneman SK, Lamberti JP, et al. Respiratory compromise as a new paradigm for the care of vulnerable hospitalized patients. Respir Care 2017;62(4):497–512. [DOI] [PubMed] [Google Scholar]
- 2. Jones DA, DeVita MA, Bellomo R. Rapid-response teams. N Engl J Med 2011;365(2):139–146. [DOI] [PubMed] [Google Scholar]
- 3. Karlsson CM, Donnino MW, Kirkegaard H, Cocchi MN, Chase M, Andersen LW; American Heart Association's Get With the Guidelines-Resuscitation® Investigators. Acute respiratory compromise in the emergency department: a description and analysis of 3571 events from the Get With the Guidelines-Resuscitation® Registry. J Emerg Med 2017;52(4):393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khanna AK, Overdyk FJ, Greening C, Di Stefano P, Buhre WF. Respiratory depression in low acuity hospital settings–Seeking answers from the PRODIGY trial. J Crit Care 2018;47:80–87. [DOI] [PubMed] [Google Scholar]
- 5. DeVita MA, Smith GB, Adam SK, Adams-Pizarro I, Buist M, Bellomo R, et al. “Identifying the hospitalised patient in crisis”–a consensus conference on the afferent limb of rapid response systems. Resuscitation 2010;81(4):375–382. [DOI] [PubMed] [Google Scholar]
- 6. Curry JP, Jungquist CR. A critical assessment of monitoring practices, patient deterioration, and alarm fatigue on inpatient wards: a review. Patient Saf Surg 2014;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andersen LW, Berg KM, Chase M, Cocchi MN, Massaro J, Donnino MW, American Heart Association's Get With The Guidelines(®)-Resuscitation Investigators. Acute respiratory compromise on inpatient wards in the United States: incidence, outcomes, and factors associated with in-hospital mortality. Resuscitation 2016;105:123–129. [DOI] [PubMed] [Google Scholar]
- 8. Andersen LW, Granfeldt A, Callaway CW, Bradley SM, Soar J, Nolan JP, et al. American Heart Association's Get With The Guidelines–Resuscitation Investigators. Association Between Tracheal Intubation During Adult In-Hospital Cardiac Arrest and Survival. JAMA 2017;317(5):494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldstein BA, Assimes T, Winkelmayer WC, Hastie T. Detecting clinically meaningful biomarkers with repeated measurements: an illustration with electronic health records. Biometrics 2015;71(2):478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cook TM, Woodall N, Harper J, Benger J; Fourth National Audit Project. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: intensive care and emergency departments. Br J Anaesth 2011;106(5):632–642. [DOI] [PubMed] [Google Scholar]
- 11. Quach JL, Downey AW, Haase M, Haase-Fielitz A, Jones D, Bellomo R. Characteristics and outcomes of patients receiving a medical emergency team review for respiratory distress or hypotension. J Crit Care 2008;23(3):325–331. [DOI] [PubMed] [Google Scholar]
- 12. Baillard C, Fosse JP, Sebbane M, Chanques G, Vincent F, Courouble P, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med 2006;174(2):171–177. [DOI] [PubMed] [Google Scholar]
- 13. Mort TC, Waberski BH, Clive J. Extending the preoxygenation period from 4 to 8 mins in critically ill patients undergoing emergency intubation. Crit Care Med 2009;37(1):68–71. [DOI] [PubMed] [Google Scholar]
- 14. Karnatovskaia LV, Lee AS, Bender SP, Talmor D, Festic E; US Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators (USCIITG–LIPS). Obstructive sleep apnea, obesity, and the development of acute respiratory distress syndrome. J Clin Sleep Med 2014;10(6):657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology 2014;120(2):268–286. [DOI] [PubMed] [Google Scholar]
- 16. Jungquist CR, Smith K, Nicely KL, Polomano RC. Monitoring hospitalized adult patients for opioid-induced sedation and respiratory depression. Am J Nurs 2017;117(3 Suppl 1):S27–S35. [DOI] [PubMed] [Google Scholar]
- 17. Höjer J, Baehrendtz S, Gustafsson L. Benzodiazepine poisoning: experience of 702 admissions to an intensive care unit during a 14-year period. J Intern Med 1989;226(2):117–122. [DOI] [PubMed] [Google Scholar]
- 18. Lee LA, Caplan RA, Stephens LS, Posner KL, Terman GW, Voepel-Lewis T, Domino KB. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology 2015;122(3):659–665. [DOI] [PubMed] [Google Scholar]
- 19. Churpek MM, Yuen TC, Winslow C, Robicsek AA, Meltzer DO, Gibbons RD, Edelson DP. Multicenter development and validation of a risk stratification tool for ward patients. Am J Respir Crit Care Med 2014;190(6):649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lynn LA, Curry JP. Patterns of unexpected in-hospital deaths: a root cause analysis. Patient Saf Surg 2011;5(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stites M, Surprise J, McNiel J, Northrop D, De Ruyter M. Continuous capnography reduces the incidence of opioid-induced respiratory rescue by hospital rapid resuscitation team. J Patient Saf 2017. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22. Whitaker DK, Benson JP. Capnography standards for outside the operating room. Curr Opin Anaesthesiol 2016;29(4):485–492. [DOI] [PubMed] [Google Scholar]
- 23. Grace RF. Pulse oximetry. Gold standard or false sense of security? Med J Aust 1994;160(10):638–644. [PubMed] [Google Scholar]
- 24. Mengelkoch LJ, Martin D, Lawler J. A review of the principles of pulse oximetry and accuracy of pulse oximeter estimates during exercise. Phys Ther 1994;74(1):40–49. [DOI] [PubMed] [Google Scholar]
- 25. Mardirossian G, Schneider RE. Limitations of pulse oximetry. Anesth Prog 1992;39(6):194–196. [PMC free article] [PubMed] [Google Scholar]
- 26. Drew BJ, Califf RM, Funk M, Kaufman ES, Krucoff MW, Laks MM, et al. ; American Heart Association; Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004;110(17):2721–2746. [DOI] [PubMed] [Google Scholar]
- 27. Stiglic G, Kocbek P, Fijacko N, Sheikh A, Pajnkihar M. Challenges associated with missing data in electronic health records: a case study of a risk prediction model for diabetes using data from Slovenian primary care. Health Informatics J 2019;25(3):951–959. [DOI] [PubMed] [Google Scholar]
- 28. Harrison GA, Jacques TC, Kilborn G, McLaws ML. The prevalence of recordings of the signs of critical conditions and emergency responses in hospital wards–the SOCCER study. Resuscitation 2005;65(2):149–157. [DOI] [PubMed] [Google Scholar]
- 29. Leuvan CH, Mitchell I. Missed opportunities? An observational study of vital sign measurements. Crit Care Resusc 2008;10(2):111–115. [PubMed] [Google Scholar]
- 30. Cretikos MA, Bellomo R, Hillman K, Chen J, Finfer S, Flabouris A. Respiratory rate: the neglected vital sign. Med J Aust 2008;188(11):657–659. [DOI] [PubMed] [Google Scholar]