Abstract
BACKGROUND:
With an increasing number of follow-up studies of acute respiratory failure survivors, there is need for a better understanding of participant retention and its reporting in this field of research. Hence, our objective was to synthesize participant retention data and associated reporting for this field.
METHODS:
Two screeners independently searched for acute respiratory failure survivorship studies within a published scoping review to evaluate subject outcomes after hospital discharge in critical illness survivors.
RESULTS:
There were 21 acute respiratory failure studies (n = 4,342 survivors) over 47 follow-up time points. Six-month follow-up (range: 2–60 months) was the most frequently reported time point, in 81% of studies. Only 1 study (5%) reported accounting for loss to follow-up in sample-size calculation. Retention rates could not be calculated for 5 (24%) studies. In 16 studies reporting on retention across all time points, retention ranged from 32% to 100%. Pooled retention rates at 3, 6, 12, and 24 months were 85%, 89%, 82%, and 88%, respectively. Retention rates did not significantly differ by publication year, participant mean age, or when comparing earlier (3 months) versus each later follow-up time point (6, 12, or 24 months).
CONCLUSIONS:
Participant retention was generally high but varied greatly across individual studies and time points, with 24% of studies reporting inadequate data to calculate retention rate. High participant retention is possible, but resources for optimizing retention may help studies retain participants. Improved reporting guidelines with greater adherence would be beneficial.
Keywords: participant retention, cohort, acute respiratory failure, meta-analysis, systematic review, follow-up studies
Introduction
The number of patients surviving acute respiratory failure (ARF) is increasing with advances in critical care medicine.1,2 These survivors often experience long-term health impairments.3–7 Consequently, there has been an increasing number of follow-up studies focused on survivors' functional outcomes after hospital discharge.8
In studies completing longitudinal follow-up, it is important to assess as many participants of those eligible for follow-up (eg, alive) at each time point (ie, high retention rates). Achieving high participant retention rates is important for preserving statistical power and maintaining internal validity and generalizability.9 There is growing interest and associated publications related to identifying effective participant-retention strategies;10–12 however, there are little data specifically evaluating participant retention rates and related reporting of such data in longitudinal studies evaluating ARF survivors. The objective of this systematic review and meta-analysis is to synthesize participant retention data and its reporting in studies of outcomes after hospital discharge for survivors of ARF.
QUICK LOOK.
Current knowledge
With more patients surviving acute respiratory failure, there is a growing number of follow-up studies focused on patient outcomes after hospital discharge. There are little data on participant retention and associated reporting in longitudinal studies of acute respiratory failure survivors.
What this paper contributes to our knowledge
Via a systematic review and meta-analysis of the acute respiratory failure literature, pooled participant retention rates at 3, 6, 12, and 24 month follow-up were > 80%; however, across all studies, participant retention rates were highly variable. Moreover, for a substantial proportion of studies, participant retention rates could not be calculated from the reported data. Also, there was variation in the nature of participant retention data reported. Standardizing collection and reporting of participant retention in longitudinal studies would help advance this field of research.
Methods
The publications included in this analysis were obtained from a previously completed comprehensive scoping review of outcomes measurement other than mortality after hospital discharge in ICU survivors.8 This scoping review searched 5 databases (PubMed, EMBASE, PsycINFO, CINAHL, and the Cochrane Controlled Trials Registry from 1970 to 2013, without language restrictions. The scoping review8 identified a total of 425 eligible papers that served as the population of studies to evaluate for eligibility for inclusion in this analysis.
From the 425 papers included in the scoping review,8 2 independent trained researchers (VR, ZW) screened for studies that specifically evaluated subjects with ARF. The researchers were not blinded to author or journal details. Studies were excluded if: (1) non-ARF subjects were included in study (ie, outside the focus of this report); (2) the ARF population was exclusively focused on neuromuscular disease or chronic pulmonary disease such as COPD (ie, a specific population that may not be generalizable to all ARF patients); or (3) there was only a single follow-up time point with consent occurring at the same time (ie, no prospective follow-up performed to evaluate participant retention).
Data abstraction was performed independently, in duplicate, by pairs of trained researchers (AA, VR, RN, ZW). Data abstractors were not blinded to author or journal details. Conflicts between reviewers were resolved, by consensus, in consultation with a senior researcher (DMN or VDD). Authors were contacted for additional data when necessary. For studies that had > 1 paper reporting on the same time point, we used the paper with the higher participant count (eg, preliminary analyses vs completed study). The following data were collected: participant retention rates at each follow-up time point; utilization of participant flow chart; modes of data collection (eg, in-person, telephone, mail); reporting of mortality during follow-up; blinding of assessors (if interventional study); accounting for loss to follow-up in sample size or power calculation; study exclusion criteria related to barriers to follow-up (eg, homelessness); and a description of participant-retention strategies. We also identified studies that focused solely on subjects with ARDS.
Risk of bias for randomized controlled trials (RCTs) was assessed using the Cochrane Risk of Bias methodology.13 For observational studies, the Newcastle-Ottawa Scale was used.14 We adapted the Newcastle-Ottawa Scale to omit the following criteria that were not applicable to this systematic review, given its focus on participant retention rates rather than a specific clinical outcome: demonstration that the outcome was not present at enrollment; assessment of the outcome; follow-up that was long enough for the outcome to occur.
Statistical Analysis
The pooled average participant retention rate was calculated as part of this meta-analysis. Follow-up time points among eligible studies were 0.5, 2, 3, 6, 12, 24, 36, 48, and 60 months. We did not include the 0.5-month (2-week) time point. However, the one 2-month study was pooled with the 3-month time point. Follow-up time points > 24 months could not be pooled due to only having one study at each time point. For studies with age reported only as median (interquartile range), the mean (SD) were estimated using established methods.15 Treatment groups or participant subgroups separately reported within a study for the same time point were tested for a statistically significant difference using the Fisher's exact test and combined when the test resulted in a nonsignificant difference (P < .05). For studies where retention rates were 100%, the Haldane-Anscombe correction was used to calculate the variance of the observed retention rate and presentation of confidence intervals.16,17
Two different approaches were used to calculate retention rates. The primary approach calculated the retention rate as the number of participants who had a study assessment (numerator) divided by the total number of participants who were eligible for follow-up at that same time point (denominator), excluding participants who died by that time point. The alternative approach excluded from the denominator participants who died and those who permanently discontinued participation in the study or were withdrawn from the study. Retention rates were not calculated if all requisite data were not reported or if mortality was combined with lost-to-follow-up data. The results using the primary approach are reported in this manuscript; the results using the alternative approach are available online (see the supplementary materials at http://www.rcjournal.com).
A linear random effects regression model for the log odds of the retention rate (logit transformation) was used to estimate the population average log odds of the retention rate for each follow-up. The model included a random intercept for each study, a set of indicators for follow-up times, and the observed variance within the study follow-up time was used as the study-specific known residual variance. The estimated average retention rate was computed by applying the inverse-logit transformation. To determine if the average retention rate varied as a function of participant (average age and percent male) or study publication characteristics, each characteristic was separately added to the model described above as a fixed effect.
Statistical heterogeneity among included studies was evaluated using the I2 statistic (with > 50% deemed to be substantial heterogeneity).18 The I2 statistic was calculated for each follow-up when there were > 2 studies reporting data.19 SAS 9.4 (SAS Institute, Cary, North Carolina) was used for all analyses. We followed the PRISMA checklist in reporting this manuscript.20 The protocol was registered with PROSPERO (CRD42018087835).
Results
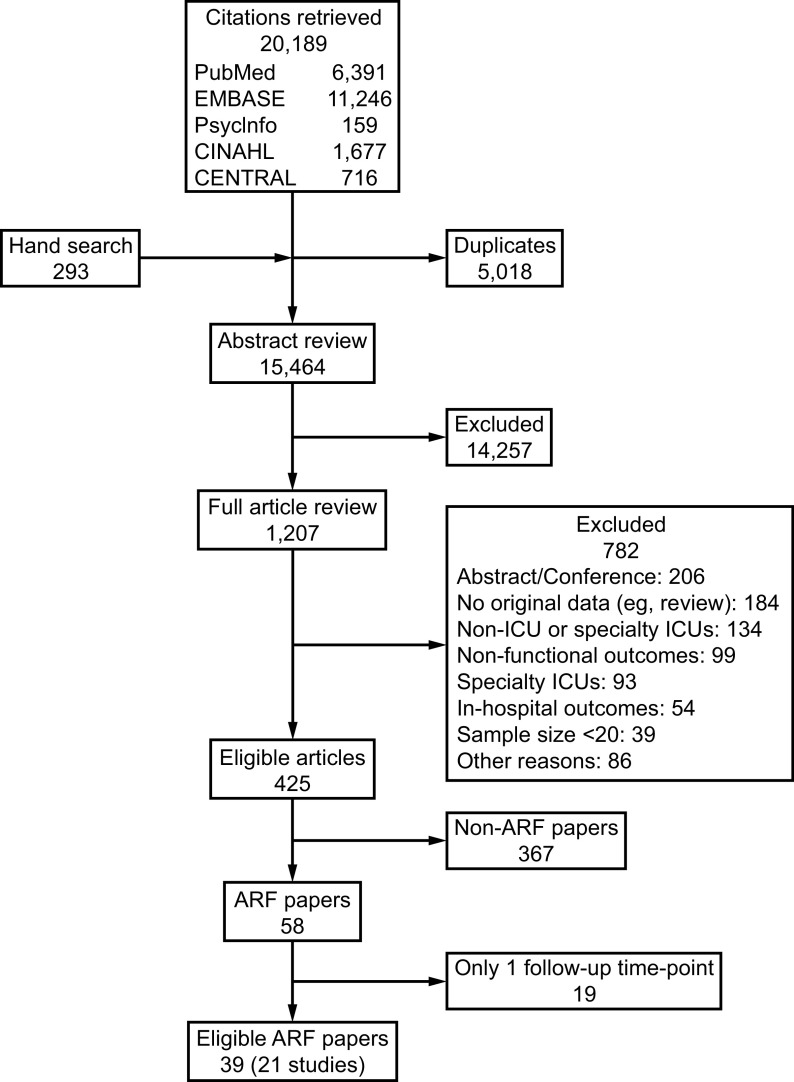
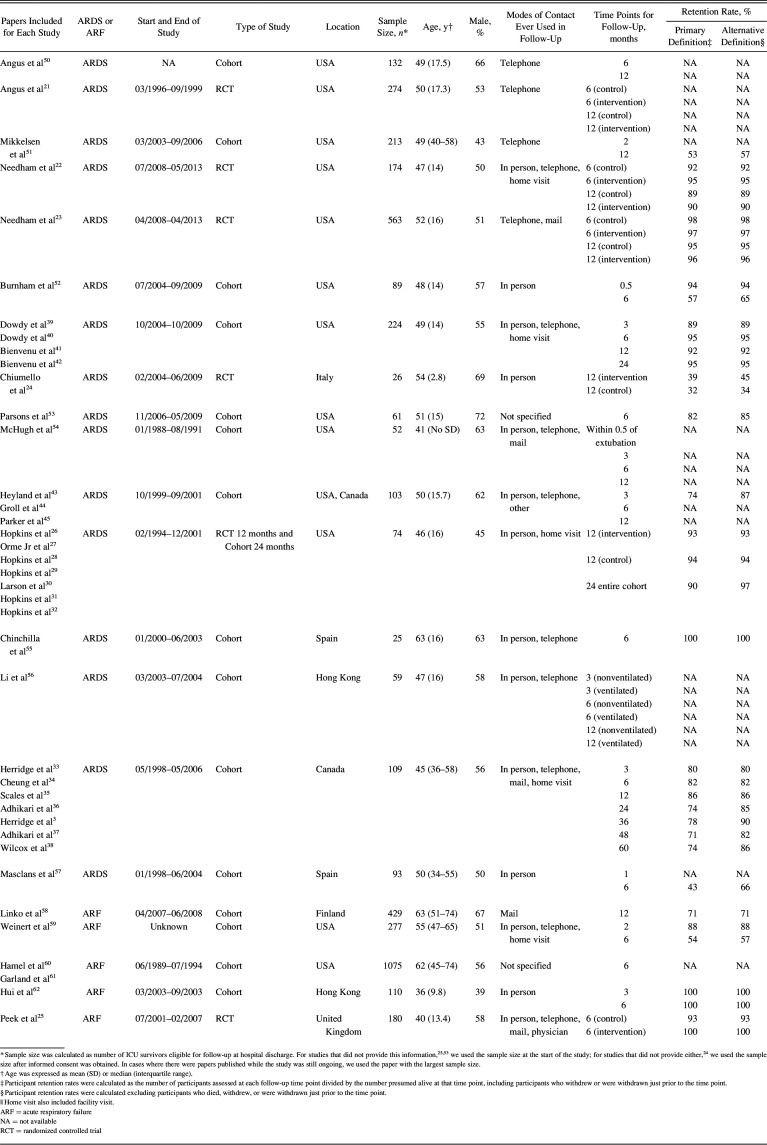
The literature search completed for the scoping review found 20,189 citations, with 15,464 unique titles and abstracts reviewed, of which 1,207 full-text articles were reviewed, yielding 425 eligible papers that reported on post-hospital functional outcomes in critical illness survivors.8 From these 425 papers, a total of 39 publications reporting on 21 unique studies (Fig. 1) met the eligibility criteria for our systematic review focused on ARF survivors; of these 21 unique studies, 16 (76%) focused exclusively on ARDS survivors. Among these 21 studies, 6 (29%) were RCTs21–32 and 15 (71%) were cohort studies (Table 1). There were a total of 4,342 ARF subjects in the included studies (the 16 ARDS studies reported on 2,271 subjects with ARDS). Thirteen (62%) of the 21 studies were conducted in United States (Table 1). The most frequent time points for follow-up was 6 months, evaluated in 17 (81%) studies, and 12 months, evaluated in 13 (62%) studies; the earliest and latest time points were 2 weeks (1 study) and 60 months (1 study).
Fig. 1.
Flow chart. ARF = acute respiratory failure.
Table 1.
Study Cohort Characteristics
Risk of Bias Assessment
Among the 6 RCTs, randomization and allocation concealment was rated as a low risk of bias in all the studies. Blinding of outcome assessments, addressing incomplete outcome data, and selective reporting were adequate in 5 of the 6 RCTs. In observational cohort studies, 13 of 15 studies (87%) had low risk for representativeness of the exposed cohort, whereas 11 (73%) had adequate follow-up (see the supplementary materials at http://www.rcjournal.com).
Retention-Related Reporting
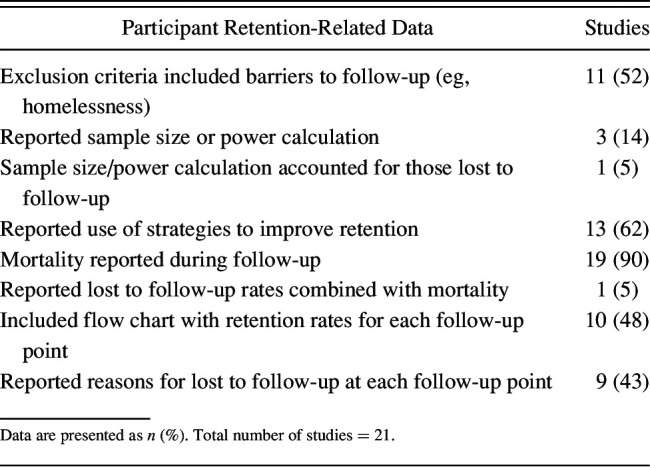
Eleven (52%) of 21 studies reported exclusion criteria related to inability to follow participants after hospital discharge, and 9 of 21 (43%) reported reasons for lost to follow-up at each time point (Table 2). Ten of 21 (48%) studies included flow charts that explicitly reported retention rates for each time point. Strategies to maximize participant retention were described in 13 of the 21 (62%) studies. Only 8 of 21 (38%) studies reported both retention strategies and retention rates,3,22,23,33–45 with 6 of these 8 studies reporting retention rates > 80% at all follow-up time points. Of 8 studies reporting participant retention rates without reporting on cohort-retention strategies, only 4 (50%) had retention rates > 80%. Three of the 21 (14%) studies reported a sample size or power calculation, of which only 1 specified a primary follow-up time point. Only 1 (5%) study reported consideration of loss to follow-up in calculating sample size or statistical power (Table 2).
Table 2.
Participant Retention-Related Data in Longitudinal Studies of Acute Respiratory Failure Survivors
Participant Retention
Participant retention rates could not be calculated for 5 of the 21 (24%) studies, representing 12 of the 47 (26%) time points. In 3 of the remaining 16 eligible studies, retention rates could not be calculated for 4 of the 35 (11%) time points. Among the 6 RCTs, we were able to calculate participant retention for 5 (83%) studies. Based on the available data from 31 time points in 16 studies, participant retention ranged from 32% to 100%, with a median (interquartile range) of 90% (74–95%). Participant retention was > 89% across available time points in 4 of the 5 RCTs reporting data.
Pooled Results
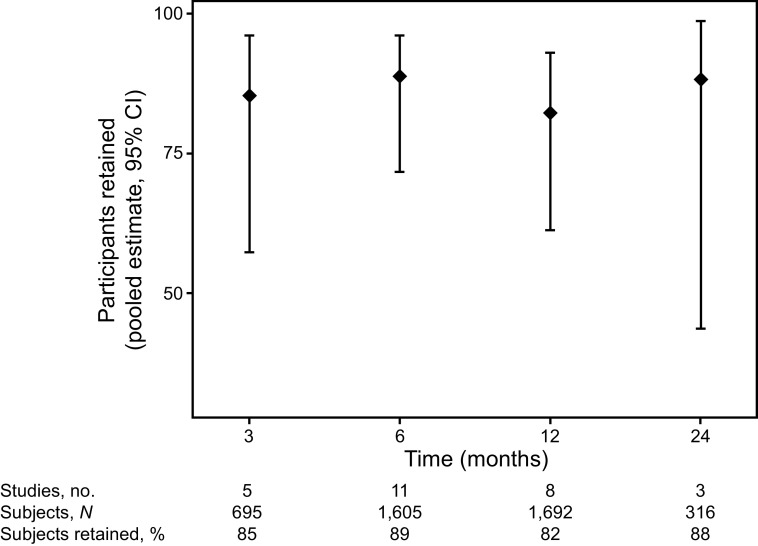
The pooled participant retention rate for each time point was 85% (95% CI 57–96%, I2 44%) at 3 months, 89% (95% CI 72–96%, I2 95%) at 6 months, 82% (95% CI 61–93%, I2 97%) at 12 months, and 88% (95% CI 44–99%, I2 75%) at 24 months (Fig. 2). As part of post hoc analyses to explore potential contributors to high heterogeneity, we pooled retention rates by following subgroups: ARDS studies, studies that conducted only in-person visits, and studies that used different modes of visits instead of or in addition to in-person visits. The I2 statistic did not qualitatively improve in these subgroups.
Fig. 2.
Pooled average retention rates in acute respiratory failure survivor follow-up studies. Retention rates were calculated as the number of participants assessed at each follow-up time point divided by the number presumed alive at that time point (this included participants who withdrew just prior to the time point). Points denote pooled average retention rates, and bars represent 95% CI.
There was no difference in retention rates across follow-up time points when comparing early (3 months) versus each later time point: 6 months (P = .61), 12 months (P = .68), and 24 months (P = .72). No qualitative differences in the studies could explain the substantial statistical heterogeneity observed at the 6-, 12-, and 24-month follow-up time points. The proportion of male participants in each study was associated with a decreased retention rate; the odds ratio for a 1% increase in male participants was 0.92 (95% CI 0.87–0.97, P = .006). Participant age and publication year were not significantly associated with retention rates. These results do not qualitatively differ when using the alternative definition for calculating retention rates, as described above. The pooled average retention rates using the second definition is in the supplementary materials (see the supplementary materials at http://www.rcjournal.com).
Discussion
In this systematic review and meta-analysis evaluating participant retention in 21 studies of ARF survivors' outcomes after hospital discharge, participant retention rates varied widely. Although the median retention rate (90%) was high, participant retention could not be calculated for 24% of studies. There was wide variability in reporting of important issues related to participant retention, such as reasons for loss to follow-up and use of strategies to maximize participant retention.
For studies in which retention rates could be calculated, pooled retention rates were high at the 3-, 6-, 12-, and 24-month time points (82–89%), but there was high variability among individual studies, with a range of 32–100% across all studies and follow-up time points. Researchers and stakeholders must be aware of potential limitations in the validity of studies with low participant retention. Participant age, publication year, and follow-up time point were not associated with retention rates. However, a greater proportion of males in studies was associated with reduced retention, which is noteworthy for evaluating any sex-specific findings from studies.
Although most studies reported some form of “lost to follow-up” data, the reporting was inconsistent and the type of data reported varied widely. For example, we were unable to calculate retention rates in 24% of studies because they either reported incomplete or no lost to follow-up data, or they combined lost to follow-up rates with mortality. Additionally, there was incomplete reporting of sample size calculations and other methodological issues relating to participant retention (eg, retention strategies utilized, participant exclusions).
Reporting guidelines do exist for RCTs and cohort studies. The CONSORT checklist for RCTs was first published in 1996,46 updated in 2001,47 and most recently revised in 2010.48 Since inception, the CONSORT checklist recommended utilization of a participant flow chart, with it being “strongly recommended” as of the 2001 update. The CONSORT checklist did not explicitly include reporting rates and reasons for participant loss and post-randomization exclusion until 2010, although the example flow chart in all 3 CONSORT versions mention “lost to follow-up.” For cohort studies, the STROBE checklist was published in 2007 and recommends reporting reasons for nonparticipation at each time point and consideration of a flow chart.49 In this systematic review, the 5 studies for which we could not calculate retention rates were all published prior to 2007 (one RCT published in 2006, 4 cohort studies published in 1994, 2000/2004, 2001, and 2006). Further improvement is needed to standardize reporting participant retention data and related issues, along with greater adherence to existing guidelines. A consensus process, including relevant stakeholders, may be useful for standardizing collection and reporting of data elements related to participant retention for all studies. Thereafter, journals may require more complete reporting of participant retention according to such consensus recommendations.
To assist with improving participant retention, there is a growing body of relevant publications and resources. A recent update to an earlier systematic review10,11 demonstrated a large increase in the number of publications related to participant-retention strategies. A total of 618 participant-retention strategies across 12 different themes, compiled from the most recent systematic review,11 are freely available as an online searchable database (https://www.improvelto.com/sysrevstrategies, Accessed February 19, 2020) to assist with understanding best practices in the field. Moreover, as part of a NIH-funded national research infrastructure project (R24HL111895), additional practical cohort-retention tools are freely available (https://www.improvelto.com/cohort-retention-tools, Accessed February 19, 2020) with a goal of providing additional research resources to assist investigators in this area.
Strengths and Limitations
To our knowledge, no prior systematic review has reported on cohort-retention rates and related methodology in ARF survivorship studies. Despite this strength, there are also potential limitations of this analysis. In an effort to reduce heterogeneity between studies and to optimize feasibility of this synthesis, we exclusively focused on studies of ARF survivors. However, this specific focus may result in limitations to precision and generalizability of these results. Despite this restriction to ARF studies, there remained substantial heterogeneity in pooled participant-retention rates beyond the 3-month follow-up time point, thus caution is advised when interpreting these results. Furthermore, studies published after 2013 were not included in this analysis because 2013 was the end date for the scoping review upon which this analysis was based. However, in comparing retention results over time (from 1970 to 2013), there was no temporal trend in retention, which may attenuate this potential limitation.
Conclusions
In this systematic review and meta-analysis evaluating 21 studies of ARF survivors at 3-, 6-, 12-, and 24-month follow-up time points, pooled participant retention rates were ≥ 82%. However, retention rates were highly variable across individual studies (range: 32–100%) and could not be calculated for nearly a quarter of the studies, with substantial differences in reporting of methodological issues related to participant retention. In addition to greater adherence to existing reporting standards for RCTs and cohort studies, additional reporting recommendations related to participant retention may be beneficial. Moreover, use of existing resources and best practices for optimizing participant retention data may benefit some acute respiratory survivorship studies.
Footnotes
This project was supported through a grant from the National Heart, Lung and Blood Institute (R24HL111895). The authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
References
- 1. Spragg RG, Bernard GR, Checkley W, Curtis JR, Gajic O, Guyatt G, et al. Beyond mortality: future clinical research in acute lung injury. Am J Respir Crit Care Med 2010;181(10):1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Crit Care Med 2010;38(10):1947–1953. [DOI] [PubMed] [Google Scholar]
- 3. Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011;364(14):1293–1304. [DOI] [PubMed] [Google Scholar]
- 4. Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med 2014;42(4):849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. Long-term cognitive impairment after critical illness. N Engl J Med 2013;369(14):1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med 2009;35(5):796–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med 2015;43(5):1121–1129. [DOI] [PubMed] [Google Scholar]
- 8. Turnbull AE, Rabiee A, Davis WE, Nasser MF, Venna VR, Lolitha R, et al. Outcome measurement in ICU survivorship research from 1970 to 2013: a scoping review of 425 publications. Crit Care Med 2016;44(7):1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fewtrell MS, Kennedy K, Singhal A, Martin RM, Ness A, Hadders-Algra M, et al. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Arch Dis Child 2008;93(6):458–461. [DOI] [PubMed] [Google Scholar]
- 10. Robinson KA, Dennison CR, Wayman DM, Pronovost PJ, Needham DM. Systematic review identifies number of strategies important for retaining study participants. J Clin Epidemiol 2007;60(8):757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robinson KA, Dinglas VD, Sukrithan V, Yalamanchilli R, Mendez-Tellez PA, Dennison-Himmelfarb C, et al. Updated systematic review identifies substantial number of retention strategies: using more strategies retains more study participants. J Clin Epidemiol 2015;68(12):1481–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abshire M, Dinglas VD, Cajita MI, Eakin MN, Needham DM, Himmelfarb CD. Participant retention practices in longitudinal clinical research studies with high retention rates. BMC Med Res Methodol 2017;17(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wells G, Shea B, O'Connell D. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in metaanalyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed December 25, 2019.
- 15. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haldane J. The mean and variance of chi-square, when used as a test of homogeneity, when expectations are small. Biometrika 1940;31(3-4):346–355. [Google Scholar]
- 17. Anscombe FJ. On estimating binomial response relations. Biometrika 1956;43(3-4):461–464. [Google Scholar]
- 18. Sedgwick P. Meta-analyses: heterogeneity and subgroup analysis. BMJ 2013;346:f4040. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151(4):264–269. [DOI] [PubMed] [Google Scholar]
- 21. Angus DC, Clermont G, Linde-Zwirble WT, Musthafa AA, Dremsizov TT, Lidicker J, et al. Healthcare costs and long-term outcomes after acute respiratory distress syndrome: a phase III trial of inhaled nitric oxide. Crit Care Med 2006;34(12):2883–2890. [DOI] [PubMed] [Google Scholar]
- 22. Needham DM, Dinglas VD, Morris PE, Jackson JC, Hough CL, Mendez-Tellez PA, et al. Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic versus full enteral feeding. EDEN trial follow-up. Am J Respir Crit Care Med 2013;188(5):567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Needham DM, Dinglas VD, Bienvenu OJ, Colantuoni E, Wozniak AW, Rice TW, et al. One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: prospective follow-up of EDEN randomised trial. BMJ 2013;346:f1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chiumello D, Taccone P, Berto V, Marino A, Migliara G, Lazzerini M, et al. Long-term outcomes in survivors of acute respiratory distress syndrome ventilated in supine or prone position. Intensive Care Med 2012;38(2):221–229. [DOI] [PubMed] [Google Scholar]
- 25. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374(9698):1351–1363. [DOI] [PubMed] [Google Scholar]
- 26. Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson-Lohr V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med 1999;160(1):50–56. [DOI] [PubMed] [Google Scholar]
- 27. Orme J, Jr, Romney JS, Hopkins RO, Pope D, Chan KJ, Thomsen G, et al. Pulmonary function and health-related quality of life in survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med 2003;167(5):690–694. [DOI] [PubMed] [Google Scholar]
- 28. Hopkins RO, Weaver LK, Chan KJ, Orme JF. Quality of life, emotional, and cognitive function following acute respiratory distress syndrome. J Int Neuropsychol Soc 2004;10(7):1005–1017. [DOI] [PubMed] [Google Scholar]
- 29. Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF, Jr. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med 2005;171(4):340–347. [DOI] [PubMed] [Google Scholar]
- 30. Larson MJ, Weaver LK, Hopkins RO. Cognitive sequelae in acute respiratory distress syndrome patients with and without recall of the intensive care unit. J Int Neuropsychol Soc 2007;13(4):595–605. [DOI] [PubMed] [Google Scholar]
- 31. Hopkins RO, Key CW, Suchyta MR, Weaver LK, Orme JF, Jr. Risk factors for depression and anxiety in survivors of acute respiratory distress syndrome. Gen Hosp Psychiatry 2010;32(2):147–155. [DOI] [PubMed] [Google Scholar]
- 32. Hopkins RO, Suchyta MR, Snow GL, Jephson A, Weaver LK, Orme JF. Blood glucose dysregulation and cognitive outcome in ARDS survivors. Brain Inj 2010;24(12):1478–1484. [DOI] [PubMed] [Google Scholar]
- 33. Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al Saidi F, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 2003;348(8):683–693. [DOI] [PubMed] [Google Scholar]
- 34. Cheung AM, Tansey CM, Tomlinson G, Diaz-Granados N, Matté A, Barr A, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med 2006;174(5):538–544. [DOI] [PubMed] [Google Scholar]
- 35. Scales DC, Tansey CM, Matte A, Herridge MS. Difference in reported pre-morbid health-related quality of life between ARDS survivors and their substitute decision makers. Intensive Care Med 2006;32(11):1826–1831. [DOI] [PubMed] [Google Scholar]
- 36. Adhikari NKJ, McAndrews MP, Tansey CM, Matté A, Pinto R, Cheung AM, et al. Self-reported symptoms of depression and memory dysfunction in survivors of ARDS. Chest 2009;135(3):678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Adhikari NKJ, Tansey CM, McAndrews MP, Matté A, Pinto R, Cheung AM, et al. Self-reported depressive symptoms and memory complaints in survivors five years after ARDS. Chest 2011;140(6):1484–1493. [DOI] [PubMed] [Google Scholar]
- 38. Wilcox ME, Patsios D, Murphy G, Kudlow P, Paul N, Tansey CM, et al. Radiologic outcomes at 5 years after severe ARDS. Chest 2013;143(4):920–926. [DOI] [PubMed] [Google Scholar]
- 39. Dowdy DW, Dinglas V, Mendez-Tellez PA, Bienvenu OJ, Sevransky J, Dennison CR, et al. Intensive care unit hypoglycemia predicts depression during early recovery from acute lung injury. Crit Care Med 2008;36(10):2726–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dowdy DW, Bienvenu OJ, Dinglas VD, Mendez-Tellez PA, Sevransky J, Shanholtz C, et al. Are intensive care factors associated with depressive symptoms 6 months after acute lung injury? Crit Care Med 2009;37(5):1702–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, Dinglas VD, Shanholtz C, Husain N, et al. Depressive symptoms and impaired physical function after acute lung injury: a 2-year longitudinal study. Am J Respir Crit Care Med 2012;185(5):517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bienvenu OJ, Gellar J, Althouse BM, Colantuoni E, Sricharoenchai T, Mendez-Tellez PA, et al. Post-traumatic stress disorder symptoms after acute lung injury: a 2-year prospective longitudinal study. Psychol Med 2013;43(12):2657–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heyland DK, Groll D, Caeser M. Survivors of acute respiratory distress syndrome: relationship between pulmonary dysfunction and long-term health-related quality of life. Crit Care Med 2005;33(7):1549–1556. [DOI] [PubMed] [Google Scholar]
- 44. Groll DL, Heyland DK, Caeser M, Wright JG. Assessment of long-term physical function in acute respiratory distress syndrome (ARDS) patients: comparison of the Charlson Comorbidity Index and the Functional Comorbidity Index. Am J Phys Med Rehabil 2006;85(7):574–581. [DOI] [PubMed] [Google Scholar]
- 45. Parker CM, Heyland DK, Groll D, Caeser M. Mechanism of injury influences quality of life in survivors of acute respiratory distress syndrome. Intensive Care Med 2006;32(11):1895–1900. [DOI] [PubMed] [Google Scholar]
- 46. Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276(8):637–639. [DOI] [PubMed] [Google Scholar]
- 47. Moher D, Schulz KF, Altman D, CONSORT Group (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 2001;285(15):1987–1991. [DOI] [PubMed] [Google Scholar]
- 48. Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010;152(11):726–732. [DOI] [PubMed] [Google Scholar]
- 49. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370(9596):1453–1457. [DOI] [PubMed] [Google Scholar]
- 50. Angus DC, Musthafa AA, Clermont G, Griffin MF, Linde-Zwirble WT, Dremsizov TT, et al. Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;163(6):1389–1394. [DOI] [PubMed] [Google Scholar]
- 51. Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med 2012;185(12):1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Burnham EL, Hyzy RC, Paine R, Coley C, Kelly AM, Quint LE, et al. Chest CT features are associated with poorer quality of life in acute lung injury survivors. Crit Care Med 2013;41(12):445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Parsons EC, Kross EK, Caldwell ES, Kapur VK, McCurry SM, Vitiello MV, et al. Post-discharge insomnia symptoms are associated with quality of life impairment among survivors of acute lung injury. Sleep Med 2012;13(8):1106–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McHugh LG, Milberg JA, Whitcomb ME, Schoene RB, Maunder RJ, Hudson LD. Recovery of function in survivors of the acute respiratory distress syndrome. Am J Respir Crit Care Med 1994;150(1):90–94. [DOI] [PubMed] [Google Scholar]
- 55. Ortiz Chinchilla D, Jam Gatell MR. Long term of quality of life and mortality in acute respiratory distress syndrome (ARDS) patients. Enferm Intensiva 2003;14(3):88–95. [DOI] [PubMed] [Google Scholar]
- 56. Li TS, Gomersall CD, Joynt GM, Chan DPS, Leung P, Hui DSC. Long-term outcome of acute respiratory distress syndrome caused by severe acute respiratory syndrome (SARS): an observational study. Crit Care Resusc J Australas Acad Crit Care Med 2006;8(4):302–308. [PubMed] [Google Scholar]
- 57. Masclans JR, Roca O, Muñoz X, Pallisa E, Torres F, Rello J, et al. Quality of life, pulmonary function, and tomographic scan abnormalities after ARDS. Chest 2011;139(6):1340–1346. [DOI] [PubMed] [Google Scholar]
- 58. Linko R, Suojaranta-Ylinen R, Karlsson S, Ruokonen E, Varpula T, Pettilä V, et al. One-year mortality, quality of life and predicted life-time cost-utility in critically ill patients with acute respiratory failure. Crit Care 2010;14(2):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Weinert C, Meller W. Epidemiology of depression and antidepressant therapy after acute respiratory failure. Psychosomatics 2006;47(5):399–407. [DOI] [PubMed] [Google Scholar]
- 60. Hamel MB, Phillips RS, Davis RB, Teno J, Connors AF, Desbiens N, et al. Outcomes and cost-effectiveness of ventilator support and aggressive care for patients with acute respiratory failure due to pneumonia or acute respiratory distress syndrome. Am J Med 2000;109(8):614–620. [DOI] [PubMed] [Google Scholar]
- 61. Garland A, Dawson NV, Altmann I, Thomas CL, Phillips RS, Tsevat J, et al. Outcomes up to 5 years after severe, acute respiratory failure. Chest 2004;126(6):1897–1904. [DOI] [PubMed] [Google Scholar]
- 62. Hui DS, Joynt GM, Wong KT, Gomersall CD, Li TS, Antonio G, et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]