Abstract
Background
A vaccine against COVID-19 is urgently needed for older adults, in whom morbidity and mortality due to the disease are increased. We aimed to assess the safety, tolerability, and immunogenicity of a candidate COVID-19 vaccine, CoronaVac, containing inactivated SARS-CoV-2, in adults aged 60 years and older.
Methods
We did a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial of CoronaVac in healthy adults aged 60 years and older in Renqiu (Hebei, China). Vaccine or placebo was given by intramuscular injection in two doses (days 0 and 28). Phase 1 comprised a dose-escalation study, in which participants were allocated to two blocks: block 1 (3 μg inactivated virus in 0·5 mL of aluminium hydroxide solution per injection) and block 2 (6 μg per injection). Within each block, participants were randomly assigned (2:1) using block randomisation to receive CoronaVac or placebo (aluminium hydroxide solution only). In phase 2, participants were randomly assigned (2:2:2:1) using block randomisation to receive either CoronaVac at 1·5 μg, 3 μg, or 6 μg per dose, or placebo. All participants, investigators, and laboratory staff were masked to treatment allocation. The primary safety endpoint was adverse reactions within 28 days after each injection in all participants who received at least one dose. The primary immunogenicity endpoint was seroconversion rate at 28 days after the second injection (which was assessed in all participants who had received the two doses of vaccine according to their random assignment, had antibody results available, and did not violate the trial protocol). Seroconversion was defined as a change from seronegative at baseline to seropositive for neutralising antibodies to live SARS-CoV-2 (positive cutoff titre 1/8), or a four-fold titre increase if the participant was seropositive at baseline. This study is ongoing and is registered with ClinicalTrials.gov (NCT04383574).
Findings
Between May 22 and June 1, 2020, 72 participants (24 in each intervention group and 24 in the placebo group; mean age 65·8 years [SD 4·8]) were enrolled in phase 1, and between June 12 and June 15, 2020, 350 participants were enrolled in phase 2 (100 in each intervention group and 50 in the placebo group; mean age 66·6 years [SD 4·7] in 349 participants). In the safety populations from both phases, any adverse reaction within 28 days after injection occurred in 20 (20%) of 100 participants in the 1·5 μg group, 25 (20%) of 125 in the 3 μg group, 27 (22%) of 123 in the 6 μg group, and 15 (21%) of 73 in the placebo group. All adverse reactions were mild or moderate in severity and injection site pain (39 [9%] of 421 participants) was the most frequently reported event. As of Aug 28, 2020, eight serious adverse events, considered unrelated to vaccination, have been reported by seven (2%) participants. In phase 1, seroconversion after the second dose was observed in 24 of 24 participants (100·0% [95% CI 85·8–100·0]) in the 3 μg group and 22 of 23 (95·7% [78·1–99·9]) in the 6 μg group. In phase 2, seroconversion was seen in 88 of 97 participants in the 1·5 μg group (90·7% [83·1–95·7]), 96 of 98 in the 3 μg group (98·0% [92·8–99·8]), and 97 of 98 (99·0% [94·5–100·0]) in the 6 μg group. There were no detectable antibody responses in the placebo groups.
Interpretation
CoronaVac is safe and well tolerated in older adults. Neutralising antibody titres induced by the 3 μg dose were similar to those of the 6 μg dose, and higher than those of the 1·5 μg dose, supporting the use of the 3 μg dose CoronaVac in phase 3 trials to assess protection against COVID-19.
Funding
Chinese National Key Research and Development Program and Beijing Science and Technology Program.
Introduction
The ongoing COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly spread across the world and led to more than 94 million infections and more than 2 million deaths worldwide as of Jan 19, 2021.1 Studies have shown that individuals aged 60 years or older, and especially those with underlying chronic conditions, have an increased risk of severe illness and death compared with younger people, and that this risk increases with age.2, 3, 4
Research in context.
Evidence before this study
We searched PubMed and the American Medical Association website on Dec 1, 2020, for published research articles, with no language or date restrictions, using the search terms “SARS-CoV-2”, “COVID-19”, “vaccine”, and “clinical trial”. We identified a phase 1/2 study of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV, developed by Sinopharm (Beijing, China), which showed that the vaccine was safe, tolerable, and immunogenic in healthy people in China. Two-dose immunisations (on days 0 and 28) at all doses (2 μg, 4 μg, and 8 μg) in two age groups (18–59 years and ≥60 years) induced neutralising antibodies in 100% of vaccine recipients. A phase 2 clinical trial of another inactivated Sinopharm vaccine in healthy adults aged 18–59 years showed a seroconversion rate of 97·6% at 14 days after a day 0 and 21 vaccination schedule. Additionally, a phase 2 study of another inactivated vaccine developed by the Institute of Medical Biology, Chinese Academy of Medical Sciences, showed that the vaccine was safe and induced neutralising antibody responses in adults aged 18–59 years. Several studies have shown neutralising antibody responses in older adults using other vaccine platforms, including two mRNA vaccines (Moderna and Pfizer/BioNTech, USA), one adenovirus type-5 vectored vaccine (CanSino Biological/Beijing Institute of Biotechnology, China), and one chimpanzee adenovirus-vectored vaccine (AstraZeneca, UK). We previously assessed CoronaVac, an inactivated vaccine developed by Sinovac Life Sciences, in adults aged 18–59 years, and showed that it was safe and well tolerated. Seroconversion rates ranged from 92% to 100% after two doses of CoronaVac (3 μg and 6 μg) with two immunisation schedules (on days 0 and 14, or on days 0 and 28).
Added value of this study
This is the first report of an inactivated SARS-CoV-2 vaccine, CoronaVac, tested in older adults (aged ≥60 years). We used a phase 1/2 study design to assess the safety of two different doses (3 μg and 6 μg) in a dose-escalation study with a two-dose vaccination schedule (days 0 and 28) before expanding the study to a larger cohort to explore immunogenicity in healthy older people. The neutralising antibody responses observed in those who received the 3 μg or 6 μg dose were higher than those in people who received the 1·5 μg dose, and were similar to the responses among adults aged 18–59 years who received the 3 μg or 6 μg dose.
Implications of all the available evidence
People older than 60 years have an increasing risk of severe illness and death from COVID-19, especially those with underlying chronic conditions. The response to vaccines is usually reduced in older adults due to immune senescence. Our findings indicate that CoronaVac is well tolerated and immunogenic in healthy adults aged 60 years and older, and neutralising antibody responses to live SARS-CoV-2 are not reduced in this population. Further studies of the effectiveness of this vaccine for prevention of COVID-19 in older adults are needed.
To control the pandemic and reduce the burden of COVID-19 worldwide, effective and safe COVID-19 vaccines are urgently needed. The response to vaccines is usually reduced in older adults because of immune senescence,4, 5, 6, 7 the age-related changes that affect many of the cellular and molecular elements of both the innate and adaptive immune systems. Therefore, testing the effectiveness of COVID-19 vaccines in this population is necessary.
Researchers around the world have been racing to develop COVID-19 vaccines since the outbreak began, with more than 64 candidate vaccines in the clinical evaluation stage and another 173 vaccines in preclinical evaluation as of Jan 15, 2021.8 Studies have shown that the neutralising antibody responses can be induced in older adults with use of different vaccine platforms, including mRNA,9, 10 adenovirus vectors,11, 12 and inactivated virus.13 The interim efficacy analyses from four phase 3 trials have shown different vaccines to be highly effective against COVID-19 in adults aged 16 years or older, including two mRNA vaccines with efficacies of 95%,14, 15 and two adenovirus-vectored vaccines with efficacies of 70%16 and 91%.17
Purified inactivated viruses have traditionally been used for vaccine development, and currently eight inactivated COVID-19 candidate vaccines are in clinical evaluation.8 Although the results of efficacy against COVID-19 are not yet available, several studies have shown that the inactivated vaccines can induce neutralising antibody responses and have good safety profiles.13, 18, 19, 20 CoronaVac is an inactivated SARS-CoV-2 vaccine developed by Sinovac Life Sciences (Beijing, China). The results of a preclinical study showed that CoronaVac induced good neutralising antibody responses in animals and provided partial or complete protection from severe interstitial pneumonia in macaques following SARS-CoV-2 challenge, without observable antibody-dependent enhancement of infection.21 We previously reported the results of our phase 1/2 clinical trial of CoronaVac in participants aged 18–59 years, which showed that CoronaVac was well tolerated and induced humoral responses against SARS-CoV-2.20 Here we report the safety, tolerability, and immunogenicity of CoronaVac among healthy adults aged 60 years and older.
Methods
Study design and participants
In our initial phase 1/2 trial of CoronaVac in participants aged 18–59 years old, doses of 3 μg and 6 μg were evaluated,20 and the preliminary safety results supported the expansion of the trial to older adults. We subsequently did a single-centre, randomised, double-blind, placebo-controlled, phase 1/2 trial to evaluate the safety, tolerability, and immunogenicity of CoronaVac in adults aged 60 years and older. Three different doses—1·5 μg, 3 μg, and 6 μg—were used in this study. Because the 1·5 μg dose was not evaluated in the initial human trial, and the number of participants in phase 1 of the current study was small, the dose of 1·5 μg was only given to participants in the phase 2 trial of those aged 60 years or older. This trial was run at Hebei Provincial Center for Disease Control and Prevention (CDC) in Renqiu (Hebei, China).
Phase 1 of the trial was a dose-escalation study of 72 participants. The first 36 participants (block 1) were randomly assigned to receive either 3 μg vaccine or placebo. After 7 days of follow-up for safety after the first dose, another 36 participants (block 2) were randomly assigned to receive either 6 μg vaccine or placebo. Phase 2 was initiated only after all the participants in phase 1 had finished and passed a 7-day safety observation period after the first dose, as confirmed by the data monitoring committee. The required safety criteria in both blocks were no life-threatening adverse events and no more than 15% of vaccinated participants reporting severe adverse events. In phase 2, vaccine doses of 1·5 μg, 3 μg, or 6 μg were compared with placebo.
Eligible participants were healthy adults aged 60 years or older. The key exclusion criteria included high-risk epidemiological history within 14 days before enrolment (eg, travel to or residence in Wuhan and surrounding areas or other communities with reports of COVID-19 cases, or contact with someone infected with SARS-CoV-2), history of severe acute respiratory syndrome or SARS-CoV-2 infection, axillary temperature of more than 37·0°C, or a history of allergy to any vaccine component. A complete list of exclusion criteria is provided in the protocol, which is available online.
Written informed consent was obtained from each participant before enrolment. The clinical trial protocol and informed consent form were approved by the Ethics Committee of Hebei CDC (IRB2020–006). The study was conducted in accordance with the requirements of Good Clinical Practice of China and the International Conference on Harmonisation.
Randomisation and masking
In phase 1, participants in block 1 and block 2 were randomly assigned (2:1) to receive either vaccine or placebo. In phase 2, participants were randomly assigned (2:2:2:1) to either 1·5 μg, 3 μg, or 6 μg of vaccine or placebo. The randomisation codes for phases 1 and 2 were generated by the randomisation statistician, using block randomisation and SAS software (version 9.4). The randomisation code was assigned to each participant in sequence in order of enrolment, and participants received the study vaccine or placebo labelled with the same code. The vaccine and placebo were completely identical in appearance, and all participants, investigators, and laboratory staff were masked to group allocation.
Procedures
CoronaVac is an inactivated vaccine candidate against COVID-19. To prepare the vaccine, SARS-CoV-2 (CN02 strain) was propagated in African green monkey kidney cells (WHO Vero 10-87 cells). At the end of the incubation period, the virus was harvested, inactivated with β-propiolactone, concentrated, purified, and finally adsorbed onto aluminium hydroxide. The aluminium hydroxide complex was then diluted in sodium chloride, phosphate-buffered saline, and water before being sterilised and filtered for injection. The placebo consisted only of the aluminium hydroxide solution with no virus. Both the vaccine and placebo were prepared in a Good Manufacturing Practice-accredited facility of Sinovac Life Sciences that is periodically inspected by the National Medical Products Administration committee for compliance. The production process of the vaccine in this trial was a highly automated bioreactor (ReadyToProcess WAVE 25; GE, Umea, Sweden), which was consistent with the production process of vaccine used in the phase 2 trial of adults aged 18–59 years.20 Vaccine doses of 1·5 μg, 3 μg, or 6 μg in 0·5 mL of aluminium hydroxide solution per injection and placebo in ready-to-use syringes were administered intramuscularly to participants on days 0 and 28.
For the first 7 days after each dose, participants were required to record injection site adverse events (eg, pain, swelling, or redness) and systemic adverse events (eg, allergic reaction, cough, or fever) on diary cards. On days 8 and 28, participants visited the study site for assessment by the study investigators (all medical practitioners) who conducted face-to-face interviews to confirm safety. Between visits, safety data were collected by spontaneous recording and reporting of adverse events by participants. The serious adverse events reported in this Article were collected from May 22 to Aug 28, 2020 and follow-up will continue until 12 months after the second dose. The reported adverse events were graded according to the China National Medical Products Administration guidelines.22 The causal relationship between adverse events and vaccination was determined by the investigators.
Blood samples were collected on days 0, 28, and 56 from participants in phase 1, and on days 0 and 56 in phase 2 to evaluate the neutralising antibody titres. The neutralising antibody titres to live SARS-CoV-2 (virus strain SARS-CoV-2/human/CHN/CN1/2020, GenBank number MT407649.1) were quantified using a micro cytopathogenic effect assay.23 Serum samples were inactivated at 56°C for 30 min and serially diluted with cell culture medium in two-fold steps. The diluted serum samples were incubated with equal volumes (50 μL) of live SARS-CoV-2 suspension, with a 50% cell culture infective dose of 100 for 2 h at 37·0°C. Vero cells (1·0 × 105 to 2·0 × 105 cells per mL) were then added to the serum–virus suspensions in microplates in duplicate and incubated at 36·5°C for 5 days. Cytopathic effects were recorded under microscopes and the neutralising antibody titre was calculated by the dilution number of the 50% protective condition. Detection was done by the National Institute for Food and Drug Control. Further information on the method is provided in the appendix (p 1).
Outcomes
The primary safety endpoint was any vaccine-related adverse event (adverse reaction) within 28 days after the administration of each dose of vaccine or placebo. The primary immunogenicity endpoint was the seroconversion rate of neutralising antibodies to live SARS-CoV-2 at day 28 after the second dose. Secondary endpoints were severe adverse events and geometric mean titre (GMT) of neutralising antibodies to live SARS-CoV-2, as well as seropositive rates and geometric mean increase. Seroconversion was defined as a change from seronegative at baseline to seropositive, or a four-fold titre increase if the participant was seropositive at baseline. The positive cutoff of the titre for neutralising antibodies to live SARS-CoV-2 was 1/8.
Statistical analysis
We assessed the safety endpoints in the safety population, which included all participants who received at least one dose of vaccine or placebo. We assessed the immunogenicity endpoints in the per-protocol population, which included all participants who had received the two doses of vaccine according to their random assignment, had antibody results available, and did not violate the trial protocol.
We did not determine the sample sizes on the basis of a statistical power calculation, but followed the requirements of the China National Medical Products Administration and Chinese Technical Guidelines for Clinical Trials of Vaccines—ie, recruitment of at least 20–30 participants in phase 1 and 300 participants in phase 2.
We used the Pearson χ2 test or Fisher's exact test for the analysis of categorical outcomes. We calculated 95% CIs for all categorical outcomes using the Clopper-Pearson method. We calculated GMTs and corresponding 95% CIs on the basis of standard normal distribution of the log-transformation antibody titre. We used the ANOVA method to compare the log-transformed antibody titre. When the comparison among all groups showed a significant difference, we did pairwise comparisons. Hypothesis testing was two-sided and we considered p values of less than 0·05 to be significant.
An independent data monitoring committee consisting of one independent statistician, one clinician, and one epidemiologist was established before commencement of the study. Safety data were assessed and reviewed by the committee to ensure further proceeding of the study.
We used SAS (version 9.4) for all analyses. This trial is registered with ClinicalTrials.gov (NCT04383574).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All the authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Result
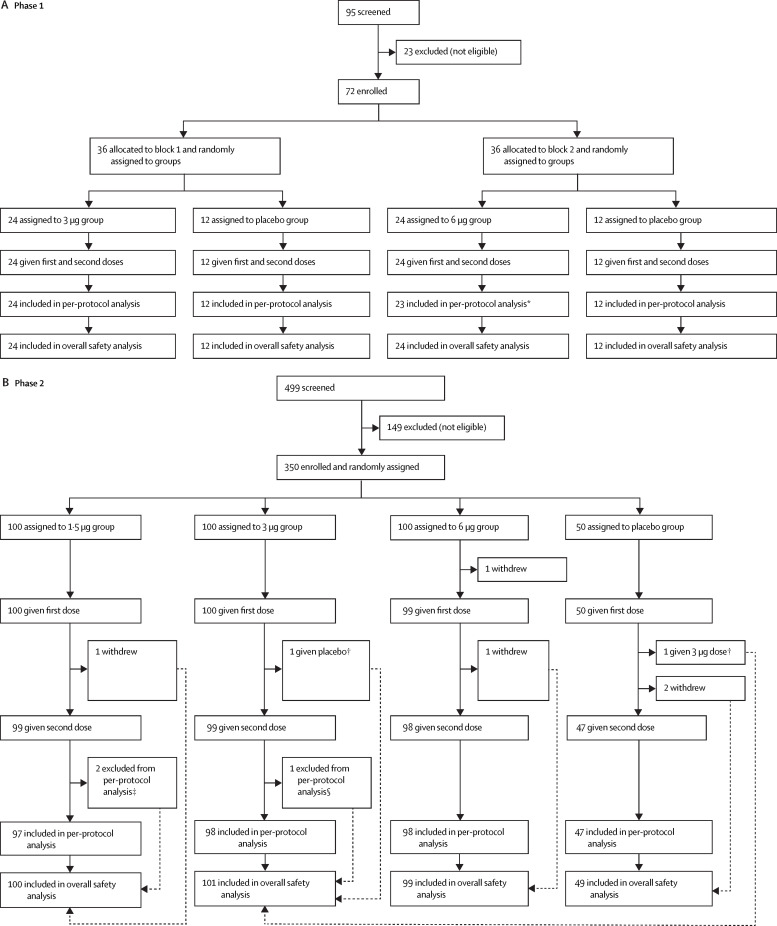
Between May 22 and June 1, 2020, 95 individuals were screened and 72 were enrolled in phase 1. Between June 12 and June 15, 2020, 499 individuals were screened and 350 were enrolled in phase 2. 421 (>99%) of 422 enrolled participants received at least one dose of vaccine or placebo (72 in phase 1 and 349 in phase 2) and were included in the safety population (figure ). 71 (99%) participants in phase 1 and 340 (97%) in phase 2 were eligible for the immunogenicity evaluation at day 28 after the second dose (per-protocol population; figure). The demographic characteristics of participants in the safety population were similar across treatment groups in terms of sex, mean age, height, weight, and ethnicity (table 1 ). The mean age of study participants in phase 1 was 65·8 years (SD 4·8), including 39 (54%) participants aged 60–64 years, 18 (25%) aged 65–69 years, and 15 (21%) aged 70 years or older. In phase 2, the mean age of the 349 treated participants was 66·6 years (SD 4·7), including 132 (38%) aged 60–64 years, 125 (36%) aged 65–69 years, and 92 (26%) aged 70 years or older.
Figure.
Trial profile
*One participant was excluded from the per-protocol analysis because he received immunoglobulin within 7 days after the second dose. †One participant in the 3 μg group and one in the placebo group were mistakenly given each other's vaccine at the second dose. In the overall safety analysis, both participants were analysed as part of the 3 μg group. In the safety analysis of the second dose, each participant was analysed in the group corresponding to what they had actually received at the second dose. Both participants were excluded from the per-protocol immunogenicity evaluation. ‡Two participants in the 1·5 μg group were excluded from the per-protocol analysis (one did not have a blood sample taken 28 days after the second dose, and one was found not to meet the eligibility criteria after enrolment). §One participant in the 3 μg group was excluded from the per-protocol analysis because the second dose was given outside of the specified time window.
Table 1.
Baseline demographic characteristics
|
Phase 1 |
Phase 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| 3 μggroup (n=24) | 6 μggroup (n=24) | Placebo group (n=24) | 1·5 μg group (n=100) | 3 μggroup (n=100) | 6 μggroup (n=99) | Placebo group (n=50) | ||
| Age, years | ||||||||
| Mean (SD) | 65·6 (4·3) | 67·5 (5·5) | 64·2 (4·2) | 66·8 (4·6) | 66·5 (4·9) | 66·2 (4·4) | 67·4 (4·9) | |
| 60–64 | 12 (50%) | 9 (38%) | 18 (75%) | 38 (38%) | 39 (39%) | 39 (39%) | 16 (32%) | |
| 65–69 | 6 (25%) | 8 (33%) | 4 (17%) | 35 (35%) | 33 (33%) | 40 (40%) | 17 (34%) | |
| ≥70 | 6 (25%) | 7 (29%) | 2 (8%) | 27 (27%) | 28 (28%) | 20 (20%) | 17 (34%) | |
| Sex | ||||||||
| Male | 11 (46%) | 13 (54%) | 13 (54%) | 49 (49%) | 49 (49%) | 44 (44%) | 27 (54%) | |
| Female | 13 (54%) | 11 (46%) | 11 (46%) | 51 (51%) | 51 (51%) | 55 (56%) | 23 (46%) | |
| Han ethnicity | 24 (100%) | 24 (100%) | 24 (100%) | 99 (99%) | 100 (100%) | 99 (100%) | 50 (100%) | |
| Height, m | 1·6 (0·1) | 1·7 (0·1) | 1·6 (0·8) | 1·6 (0·1) | 1·6 (0·1) | 1·6 (0·1) | 1·6 (0·1) | |
| Weight, kg | 69·6 (7·5) | 66·9 (9·0) | 68·8 (7·0) | 66·8 (10·2) | 67·2 (11·2) | 66·1 (9·8) | 64·9 (11·5) | |
Data are mean (SD) or n (%).
The safety data of the phase 1 and phase 2 trial were combined for analysis because the same batches of vaccine and placebo and the same safety observation method were used. 87 (21%) of 421 participants reported at least one adverse reaction within 28 days of either vaccination, and the proportions of patients with any adverse reaction were similar across groups (table 2 ). All adverse reactions were either mild (grade 1) or moderate (grade 2) in severity. Most adverse reactions occurred within 7 days after vaccination and participants recovered within 48 h. The most common reactions were injection site pain (39 [9%] participants) and fever (14 [3%]). Except for a slightly higher incidence of headache and mucocutaneous eruption in the 6 μg group and a slightly higher incidence of hypoesthesia in the placebo group, there were no significant differences in the incidence of other injection site or systemic events among the four groups (table 2). As of Aug 28, 2020, eight serious adverse events have been reported by seven (2%) participants: four (4%) of 100 participants in the 1·5 μg group, one (1%) of 125 participants in the 3 μg group, two (2%) of 123 participants in the 6 μg group, and none of the 73 participants in the placebo group (p=0·240). All serious adverse events were considered to be unrelated to either the vaccine or the placebo (appendix p 10). The 6-month follow-up analysis is not yet complete, but no vaccine-related serious adverse events have been reported as of Jan 20, 2021.
Table 2.
Adverse reactions reported within 28 days after the first and second doses of vaccine or placebo in phase 1 and phase 2
| 1·5 μg group (n=100) | 3 μg group (n=125) | 6 μg group (n=123) | Placebo group (n=73) | p value* | ||
|---|---|---|---|---|---|---|
| Any adverse reaction | 20 (20%) | 25 (20%) | 27 (22%) | 15 (21%) | 0·981 | |
| Grade 1 | 16 (16%) | 24 (19%) | 23 (19%) | 13 (18%) | 0·943 | |
| Grade 2 | 7 (7%) | 4 (3%) | 7 (6%) | 4 (5%) | 0·596 | |
| Local reactions | 13 (13%) | 15 (12%) | 13 (11%) | 3 (4%) | 0·212 | |
| Pain | 11 (11%) | 14 (11%) | 11 (9%) | 3 (4%) | 0·335 | |
| Erythema | 2 (2%) | 0 | 1 (1%) | 1 (1%) | 0·434 | |
| Pruritus | 1 (1%) | 1 (1%) | 1 (1%) | 0 | 1·000 | |
| Swelling | 0 | 1 (1%) | 1 (1%) | 0 | 1·000 | |
| Systemic reactions | 10 (10%) | 13 (10%) | 18 (15%) | 12 (16%) | 0·459 | |
| Fever | 4 (4%) | 4 (3%) | 5 (4%) | 1 (1%) | 0·783 | |
| Fatigue | 4 (4%) | 4 (3%) | 4 (3%) | 1 (1%) | 0·829 | |
| Diarrhoea | 2 (2%) | 2 (2%) | 0 | 2 (3%) | 0·323 | |
| Muscle pain | 1 (1%) | 2 (2%) | 1 (1%) | 2 (3%) | 0·738 | |
| Nausea | 1 (1%) | 1 (1%) | 0 | 3 (4%) | 0·063 | |
| Headache | 0 | 0 | 5 (4%) | 0 | 0·0064 | |
| Mucocutaneous eruption | 0 | 0 | 5 (4%) | 0 | 0·0064 | |
| Cough | 1 (1%) | 1 (1%) | 1 (1%) | 1 (1%) | 1·000 | |
| Anorexia | 0 | 1 (1%) | 2 (2%) | 0 | 0·707 | |
| Hypoesthesia | 0 | 0 | 0 | 2 (3%) | 0·030 | |
| Dizziness | 1 (1%) | 0 | 0 | 1 (1%) | 0·168 | |
| Abdominal distention | 0 | 2 (2%) | 0 | 0 | 0·341 | |
| Oral hypoesthesia | 0 | 0 | 0 | 1 (1%) | 0·173 | |
| Peripheral oedema | 0 | 0 | 0 | 1 (1%) | 0·173 | |
| Abdominal pain | 0 | 0 | 0 | 1 (1%) | 0·173 | |
| Vomiting | 1 (1%) | 0 | 0 | 0 | 0·411 | |
| Drowsiness | 0 | 0 | 1 (1%) | 0 | 0·703 | |
| Joint pains | 0 | 0 | 0 | 1 (1%) | 0·173 | |
| Rash | 0 | 0 | 1 (1%) | 0 | 0·703 | |
| Raised blood pressure | 0 | 1 (1%) | 0 | 0 | 1·000 | |
| Hypersensitivity | 0 | 1 (1%) | 0 | 0 | 1·000 | |
| Palpitation | 0 | 0 | 0 | 1 (1%) | 0·173 | |
Data are n (%), representing the total number of participants who had adverse reactions (ie, adverse events related to vaccination). Results are broken down by phase and dose in the appendix (pp 3–9).
For differences across all groups.
In phase 1, none of the participants had any detectable neutralising antibody response against live SARS-CoV-2 at baseline (appendix p 11). The GMTs and seroconversion rates of neutralising antibodies to live SARS-CoV-2 at day 28 showed no significant difference between the 3 μg group and 6 μg group after either the first vaccination (seroconversion 54·2% in the 3 μg group vs 62·5% in the 6 μg group) or second vaccination (100·0% vs 95·7%; table 3 ). Neutralising antibodies in all placebo recipients were negative after vaccination (appendix p 11). In an exploratory analysis by age, seroconversion rates at day 28 after the second dose of either 3 μg or 6 μg vaccine were higher than 88% in participants aged 60–64 years, 65–69 years, and 70 years or older, with the GMTs ranging from 33·3 to 89·1 (table 3, appendix p 13).
Table 3.
Neutralising antibody responses to live SARS-CoV-2 28 days after each dose in the phase 1 trial
|
Day 28 after first dose |
Day 28 after second dose |
|||||
|---|---|---|---|---|---|---|
| 3 μg group | 6 μg group | p value | 3 μg group | 6 μg group | p value | |
| Seroconversion rate | ||||||
| Total | 13/24 (54·2% [32·8–74·5]) | 15/24 (62·5% [40·6–81·2]) | 0·558 | 24/24 (100·0% [85·8–100·0]) | 22/23 (95·7% [78·1–99·9]) | 0·489 |
| 60–64 years | 8/12 (66·7% [34·9–90·1]) | 5/9 (55·6% [21·2–86·3]) | 0·673 | 12/12 (100·0% [73·5–100·0]) | 8/9 (88·9% [51·8–99·7]) | 0·429 |
| 65–69 years | 3/6 (50·0% [11·8–88·2]) | 5/8 (62·5% [24·5–91·5]) | 1·000 | 6/6 (100·0% [54·1–100·0]) | 7/7 (100·0% [59·0–100·0]) | 1·000 |
| ≥70 years | 2/6 (33·3% [4·3–77·7]) | 5/7 (71·4% [29·0–96·3]) | 0·286 | 6/6 (100·0% [54·1–100·0]) | 7/7 (100·0% [59·0–100·0]) | 1·000 |
| Geometric mean titre | ||||||
| Total | 6·9 (4·6–10·2) | 9·1 (6·4–13·0) | 0·278 | 54·9 (38·6–78·2) | 64·4 (41·5–99·7) | 0·560 |
| 60–64 years | 10·0 (5·7–17·8) | 9·7 (4·9–19·4) | 0·934 | 69·5 (38·9–124·2) | 43·9 (15·9–120·7) | 0·355 |
| 65–69 years | 5·5 (2·3–13·1) | 7·4 (3·2–17·4) | 0·562 | 56·5 (35·7–89·6) | 76·5 (32·5–178·4) | 0·485 |
| ≥70 years | 4·0 (1·6–10·0) | 10·5 (6·0–18·4) | 0·039 | 33·3 (13·4–82·8) | 89·1 (57·6–137·8) | 0·025 |
Seroconversion rates are n/N (% [95% CI]). Geometric mean titres are shown with 95% CIs.
In phase 2, one participant in the 6 μg group was seropositive for SARS-CoV-2 neutralising antibody at baseline (appendix p 12). After the second dose of vaccine, there was no significant difference in seroconversion rate or GMT between the 3 μg group (seroconversion 98·0%) and the 6 μg group (99·0%; table 4 ). However, the seroconversion rates and GMTs of the 3 μg and 6 μg groups were significantly higher than those of the 1·5 μg group (table 4). All placebo recipients were negative for SARS-CoV-2 neutralising antibodies after vaccination (appendix p 12). In an exploratory analysis by age, seroconversion rates at day 28 after the second dose were higher than 94% in the 3 μg and 6 μg groups for participants aged 60–64 years, 65–69 years, and 70 years or older, with GMTs ranging from 36·4 to 55·2 (table 4; appendix p 13).
Table 4.
Neutralising antibody responses to live SARS-CoV-2 28 days after the second dose in the phase 2 trial
| 1·5 μg group | 3 μg group | 6 μg group |
p value |
|||
|---|---|---|---|---|---|---|
| 1·5 μg vs 3 μg | 1·5 μg vs 6 μg | 3 μg vs 6 μg | ||||
| Seroconversion rate | ||||||
| Total | 88/97 (90·7% [83·1–95·7]) | 96/98 (98·0% [92·8–99·8]) | 97/98 (99·0% [94·5–100·0]) | 0·029 | 0·010 | 1·000 |
| 60–64 years | 34/36 (94·4% [81·3–99·3]) | 35/37 (94·6% [81·8–99·3]) | 38/38 (100·0% [90·8–100·0]) | 1·000 | 0·233 | 0·240 |
| 65–69 years | 29/35 (82·9% [66·4–93·4]) | 33/33 (100·0% [89·4–100·0]) | 40/40 (100·0% [91·2–100·0]) | 0·025 | 0·0081 | 1·000 |
| ≥70 years | 25/26 (96·2% [80·4–99·9]) | 28/28 (100·0% [87·7–100·0]) | 19/20 (95·0% [75·1–99·9]) | 0·482 | 1·000 | 0·417 |
| Geometric mean titre | ||||||
| Total | 23·4 (19·4–28·3) | 42·2 (35·2–50·6) | 49·9 (42·2–58·9) | <0·0001 | <0·0001 | 0·181 |
| 60–64 years | 26·5 (20·2–34·7) | 36·4 (26·2–50·6) | 55·2 (43·4–70·1) | 0·135 | 0·0001 | 0·041 |
| 65–69 years | 21·1 (14·1–31·6) | 44·5 (33·0–60·0) | 50·4 (37·9–67·0) | 0·0039 | 0·0005 | 0·545 |
| ≥70 years | 22·7 (16·8–30·7) | 48·2 (34·3–67·6) | 40·2 (26·7–60·7) | 0·0014 | 0·021 | 0·485 |
Seroconversion rates are n/N (% [95% CI]). Geometric mean titres are shown with 95% CIs.
Discussion
We found that the two doses of CoronaVac were safe and well tolerated at doses of 1·5 μg, 3 μg, and 6 μg among adults aged 60 years and older. The incidence of adverse reactions in different dose groups was similar, indicating that there was no dose-related aggravation concern with regard to safety. Moreover, most adverse reactions were mild and transient, and injection site pain was the most reported symptom. The results were similar to our study of adults aged 18–59 years.20 Our findings were also similar to the results of other inactivated COVID-19 vaccines in younger and older adults.13, 18, 19
None of the serious adverse events reported during the trial was related to vaccination. In our study, one case of pancreatitis was reported in the 3 μg group and was deemed to be unrelated to the receipt of the vaccine. Vaccine-related pancreatitis has been reported after the administration of other vaccines, such as vaccines against hepatitis A and hepatitis B (combined), hepatitis A, human papillomavirus (HPV), and measles, mumps, and rubella.24, 25 Among them, the combined hepatitis A and hepatitis B vaccine and the HPV vaccine contain an aluminium adjuvant. Bizjak and colleagues24 pointed out that in conjunction with an aluminium adjuvant, the induction of immunity through molecular mimicry might culminate in the production of cytotoxic autoantibodies with a particular affinity for pancreatic acinar cells. Vaccine-induced pancreatitis is likely to be an underdiagnosed condition and can often be masked by the incidental presence of more commonly recognised causes, or it might simply be misdiagnosed as idiopathic pancreatitis.24, 25 We will carefully monitor the occurrence of pancreatitis in ongoing and future studies and through pharmacovigilance.
CoronaVac was immunogenic in adults aged 60 years and older. The neutralising antibody responses observed in the older adults who had received two vaccine doses of 3 μg or 6 μg were similar, and exceeded the response to the 1·5 μg dose. Phase 1 data showed that seroconversion rates and GMTs of neutralising antibodies were low before the second vaccination, which provides evidence for a two-dose immunisation schedule. In this study, the seroconversion rates in those who received 3 μg or 6 μg doses were over 95% after the two-dose vaccination, with GMTs ranging from 42·2 to 64·4—similar to the responses among adults aged 18–59 years who received 3 μg (seroconversion 97%; GMT 44·1) or 6 μg doses (100%; 65·4) of vaccine with the same immunisation schedule.20 Thus, the preliminary results indicate that the responses to CoronaVac are not reduced in older adults.
In an exploratory analysis stratified by age, we did not observe significant differences in neutralising antibody responses after the second vaccination between age groups (60–64 years, 65–69 years, and ≥70 years) after the same doses of vaccine were given (appendix p 13). GMTs in phase 1 increased with age in recipients of the 3 μg dose, whereas they decreased with age in recipients of the 6 μg dose, although these trends were not statistically significant. By contrast, in phase 2, opposite trends in GMTs to those of phase 1 were observed (appendix p 13). In each age group, GMTs did not differ significantly between recipients of the 3 μg and 6 μg doses after the second injection, except in the group aged 70 years and older in phase 1 (p=0·025) and the group aged 60–64 years in phase 2 (p=0·041). Small sample sizes and large differences in immune responses to vaccination across older individuals might account for these differences. We will assess the relationship between the immune response to CoronaVac and age in ongoing phase 3 trials (NCT004456959 and NCT04617483).
Although the correlates of protection have not yet been established for any COVID-19 vaccines in development, neutralising antibodies are evaluated in all clinical trials because of evidence of their association with protection against COVID-19 in animal challenge experiments.21, 26 Live virus neutralisation assays are laborious, time-consuming, and require biosafety level 3 conditions. Development of other assays to evaluate the antibody titres is necessary. In this study, we used only a neutralising antibody assay, which was a limitation of the study design. However, we assessed neutralising antibodies to live SARS-CoV-2 and anti-receptor-binding domain (anti-RBD) IgG antibodies in the study of adults aged 18–59 years, and a strong correlation between neutralising antibodies and anti-RBD IgG antibodies was found.20
This study has some further limitations. First, although much evidence supports the important role of a T cell response to COVID-19,27 and such responses have been observed with use of mRNA vaccines and adenoviral-vectored vaccines,9, 10, 11, 12, 28, 29 those tests were not done in this study. However, we detected IFN-γ as an indicator of T-cell responses following vaccination in our phase 1 trial among adults aged 18–59 years, and the results showed that the responses induced by CoronaVac were low.20 We will also assess the responses of type 1 and type 2 T-helper cells by CoronaVac in the ongoing phase 3 trial (NCT004456959). Second, at the time of the report, long-term immunogenicity and safety could not be evaluated, although the participants will be followed up for at least 1 year. Further results will be reported when data are available. Third, the study population were healthy older adults, and most of them were of Han ethnicity. Further studies are required to assess the efficacy of CoronaVac in various populations, including older people with chronic underlying diseases, and with ethnic and geographical diversity. Fourth, only a two-dose immunisation schedule with an interval of 28 days was used in this clinical trial. Antibody responses against viral infections such as SARS-CoV-2 infection, hepatitis A, and hepatitis B, could be induced within a relatively short time (1–2 weeks), which might be suitable for emergency use.20, 30 In our ongoing phase 3 clinical trial, a rapid immunisation schedule (with injections on days 0 and 14) is also being used in older adults to assess the efficacy of CoronaVac. Finally, the calculated p values presented in this study cannot support any powerful statistical conclusions, and are only for reference and should be interpreted with caution.
In conclusion, CoronaVac was well tolerated and induced humoral responses in adults aged 60 years and older, which supports the use of this vaccine in an older population. Among the three doses evaluated, the neutralising antibody titres induced by the 3 μg dose were similar to those of the 6 μg dose, and higher than those of the 1·5 μg dose. Combined with the safety and production capacity, the 3 μg dose of CoronaVac with a two-dose immunisation schedule is being used in the ongoing phase 3 trials to assess protection against COVID-19.
Data sharing
The individual participant-level data that underlie the results reported in this Article will be shared after de-identification (text, tables, figures, and appendices). This clinical trial is ongoing, and all the individual participant data cannot be available until the immune persistence evaluation is conducted. The data will be available immediately after publication and finalisation of the completed clinical study report for at least 1 year. Supporting clinical documents including the study protocol and statistical analysis plan and the informed consent form will be available immediately following the publication of the current Article for at least 1 year. Information on how to access the supporting clinical documents is available online. Researchers who provide a scientifically sound proposal will be allowed access to the de-identified individual participant data. Proposals should be sent to the corresponding authors. These proposals will be reviewed and approved by the sponsor, investigators, and collaborators on the basis of scientific merit. To gain access, data requestors will need to sign a data access agreement.
Acknowledgments
Acknowledgments
This study was funded by the National Key Research and Development Program (2020YFC0849600) and the Beijing Science and Technology Program (Z201100005420023).
Contributors
YZ is the principal investigator. YZ, YH, ML, and WYi designed the trial and study protocol. ZW, WYa, and GC contributed to the literature search. All authors had access to data and YH and YZ verified the data. ZW and WYa wrote the first draft of the manuscript. WYi, YZ, YH, ZJ, and GZ contributed to the data interpretation and revision of the manuscript. LW monitored the trial. HJ, PC, and YD were responsible for the site work including recruitment, follow-up, and data collection, and ZW was the site coordinator. ZC and MX were responsible for the laboratory analysis.
Declaration of interests
GC is an employee of Sinovac Life Sciences. YH, WYa, GC, LW, and WYi are employees of Sinovac Biotech. All other authors declare no competing interests.
Supplementary Material
References
- 1.WHO WHO coronavirus disease (COVID-19) dashboard. 2020. https://covid19.who.int/
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Mao B, Liang S, et al. Association between age and clinical characteristics and outcomes of COVID-19. Eur Respir J. 2020;55 doi: 10.1183/13993003.01112-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID-19 and older adults: what we know. J Am Geriatr Soc. 2020;68:926–929. doi: 10.1111/jgs.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikolich-Zugich J, Knox KS, Rios CT, Natt B, Fain MJ. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. GeroScience. 2020;42:505–514. doi: 10.1007/s11357-020-00186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trzewikoswki de Lima G, De Gaspari E. Study of the immune response in the elderly: is it necessary to develop a vaccine against Neisseria meningitidis for the aged? J Aging Res. 2019;2019 doi: 10.1155/2019/9287121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maijó M, Clements SJ, Ivory K, Nicoletti C, Carding SR. Nutrition, diet and immunosenescence. Mech Ageing Dev. 2014;136–137:116–128. doi: 10.1016/j.mad.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 8.WHO Draft landscape of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 9.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh EE, Frenck RW, Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu F-C, Guan X-H, Li Y-H, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime–boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2020 doi: 10.1056/nejmc2032195. published online Dec 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfizer Our progress in developing a potential COVID-19 vaccine. 2020. https://www.pfizer.com/science/coronavirus/vaccine
- 16.AstraZeneca AZD1222 vaccine met primary efficacy endpoint in preventing COVID-19. 2020. https://www.astrazeneca.com/media-centre/press-releases/2020/azd1222hlr.html
- 17.Gamaleya National Center of Epidemiology and Microbiology Sputnik V: general information. 2020. https://sputnikvaccine.com/about-vaccine/
- 18.Che Y, Liu X, Pu Y, et al. Randomized, double-blinded and placebo-controlled phase II trial of an inactivated SARS-CoV-2 vaccine in healthy adults. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1703. published online Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30843-4. published online Nov 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Q, Bao L, Mao H, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.China National Medical Products Administration Guidelines for grading standards of adverse events in clinical trials of preventive vaccines. 2019. https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20191231111901460.html
- 23.Kavanagh K, Robertson C, McMenamin J. Estimates of influenza vaccine effectiveness in primary care in Scotland vary with clinical or laboratory endpoint and method—experience across the 2010/11 season. Vaccine. 2013;31:4556–4563. doi: 10.1016/j.vaccine.2013.07.056. [DOI] [PubMed] [Google Scholar]
- 24.Bizjak M, Bruck O, Praprotnik S, Dahan S, Shoenfeld Y. Pancreatitis after human papillomavirus vaccination: a matter of molecular mimicry. Immunol Res. 2017;65:164–167. doi: 10.1007/s12026-016-8823-9. [DOI] [PubMed] [Google Scholar]
- 25.Shlomovitz E, Davies W, Cairns E, Brintnell WC, Goldszmidt M, Dresser GK. Severe necrotizing pancreatitis following combined hepatitis A and B vaccination. CMAJ. 2007;176:339–342. doi: 10.1503/cmaj.060360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J, Tostanoski LH, Peter L, Mercado NB, Barouch DH. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sauer K, Harris T. An effective COVID-19 vaccine needs to engage T cells. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.581807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nothdurft HD, Dietrich M, Zuckerman JN, et al. A new accelerated vaccination schedule for rapid protection against hepatitis A and B. Vaccine. 2002;20:1157–1162. doi: 10.1016/s0264-410x(01)00432-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The individual participant-level data that underlie the results reported in this Article will be shared after de-identification (text, tables, figures, and appendices). This clinical trial is ongoing, and all the individual participant data cannot be available until the immune persistence evaluation is conducted. The data will be available immediately after publication and finalisation of the completed clinical study report for at least 1 year. Supporting clinical documents including the study protocol and statistical analysis plan and the informed consent form will be available immediately following the publication of the current Article for at least 1 year. Information on how to access the supporting clinical documents is available online. Researchers who provide a scientifically sound proposal will be allowed access to the de-identified individual participant data. Proposals should be sent to the corresponding authors. These proposals will be reviewed and approved by the sponsor, investigators, and collaborators on the basis of scientific merit. To gain access, data requestors will need to sign a data access agreement.