Abstract
The COVID-19 pandemic is unlikely to end until there is global roll-out of vaccines that protect against severe disease and preferably drive herd immunity. Regulators in numerous countries have authorised or approved COVID-19 vaccines for human use, with more expected to be licensed in 2021. Yet having licensed vaccines is not enough to achieve global control of COVID-19: they also need to be produced at scale, priced affordably, allocated globally so that they are available where needed, and widely deployed in local communities. In this Health Policy paper, we review potential challenges to success in each of these dimensions and discuss policy implications. To guide our review, we developed a dashboard to highlight key characteristics of 26 leading vaccine candidates, including efficacy levels, dosing regimens, storage requirements, prices, production capacities in 2021, and stocks reserved for low-income and middle-income countries. We use a traffic-light system to signal the potential contributions of each candidate to achieving global vaccine immunity, highlighting important trade-offs that policy makers need to consider when developing and implementing vaccination programmes. Although specific datapoints are subject to change as the pandemic response progresses, the dashboard will continue to provide a useful lens through which to analyse the key issues affecting the use of COVID-19 vaccines. We also present original data from a 32-country survey (n=26 758) on potential acceptance of COVID-19 vaccines, conducted from October to December, 2020. Vaccine acceptance was highest in Vietnam (98%), India (91%), China (91%), Denmark (87%), and South Korea (87%), and lowest in Serbia (38%), Croatia (41%), France (44%), Lebanon (44%), and Paraguay (51%).
Introduction
The COVID-19 pandemic has caused substantial excess mortality and plunged national economies into deep recessions.1 Although the spread of the virus can be mitigated through physical distancing, face coverings, and testing and tracing—and potentially with therapeutics—the risk of outbreaks and disruption to economic and social life will probably remain until effective vaccines are administered to large portions of the global population to prevent hospitalisation and severe disease, and preferably achieve herd immunity to halt transmission of the virus.
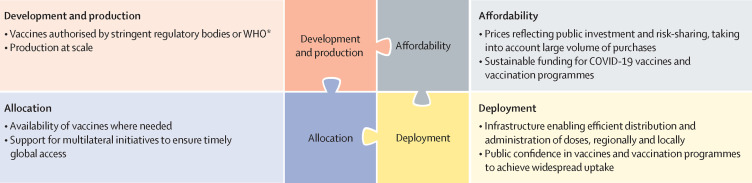
Several COVID-19 vaccines have now been authorised or approved for human use, with many more in the late stages of clinical development. Yet having licensed vaccines is not enough to achieve global control of COVID-19: they also need to be produced at scale, priced affordably, allocated globally so that they are available where needed, and widely deployed in local communities (figure 1 ). These four dimensions of the global vaccination challenge are closely related, and the development and production steps have important implications for pricing, allocation, and public confidence.
Figure 1.
Four dimensions of an effective global immunisation strategy against COVID-19
*Stringent regulatory bodies can approve vaccines or authorise their use in emergencies (eg, emergency use authorisation during public health crises, such as pandemics); WHO can grant emergency use listing (comparable to emergency use authorisation by a stringent body) or prequalification (comparable to approval by a stringent body). WHO publishes a list of stringent regulatory authorities.2
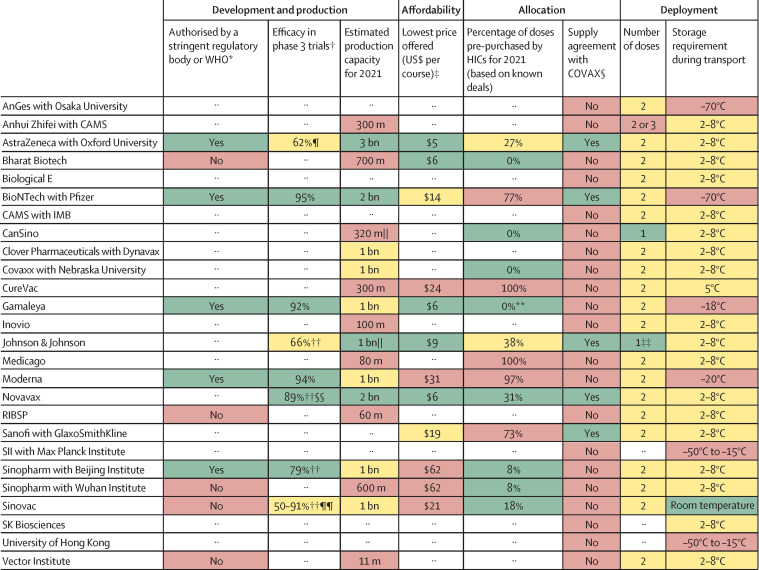
In this Health Policy paper, we review potential challenges to success in each of these dimensions and discuss policy implications. To guide our review, we developed a dashboard (figure 2 ) to highlight the key characteristics of 26 leading vaccine candidates, based on the target product profiles for COVID-19 vaccines set by WHO.4 We focused on characteristics that distinguish individual vaccine candidates from one another. We used a traffic-light system to signal the potential contributions of each candidate to achieving global vaccine immunity, with the colour red indicating high risks to achieving widespread immunity, amber indicating medium risk, and green indicating little or no risk. Appendix 1 outlines the methodology for constructing the dashboard, including the criteria for assigning a green, amber, or red light for each characteristic. Although specific datapoints and their corresponding traffic-light categorisations are subject to change as the pandemic response progresses, the dashboard will continue to provide a useful lens through which to analyse the key issues affecting the use of COVID-19 vaccines.
Figure 2.
Key characteristics of leading vaccine candidates with traffic-light system signalling potential for achieving global vaccine immunity
The sources and methodology are documented in appendix 1, including the criteria for assigning a green, amber, or red light for each characteristic. Candidates shown in this figure have been approved or authorised on an emergency basis for human use in one or more countries, are in phase 3 clinical testing, or are under contract with CEPI or the COVAX Facility, as of Feb 3, 2021. Where there are no entries, either the data are unavailable or it is too early to know (eg, for vaccines in the early stages of development). Both Institut Pasteur (in collaboration with Merck) and the University of Queensland were developing COVID-19 vaccine candidates with funding from CEPI, but these clinical trials have been discontinued. CAMS=Chinese Academy of Medical Sciences. CEPI=Coalition for Epidemic Preparedness Innovations. HIC=high-income country. IMB=Institute of Medical Biology (China). RIBSP=Research Institute for Biological Safety Problems (Kazakhstan). SII=Serum Institute of India. *Only for vaccines that have been approved or granted emergency authorisation by at least one regulatory body; WHO publishes a list of stringent regulatory authorities,2 and can itself grant emergency use listing or prequalification for vaccines. †Clinical trial designs, including efficacy endpoints, differed for the various vaccine candidates; the efficacy figures might therefore not be perfectly comparable. Some of these results are interim analyses from phase 3 studies. Due to the emergence of new variants of the virus, the conditions under which trials take place vary, and not all vaccines are tested against the same variants. ‡These prices are the lowest the developers offered to any country or purchasing bloc; median prices for a range of countries are presented in figure 3. §The COVAX Facility has first right of refusal for a potential combined total of more than 1 billion doses in 2021 of vaccine candidates being developed by CEPI-funded companies: Biological E, Clover Pharmaceuticals, CureVac, Inovio, Moderna, Novavax, Oxford University/AstraZeneca, SK Biosciences, and the University of Hong Kong.3 ¶This was the result in the main efficacy analysis for participants receiving two standard doses, as specified in the protocol. The result in the out-of-protocol arm (a half dose followed by a standard dose) was 90%. This first-generation vaccine might offer less protection against a strain of SARS-CoV-2 first identified in South Africa. ||For the assignment of risk levels, we treated a single dose of a one-dose vaccine as equivalent to two doses of a two-dose vaccine. **One HIC (Hungary) has purchased 2 million doses, corresponding to 0·4% of all purchased doses; due to rounding, the figure presented in the dashboard is 0%. ††These interim phase 3 results have not been published in peer-reviewed journals; the figures were sourced from press releases by companies or researchers running the clinical trials. ‡‡The developer is also testing a two-dose version. §§This was the efficacy reported from a phase 3 trial in the UK; Novavax reported a lower efficacy level in a smaller phase 2b clinical trial in South Africa (49%). These results have not yet been published in peer-reviewed journals. ¶¶Sinovac and its research partners have reported a range of efficacy levels on the basis of phase 3 trials in Brazil (50%), Indonesia (65%), Turkey (91%), and the United Arab Emirates (86%), but none of these results have been published in peer-reviewed journals.
Development and production
Several manufacturers have successfully developed COVID-19 vaccines in less than 12 months—an extraordinary achievement, given it typically takes a decade or longer to develop new vaccines.5, 6, 7, 8 The world now needs more doses of COVID-19 vaccines than it has done for any other vaccine in history to inoculate enough people for global vaccine immunity.
Vaccines often suffer from underinvestment,9 but that has not been the case in this pandemic. As of Feb 3, 2021, there were 289 experimental COVID-19 vaccines in development, 66 of which were in different phases of clinical testing, including 20 in phase 3. Only five of these 66 vaccines—those developed by AstraZeneca in partnership with Oxford University, BioNTech in partnership with Pfizer, Gamaleya, Moderna, and Sinopharm in partnership with the Beijing Institute—have been authorised by stringent regulatory authorities (as per WHO criteria of such authorities2) or WHO (figure 2). Another five—from China, India, Kazakhstan, and Russia—have received approval or been authorised for emergency use by other regulatory agencies; some of the organisations developing these vaccines have submitted documentation to WHO for emergency use listing or prequalification, but these submissions are still under review.10 Additional vaccines from Novavax and Johnson & Johnson are expected to be authorised on the basis of positive interim phase 3 results. Several vaccines have shown high levels of efficacy (ie, more than 70%) in clinical trials, although not all developers have published their results; most of the authorised vaccines have been shown to provide strong protection against hospitalisations and deaths due to COVID-19.
Whereas public support for basic research and early-stage drug development is widespread,11 the urgent need to develop COVID-19 vaccines and scale up supply has inspired new ways of aiding research, development, and production activities and enlisting broad participation among private companies.12 Governments and non-profit organisations have financed clinical trials, invested in the building and expansion of production facilities, and established contract manufacturing and distribution networks to enable the rapid roll-out of successful vaccines.13
The table summarises publicly available data on investments by governments and non-profit organisations into the research, development, and production of advanced COVID-19 vaccine candidates (appendix 2). In total, developers have received approximately $10 billion in public and non-profit funding for their vaccine candidates, although this number is probably an underestimate, given the scarcity of data on some of these projects. The top five companies have each received between $957 million and $2·1 billion in funding commitments, mostly from the US Government and the Coalition for Epidemic Preparedness Innovations (CEPI). The Chinese and Russian Governments have invested in several vaccine candidates being developed by private companies or state-owned enterprises. Because many funding arrangements are confidential, details regarding the specific breakdown of spending are unclear.
Table.
Public and non-profit funding for the research, development, and production of leading vaccine candidates
| Technology | Known public and non-profit funding, US$ | Funders | |
|---|---|---|---|
| Sanofi with GlaxoSmithKline | Protein subunit | $2·1 billion | US Government |
| Novavax | Protein subunit | $2·1 billion | Bill & Melinda Gates Foundation, CEPI, US Government |
| AstraZeneca with Oxford University | Non-replicating viral vector | $1·7 billion | CEPI, UK Government, US Government |
| Johnson & Johnson | Non-replicating viral vector | $1·5 billion | US Government |
| Moderna | mRNA | $957 million | CEPI, Dolly Parton COVID-19 Research Fund, US Government |
| BioNTech with Pfizer | mRNA | $445 million | German Government |
| Clover Pharmaceuticals with Dynavax | Protein subunit | $430 million | Bill & Melinda Gates Foundation, CEPI |
| CureVac | mRNA | $348 million | CEPI, German Government |
| Sinopharm with Wuhan Institute | Inactivated virus | $142 million | Chinese Government |
| Medicago | Virus-like particle | $137 million | Canadian Government |
| Inovio | DNA | $107 million | Bill & Melinda Gates Foundation, CEPI, US Government |
| Covaxx with Nebraska University | Protein subunit | $15 million | Taiwanese Government |
| SK Biosciences | Protein subunit | $14 million | Bill & Melinda Gates Foundation, CEPI |
| Biological E | Protein subunit | $9 million | Bill & Melinda Gates Foundation, CEPI, Indian Government |
| University of Hong Kong | Replicating viral vector | $4 million | CEPI, Hong Kong Government |
| CAMS with IMB | Inactivated virus | $3 million | Chinese Government, Jack Ma Foundation |
| AnGes with Osaka University | DNA | Unknown | Japanese Government |
| Anhui Zhifei with CAMS | Protein subunit | Unknown | Chinese Government |
| Bharat Biotech | Inactivated virus | Unknown | Indian Government |
| CanSino | Non-replicating viral vector | Unknown | Unknown |
| Gamaleya | Non-replicating viral vector | Unknown | Russian Government |
| RIBSP | Inactivated virus | Unknown | Kazakh Government |
| SII with Max Planck Institute | Live attenuated virus | Unknown | Unknown |
| Sinopharm with Beijing Institute | Inactivated virus | Unknown | Chinese Government |
| Sinovac | Inactivated virus | Unknown | Unknown |
| Vector Institute | Protein subunit | Unknown | Russian Government |
Data are as of Feb 3, 2021. The sources and methodology are outlined in appendix 2, which also includes more information about the funding arrangements. In brief, for developers with COVID-19 vaccines that have been approved or authorised for human use in one or more countries, are in phase 3 clinical testing, or are under contract with CEPI or the COVAX Facility, we searched press releases from developers and funders, as well as financial reports filed by developers with regulators in various countries, for information on public and non-profit funding. We did not count funds provided to licensees that produce and distribute vaccines on behalf of lead developers or to contract development and manufacturing organisations, nor did we count loans (ie, debt financing) from international financial institutions (eg, European Investment Bank) or national governments. We included pre-purchase agreements between governments and companies where it appeared as though a substantial portion of the funding went towards late-stage development (ie, phase 1–3 trials) or scaling up production at risk before the completion of clinical testing. CAMS=Chinese Academy of Medical Sciences. CEPI=Coalition for Epidemic Preparedness Innovation. IMB=Institute of Medical Biology (China). RIBSP=Research Institute for Biological Safety Problems (Kazakhstan). SII=Serum Institute of India.
Attention has now turned to expanding production capacity to promote the widespread roll-out of successful vaccines, as well as efficiently distributing them to administration facilities. Companies with leading candidates have reported widely different supply capabilities up to the end of 2021 (figure 2). Nine developers have said they will be able to produce at most 700 million doses each this year, while ten other manufacturers have set production targets of 1 billion doses each or more. No single company will be able to supply all countries in this period, even if they meet these estimated production figures.
Scaling up production to meet global demand is a monumental challenge.14, 15 Before this pandemic, there were no existing networks of contract manufacturers for several of the leading vaccine candidates that feature novel technologies, including those relying on mRNA delivery platforms. Additionally, the volume of vaccines that is needed places pressure on global supply chains for inputs, such as glass vials, syringes, and stabilising agents.
The production of COVID-19 vaccines is limited by the highly concentrated state of global vaccine manufacturing capacity,16 and the relationships established between lead developers and contract manufacturers. A successful solution to the production bottleneck would probably require widespread technology transfer to enable the expansion of manufacturing capacity. Currently, few countries have the domestic capacity to rapidly produce COVID-19 vaccines on their own and instead will need companies to actively share knowledge, technology, and data with domestic manufacturers.17 Some of the lead developers of COVID-19 vaccines have collaboration agreements with manufacturers in middle-income countries—AstraZeneca has such agreements with the Serum Institute of India, Fiocruz in Brazil, mAbxience Buenos Aires in Argentina, and Siam Bioscience in Thailand; Johnson & Johnson has an agreement with Aspen Pharmacare in South Africa; and Novavax with the Serum Institute of India—although the terms of these partnerships, including the extent to which the licensed manufacturers can negotiate their own supply arrangements with countries, are unclear.
Affordability
Mechanisms are needed to ensure the affordability and sustainable financing of COVID-19 vaccines in low-income and middle-income countries, which are home to about 85% of the global population and which might lack the resources to buy adequate quantities of vaccines.18, 19 Even in high-income countries, it is important to ensure access to COVID-19 vaccines for poor and marginalised populations.
Pricing
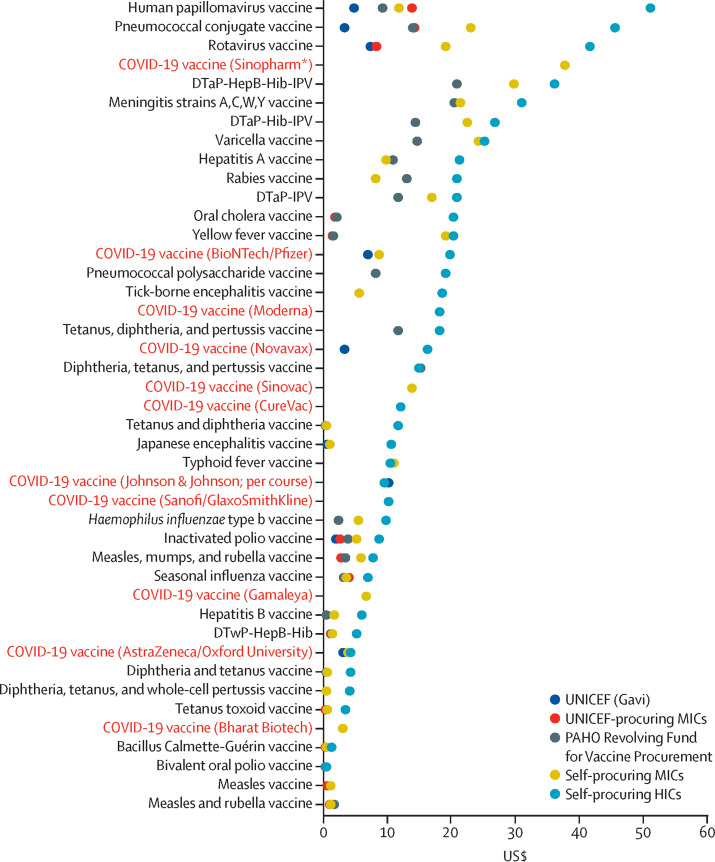
Companies have gradually been disclosing the prices they are offering to countries of different income levels, with marked variation in the lowest price per course (figure 2). Some companies such as AstraZeneca and Johnson & Johnson, which are benefiting heavily from public-sector investments, have pledged to sell their vaccines globally at low prices. Both companies have committed to maintaining these prices during the pandemic,20, 21 although more clarity is needed on how it will be determined that the pandemic is over, as well as on post-pandemic pricing models. These factors have implications for the durability of vaccination campaigns, especially if yearly injections become necessary. Other companies are charging considerably more, with some companies setting prices that are among the highest of any in existence for vaccines (figure 3 ). Some manufacturers are also planning to sell COVID-19 vaccines at a premium in private markets in countries such as Bangladesh, Brazil, and India.23, 24, 25 There are concerns that wealthier patients in these countries might gain quicker access to vaccines through these markets than poorer patients will.
Figure 3.
Median price per dose for existing vaccines and for leading COVID-19 vaccine candidates by procurement or country income group
Data obtained from the WHO Global Vaccine Market Report.22 Data for non-COVID-19 vaccines are as of 2018; data for COVID-19 vaccines are as of Feb 3, 2021. Prices were not available for all procurement or income groups for all vaccines. Appendix 1 outlines the sources for all COVID-19 vaccine prices, which were obtained from press releases, investor documents, and media reports. The prices reported for COVID-19 vaccines are median prices for each country group; these prices might therefore not match those reported in figure 2, which show the lowest price offered. DTap–HepB–Hib–IPV=diphtheria, tetanus, acellular pertussis–hepatitis B–Haemophilus influenza type b–inactivated polio vaccine. DTap–Hib–IPV=diphtheria, tetanus, acellular pertussis–H influenza type b–inactivated polio vaccine. DTap–IPV=diptheria, tetanus, acellular pertussis–inactivated polio vaccine. DTwP–HepB–Hib=diphtheria, tetanus, whole-cell pertussis–hepatitis B–H influenza type b vaccine. HIC=high-income country. MIC=middle-income country. PAHO=Pan American Health Organization. *Sinopharm is charging the same price for both of its vaccine candidates.
Multiple factors could be driving the observed variation in prices. These include, for example, differences in technological platforms and the associated development and manufacturing costs; the amount of public funding that developers received; companies' approaches towards licensing and the establishment of production networks; the extent to which COVID-19 vaccines fit into pharmaceutical companies' overall profit-making strategies; the presence of intellectual property rights; funders' demands (eg, CEPI's access conditions); and political pressure on companies to keep prices low.
To illustrate how the prices of COVID-19 vaccines compare with those of other vaccines, figure 3 shows the median price per dose of existing vaccines by procurement or income group, as of the end of 2018. Generally, countries covered by Gavi, the Vaccine Alliance (a major buyer of vaccines for low-income countries), paid the lowest prices per dose (median across all vaccines $0·57 [IQR 0·16–1·90]), followed by countries covered by UNICEF (median $0·80 [IQR 0·16–2·80]) and the Pan American Health Organization (median $3·50 [IQR 0·87–13·0]), self-procuring middle-income countries (median $5·30 [IQR 0·79–18·30]), and self-procuring high-income countries (median $16·3 [IQR 6·5–22·0]).22 Many self-procuring middle-income countries, which receive little external assistance, have historically been charged vaccine prices that are largely unrelated to income levels.26
Vaccine prices are especially important for COVID-19, on account of the volumes demanded. Countries are aiming to administer COVID-19 vaccines to nearly their entire populations, making these vaccines potentially unaffordable for many governments, even at low prices per dose. Depending on the duration of protection offered by these vaccines, as well as the potential need for modified vaccines that protect against new variants, these purchases could become recurring expenses.
Sustainable funding
To fund COVID-19 vaccines and vaccination programmes, including the costs of distribution, administration, record-keeping, and surveillance, governments will need substantial national revenue generation or external aid. Experiences with mass drug administration in previous health crises, such as during the HIV/AIDS epidemic, have shown that, even when pharmaceutical products are inexpensive or free, countries need financial support to both purchase and deploy them.27, 28
These financial pressures are coming at a time when many economies are in crisis due to the pandemic. If governments in resource-constrained settings divert resources from other vaccination programmes or essential health-care services to pay for COVID-19 vaccines and vaccination programmes, health budgets could be distorted with long-term adverse consequences for health and economic development.
Major donors and lenders, such as the World Bank and other multilateral development banks, have earmarked billions of dollars in funds for COVID-19 vaccination programmes in low-income and middle-income countries.29, 30 These funds can be used to buy vaccines that have been authorised by stringent regulatory bodies or WHO. The G20 group of high-income countries' Debt Service Suspension Initiative might provide additional fiscal space too, by allowing the world's poorest countries to spread repayment of debt owed to other countries over extended periods. Although this initiative does not address debt owed to private creditors, the hope is that the temporary suspension of some repayments could release resources for more countries to better meet the costs of obtaining and administering vaccines.31
Global allocation
In addition to the development and affordability of vaccines, an essential pillar of the vaccination challenge is ensuring that enough doses are available globally. Current decisions regarding allocation are being made in the context of constrained supply, with demand exceeding current and projected levels of output.16, 32 Scarcity in supply coupled with the large volumes of pre-orders made by richer countries creates challenges to achieving timely, universal access. Billions of individuals around the world might not have access to COVID-19 vaccines in 2021, which could prolong the pandemic and raise the risk of further mutations of the virus emerging, possibly undermining the efficacy of existing vaccines.
COVAX approach to global allocation
Uneven access to vaccines would not be unprecedented. During the 2009 H1N1 influenza pandemic, rich countries bought up most of the global supply of pandemic influenza vaccines, leaving inadequate amounts for resource-poor countries, many of which were among the world's worst affected.33, 34 Some countries went as far as to block locally manufactured vaccine doses from being exported elsewhere,35 something that EU member states are considering in the present pandemic too.
To avoid a repeat of the H1N1 scenario, in April, 2020, WHO announced the creation of a global allocation mechanism, the COVID-19 Vaccine Global Access (COVAX) Facility, coordinated jointly with CEPI and Gavi. COVAX is a pooled procurement initiative that, in addition to seeking to secure low prices, aims to provide all countries with access to a diversified portfolio of vaccines during the acute phase of the pandemic in 2021. High-income, self-financing countries can purchase vaccines from COVAX at an estimated average price of $11 per dose, whereas 92 low-income and middle-income countries can receive them at considerably lower prices ($1·6–2·0 per dose), subsidised through official development assistance.36
At the core of the COVAX approach to global allocation is that vaccination should proceed in stages, with priority given to protecting older adults, health-care workers, and other high-risk individuals, before proceeding to vaccinate wider sections of the population.37 According to the COVAX model, all participating countries would initially receive enough stock for 20% of their populations, after which distribution would adhere to the WHO framework for allocating COVID-19 vaccines internationally on the basis of need.37 The overarching logic of COVAX is that no country should vaccinate more than 20% of its population until all countries have vaccinated 20% of their populations, in accordance with principles of global equality. Others have suggested alternative allocation frameworks, although all share their roots in principles of fairness and ethical distribution.38, 39, 40, 41, 42
Threats to equitable allocation
For COVAX to succeed, it needs substantial funding to purchase vaccines. As of February, 2021, governments and other partners have committed around $4 billion in funding for COVAX,43 but Gavi and WHO estimate that a further $6·8 billion will be needed for COVAX to procure and deliver at least 2 billion doses by the end of 2021.3, 44
A greater threat to equitable allocation comes from national procurement strategies that might leave COVAX with inadequate supply.45, 46, 47, 48, 49, 50, 51 Many high-income countries have opted not to purchase their vaccines via COVAX and instead have sought to gain priority access to abundant quantities of COVID-19 vaccines by striking advance purchase agreements with developers. The goal of such agreements is to secure access to enough vaccines to inoculate most, if not all, of countries' adult populations in 2021. Securing large quantities of vaccines in this way amounts to countries placing widespread inoculation of their own populations ahead of the vaccination of health-care workers and high-risk populations in poorer countries. On the basis of public records, governments in high-income countries, representing 16% of the global population, have struck pre-orders covering at least 4·2 billion doses of COVID-19 vaccines. These countries have secured at least 70% of doses available in 2021 of five leading vaccine candidates, on the basis of known deals (figure 2).
Although the pattern of purchasing vaccines directly from developers and not via COVAX began with high-income countries (including the EU as a unified buyer), numerous other countries have followed suit. This dynamic is self-reinforcing: as more countries procure doses directly, concerns about the reliability of COVAX's supply heighten, thus creating greater incentives for countries to procure doses on their own. The incentives to procure vaccines this way increases further after positive trial results are announced, which reduces the risk of purchasing in advance for the successful vaccines. As of Feb 3, 2021, at least 62 countries or blocs of countries had signed purchase agreements with manufacturers.52
But not all countries can procure enough COVID-19 vaccines on their own. Instead, most countries are counting on COVAX, which has reached agreements with five companies for about 2 billion doses (figure 2).3 This amount could allow COVAX to achieve the goal of vaccinating 20% of the populations of participating countries. However, because it is unclear which vaccines will be distributed to which countries at what time, it is challenging for governments reliant on COVAX to plan vaccination programmes. Similarly, uncertainty about COVAX supply complicates governments' decisions about how to acquire the best vaccine portfolios for their populations, including doses beyond those covered by COVAX.
Apart from the cross-country equity concerns raised by a scenario of low-income countries vaccinating 20% of their population after much wider (if not universal) vaccination in high-income countries, there is uncertainty about the supply earmarked for COVAX. Many of the doses secured by COVAX are of vaccines that, as of February, 2021, are just completing clinical trials and might not be available for months to come.3 COVAX might also gain access to vaccines being developed by CEPI-funded companies that are not as far along in trials, and it might negotiate further agreements with other suppliers. Yet overall, COVAX's supply is precarious and depends on what happens to the vaccines in clinical trials, how much of the successful candidates can be produced quickly, and how much of the output is left for COVAX after sales to national governments.
Although COVAX was created to achieve equality in the initial stages of vaccination, as all countries inoculate the first 20% of their populations, it is unlikely to achieve that goal. Instead, what COVAX can hopefully achieve is to help countries procure doses at lower prices and thus launch their vaccination campaigns earlier than they would without external assistance. With additional funding, COVAX could probably compete better in the global scramble for vaccines and secure a place further towards the front of the queue.
Given the scarce supply of some of the vaccines developed in Europe and the USA, governments in Latin America, Africa, the Middle East, and Asia have turned increasingly towards vaccines developed by Chinese, Indian, and Russian manufacturers.53, 54 These vaccines, which are far along in the development process, might relax the global supply constraint. To the extent that high-income countries continue to refrain from purchasing these products, their emergence might allow low-income and middle-income countries to also procure abundant doses to achieve national vaccination goals. Although few of these vaccines have been authorised by WHO or WHO-classified stringent regulatory authorities, as they do so, these vaccines could also contribute to the COVAX portfolio.
Deployment
Beyond issues related to determining which countries will get vaccine doses when and at what prices, it is essential to ensure the smooth deployment of COVID-19 vaccines. The rapid pace of production and development has shortened the time available for national, regional, and local health officials to plan training and preparedness for COVID-19 vaccination programmes.
Logistical and administrative challenges
Robust data infrastructure will be needed for local authorities to identify eligible individuals by priority group, send invitations, arrange transport for older patients and patients with disabilities, and recall individuals to receive the second doses of some vaccines. Several of the leading vaccine candidates require ultra-cold chains and have short shelf-lives once they are removed from storage. The mRNA vaccine by BioNTech and Pfizer, for instance, must be administered within 5 days of leaving ultra-low temperature conditions (–70°C);55 similar, if less extreme, requirements apply to Moderna's mRNA vaccine. Strong coordination will be needed between workers at central depots and local vaccinators to ensure the timely and efficient distribution of mRNA vaccine batches to areas without freezers.
Many low-income and middle-income countries will face barriers in delivering vaccination programmes to their entire adult populations, ensuring completion of two-dose vaccination schedules, and maintaining cold or ultra-cold supply chains. As of 2018, 74 of 194 WHO member states had no adult vaccination programme for any disease; fewer than 11% of countries in Africa and South Asia reported having any such programme.56 These countries might lack immunisation registries for adults and the storage, delivery, and waste management systems needed to administer vaccines at this scale.56 It is worth noting that Gavi and its partners established ultra-cold supply chains in several sub-Saharan African countries after the 2013–14 Ebola epidemic to deploy an Ebola vaccine developed by Merck that had to be kept at −60 to −80°C.57, 58 However, this infrastructure was set up on a much smaller scale than what is currently needed and would be prohibitively expensive for the global administration of vaccines during this pandemic.
Several vaccines that only require refrigeration during transport have been authorised for human use, while a few single-dose products are in clinical development (figure 2); one in particular—that developed by Johnson & Johnson—has shown promising interim phase 3 results. The availability of one-dose vaccines that can be kept refrigerated or at room temperature would greatly simplify the logistical and administrative challenges associated with COVID-19 vaccination programmes. Moreover, as scientific understanding of the properties of new vaccines improves, such as the thermal stability of mRNA vaccines, or new ways of formulating these vaccines are developed, logistical barriers might be lowered. Such a development would make it easier to deploy these vaccines in resource-poor countries. Indeed, CureVac has an experimental mRNA vaccine in late-stage clinical development that can be kept refrigerated. The product profiles of COVID-19 vaccines can help governments decide which vaccines to procure; these profiles, alongside any constraints reported by governments, can also help inform COVAX's allocation decisions and might become increasingly important as additional, differentiated vaccines are authorised.
Beyond technical issues related to data and storage infrastructure, vaccination schedules, and other logistical matters, there are steps that governments can take to promote accountability, which might make COVID-19 vaccination campaigns more effective. These steps include transparency and clear communication on the part of government officials about timelines, prioritisation of different groups, choice of vaccine products, and design of administration schedules. Country-level monitoring and evaluation systems might be required to track vaccine roll-out, which can help support the efficient running of campaigns, as well as continued population adherence to non-pharmaceutical interventions, such as physical distancing and face coverings, as vaccination programmes are established and scaled up.
Vaccine hesitancy
Deployment can also be hampered by vaccine hesitancy,59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69 potentially leading to refusal or delayed acceptance of COVID-19 vaccines. Hesitancy is prevalent in low-income and high-income countries alike, with sceptics found in all socioeconomic, religious, and ethnic groups.
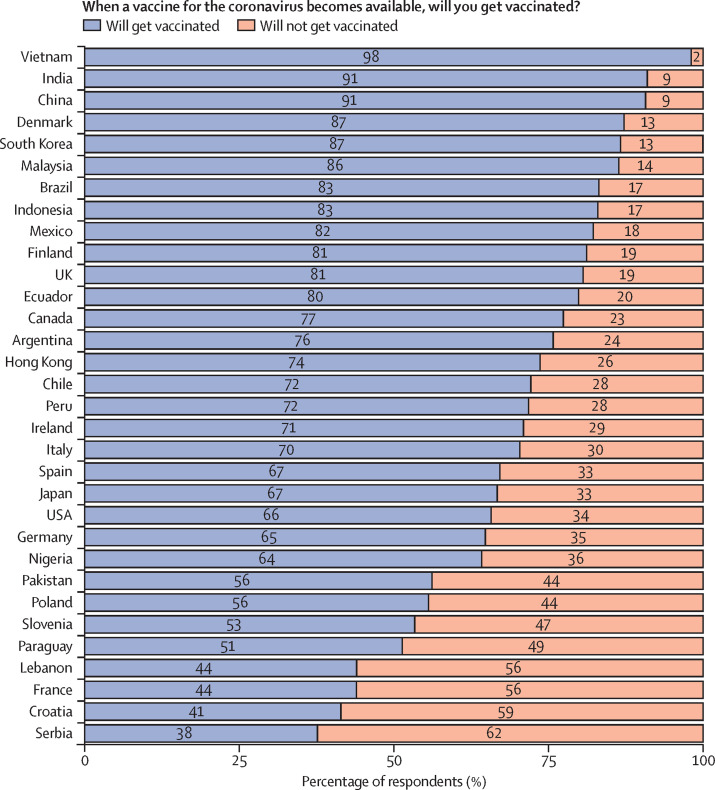
Figure 4 presents original data from a 32-country survey (n=26 758) of potential acceptance of COVID-19 vaccines conducted between Oct 21 and Dec 16, 2020 (appendix 3). The share of respondents who said they would definitely or probably get vaccinated when a COVID-19 vaccine becomes available was highest in Vietnam (98%), followed by India and China (both at 91%), and Denmark and South Korea (both at 87%). The country that reported the lowest number of people who would definitely or probably get vaccinated was Serbia (38%), followed by Croatia (41%), France and Lebanon (both at 44%), and Paraguay (51%).
Figure 4.
Survey of potential acceptance of COVID-19 vaccines
Data were jointly collected by the polling company ORB International and the Vaccine Confidence Project (London School of Hygiene & Tropical Medicine) between Oct 21 and Dec 16, 2020. Samples were random and nationally representative of the adult population in 30 of the 32 countries. Each respondent was asked, in the local language: “When a vaccine for the coronavirus becomes available, will you get vaccinated?” The possible responses were “definitely will”, “unsure but probably will”, “unsure but probably will not”, or “definitely will not”. In this figure, the category “will not get vaccinated” included respondents who said they “definitely will not” or “probably will not” get vaccinated, and the category “will get vaccinated” included respondents who said they “definitely will” or “probably will” get vaccinated. Appendix 3 describes the survey methodology.
Numerous other surveys of COVID-19 vaccine acceptance were done between March and October, 2020.70, 71, 72, 73, 74, 75 Although it is not possible to directly compare the results of all existing surveys because of differences in the countries included, and in questionnaires and methodologies used, these surveys overall seem to suggest that willingness to vaccinate against COVID-19 has declined globally between the early months of the pandemic and December, 2020, although rates tend to fluctuate.
At least three issues are contributing to COVID-19 vaccine hesitancy. First, the speed at which vaccines have been developed, which reflects the unprecedented amount of funding from governments and non-profit groups, has raised concerns that the trials were rushed and regulatory standards relaxed,76 concerns that were similarly reported during the H1N1 influenza pandemic.77 Second, there are no previously approved mRNA vaccines, which has also sparked hesitancy given the novelty of the approach. Third, conspiracy theories about COVID-19 vaccines are being widely circulated on unregulated social media platforms,78, 79, 80 sometimes by highly organised anti-vaccination groups.81, 82, 83
The evidence for measures to mitigate vaccine hesitancy and refusal is mixed, in part due to the wide range of strategies that have been used across settings for different vaccines and target groups.84 Common elements across successful strategies include: (1) initiatives to increase vaccination knowledge and awareness; (2) community engagement, including involvement of religious and other influential leaders, to understand concerns, build trust, and manage rumours and misinformation; and (3) making vaccines available in convenient and accessible locations.65, 85, 86, 87 Having robust pharmacovigilance systems alongside compensation schemes for severe adverse events might help build confidence in vaccine safety in post-approval periods, especially in resource-poor countries with imperfect consumer protection systems.88, 89 Moreover, disadvantaged groups, many of which have suffered historical neglect and abuse,90 often report lower levels of trust in the medical community91, 92 and lower uptake of health-care interventions, including vaccines, than the general population.93, 94, 95, 96 Additional efforts are needed to build trust among these groups.
Vaccine confidence might also be strengthened as more manufacturers obtain authorisation from stringent regulatory authorities or WHO and by these bodies clearly communicating to the public the rationale behind their decisions. The approval of experimental COVID-19 vaccines by Chinese, Indian, and Russian regulators before the conduct of phase 3 trials has generated widespread consternation among regulators and scientists in other countries because of the scarcity of safety and efficacy data and concerns that it could weaken confidence in vaccines.54, 97, 98, 99, 100, 101 The European Medicines Agency has also been subject to lobbying from several EU governments, who have urged the regulator to grant authorisation for the vaccine by AstraZeneca and Oxford University as soon as possible to expedite vaccination programmes.102 Authorisations that are perceived to be premature might undermine trust in regulators, vaccines, and vaccination programmes.
Discussion
Many commentators have called for a cooperative approach to vaccine allocation and deployment.47, 48 In doing so, appeals to values of fairness and solidarity are common. By contrast, the widespread disregard for a global approach to vaccine allocation shown by national governments misses an opportunity to maximise the common good by reducing the global death toll,103 supporting widespread economic recovery,104 and mitigating supply chain disruptions.48 More equitable distribution of COVID-19 vaccines would help contain the pandemic sooner, and thus minimise the risk of new variants of the virus arising, against which existing vaccines might be less effective.
In this Health Policy paper, we have stressed the interactions among the four dimensions involved in the global COVID-19 vaccination challenge. It is not enough to have new vaccines developed; they must be affordable, accessible, trusted, and, to maximise impact, used efficiently.
Governments and other vaccine purchasers must now decide which vaccines to procure, as well as how to secure funding for COVID-19 vaccines and vaccination programmes. To reach these decisions, government officials and partners in international organisations will need to assess the suitability of various vaccines for their respective health systems and populations—for example, in terms of availability, affordability, efficacy, and dosing and storage requirements.
The dashboard highlights the trade-offs associated with leading COVID-19 vaccines in relation to these dimensions (figure 2). Multiple vaccines, for instance, are highly efficacious—exceeding WHO targets of a minimum of 50% and preferably 70% efficacy—but require ultra-cold storage during transport or have little reserved capacity for low-income and middle-income countries. Although all currently authorised or approved vaccines require two doses, single-dose vaccines that can be stored at refrigerated temperatures are in the late stages of clinical development, with one by Johnson & Johnson likely to be authorised; these vaccines would be easier to deploy in resource-constrained settings, which might lack infrastructure for delivering and administering two-dose vaccines reliably.
Differences in product characteristics might become particularly salient in 2021, while vaccines remain in short supply. If additional vaccines are successful in clinical testing and developers meet their production targets, then COVAX could allocate vaccines, in part, on the basis of their suitability for local conditions. For instance, should single-dose vaccines that can be stored in refrigerators become available, which seems increasingly likely given the promising interim results by Johnson & Johnson, then these could be prioritised for distribution in low-income and middle-income countries that lack ultra-cold supply chains or national vaccine registries for two-dose regimens.
The dynamics of production and development have important implications for each of the other dimensions. Governments and non-profit groups have committed unprecedented sums towards the development of COVID-19 vaccines and the infrastructure to produce them at scale, which has helped companies develop new vaccines in record time. But affordability remains a concern, given the volume of doses that countries will need to purchase and the additional expenditures that distributing and delivering vaccines entails. The extensive involvement of public funders in the development and production of COVID-19 vaccines provides them with opportunities to make these vaccines globally affordable. External funders that have invested in companies developing the vaccines and who share the financial risks could try to influence the pricing of these products, as CEPI has aimed to do with uncertain levels of success.106, 107 Funders could also negotiate clear timelines for the recovery of research, development, and production costs by companies; for example, initial doses might be sold at higher prices in the first year in high-income countries and then sold closer to their marginal cost in subsequent years.108 Determining these prices will require governments to audit the financial records of vaccine makers.
These allocation challenges also relate to production: conflicts over priority access to scarce vaccine doses could be made less acute with greater output (ie, with reduced scarcity of vaccine doses). To that end, WHO has called for member states, manufacturers, and other organisations to commit to sharing knowledge, intellectual property, and data related to COVID-19 health technologies, through the COVID-19 Technology Access Pool (C-TAP). Similarly, several countries have proposed to suspend World Trade Organization rules on intellectual property rights during the pandemic, suggesting that doing so could facilitate scale-up. Yet, as of February, 2021, no manufacturers of leading vaccine candidates have engaged with C-TAP, and the World Trade Organisation reform proposal has not gained traction.
In this domain too, the extensive public role in funding vaccine development potentially provides opportunities. Funders could encourage vaccine developers receiving public support to share their technologies and know-how systematically and widely to expand global production. Funders could also work with developers to alleviate supply chain constraints and accelerate the scaling up of production. To the extent that international control of COVID-19 is regarded as a priority for individual countries, governments might have an incentive to exercise these levers.
Public confidence and trust in COVID-19 vaccines and those who deliver them to ensure uptake are as important as the vaccines' safety, efficacy, and affordability. Policy makers should urgently engage with communities to improve confidence in vaccines and combat misinformation and rumours around COVID-19. Post-marketing surveillance is important to build confidence during vaccine roll-out. Developing successful, locally tailored strategies requires an understanding of contextual and historical influences of vaccine hesitancy and refusal.7
Equally, vaccine manufacturers should aim for maximum transparency and scrutiny of their clinical trial data to build public trust. Regulatory bodies safeguard public health by assessing whether the benefits of pharmaceuticals outweigh their risks. Regulatory decisions and their rationale should be clearly communicated to the public to provide reassurance that authorised products are safe and efficacious. It is in the interest of vaccine developers to seek approval or emergency use authorisation from a stringent regulatory body or WHO: only vaccines that have gone through one of these regulatory pathways will be eligible for purchase through COVAX or through funds made available by major development banks.
Conclusion
The societal value of safe and effective COVID-19 vaccines is enormous. Yet new vaccines will mean little to individuals around the world if they are unable to get vaccinated in a timely manner. This objective requires vaccines to be affordable and available to countries around the world, and governments to have the administrative and political capacities to deliver them locally. In this Health Policy paper, we have discussed the development and production, affordability, allocation, and deployment of COVID-19 vaccines, as well as the interactions between these dimensions of the global vaccination challenge. The distinct characteristics of leading COVID-19 vaccines across each of these dimensions generate trade-offs, which mean that both globally and nationally, the availability of diversified sets of vaccine options is likely to be needed to bring the global pandemic under control.
Acknowledgments
Acknowledgments
We thank Wunan Shi for assistance in collecting data from Chinese-language sources on the costs of development and production for experimental COVID-19 vaccines being developed by Chinese companies. We thank Alex de Figueiredo for assistance with analysing and presenting the results of the COVID-19 vaccine acceptance survey. Neither individual received compensation for their role in the study. There was no funding source for this study. The data on COVID-19 vaccine acceptance in 32 countries presented in this paper were collected by the polling company Orb International, a member of the Worldwide Independent Network and Gallup International Association, following industry ethics guidelines. Orb International is the polling partner of the Vaccine Confidence Project at London School of Hygiene & Tropical Medicine for surveys on COVID-19 vaccine acceptance, and Orb International has data collection agreements in place in each country. The raw data are available from the authors upon request.
Declaration of interests
MS-K reports receiving grants from Health Action International, outside the submitted work. AJP is Chair of the UK Department of Health & Social Care's Joint Committee on Vaccination & Immunisation (JCVI) but does not participate in policy advice on coronavirus vaccines. He is also a member of the WHO Strategic Advisory Group of Experts (SAGE) and Chief Investigator of the clinical trials for vaccine candidate AZD1222 against COVID-19, sponsored by the University of Oxford. The University of Oxford has entered into a partnership with AstraZeneca on vaccine development for candidate AZD1222. The trials are funded by UK Research and Innovation (MC_PC_19055), Engineering and Physical Sciences Research Council (EP/R013756/1), the Coalition for Epidemic Preparedness Innovations (CEPI), the National Institute for Health Research (NIHR), the NIHR Oxford Biomedical Research Centre, and the German Center for Infection Research (DZIF). HJL reports receiving grants from Merck and GlaxoSmithKline, and honoraria from Merck (for serving on a vaccine confidence advisory board) and GlaxoSmithKline (for speaking at staff training sessions). MJ reports receiving grants from the Bill & Melinda Gates Foundation (INV-016832), European Commission's Horizon 2020 programme (101003688), and National Institute for Health Research (NIHR200929, NIHR200908), outside the submitted work. All other authors declare no competing interests.
Author contributions
OJW, KCS, and MJ conceived of and designed the manuscript. OJW and MS-K collected and analysed the data. OJW drafted the manuscript. All authors had full access to all the data in the study, contributed to revisions to the article, and had final responsibility for the decision to submit for publication.
Supplementary Materials
References
- 1.International Monetary Fund A crisis like no other, an uncertain recovery. June, 2020. https://www.imf.org/en/Publications/WEO/Issues/2020/06/24/WEOUpdateJune2020
- 2.WHO List of stringent regulatory authorities (SRAs) June 20, 2020. http://www.who.int/medicines/regulation/sras/en/
- 3.WHO COVAX announces additional deals to access promising COVID-19 vaccine candidates; plans global rollout starting Q1 2021. Dec 18, 2020. https://www.who.int/news/item/18-12-2020-covax-announces-additional-deals-to-access-promising-covid-19-vaccine-candidates-plans-global-rollout-starting-q1-2021
- 4.WHO Target product profiles for COVID-19 vaccines, version 3. April 29, 2020. https://www.who.int/publications/m/item/who-target-product-profiles-for-covid-19-vaccines
- 5.Hanney SR, Wooding S, Sussex J, Grant J. From COVID-19 research to vaccine application: why might it take 17 months not 17 years and what are the wider lessons? Health Res Policy Sys. 2020;18:61. doi: 10.1186/s12961-020-00571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis MM, Butchart AT, Wheeler JRC, Coleman MS, Singer DC, Freed GL. Failure-to-success ratios, transition probabilities and phase lengths for prophylactic vaccines versus other pharmaceuticals in the development pipeline. Vaccine. 2011;29:9414–9416. doi: 10.1016/j.vaccine.2011.09.128. [DOI] [PubMed] [Google Scholar]
- 7.Pronker ES, Weenen TC, Commandeur H, Claassen EHJHM, Osterhaus ADME. Risk in vaccine research and development quantified. PLoS One. 2013;8 doi: 10.1371/journal.pone.0057755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Struck MM. Vaccine R&D success rates and development times. Nat Biotechnol. 1996;14:591–593. doi: 10.1038/nbt0596-591. [DOI] [PubMed] [Google Scholar]
- 9.Xue QC, Ouellette LL. Innovation policy and the market for vaccines. J Law Biosci. 2020;7:a026. doi: 10.1093/jlb/lsaa026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process. Jan 11, 2021. https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVID_VAX.pdf
- 11.Galkina Cleary E, Beierlein JM, Khanuja NS, McNamee LM, Ledley FD. Contribution of NIH funding to new drug approvals 2010–16. Proc Natl Acad Sci USA. 2018;115:2329–2334. doi: 10.1073/pnas.1715368115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampat BN, Shadlen KC. The COVID-19 innovation system. Health Aff (Millwood) 2021 doi: 10.1377/hlthaff.2020.02097. published online Feb 4. [DOI] [PubMed] [Google Scholar]
- 13.Policy Cures Research COVID-19 R&D tracker. Oct 1, 2020. https://www.policycuresresearch.org/covid-19-r-d-tracker
- 14.Amanat F, Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52:583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, Wu Q, Yang J, et al. Global, regional, and national estimates of target population sizes for covid-19 vaccination: descriptive study. BMJ. 2020;371 doi: 10.1136/bmj.m4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coalition for Epidemic Preparedness Innovations Manufacturing survey results analysis. June 29, 2020. https://cepi.net/wp-content/uploads/2020/08/CEPI_Survey-of-global-drug-substance-and-drug-product-landscape-June-2020_RELEASED-1.pdf
- 17.Price WN, II, Rai AK, Minssen T. Knowledge transfer for large-scale vaccine manufacturing. Science. 2020;369:912–914. doi: 10.1126/science.abc9588. [DOI] [PubMed] [Google Scholar]
- 18.Crager SE. Improving global access to new vaccines: intellectual property, technology transfer, and regulatory pathways. Am J Public Health. 2018;108:S414–S420. doi: 10.2105/AJPH.2014.302236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahonkhai V, Martins SF, Portet A, Lumpkin M, Hartman D. Speeding access to vaccines and medicines in low- and middle-income countries: a case for change and a framework for optimized product market authorization. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US House Subcommittee on Oversight and Investigations of the Committee on Energy and Commerce Pathway to a vaccine: efforts to develop a safe, effective and accessible COVID-19 vaccine. July 21, 2020. https://energycommerce.house.gov/committee-activity/hearings/hearing-on-pathway-to-a-vaccine-efforts-to-develop-a-safe-effective-and
- 21.Kemp A, AstraZeneca AstraZeneca to supply Europe with up to 400 million doses of Oxford University's vaccine at no profit. June 13, 2020. https://www.astrazeneca.com/media-centre/press-releases/2020/astrazeneca-to-supply-europe-with-up-to-400-million-doses-of-oxford-universitys-vaccine-at-no-profit.html
- 22.WHO Global vaccine market report. December, 2019. https://www.who.int/immunization/programmes_systems/procurement/mi4a/platform/module2/2019_Global_Vaccine_Market_Report.pdf?ua=1
- 23.Findlay S. Financial Times; 2020. Covid vaccines will be available for private purchase in India.https://www.ft.com/content/224b13fb-1d7d-4250-a6c6-1535b30496bc [Google Scholar]
- 24.Das KN. Reuters; Jan 12, 2021. Bangladesh's Beximco could start private sales of AstraZeneca vaccine next month.https://www.reuters.com/article/health-coronavirus-bangladesh-vaccine/exclusive-bangladeshs-beximco-could-start-private-sales-of-astrazeneca-vaccine-next-month-idUSKBN29H1YD [Google Scholar]
- 25.Simoes E, Boadle A. Reuters; Jan 4, 2021. Private Brazilian clinics to buy COVID-19 vaccine from India's Bharat.https://www.reuters.com/article/health-coronavirus-brazil-india/private-brazilian-clinics-to-buy-covid-19-vaccine-from-indias-bharat-idUKL1N2JF0U8 [Google Scholar]
- 26.Herlihy N, Hutubessy R, Jit M. Current global pricing for human papillomavirus vaccines brings the greatest economic benefits to rich countries. Health Aff (Millwood) 2016;35:227–234. doi: 10.1377/hlthaff.2015.1411. [DOI] [PubMed] [Google Scholar]
- 27.Hecht R, Stover J, Bollinger L, Muhib F, Case K, de Ferranti D. Financing of HIV/AIDS programme scale-up in low-income and middle-income countries, 2009–31. Lancet. 2010;376:1254–1260. doi: 10.1016/S0140-6736(10)61255-X. [DOI] [PubMed] [Google Scholar]
- 28.Hogan DR, Baltussen R, Hayashi C, Lauer JA, Salomon JA. Cost effectiveness analysis of strategies to combat HIV/AIDS in developing countries. BMJ. 2005;331:1431–1437. doi: 10.1136/bmj.38643.368692.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Bank World Bank COVID-19 response. Oct 13, 2020. https://www.worldbank.org/en/news/factsheet/2020/10/14/world-bank-covid-19-response
- 30.Duggan J, Morris S, Sandefur J, Yang G. Is the World Bank's COVID crisis lending big enough, fast enough? New evidence on loan disbursements. Oct 12, 2020. https://www.cgdev.org/publication/world-banks-covid-crisis-lending-big-enough-fast-enough-new-evidence-loan-disbursements
- 31.World Bank COVID 19: debt service suspension initiative. Jan 12, 2021. https://www.worldbank.org/en/topic/debt/brief/covid-19-debt-service-suspension-initiative
- 32.Khamsi R. If a coronavirus vaccine arrives, can the world make enough? Nature. 2020;580:578–580. doi: 10.1038/d41586-020-01063-8. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S, Quinn SC, Kim KH, Hilyard KM. US public support for vaccine donation to poorer countries in the 2009 H1N1 pandemic. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO Report of the WHO Pandemic Influenza A(H1N1) Vaccine Deployment initiative. 2012. https://www.who.int/influenza_vaccines_plan/resources/h1n1_deployment_report.pdf
- 35.Fidler DP. Negotiating equitable access to influenza vaccines: global health diplomacy and the controversies surrounding avian influenza H5N1 and pandemic influenza H1N1. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gavi. the Vaccine Alliance COVAX explained. Sept 12, 2020. https://www.gavi.org/vaccineswork/covax-explained
- 37.WHO Fair allocation mechanism for COVID-19 vaccines through the COVAX Facility. Sept 9, 2020. https://www.who.int/publications/m/item/fair-allocation-mechanism-for-covid-19-vaccines-through-the-covax-facility
- 38.Emanuel EJ, Persad G, Kern A, et al. An ethical framework for global vaccine allocation. Science. 2020;369:1309–1312. doi: 10.1126/science.abe2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Salwi S, Drolet BC. Multivalue ethical framework for fair global allocation of a COVID-19 vaccine. J Med Ethics. 2020;46:499–501. doi: 10.1136/medethics-2020-106516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bill & Melinda Gates Foundation Proposed principles to guide global allocation of pandemic vaccine. 2009. https://docs.gatesfoundation.org/Documents/Pandemic-Flu-Principles-2009.pdf
- 41.Yamada T. Poverty, wealth, and access to pandemic influenza vaccines. N Engl J Med. 2009;361:1129–1131. doi: 10.1056/NEJMp0906972. [DOI] [PubMed] [Google Scholar]
- 42.Herzog LM, Norheim OF, Emanuel EJ, McCoy MS. COVAX must go beyond proportional allocation of COVID vaccines to ensure fair and equitable access. BMJ. 2021;372 doi: 10.1136/bmj.m4853. [DOI] [PubMed] [Google Scholar]
- 43.WHO Access to COVID-19 tools funding commitment tracker. Jan 18, 2021. https://www.who.int/publications/m/item/access-to-covid-19-tools-tracker
- 44.Gavi. the Vaccine Alliance COVAX, the act-accelerator vaccines pillar. 2020. https://www.gavi.org/sites/default/files/covid/COVAX-Pillar-background.pdf
- 45.Usher AD. COVID-19 vaccines for all? Lancet. 2020;395:1822–1823. doi: 10.1016/S0140-6736(20)31354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.The Lancet Global governance for COVID-19 vaccines. Lancet. 2020;395 doi: 10.1016/S0140-6736(20)31405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bollyky TJ, Gostin LO, Hamburg MA. The equitable distribution of COVID-19 therapeutics and vaccines. JAMA. 2020;323:2462–2463. doi: 10.1001/jama.2020.6641. [DOI] [PubMed] [Google Scholar]
- 48.Bollyky TJ, Bown CP. The tragedy of vaccine nationalism. Foreign Affairs. July 27, 2020. https://www.foreignaffairs.com/articles/united-states/2020-07-27/vaccine-nationalism-pandemic
- 49.Yamey G, Schäferhoff M, Hatchett R, Pate M, Zhao F, McDade KK. Ensuring global access to COVID-19 vaccines. Lancet. 2020;395:1405–1406. doi: 10.1016/S0140-6736(20)30763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.So AD, Woo J. Reserving coronavirus disease 2019 vaccines for global access: cross sectional analysis. BMJ. 2020;371 doi: 10.1136/bmj.m4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torjesen I. Covid-19: pre-purchasing vaccine-sensible or selfish? BMJ. 2020;370 doi: 10.1136/bmj.m3226. [DOI] [PubMed] [Google Scholar]
- 52.Duke University Global Health Innovation Center COVID-19: launch and scale speedometer. https://launchandscalefaster.org/COVID-19
- 53.Cyranoski D. Arab nations first to approve Chinese COVID vaccine—despite lack of public data. Nature. 2020;588:548. doi: 10.1038/d41586-020-03563-z. [DOI] [PubMed] [Google Scholar]
- 54.Cyranoski D. What China's speedy COVID vaccine deployment means for the pandemic. Nature. 2020;586:343–344. doi: 10.1038/d41586-020-02807-2. [DOI] [PubMed] [Google Scholar]
- 55.UK Medicines & Healthcare products Regulatory Agency Conditions of authorisation for Pfizer/BioNTech COVID-19 vaccine. Jan 28, 2021. https://www.gov.uk/government/publications/regulatory-approval-of-pfizer-biontech-vaccine-for-covid-19/conditions-of-authorisation-for-pfizerbiontech-covid-19-vaccine
- 56.Williams SR, Driscoll AJ, LeBuhn HM, Chen WH, Neuzil KM, Ortiz JR. National routine adult immunization programs among World Health Organization member states: an assessment of health systems to deploy future SARS-CoV-2 vaccines. MedRxiv. 2020 doi: 10.1101/2020.10.16.20213967. published online Dec 16, 2020. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jusu MO, Glauser G, Seward JF, et al. Rapid establishment of a cold chain capacity of -60°C or colder for the STRIVE Ebola vaccine trial during the Ebola outbreak in Sierra Leone. J Infect Dis. 2018;217(suppl 1):S48–S55. doi: 10.1093/infdis/jix336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gavi. the Vaccine Alliance From biodefence to the DRC: how the Ebola vaccine became one of the fastest vaccines to license in history. Nov 18, 2020. https://www.gavi.org/vaccineswork/biodefence-drc-how-ebola-vaccine-became-one-fastest-vaccines-license-history
- 59.Trogen B, Oshinsky D, Caplan A. Adverse consequences of rushing a SARS-CoV-2 vaccine: implications for public trust. JAMA. 2020;323:2460–2461. doi: 10.1001/jama.2020.8917. [DOI] [PubMed] [Google Scholar]
- 60.Schaffer DeRoo S, Pudalov NJ, Fu LY. Planning for a COVID-19 vaccination program. JAMA. 2020;323:2458–2459. doi: 10.1001/jama.2020.8711. [DOI] [PubMed] [Google Scholar]
- 61.MacDonald NE, Eskola J, Liang X, et al. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33:4161–4164. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 62.Lurie N, Sharfstein JM, Goodman JL. The development of COVID-19 vaccines. JAMA. 2020;324:439–440. doi: 10.1001/jama.2020.12461. [DOI] [PubMed] [Google Scholar]
- 63.Gostin LO, Salmon DA. The dual epidemics of COVID-19 and influenza: vaccine acceptance, coverage, and mandates. JAMA. 2020;324:335–336. doi: 10.1001/jama.2020.10802. [DOI] [PubMed] [Google Scholar]
- 64.de Figueiredo A, Simas C, Karafillakis E, Paterson P, Larson HJ. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020;396:898–908. doi: 10.1016/S0140-6736(20)31558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Larson HJ, Cooper LZ, Eskola J, Katz SL, Ratzan S. Addressing the vaccine confidence gap. Lancet. 2011;378:526–535. doi: 10.1016/S0140-6736(11)60678-8. [DOI] [PubMed] [Google Scholar]
- 66.Karafillakis E, Dinca I, Apfel F, et al. Vaccine hesitancy among healthcare workers in Europe: a qualitative study. Vaccine. 2016;34:5013–5020. doi: 10.1016/j.vaccine.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 67.Karafillakis E, Larson HJ. The benefit of the doubt or doubts over benefits? A systematic literature review of perceived risks of vaccines in European populations. Vaccine. 2017;35:4840–4850. doi: 10.1016/j.vaccine.2017.07.061. [DOI] [PubMed] [Google Scholar]
- 68.Larson HJ, Jarrett C, Eckersberger E, Smith DMD, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012. Vaccine. 2014;32:2150–2159. doi: 10.1016/j.vaccine.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 69.Larson HJ, de Figueiredo A, Xiahong Z, et al. The state of vaccine confidence 2016: global insights through a 67-country survey. EBioMedicine. 2016;12:295–301. doi: 10.1016/j.ebiom.2016.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thigpen CL, Funk C. Most Americans expect a COVID-19 vaccine within a year; 72% say they would get vaccinated. May 21, 2020. https://www.pewresearch.org/fact-tank/2020/05/21/most-americans-expect-a-covid-19-vaccine-within-a-year-72-say-they-would-get-vaccinated/
- 71.King's College London Who's least likely to say they'll get a COVID-19 vaccine? Aug 9, 2020. https://www.kcl.ac.uk/news/whos-least-likely-to-say-theyll-get-a-covid-19-vaccine
- 72.Peretti-Watel P, Seror V, Cortaredona S, et al. A future vaccination campaign against COVID-19 at risk of vaccine hesitancy and politicisation. Lancet Infect Dis. 2020;20:769–770. doi: 10.1016/S1473-3099(20)30426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Szilagyi PG, Thomas K, Shah MD, et al. National trends in the US public's likelihood of getting a COVID-19 vaccine—April 1 to December 8, 2020. JAMA. 2021;325:396–398. doi: 10.1001/jama.2020.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lazarus JV, Ratzan SC, Palayew A, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2020 doi: 10.1038/s41591-020-1124-9. published online Oct 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwarzinger M, Watson V, Arwidson P, Alla F, Luchini S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Health. 2021 doi: 10.1016/S2468-2667(21)00012-8. published online Feb 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shah A, Marks PW, Hahn SM. Unwavering regulatory safeguards for COVID-19 vaccines. JAMA. 2020 doi: 10.1001/jama.2020.15725. published online Aug 7. [DOI] [PubMed] [Google Scholar]
- 77.Center for Health Security The public's role in COVID-19 vaccination: planning recommendations informed by design thinking and the social, behavioral, and communication sciences. July, 2020. https://www.centerforhealthsecurity.org/our-work/pubs_archive/pubs-pdfs/2020/200709-The-Publics-Role-in-COVID-19-Vaccination.pdf
- 78.Ball P, Maxmen A. The epic battle against coronavirus misinformation and conspiracy theories. Nature. 2020;581:371–374. doi: 10.1038/d41586-020-01452-z. [DOI] [PubMed] [Google Scholar]
- 79.Islam MS, Sarkar T, Khan SH, et al. COVID-19-related infodemic and its impact on public health: a global social media analysis. Am J Trop Med Hyg. 2020;103:1621–1629. doi: 10.4269/ajtmh.20-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gavi. the Vaccine Alliance How creative communication strategies are helping fight COVID-19 misinformation in DRC. Aug 4, 2020. https://www.gavi.org/vaccineswork/how-creative-communication-strategies-helping-fight-covid-19-misinformation-drc
- 81.Johnson NF, Velásquez N, Restrepo NJ, et al. The online competition between pro- and anti-vaccination views. Nature. 2020;582:230–233. doi: 10.1038/s41586-020-2281-1. [DOI] [PubMed] [Google Scholar]
- 82.Burki T. The online anti-vaccine movement in the age of COVID-19. Lancet Digit Health. 2020;2:e504–e505. doi: 10.1016/S2589-7500(20)30227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ball P. Anti-vaccine movement could undermine efforts to end coronavirus pandemic, researchers warn. Nature. 2020;581:251. doi: 10.1038/d41586-020-01423-4. [DOI] [PubMed] [Google Scholar]
- 84.Dubé E, Gagnon D, MacDonald NE. Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33:4191–4203. doi: 10.1016/j.vaccine.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 85.Jarrett C, Wilson R, O'Leary M, Eckersberger E, Larson HJ. Strategies for addressing vaccine hesitancy—a systematic review. Vaccine. 2015;33:4180–4190. doi: 10.1016/j.vaccine.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 86.Ghinai I, Willott C, Dadari I, Larson HJ. Listening to the rumours: what the northern Nigeria polio vaccine boycott can tell us ten years on. Glob Public Health. 2013;8:1138–1150. doi: 10.1080/17441692.2013.859720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Larson HJ. Oxford University Press; Oxford: 2020. Stuck: how vaccine rumors start—and why they don't go away. [Google Scholar]
- 88.Chandler RE. Optimizing safety surveillance for COVID-19 vaccines. Nat Rev Immunol. 2020;20:451–452. doi: 10.1038/s41577-020-0372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Halabi S, Heinrich A, Omer SB. No-fault compensation for vaccine injury—the other side of equitable access to COVID-19 vaccines. N Engl J Med. 2020;383:e125. doi: 10.1056/NEJMp2030600. [DOI] [PubMed] [Google Scholar]
- 90.Gamble VN. Under the shadow of Tuskegee: African Americans and health care. Am J Public Health. 1997;87:1773–1778. doi: 10.2105/ajph.87.11.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Halbert CH, Armstrong K, Gandy OH, Jr, Shaker L. Racial differences in trust in health care providers. Arch Intern Med. 2006;166:896–901. doi: 10.1001/archinte.166.8.896. [DOI] [PubMed] [Google Scholar]
- 92.Corbie-Smith G, Thomas SB, St George DMM. Distrust, race, and research. Arch Intern Med. 2002;162:2458–2463. doi: 10.1001/archinte.162.21.2458. [DOI] [PubMed] [Google Scholar]
- 93.Uscher-Pines L, Maurer J, Harris KM. Racial and ethnic disparities in uptake and location of vaccination for 2009-H1N1 and seasonal influenza. Am J Public Health. 2011;101:1252–1255. doi: 10.2105/AJPH.2011.300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wilson SE, Chung H, Schwartz KL, et al. Rotavirus vaccine coverage and factors associated with uptake using linked data: Ontario, Canada. PLoS One. 2018;13 doi: 10.1371/journal.pone.0192809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.MacDonald SE, Bell CA, Simmonds KA. Coverage and determinants of uptake for privately funded rotavirus vaccine in a Canadian birth cohort, 2008-2013. Pediatr Infect Dis J. 2016;35:e177–e179. doi: 10.1097/INF.0000000000001125. [DOI] [PubMed] [Google Scholar]
- 96.Lovie-Toon YG, Hall KK, Chang AB, Anderson J, O'Grady KF. Immunisation timeliness in a cohort of urban Aboriginal and Torres Strait Islander children. BMC Public Health. 2016;16 doi: 10.1186/s12889-016-3825-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Callaway E. Russia's fast-track coronavirus vaccine draws outrage over safety. Nature News. Aug 11, 2020. https://www.nature.com/articles/d41586-020-02386-2 [DOI] [PubMed]
- 98.Murphy F. Inside China's response to COVID. Nature. 2020;588:S49–S51. doi: 10.1038/d41586-020-03361-7. [DOI] [PubMed] [Google Scholar]
- 99.Vaidyanathan G. Vaccine makers in Asia rush to test jabs against fast-spreading COVID variant. Nature News. Jan 12, 2021. https://www.nature.com/articles/d41586-021-00041-y [DOI] [PubMed]
- 100.Pulla P. Science Magazine; Jan 5, 2021. Scientists criticize ‘rushed’ approval of Indian COVID-19 vaccine without efficacy data.https://www.sciencemag.org/news/2021/01/scientists-criticize-rushed-approval-indian-covid-19-vaccine-without-efficacy-data [Google Scholar]
- 101.Thiagarajan K. Covid-19: India is at centre of global vaccine manufacturing, but opacity threatens public trust. BMJ. 2021;372:n196. doi: 10.1136/bmj.n196. [DOI] [PubMed] [Google Scholar]
- 102.Murphy F. ‘Every week counts’ as Austria, Greece, Denmark seek quick EU OK for AstraZeneca vaccine: Kurz. Jan 8, 2021. https://www.reuters.com/article/idUSKBN29N0XV
- 103.Chinazzi M, Davis JT, Dean NE, et al. Estimating the effect of cooperative versus uncooperative strategies of COVID-19 vaccine allocation: a modeling study. Sept 14, 2020. https://www.networkscienceinstitute.org/publications/estimating-the-effect-of-cooperative-versus-uncooperative-strategies-of-covid-19-vaccine-allocation-a-modeling-study
- 104.Çakmakli C, Demiralp S, Kalemli-Özcan Ş, Yeşiltaş S, Yildirim MA. The economic case for global vaccinations: an epidemiological model with international production networks. Jan 25, 2021. https://iccwbo.org/publication/the-economic-case-for-global-vaccinations/
- 106.Usher AD. CEPI criticised for lack of transparency. Lancet. 2021;397:265–266. doi: 10.1016/S0140-6736(21)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Coalition for Epidemic Preparedness Innovations Summary of equitable access provisions in CEPI's COVID-19 vaccine development agreements. Dec 17, 2020. https://cepi.net/wp-content/uploads/2020/12/Enabling-equitable-access-to-COVID19-vaccines-v1-17Dec2020.pdf
- 108.Berndt ER, Glennerster R, Kremer MR, et al. Advance market commitments for vaccines against neglected diseases: estimating costs and effectiveness. Health Econ. 2007;16:491–511. doi: 10.1002/hec.1176. [DOI] [PubMed] [Google Scholar]
Uncited Reference
- 105.Baric RS. Emergence of a Highly Fit SARS-CoV-2 Variant. N Engl J Med. 2020;383:2684–2686. doi: 10.1056/NEJMcibr2032888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.