Abstract
Background
Patients with severe COVID-19 develop a life-threatening hyperinflammatory response to the virus. Interleukin (IL)-1 or IL-6 inhibitors have been used to treat this patient population, but the comparative effectiveness of these different strategies remains undetermined. We aimed to compare IL-1 and IL-6 inhibition in patients admitted to hospital with COVID-19, respiratory insufficiency, and hyperinflammation.
Methods
This cohort study included patients admitted to San Raffaele Hospital (Milan, Italy) with COVID-19, respiratory insufficiency, defined as a ratio of the partial pressure of oxygen to the fraction of inspired oxygen of 300 mm Hg or less, and hyperinflammation, defined as serum C-reactive protein concentration of 100 mg/L or more or ferritin concentration of 900 ng/mL or more. The primary endpoint was survival, and the secondary endpoint was a composite of death or mechanical ventilation (adverse clinical outcome). Multivariable Cox regression analysis was used to compare clinical outcomes of patients receiving IL-1 inhibition (anakinra) or IL-6 inhibition (tocilizumab or sarilumab) with those of patients who did not receive interleukin inhibitors, after accounting for baseline differences. All patients received standard care. Interaction tests were used to assess the probability of survival according to C-reactive protein or lactate dehydrogenase concentrations.
Findings
Of 392 patients included between Feb 25 and May 20, 2020, 275 did not receive interleukin inhibitors, 62 received the IL-1 inhibitor anakinra, and 55 received an IL-6 inhibitor (29 received tocilizumab and 26 received sarilumab). In the multivariable analysis, compared with patients who did not receive interleukin inhibitors, patients treated with IL-1 inhibition had a significantly reduced mortality risk (hazard ratio [HR] 0·450, 95% CI 0·204–0·990, p=0·047), but those treated with IL-6 inhibition did not (0·900, 0·412–1·966; p=0·79). In the multivariable analysis, there was no difference in adverse clinical outcome risk in patients treated with IL-1 inhibition (HR 0·866, 95% CI 0·482–1·553; p=0·63) or IL-6 inhibition (0·882, 0·452–1·722; p=0·71) relative to patients who did not receive interleukin inhibitors. For increasing C-reactive protein concentrations, patients treated with IL-6 inhibition had a significantly reduced risk of mortality (HR 0·990, 95% CI 0·981–0·999; p=0·031) and adverse clinical outcome (0·987, 0·979–0·995; p=0·0021) compared with patients who did not receive interleukin inhibitors. For decreasing concentrations of serum lactate dehydrogenase, patients treated with an IL-1 inhibitor and patients treated with IL-6 inhibitors had a reduced risk of mortality; increasing concentrations of lactate dehydrogenase in patients receiving either interleukin inhibitor were associated with an increased risk of mortality (HR 1·009, 95% CI 1·003–1·014, p=0·0011 for IL-1 inhibitors and 1·006, 1·001–1·011, p=0·028 for IL-6 inhibitors) and adverse clinical outcome (1·006, 1·002–1·010, p=0·0031 for IL-1 inhibitors and 1·005, 1·001–1·010, p=0·016 for IL-6 inhibitors) compared with patients who did not receive interleukin inhibitors.
Interpretation
IL-1 inhibition, but not IL-6 inhibition, was associated with a significant reduction of mortality in patients admitted to hospital with COVID-19, respiratory insufficiency, and hyperinflammation. IL-6 inhibition was effective in a subgroup of patients with markedly high C-reactive protein concentrations, whereas both IL-1 and IL-6 inhibition were effective in patients with low lactate dehydrogenase concentrations.
Funding
None.
Introduction
As of Feb 2, 2021, COVID-19, which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has affected 102 817 575 people worldwide, causing the death of 2 227 420 (see the WHO COVID-19 dashboard). A subset of patients with severe COVID-19 develop a life-threatening hyperinflammatory response to the virus, which resembles the cytokine storm that develops after chimeric antigen receptor T-cell treatment or in macrophage activation syndrome, with release of interleukin (IL)-1β, IL-6, IL-18, and interferon-γ.1, 2 To reduce deaths among patients with COVID-19 and hyperinflammation,2 treatment with cytokine-blocking biological agents has been proposed, with IL-6 and IL-1 as the most promising targets.1 Observational studies evaluating IL-6 inhibition with tocilizumab and sarilumab yielded conflicting results;3, 4, 5 later controlled trials of tocilizumab showed marginal or no efficacy.6, 7, 8 IL-1 inhibition with anakinra improved clinical outcomes of patients with severe COVID-19 in observational studies,9, 10 but results from controlled investigations of IL-1 inhibition in COVID-19 are not yet available.
Research in context.
Evidence before this study
A subset of patients with severe COVID-19 develop a maladaptive, systemic hyperinflammatory response to the virus, which is associated with a poor prognosis. Since the beginning of the pandemic, inhibition of pro-inflammatory interleukins with available biological agents has emerged as an attractive therapeutic opportunity, as documented by an increasing number of publications. We searched PubMed, Embase, Cochrane Review, and SCOPUS for research articles published in English up to Oct 31, 2020, using the search terms “COVID-19rldquo;, “cytokine inhibition”, “interleukin inhibition”, “therapy”, “hyperinflammation”, and “biological agents”. Our search showed that most investigations evaluated IL-6 inhibition or IL-1 inhibition; currently, controlled evidence indicates that IL-6 inhibition has marginal or no efficacy for COVID-19, whereas observational evidence suggests that IL-1 inhibition might be beneficial. No evidence is available on the comparative effectiveness of these different treatment strategies.
Added value of this study
To our knowledge, our study is the first to evaluate the clinical effectiveness of both IL-1 inhibition (anakinra) and IL-6 inhibition (tocilizumab or sarilumab) compared with standard management in a large and homogeneous cohort of patients with COVID-19, respiratory insufficiency, and hyperinflammation. We found that IL-1 inhibition, but not IL-6 inhibition, significantly reduced mortality in patients with COVID-19. In addition, this study showed that changes in serum parameters such as C-reactive protein and lactate dehydrogenase might be useful to identify those patients who are more likely to respond better to cytokine inhibitors.
Implications of all the available evidence
This study reveals differences in global outcomes of COVID-19 patients treated with IL-1 versus IL-6 inhibition, while offering a better understanding of the patient's profile associated with response to treatment with biologics. These findings are useful to instruct the current management of patients with COVID-19 as well as the design of controlled investigations evaluating cytokine inhibitors in COVID-19.
Currently, available evidence overall argues against the use of IL-6 inhibitors to treat COVID-19. However, crucial questions remain unanswered pertaining to the role and therapeutic potential of IL-1 inhibition in COVID-19—specifically, whether IL-1 inhibition confers an advantage over standard management, whether IL-1 inhibition is more effective than IL-6 inhibition, and how to identify those individuals who are most likely to benefit (or to deteriorate) upon treatment.
To address these questions, we aimed to evaluate the clinical outcomes of patients with COVID-19 and hyperinflammation treated with IL-1 inhibition (receptor blockade) or IL-6 inhibition (monoclonal antibodies), compared with patients with COVID-19 and hyperinflammation who were concomitantly admitted to hospital at the same institution but did not receive interleukin inhibitors.
Methods
Study design and participants
This cohort study was done in San Raffaele Hospital (Milan, Italy), a tertiary health-care centre, which was designated as a COVID-19 hub by Italian health authorities. All patients were enrolled between Feb 25 and May 20, 2020, which corresponds to the duration of the lockdown in Italy. Upon written consent from patients, we collected clinical data on demographics, comorbidities, baseline clinical features and laboratory data, interventions, and outcomes from all patients admitted to hospital with COVID-19, respiratory insufficiency, and hyperinflammation daily through a dedicated electronic case report form, according to a predefined institutional protocol (COVID-BioB Study, Ethical Committee approval number 34/int/2020, ClinicalTrials.gov NCT04318366).11 Written informed consent was obtained before the off-label use of anakinra, tocilizumab, or sarilumab.
Patients with COVID-19, which was diagnosed with quantitative RT-PCR and chest x-ray or CT scan, were included in the analysis if they had respiratory failure, defined as a ratio of the partial pressure of oxygen to the fraction of inspired oxygen of 300 mm Hg of less, and hyperinflammation, defined as elevation of the serum inflammation marker C-reactive protein (≥100 mg/L) or ferritin (≥900 ng/mL). Treatment with anakinra, tocilizumab, or sarilumab was initiated as soon as patients first fulfilled these criteria. Patients who fulfilled the inclusion criteria but did not receive interleukin inhibitors were included as comparators. Patients who died or had an adverse clinical outcome (defined as a composite of death or mechanical ventilation) within 24 h of fulfilling these criteria and were consequently unable to receive IL-1 or IL-6 treatment were excluded to avoid a potential immortal bias favouring IL-1 or IL-6 treatment over standard management.12 Some of the patients treated with interleukin inhibitors included in this study had been described in previous smaller studies from our group, each evaluating a single biological agent (anakinra, tocilizumab, or sarilumab) compared with standard management in COVID-19.4, 5, 9
Procedures
During the study period, patients admitted to our hospital with COVID-19 were distributed into seven dedicated wards, each aiding approximately 40 inpatients. An institutional scientific committee decided that treatment with interleukin inhibitors would be offered to patients with hyperinflammation pending evaluation by rheumatologists (GC, GDL, ED-T, and CC). However, at the time of this study, there was no available evidence indicating that either IL-1 inhibition or IL-6 inhibition would confer incremental benefits over standard management in patients with COVID-19. In this scenario, two rheumatologists (GC and GDL) decided to administer anakinra, whereas the remaining two (ED-T and CC) administered IL-6-blocking therapies. The comparator group consisted of patients with COVID-19 and hyperinflammation who either refused experimental treatment with interleukin inhibitors or escaped monitoring by the four rheumatologists due to the high-intensity situation.
All patients received institutional standard of care, which at that time comprised hydroxychloroquine, an antiviral drug, and empirical antibiotic coverage (the complete clinical management protocol is provided in the appendix p 1). Anakinra was administered off-label intravenously at a dose of 5 mg/kg twice daily (total daily dose of 10 mg/kg) until clinical benefit, defined as sustained improvement of respiratory parameters and serum C-reactive protein (75% reduction compared with baseline). Tocilizumab was administered off-label intravenously as a single dose of 400 mg, which was repeated after 24 h if the respiratory function further worsened. Sarilumab was administered off-label as a single dose of 400 mg intravenously. For the purpose of this study, patients treated with tocilizumab or sarilumab were grouped together because the two monoclonal antibodies share an identical mechanism of action (IL-6 receptor blockade). We also evaluated the possible contribution of concomitant glucocorticoid therapy, which was administered to some patients, since this treatment reportedly reduced 28-day mortality in a trial of patients with COVID-19 published after completion of this study.13 Since glucocorticoid administration did not follow a standard regimen at the time of this study, the doses and duration of treatment differed among patients; therefore, the dose is provided as cumulative methylprednisolone equivalent.
Outcomes
The primary outcome of the study was survival. The secondary outcome was adverse clinical outcome (a composite of death or mechanical ventilation). Changes in clinical outcomes were evaluated daily from enrolment (defined as first fulfilment of eligibility criteria or first administration of interleukin inhibitors) to discharge from hospital, admission to an intensive care unit (ICU), or death, whichever came first.
Statistical analysis
Statistical analyses as well as reporting and interpretation of the results were conducted according to established guidelines.14 Medians and IQRs are reported for continuous variables, frequencies and proportions are reported for categorical variables. We used Mann-Whitney and χ2 tests to compare the significance of differences in the distribution of continuous or categorical variables, respectively, between patients treated without interleukin inhibitors and patients treated with IL-1 inhibition or IL-6 inhibition. We used the Kaplan-Meier method to evaluate the 28-day survival rate.
To account for baseline clinical differences among different treatment groups, we did a multivariable Cox regression analysis to assess the effect of interleukin inhibitors on mortality and adverse clinical outcome. Covariates were selected according to a clinical criterion among established predictors of COVID-19 mortality15 and consisted of age, sex, C-reactive protein, respiratory support at baseline (as an indicator of the severity of respiratory failure), alanine aminotransferase, creatinine, lactate dehydrogenase, history of diabetes, treatment with glucocorticoids, ICU admission (occurring after 24 h of study enrolment and included only in models predicting mortality), and day of hospital admission (to account for possible changes in COVID-19 mortality and adverse outcomes over time).
We tested the hypothesis that the effect of interleukin inhibitors varied in specific subgroups of patients using interaction terms in a Cox regression analysis. For interaction tests, we selected C-reactive protein and lactate dehydrogenase, which were clinically indicative of the severity of the inflammatory response in the lung and had emerged as independently associated with a risk of mortality and adverse clinical outcome in the multivariable regression analysis; variables that were not available for all patients were not included (ie, ferritin). We used regression-derived coefficients to estimate the 28-day survival probability. We used local polynomial smoothing method to graphically explore survival probability according to C-reactive protein or lactate dehydrogenase concentrations.16
Patients with missing data in variables relevant to these analyses were excluded. All statistical tests were done with the RStudio graphical interface (version 0.98) for R software environment (version 3.0.2) with the following libraries, packages, and scripts: Hmisc, plyr, stats, MatchIt, rms, and graphics. All tests were two sided with a significance level set at a p value of less than 0·05.
Role of the funding source
There was no funding source for this study.
Results
Among the 954 patients admitted to hospital with COVID-19 between Feb 25 and May 20, 2020, 478 patients fulfilled the criteria for this study. Of these patients, 70 patients were excluded because they were admitted to the ICU or died within 24 h of fulfilment of these criteria. 16 patients were excluded due to missing variables (figure 1 ). 392 patients were included in the study; 375 (96%) were white (table 1 ). 275 (70%) did not receive interleukin inhibitors, 62 (16%) received IL-1 inhibitors (all anakinra), and 55 (14%) received IL-6 inhibitors (29 received tocilizumab and 26 received sarilumab). Among the 29 patients treated with tocilizumab, nine received a repeated dose. Availability of interleukin inhibitors was ensured throughout the study, and no patient was refused treatment with interleukin inhibitors due to reduced availability. 67 patients received glucocorticoids (median cumulative methylprednisolone-equivalent dose: 440 mg/kg [IQR 300–665]; 47 mg/kg per day [IQR 30–60]); of these patients, 54 did not receive interleukin inhibitors, seven received IL-1 inhibitors, and six received IL-6 inhibitors (appendix p 3).
Figure 1.
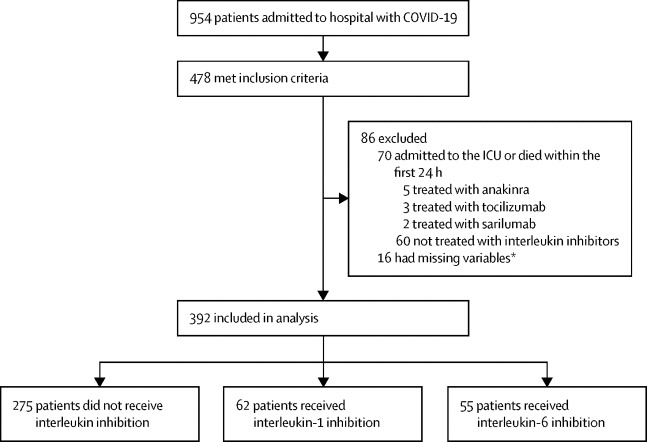
Study overview
ICU=intensive care unit. *Missing variables included laboratory values or information on medical history selected as covariates in the multivariable Cox regression analysis.
Table 1.
Baseline characteristics of patients with COVID-19, respiratory failure, and hyperinflammation
| Overall population (n=392) | No interleukin inhibitors (n=275) | IL-1 inhibitor (n=62) | IL-6 inhibitor (n=55) | ||
|---|---|---|---|---|---|
| Age, years | 67 (56–77) | 68 (58–79) | 63 (52–73) | 58 (52–74) | |
| Sex | |||||
| Male | 301 (77%) | 201 (73%) | 52 (84%) | 48 (87%) | |
| Female | 91 (23%) | 74 (27%) | 10 (16%) | 7 (13%) | |
| Ethnicity | |||||
| White | 375 (96%) | 265 (96%) | 57 (92%) | 53 (96%) | |
| Non-white | 17 (4%) | 10 (4%) | 5 (8%) | 2 (4%) | |
| C-reactive protein, mg/L | 129 (100–171) | 127 (91–169) | 143 (105–172) | 130 (100–195) | |
| Ferritin*, ng/mL | 1295 (943–2359) | 1239 (841–1887) | 1459 (946–2761) | 1727 (1151–2757) | |
| Lymphocytes, 109 cells per L | 0·9 (0·6–1·2) | 0·9 (0·6–1·1) | 0·9 (0·7–1·3) | 0·8 (0·6–1·1) | |
| Platelets, 109 cells per L | 228 (170–297) | 228 (165–297) | 238 (190–296) | 219 (176–299) | |
| Creatinine, mg/dL | 1·01 (0·8–1·31) | 1·03 (0·85–1·36) | 0·96 (0·83–1·34) | 1·00 (0·86–1·14) | |
| Alanine aminotransferase, U/L | 40 (25–61) | 37 (23–59) | 45 (27–64) | 45 (30–62) | |
| Lactate dehydrogenase, U/L | 396 (314–515) | 369 (300–479) | 430 (329–532) | 458 (414–542) | |
| Respiratory support | |||||
| Oxygen | 307 (78%) | 242 (88%) | 36 (58%) | 29 (53%) | |
| Non-invasive mechanical ventilation | 85 (22%) | 33 (12%) | 26 (42%) | 26 (47%) | |
| Cardiovascular disease | |||||
| No | 273 (70%) | 175 (64%) | 52 (84%) | 46 (84%) | |
| Yes | 119 (30%) | 100 (36%) | 10 (16%) | 9 (16%) | |
| History of neoplasia | |||||
| No | 326 (83%) | 218 (79%) | 57 (92%) | 51 (93%) | |
| Yes | 66 (17%) | 57 (21%) | 5 (8%) | 4 (7%) | |
| Diabetes | |||||
| No | 320 (82%) | 219 (80%) | 50 (81%) | 51 (93%) | |
| Yes | 72 (18%) | 56 (20%) | 12 (19%) | 4 (7%) | |
| Month of admission | |||||
| February | 9 (2%) | 9 (3%) | 0 | 0 | |
| March | 323 (82%) | 223 (81%) | 46 (74%) | 54 (98%) | |
| April | 56 (14%) | 39 (14%) | 16 (26%) | 1 (2%) | |
| May | 4 (1%) | 4 (2%) | 0 | 0 | |
Data are median (IQR) or n (%). IL=interleukin.
Data missing for 96 patients in the no interleukin inhibitor group, five patients in the IL-1 inhibitor group, and 11 patients in the IL-6 inhibitor group.
Compared with patients who did not receive interleukin inhibitors, patients treated with IL-1 inhibitors were younger, presented with higher lactate dehydrogenase concentrations at baseline, and were more frequently on non-invasive mechanical ventilation; they also less frequently had a history of cardiovascular diseases and malignancies (appendix p 4). Compared with patients who did not receive interleukin inhibitors, patients treated with IL-6 inhibitors were younger, were more likely to be male, were admitted earlier during the pandemic, had higher ferritin and higher lactate dehydrogenase concentrations at baseline, and were more frequently on non-invasive mechanical ventilation; they also less frequently had a history of cardiovascular diseases, diabetes, and malignancies (appendix p 5).
The 28-day survival rate was 75% (95% CI 68–80) in the overall population, 68% (61–75) in patients who did not receive any interleukin inhibitor, 86% (74–100) in patients treated with IL-1 inhibitors, and 82% (69–97) in patients treated with IL-6 inhibitors. In the multivariable analysis accounting for all the available covariates, patients treated with IL-1 inhibition had a lower mortality risk than patients who did not receive interleukin inhibitors (hazard ratio [HR] 0·450, 95% CI 0·204–0·990; p=0·047; table 2 ). Conversely, no evidence of a different mortality risk was recorded in patients treated with IL-6 inhibition relative to patients who did not receive interleukin inhibitors (HR 0·900, 95% CI 0·412–1·966; p=0·79; table 2).
Table 2.
Multivariable models predicting mortality and adverse clinical outcome in patients diagnosed with COVID-19, respiratory failure, and hyperinflammation
|
Mortality (Cox regression analysis) |
Adverse clinical outcome*(Cox regression analysis) |
||||
|---|---|---|---|---|---|
| HR (95%CI) | p value | HR (95%CI) | p value | ||
| Treatment | |||||
| No interleukin inhibitors | 1 (ref) | .. | 1 (ref) | .. | |
| IL-1 inhibitor | 0·450 (0·204–0·990) | 0·047 | 0·866 (0·482–1·553) | 0·63 | |
| IL-6 inhibitor | 0·900 (0·412–1·966) | 0·79 | 0·882 (0·452–1·722) | 0·71 | |
| Age | 1·081 (1·054–1·109) | <0·0001 | 1·031 (1·013–1·050) | 0·0007 | |
| Sex | |||||
| Female | 1 (ref) | .. | 1 (ref) | .. | |
| Male | 1·029 (0·608–1·739) | 0·92 | 1·176 (0·726–1·905) | 0·51 | |
| Respiratory support | |||||
| Oxygen | 1 (ref) | .. | 1 (ref) | .. | |
| Non-invasive mechanical ventilation | 0·908 (0·459–1·799) | 0·78 | 0·771 (0·443–1·344) | 0·36 | |
| C-reactive protein, mg/L | 1·003 (1·001–1·006) | 0·020 | 1·005 (1·002–1·007) | 0·0002 | |
| Alanine aminotransferase, U/L | 0·995 (0·992–0·997) | <0·0001 | 0·995 (0·993–0·997) | <0·0001 | |
| Creatinine, mg/dL | 1·212 (1·085–1·355) | 0·0007 | 1·101 (0·991–1·223) | 0·074 | |
| Lactate dehydrogenase, U/L | 1·002 (1·001–1·003) | <0·0001 | 1·002 (1·001–1·003) | <0·0001 | |
| Diabetes | |||||
| No | 1 (ref) | .. | 1 (ref) | .. | |
| Yes | 1·657 (1·025–2·680) | 0·039 | 1·836 (1·182–2·852) | 0·0068 | |
| Treatment with glucocorticoids | |||||
| No | 1 (ref) | .. | 1 (ref) | .. | |
| Yes | 0·687 (0·377–1·253) | 0·22 | 0·634 (0·368–1·093) | 0·10 | |
| ICU admission† | |||||
| No | 1 (ref) | .. | .. | .. | |
| Yes | 1·864 (0·872–3·988) | 0·11 | .. | .. | |
| Days after admission of the first patient‡ | 0·984 (0·966–1·002) | 0·074 | 0·990 (0·973–1·007) | 0·24 | |
HR=hazard ratio. ICU=intensive care unit. IL=interleukin.
Adverse clinical outcome is defined as a composite of death or mechanical ventilation.
After 24 h from enrolment.
Days after admission of the first patient: day of hospital admission during the study period, accounts for possible changes in COVID-19 mortality and adverse clinical outcome.
The 28-day adverse clinical outcome-free survival rate was 69% (95% CI 63–74) in the overall population, 65% (58–73) in patients who did not receive interleukin inhibitors, 73% (62–86) in patients treated with IL-1 inhibitors, and 76% (65–89) in patients treated with IL-6 inhibitors. In the multivariable analysis accounting for all the available covariates, no evidence of a different adverse clinical outcome risk was recorded in patients treated with IL-1 inhibition (HR 0·866, 95% CI 0·482–1·553; p=0·63) or IL-6 inhibition (0·882, 0·452–1·722; p=0·71; table 2) relative to patients who did not receive interleukin inhibitors. Treatment with glucocorticoids was not associated with significant reductions in mortality and adverse clinical outcomes (table 2).
Interaction tests confirmed the hypothesis that the benefit of treatment with IL-1 and IL-6 inhibitors is more pronounced in specific subgroups of patients (table 3 ). Specifically, for increasing concentrations of serum C-reactive protein, IL-6 inhibition was associated with a lower mortality risk (HR 0·990, 95% CI 0·981–0·999; p=0·031) and lower adverse clinical outcome risk (0·987, 0·979–0·995; p=0·0021; figure 2 ) than no interleukin inhibition. Additionally, for decreasing concentrations of serum lactate dehydrogenase, patients treated with an IL‑1 inhibitor and patients treated with IL-6 inhibitors had a reduced risk of mortality; increasing concentrations of lactate dehydrogenase in patients receiving either interleukin inhibitor were associated with an increased risk of mortality (HR 1·009, 95% CI 1·003–1·014, p=0·0011 for IL-1 inhibitors and 1·006, 1·001–1·011, p=0·028 for IL-6 inhibitors) and adverse clinical outcome (1·006, 1·002–1·010, p=0·0031 for IL-1 inhibitors and 1·005, 1·001–1·010, p=0·016 for IL-6 inhibitors) compared with patients who did not receive interleukin inhibitors (figure 3 ).
Table 3.
Subgroup analysis of treatment with interleukin inhibitors in patients diagnosed with COVID-19, respiratory failure, and hyperinflammation
|
Mortality (Cox regression analysis*) |
Adverse clinical outcome (Cox regression analysis*) |
|||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Treatment and C-reactive protein | ||||
| No interleukin inhibitors | 1 (ref) | .. | 1 (ref) | .. |
| IL-1 inhibitor by C-reactive protein | 0·994 (0·985–1·004) | 0·23 | 0·997 (0·991–1·003) | 0·31 |
| IL-6 inhibitor by C-reactive protein | 0·990 (0·981–0·999) | 0·031 | 0·987 (0·979–0·995) | 0·0021 |
| Treatment and lactate dehydrogenase | ||||
| No interleukin inhibitors | 1 (ref) | .. | 1 (ref) | .. |
| IL-1 inhibitor by lactate dehydrogenase | 1·009 (1·003–1·014) | 0·0011 | 1·006 (1·002–1·010) | 0·0031 |
| IL-6 inhibitor by lactate dehydrogenase | 1·006 (1·001–1·011) | 0·028 | 1·005 (1·001–1·010) | 0·016 |
HR=hazard ratio. IL=interleukin.
Model adjusted for age, C-reactive protein, and lactate dehydrogenase.
Figure 2.
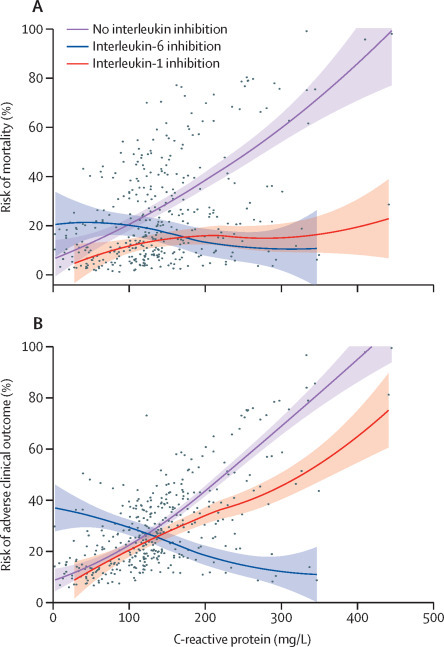
Risk of mortality (A) and adverse clinical outcome (B) according to baseline C-reactive protein in patients diagnosed with COVID-19 and hyperinflammation
Dots represent individual observations, solid lines represent multivariable model-derived probabilities, and shaded areas represent 95% CIs.
Figure 3.
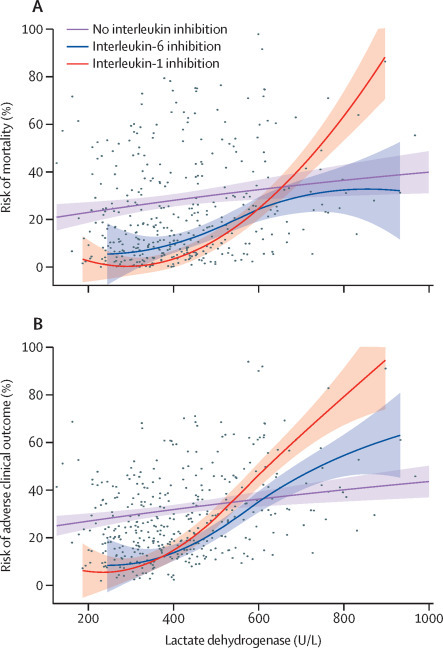
Risk of mortality (A) and adverse clinical outcome (B) according to baseline lactate dehydrogenase in patients diagnosed with COVID-19 and hyperinflammation
Dots represent individual observations, solid lines represent multivariable model-derived probabilities, and shaded areas represent 95% CIs.
Discussion
The main finding of this study is that IL-1 inhibition with anakinra, but not IL-6 inhibition with tocilizumab or sarilumab, significantly reduced mortality in the overall population of in-hospital patients with COVID-19, respiratory insufficiency, and hyperinflammation. Protective effects of IL-6 inhibition were only observed in patients with very high serum C-reactive protein at baseline, whereas both IL-1 and IL-6 inhibitors were more effective in patients with low serum lactate dehydrogenase at baseline.
Patients with severe COVID-19 develop a detrimental hyperinflammatory response to the virus, characterised by excessive release of pro-inflammatory cytokines. Targetable cytokines involved in the pathogenesis of COVID-19 include IL-1, IL-6, IL-8, tumour necrosis factor, granulocyte–macrophage colony-stimulating factor, and interferon-γ.17, 18 Available agents inhibiting IL-1 and IL-6 emerged as candidate treatments to reduce hyperinflammation and mortality in this patient population. Anakinra is a recombinant replica of the IL-1 receptor antagonist, a naturally occurring regulatory molecule that blocks the activity of both IL-1α and IL-1β, and is approved for the treatment of rheumatoid arthritis and autoinflammatory disorders at a dose of 100 mg daily subcutaneously.19 High-dose intravenous anakinra has been used off label for the treatment of macrophage activation syndrome and septic shock,20, 21 which are catastrophic conditions that share some clinical and molecular features with hyperinflammation in COVID-19. Administration of anakinra intravenously at a dose of 5 mg/kg twice daily in this study is consistent with previous use in these hyperinflammatory conditions.20, 21
Tocilizumab and sarilumab are monoclonal antibodies that share the same mechanism of action: IL-6 receptor blockade.22 Both are approved for the treatment of rheumatoid arthritis, with the first-in-class tocilizumab also approved for juvenile idiopathic arthritis and giant cell arteritis.23, 24 Use of IL-6 inhibitors in COVID-19 with hyperinflammation is based on similarities with cytokine release syndrome, a complication of chimeric antigen receptor T-cell therapy.25 Previous studies have evaluated IL-1 and IL-6 inhibition in COVID-19. Currently, controlled evidence indicates that IL-6 inhibition yields no or marginal benefit in COVID-19;6, 7, 8 the role of IL-1 inhibition has only been evaluated in observational studies suggesting moderate benefit;9, 10 and the comparative efficacy of IL-6 and IL-1 inhibition remains undetermined.
This study is the first, to our knowledge, to compare the clinical effectiveness of IL-1 inhibition and IL-6 inhibition with standard management alone in a large, homogeneous cohort of patients with severe COVID-19. Overall, patients with COVID-19 who were treated with IL-1 inhibition, but not those who were treated with IL-6 inhibition, had a lower mortality risk than patients receiving only conventional management. Notably, we also evaluated the possible contribution of concomitant glucocorticoid therapy, which reportedly reduced 28-day mortality in a trial of patients with COVID-19 receiving respiratory support.13 In our real-world setting, treatment with glucocorticoids was not a predictor of mortality or of adverse clinical outcomes;13 however, this study was done before controlled evidence on the use of corticosteroids in COVID-19 became available, and the lack of a standardised treatment protocol at the time might account for this lack of effectiveness.
Mechanistically, IL-1 is found upstream of IL-6 in inflammatory cascades.26 IL-1 induces several secondary inflammatory mediators, including, but not limited to, IL-6. Indeed, a decrease in serum IL-6 concentration typically follows effective IL-1 inhibition.26 Therefore, it is likely that effective inhibition of IL-1 in COVID-19 results in downstream suppression of IL-6, as well as other mediators. Such a hierarchical association might account for the more robust clinical benefit of IL-1 inhibition observed in our investigation.
In this study, one in two patients admitted to hospital with COVID-19 developed hyperinflammation, and these patients were more frequently male, a finding consistent with previous observations.27, 28 This high frequency of hyperinflammation delineates a critically relevant clinical problem. In this scenario, two additional findings of this study provide pragmatic and valuable information for the interpretation of available evidence from past studies and for the design of future clinical investigations.
First, IL-6 inhibition might be advantageous for patients with very high elevated serum concentrations of C-reactive protein (eg, >200 mg/L). This finding is not unexpected, given the mechanistic role of IL-6 as the main inducer of C-reactive protein production by the liver during the acute phase response.29 This finding is also in line with a study reporting a reduced risk of mortality and mechanical ventilation in patients with C-reactive protein concentrations of 200 mg/L or more who were treated with glucocorticoids.30 Second, the benefit of both IL-1 and IL-6 inhibition is more pronounced in patients with low serum lactate dehydrogenase, whereas it decreases for increasing concentrations (eg, >500 U/L). These hypothesis-generating findings suggest that the potential benefit of interleukin inhibition is highest in the early phases of disease, characterised by rampant inflammatory activation, but progressively fades in more advanced stages characterised by extensive disease burden and tissue damage. Consistent with the hypothesis of greater efficacy of anakinra in early phases of disease, previous studies have also found that early intervention with IL-1 blockade was associated with increased survival in macrophage activation syndrome.31
Limitations of this study are inherent to observational investigations, the nature of which mandates caution in interpretation of findings. Clinical differences between groups at baseline introduce the possibility of confounding (ie, the risk that observed effects could be affected by clinical or demographic features besides investigational treatments), which cannot be completely excluded even after careful adjusting by multivariable regression analysis; however, clinical differences were mixed and did not confer any study group a clear survival advantage. In addition, information on some variables with prognostic relevance in COVID-19, including ineligibility to respiratory support (non-invasive ventilation or mechanical ventilation) and D-dimer concentrations, was not available, which limits information on the effectiveness of investigational treatments in patients with altered coagulation states. The risk of a possible confounding by indication32 cannot be completely ruled out; however, this risk was minimised by the choice to entrust two different groups of rheumatologists to assign patients to specific treatments (anakinra vs tocilizumab or sarilumab), and by the fact that patients who did not receive interleukin inhibitors were not negatively selected by physicians, but rather denied consent to an off-label treatment or were retrospectively identified. Observational studies are also at risk for an immortal bias,33 but this bias was carefully minimised by the choice to exclude from our analysis all patients who died or were admitted to the ICU within 24 h of hyperinflammation being detected. In addition, tocilizumab and sarilumab share a common mechanism of action (IL-6 receptor blockade) and were considered as a common treatment strategy in this study, as the aim to compare the effectiveness of two treatment strategies (IL-1 and IL-6 inhibition) required a large sample size for statistical evaluation of findings. However, we acknowledge that slight molecular differences between these drugs might translate into differences in effectiveness. Last, we cannot exclude that a larger sample size or inclusion of patients at different disease stages might also have yielded a significant result for the primary outcome in patients treated with IL-6 inhibitors; nevertheless, our findings are in line with controlled trials of tocilizumab that indicate limited efficacy.6, 7, 8 The current investigation stands out for its novelty and uniqueness, owing to the timely and systematic comparison of two candidate therapeutic strategies for a new disease in a large and homogeneous cohort, the fulfilment of the same inclusion criteria for treatment with different interleukin inhibitors, and the management at the same institution according to the same standard treatment protocols, which together reduce risks for confounders. Moreover, the current analysis reveals new information about the profile of the optimal candidates for treatment with interleukin inhibitors, which might aid clinical decision making and instruct further study design.
In summary, IL-1 inhibition, but not IL-6 inhibition, was associated with a significant reduction of mortality in a large cohort of patients admitted to hospital with COVID-19 and hyperinflammation. IL-6 inhibition was only effective in a subgroup of patients with markedly high C-reactive protein concentrations, whereas both IL-1 inhibition and IL-6 inhibition were more effective in patients with low lactate dehydrogenase concentrations. Validation of these study findings, particularly concerning the efficacy of IL-1 inhibition in COVID-19, requires controlled investigations.
Data sharing
After publication, institutional datasets including anonymised data and data dictionary can be accessible to other bona fide investigators upon request to the corresponding author, pending approval of a written proposal by the Institutional Review Board of IRCSS San Raffaele Scientific Institute (Milan, Italy) and according to signed data access agreements between research groups and hosting institutions.
Acknowledgments
Acknowledgments
GC is supported by the Italian Association for Cancer Research under MFAG 2018 (22136 project), the Italian Ministry of Health (GR-2018-12366385), and the Foundation for Research in Rheumatology (Career Award 2020).
Contributors
GC, AL, and AT led the study and wrote the report. CC, ED-T, GDL, NF, NB, AR, AP, AS, FM, and PR-Q obtained and analysed data. PS, MT, and AC took care of patients and obtained data. AL did the statistical analysis. GL, AC, FC, AZ, and LD analysed data and drafted the report. AR, AP, PR-Q, FC, and GL had full access to raw data. GC, AL, AT, and LD had full access to, and verified, all data in the study and had final responsibility for the decision to submit for publication.
Declaration of interests
GC and LD declare consultation honoraria from Sobi, Roche, and Sanofi outside the submitted work. CC declares consultation honoraria from Roche outside the submitted work. GDL declares consultation honoraria from Sobi outside the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campochiaro C, Della-Torre E, Cavalli G, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Della-Torre E, Campochiaro C, Cavalli G, et al. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann Rheum Dis. 2020;79:1277–1285. doi: 10.1136/annrheumdis-2020-218122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermine O, Mariette X, Tharaux P-L, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavalli G, De Luca G, Campochiaro C, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2:e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zangrillo A, Beretta L, Silvani P, et al. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: facing the COVID-19 pandemic emergency. Crit Care Resusc. 2020;22:91–94. doi: 10.51893/2020.2.pov1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340 doi: 10.1136/bmj.b5087. [DOI] [PubMed] [Google Scholar]
- 13.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/nejmoa2021436. published online July 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assel M, Sjoberg DD, Catto JWF, Vickers AJ. Innovations in statistical review at European Urology. Eur Urol. 2019;75:1–2. doi: 10.1016/j.eururo.2018.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williamson EJ, Walker AJ, Bhaskaran K, et al. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larcher A, Capitanio U, De Naeyer G, et al. Is robot-assisted surgery contraindicated in the case of partial nephrectomy for complex tumours or relevant comorbidities? A comparative analysis of morbidity, renal function, and oncologic outcomes. Eur Urol Oncol. 2018;1:61–68. doi: 10.1016/j.euo.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 18.De Luca G, Cavalli G, Campochiaro C, et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet Rheumatol. 2020;2:e465–e473. doi: 10.1016/S2665-9913(20)30170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavalli G, Dinarello CA. Treating rheumatological diseases and co-morbidities with interleukin-1 blocking therapies. Rheumatology. 2015;54:2134–2144. doi: 10.1093/rheumatology/kev269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grom AA, Horne A, De Benedetti F. Macrophage activation syndrome in the era of biologic therapy. Nat Rev Rheumatol. 2016;12:259–268. doi: 10.1038/nrrheum.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaur S, Bansal Y, Kumar R, Bansal G. A panoramic review of IL-6: structure, pathophysiological roles and inhibitors. Bioorg Med Chem. 2020;28 doi: 10.1016/j.bmc.2020.115327. [DOI] [PubMed] [Google Scholar]
- 23.Yip RML, Yim CW. Role of Interleukin 6 inhibitors in the management of rheumatoid arthritis. J Clin Rheumatol. 2019 doi: 10.1097/RHU.0000000000001293. published online Dec 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubbert-Roth A, Furst DE, Nebesky JM, Jin A, Berber E. A review of recent advances using tocilizumab in the treatment of rheumatic diseases. Rheumatol Ther. 2018;5:21–42. doi: 10.1007/s40744-018-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manson JJ, Crooks C, Naja M, et al. COVID-19-associated hyperinflammation and escalation of patient care: a retrospective longitudinal cohort study. Lancet Rheumatol. 2020;2:e594–e602. doi: 10.1016/S2665-9913(20)30275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15:489–493. doi: 10.12788/jhm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eloseily EM, Weiser P, Crayne CB, et al. Benefit of anakinra in treating pediatric secondary hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. 2020;72:326–334. doi: 10.1002/art.41103. [DOI] [PubMed] [Google Scholar]
- 32.Salas M, Hofman A, Stricker BHC. Confounding by indication: an example of variation in the use of epidemiologic terminology. Am J Epidemiol. 1999;149:981–983. doi: 10.1093/oxfordjournals.aje.a009758. [DOI] [PubMed] [Google Scholar]
- 33.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167:492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
After publication, institutional datasets including anonymised data and data dictionary can be accessible to other bona fide investigators upon request to the corresponding author, pending approval of a written proposal by the Institutional Review Board of IRCSS San Raffaele Scientific Institute (Milan, Italy) and according to signed data access agreements between research groups and hosting institutions.


